Introduction
Glomerular podocytes are highly differentiated cells
that play a key role in maintaining the integrity of the glomerular
filtration barrier (1). In
glomerular diseases, podocyte damage leads to increased glomerular
barrier pore size, allowing the passage of proteins or other
mediators to the tubular lumen, which results in proteinuria and
progressive loss of kidney function (2). Accumulating evidence indicates that
hyperglycemia contributes to podocyte lesions (3). Clinical data demonstrate that podocyte
integrity is impaired, and the decreased podocyte number is
confirmed in individuals with type 1 (T1DM) and 2 diabetes mellitus
(T2DM) (4,5). In cultured podocytes, high glucose
induces apoptosis, epithelial-mesenchymal transition (EMT),
mitochondrial fission and autophagy through different signaling
pathways (6–9). However, the underlying molecular
mechanisms of high glucose-induced MPC5 podocytes dysfunction
remain to be fully elucidated.
MicroRNAs (miRs) are endogenous, noncoding, short,
single-stranded RNAs (18–25 nucleotides) that are evolutionarily
highly conserved, believed to regulate the translation of target
messenger RNAs (mRNAs) by binding to its 3′-untranslated regions
(3′-UTRs) and verified to play a role in controlling a variety of
biological processes (10).
Recently, several studies have highlighted the significance of miRs
in maintaining glucose metabolism-associated cell dysfunction
(11–13). For example, miR-130a-3p has been
shown to mediate insulin sensitivity (10), and miR-24-3p, miR-146a-5p,
miR-194-5p, miR-197-3p, miR-301a-3p and miR-375 are correlated with
residual beta cell function in children with new-onset type 1
diabetes mellitus (T1DM) (12).
Moreover, miR-27a, miR-34a, miR-195 and miR-218 promote podocyte
injury (6,14–16), in
contrast to that miR-29a, miR-30a and miR-217 protect against high
glucose-induced podocyte injury (9,17,18).
These findings suggest that the regulation of these miRs may serve
as potential therapeutic targets in high glucose-induced cell
dysfunction.
miR-130a-3p and miR-301a-3p belong to miR-130 and
miR-301 family individually, which are mainly identified in mouse
(19,20). miR-130a has been deemed a
glucose-regulated miR, and miR-130a expression is significantly
down-regulated by high glucose at 16.7 mM in pancreatic islets
compared with control group (21).
Moreover, miR-301a is involved in hyperglycemia-induced cardiac
injury (13). These results suggest
that miR-130a-3p and miR-301a-3p play a critical role in
diabetes-related complications. However, there has been no relevant
report about the post-transcriptional mechanism of
miR-130a-3p/301a-3p in high glucose-induced podocytes dysfunction.
In the present study, utilizing on-line prediction algorithms, we
had identified that miR-130a-3p and miR-301a-3p shared the same
seed site sequence of TNF gene. In vitro study on the MPC5
podocytes was designed to experimentally validate the targeting
relationship between miR-130a-3p/301a-3p and TNF-α in high
glucose-mediated podocytes dysfunction.
Materials and methods
Cell culture
Conditionally immortalized mouse podocytes (MPC5)
were obtained from Dr Peter MUNDEL (Mount Sinai School of Medicine,
New York, USA) and maintained in RPMI-1640 (Invitrogen, Carlsbad,
CA, USA) supplemented with 10% FBS (Invitrogen) at 37°C in a
humidified incubator (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 5% CO2, 95% air atmosphere. All of the experiments
were performed after 48 h incubation with RPMI-1640 medium
containing high glucose (HG, 30 mM D-glucose) or normal glucose
(NG, 5 mM D-glucose). Human embryonic kidney (HEK) 293 T cells
(American Type Culture Collection, ATCC, Manassas, USA) were
incubated in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.) and supplemented with 10% FBS, 100 µg/ml
streptomycin and 100 IU/ml penicillin (all of them from
Sigma-Aldrich, St. Louis, MO, USA). All of the experiments were
repeated with at least three different cell preparations in
triplicate.
ELISA assay
Total superoxide dismutase (SOD; cat. no: S0101) and
malondialdehyde (MDA; cat. no: S0131) were determined using assay
kits (Beyotime Institute of Biotechnology, Haimen, China),
following the manufacturer's protocol.
ROS assay
The generation of ROS in the podocytes was evaluated
using a fluorometry assay via the intracellular oxidation of
dichlorodihydrofluorescein diacetate (DCFH-DA; Sigma-Aldrich). The
cells (4×105) were incubated in a 6-well plate for 48 h,
following DCFH-DA (50 µg/ml) incubation for 30 min at 37, the cells
were harvested and washed with PBS 2 times and finally added into 1
ml PBS, which were detected and analyzed using flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). The fluorescent product
2′,7′-dichlorofluorescein (DCF) was detected at an emission
wavelength of 530 nm and excitation wavelength of 485 nm, and the
result was analyzed using the flow cytometry analysis software BD
CellQuest (v.5.1; Becton Dickinson, San Jose, CA, USA).
Caspase-3 activity assay
Podocytes (1×106) were lysated by NP-40
buffer, and the supernatant was collected for assay. In brief, 20
µl of cell lysate incubated with anti-caspase-3 antibody (cat. no:
sc-7272; dilution, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA,
USA) at 37°C for 1 h. The immunocomplexes were then incubated with
peptide substrate (2 µl of 10 mM
acetyl-Asp-Glu-Val-Asp-p-nitroanilide) in assay buffer (100 mM
Hepes, pH 7.5, 20% v/v glycerol, 5 mM dithiothreitol, and 0.5 mM
EDTA) for 2 h at 37°C. The release of p-nitroaniline was measured
at 405 nm using an ELISA reader (MD SpectraMax M5; Molecular
Devices, LLC, Sunnyvale, CA, USA).
Transfection with miR-130a-3p and
miR-301a-3p
The sequences of the miR-130-3p mimics
(5′-CAGUGCAAUGUUAAAAGGGCAU-3′), mutant miR-130-3p (5′-CAa caa
cAUGUUAAAAGGGCAU-3′), anti-miR-130-3p (antisense inhibitor of
miR-130-3p: 5′-AUGCCCUUUUAACAUUGCACUG-3′), mutant anti-miR-130-3p
(5′-AUGCCCUUUUAACAUgaa caU G-3′), miR-301a-3p mimics
(5′-CAGUGCAAUAGUAUUGUCAAAGC-3′), mutant miR-301a-3p (5′-CAa caa
cAUAGUAUUGUCAAAGC-3′), anti-miR-301a-3p (antisense inhibitor of
miR-301a-3p: 5′-GCUUUGACAAUACUAUUGCACUG-3′) and mutant
anti-miR-301a-3p (5′-GCUUUGACAAUACUAUga aca UG-3′) were
synthesized by RiboBio (Guangzhou, China). The MPC5 podocytes were
transfected using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 50 nM. At 48 h
post-transfection, the cells were harvested for analysis.
Dual-luciferase reporter gene
assay
The potential binding site between
miR-130a-3p/301a-3p and TNF-α was obtained using online predict
software Targetscan (www.targetscan.org) and miRanda (www.microrna.org), and synthesized by RiboBio. The
wild-type (WT) and mutant-type (MUT) 3′-UTR of TNF-α were inserted
into the multiple cloning sites of the luciferase expressing
pMIR-REPORT vector (Ambion; Thermo Fisher Scientific, Inc.). For
the luciferase assay, the 293 T cells (1×105) were
seeded into 24-wells and co-transfected with luciferase reporter
vectors containing the WT and MUT of TNF-α-3′-UTR (0.5 µg) and
mimics, antisense and corresponding mutant sequences of
miR-130a-3p/301a-3p (50 nM) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). The luciferase activity was
measured using a dual luciferase reporter assay kit (cat. no:
RG027; Beyotime Institute of Biotechnology) according to the
manufacturer's protocol.
Small interfering TNF-α (si-TNF-α)
design and transfections
si-TNF-α (AGATTGAGGTGAAATCTTC) was designed using
siDesigner (http://sidirect2.rnai.jp/) and
synthesized by RiboBio. MPC5 podocytes were transfected with
si-TNF-α and the scramble sequence, respectively. Moreover, cells
were treated with TNF-α inhibitor (10 µM, cat. no: SAB4502989;
Sigma-Aldrich), then the levels of ROS, SOD, MDA and caspase-3 and
the protein expression of Bcl-2 and BAX were performed after 48 h
treatment.
RNA analysis and Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA in podocyte was extracted by TRIzol
(Invitrogen) according to the manufacturer's protocol. The cDNA was
synthesized by reverse transcription reactions with 2 µg of total
RNA using moloney murine leukemia virus reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. PCR reaction mixtures were contained 12.5
µl SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), 1 µl cDNA, 300 nM of each primer, and DEPC H2O to
a final volume of 25 µl, and then RT-qPCR was performed using the
Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). The Cq (quantification cycle fluorescence value)
was calculated using SDS software, version 2.1 (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and the relative expression levels
of miRs and mRNA were calculated using the 2−ΔΔCq method
(22) and normalized to the internal
control U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH),
respectively. The primers were synthesized by Sangon Biotech
(Shanghai, China) as following: miR-130a-3p: Forward
5′-GCCCAGTGCAATGTTAAAAGGGCAT-3′ and reverse
5′-CCAGTCTCAGGGTCCGAGGTATTC-3′; miR-301a-3p: Forward
5′-ACACTCCAGCTGGGCAGTGCAATAGTATTGTC-3′ and reverse
5′-CTCAACTGGTGTCGTGGA-3′; U6: Forward 5′-CTCGCTTCGGCAGCACA-3′ and
reverse 5′-AACGCTTCACGAATTTGCGT-3′; TNF-α: Forward
5′-AAGCCCGTAGCCCACGTCGTA-3′ and reverse
5′-GCCCGCAATCCAGGCCACTAC-3′; GAPDH: Forward
5′-GCACCGTCAAGCTGAGAAC-3′ and reverse
5′-TGGTGAAGACGCCAGTGGA-3′.
Western blot analysis
Proteins were extracted with radio
immunoprecipitation assay (RIPA) buffer (cat. no: P0013B; Beyotime
Institute of Biotechnology) with protease inhibitors, and the
concentrations were determined using the Bicinchoninic Acid Kit for
Protein Determination (cat. no: BCA1-1KT; Sigma-Aldrich; Merck
KGaA). 50 µg of protein for each sample was separated on a 10%
SDS-PAGE gel and transferred to nitrocellulose membranes (Bio-Rad
Laboratories, Inc.). After blocking with 5% non-fat dry milk at
room temperature for 2 h, the membrances were incubated with the
primary antibody of TNF-α (cat. no: sc-52746; dilution: 1:1,000),
Bcl-2 (cat. no: sc-509; dilution: 1:1,000), BAX (cat. no: sc-70405;
dilution: 1:1,000) and β-actin (cat. no: sc-130301; dilution:
1:2,000; all of them from Santa Cruz Biotechnology) at room
temperature for 2 h. β-actin signals were used to correct for
unequal loading. Following three washes with TBST, the membranes
were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (cat. no: sc-516102;
dilution: 1:10,000; Santa Cruz Biotechnology) at room temperature
for 2 h and visualized by chemiluminescence (Thermo Fisher
Scientific, Inc.). Signals were analyzed with Quantity
One® software version 4.5 (Bio Rad Laboratories,
Inc.).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) for each group. All statistical analyses were performed using
PRISM v5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Inter-group differences were analyzed by one-way analysis of
variance, followed by a post hoc Tukey test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
HG induced the down-regulation of
miR-130a-3p/301a-3p and the up-regulation of TNF-α in
podocytes
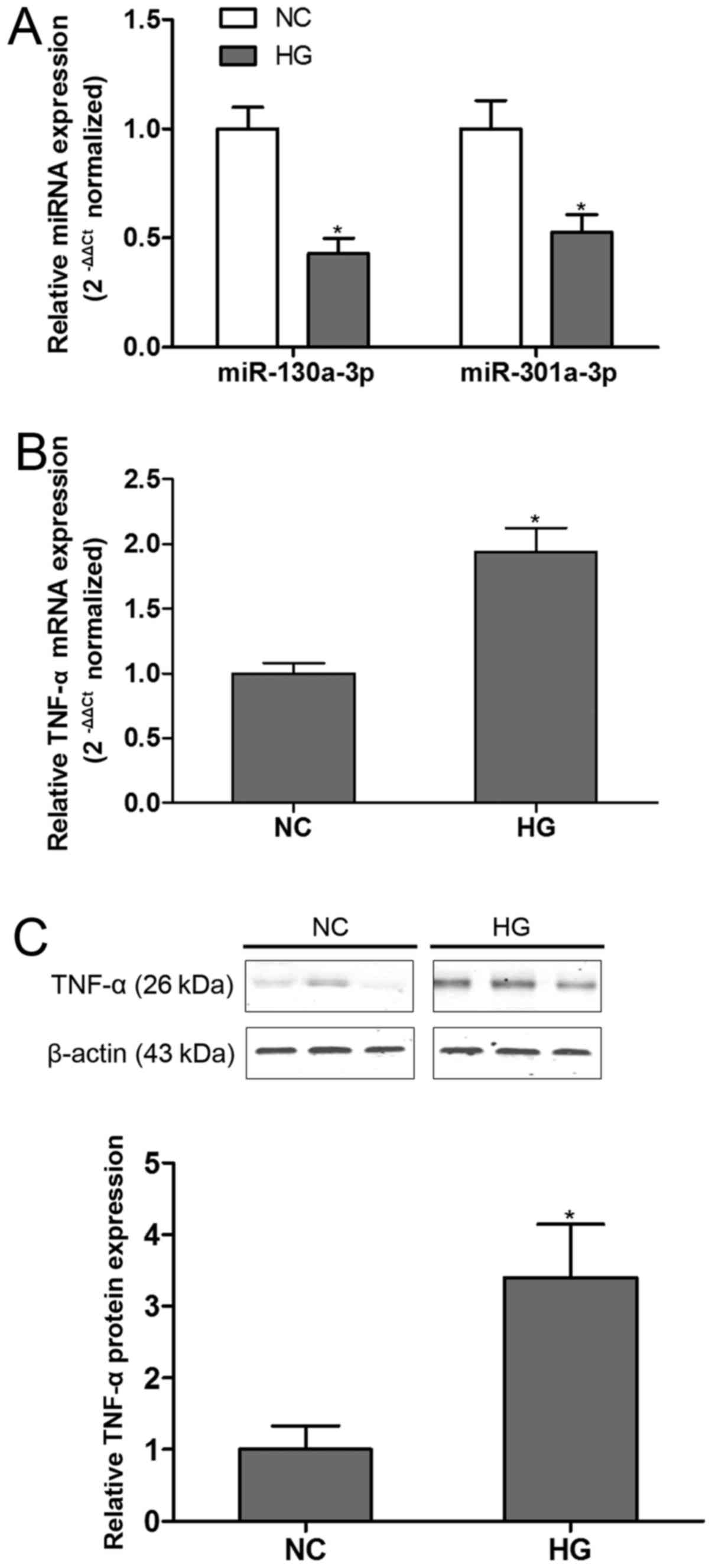
Our preliminary analysis indicated that
miR-130a-3p/301a-3p and TNF-α were closely related to HG-induced
cell dysfunction. Thus, for the first steps we focused our
experiments on the expression of miR-130a-3p/301a-3p and TNF-α in
podocytes exposure to HG. The findings demonstrated that both
miR-130a-3p and miR-301a-3p were significantly down-regulated in
HG-incubated podocytes compared with control group (Fig. 1A). On the other hand, TNF-α was found
robustly up-regualted in podocytes at both mRNA (Fig. 1B) and protein (Fig. 1C) levels in the present of HG. These
results showed the deregulation of miR-130a-3p/301a-3p and TNF-α in
HG condition.
TNF-α was a direct target gene of
miR-130a-3p/301a-3p
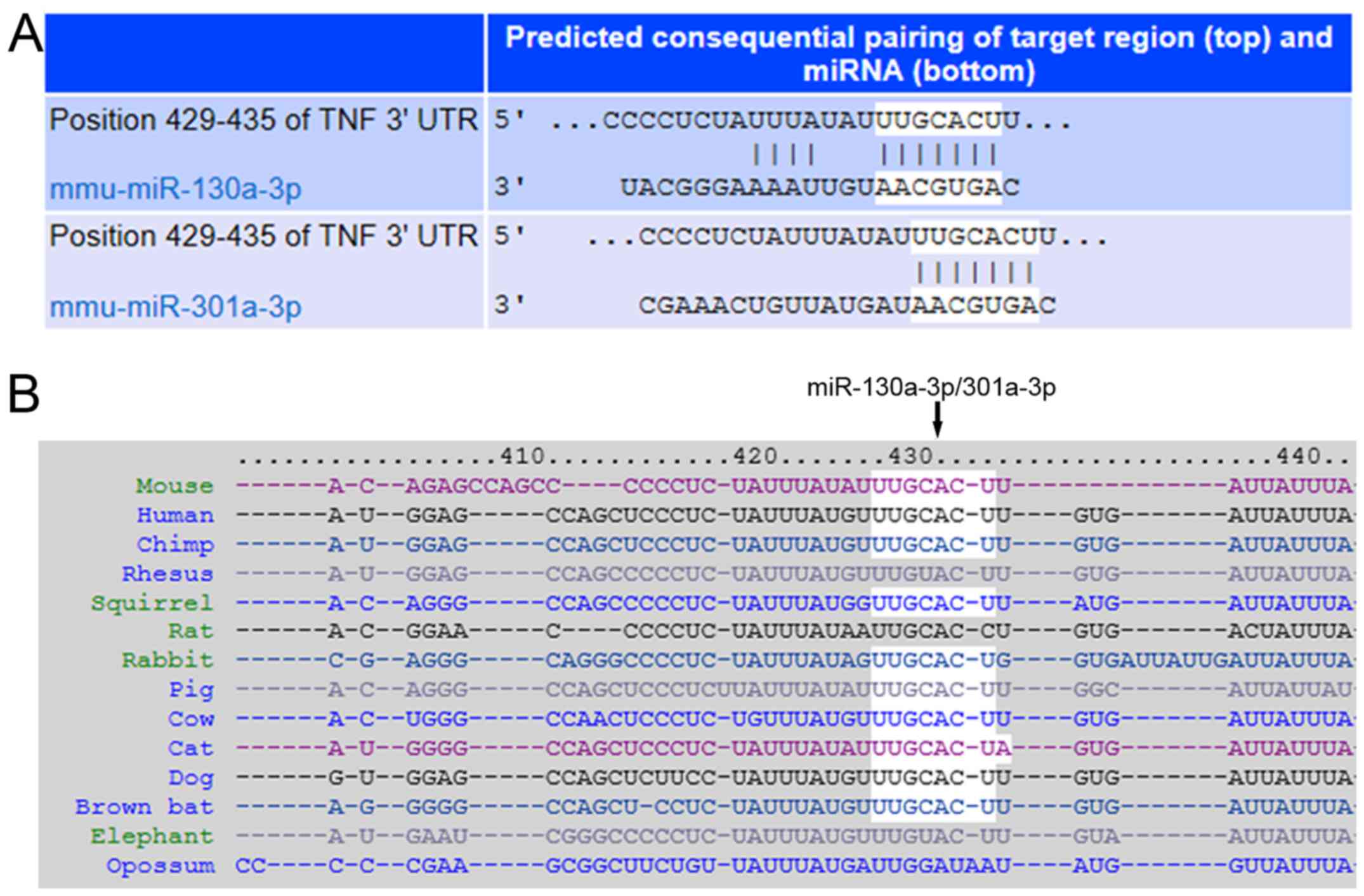
To investigate whether TNF-α was a direct target of
miR-130a-3p/301a-3p, the online predict software Targetscan and
miRanda were used for prediction. The results demonstrated that
TNF-α was a direct target gene for both miR-130a-3p and miR-301a-3p
that share the same 3′-UTR of target gene (Fig. 2A), which is highly conserved across
species (Fig. 2B).
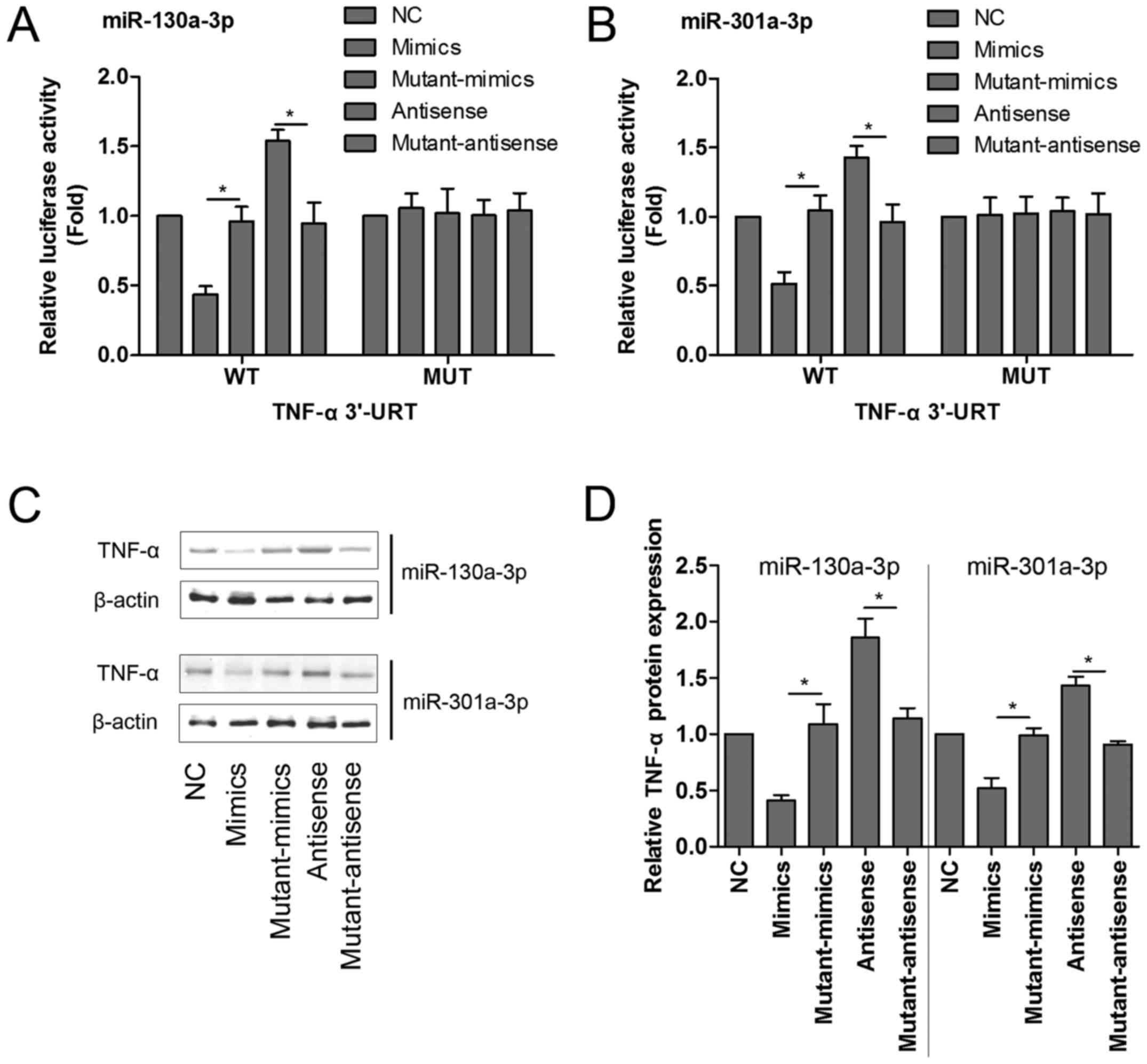
To confirm this, either the WT sequence of TNF-α or
its mutant-type (MUT) sequence was transfected into the
luciferase-reporter plasmids, and then the reporters were
co-transfected with mimics, mutant-mimics, antisense or
mutant-antisense of miR-130a-3p and miR-301a-3p into 293 T cells,
and the levels of luciferase enzyme activity were measured. As
shown in Fig. 3A and B, transfection
with miR-130a-3p or miR-301a-3p mimics significantly diminished the
luciferase enzyme activity, but miR-130a-3p or miR-301a-3p
antisense dramatically enhanced the luciferase activity in the
presence of the WT 3′-UTR of TNF-α compared with corresponding
control group. Mutant-mimics or mutant-antisense of miR-130a-3p and
miR-301a-3p had no obvious affect on the luciferase enzyme
activity.
To further determine whether TNF-α protein
expression was regulated by miR-130a-3p or miR-301a-3p, the MPC5
podocytes were transfected with mimics, mutant-mimics, antisense or
mutant-antisense of miR-130a-3p and miR-301a-3p, respectively, and
the protein quantification assay of TNF-α was performed by western
blotting. Our results showed that miR-130a-3p or miR-301a-3p mimics
significantly reduced the protein expression of TNF-α. On the
contrary, miR-130a-3p or miR-301a-3p antisense markedly increased
TNF-α protein expression compared with corresponding control group
(Fig. 3C and D).
Overexpressed miR-130a-3p/301a-3p
inhibited HG-induced MPC5 podocytes dysfunction
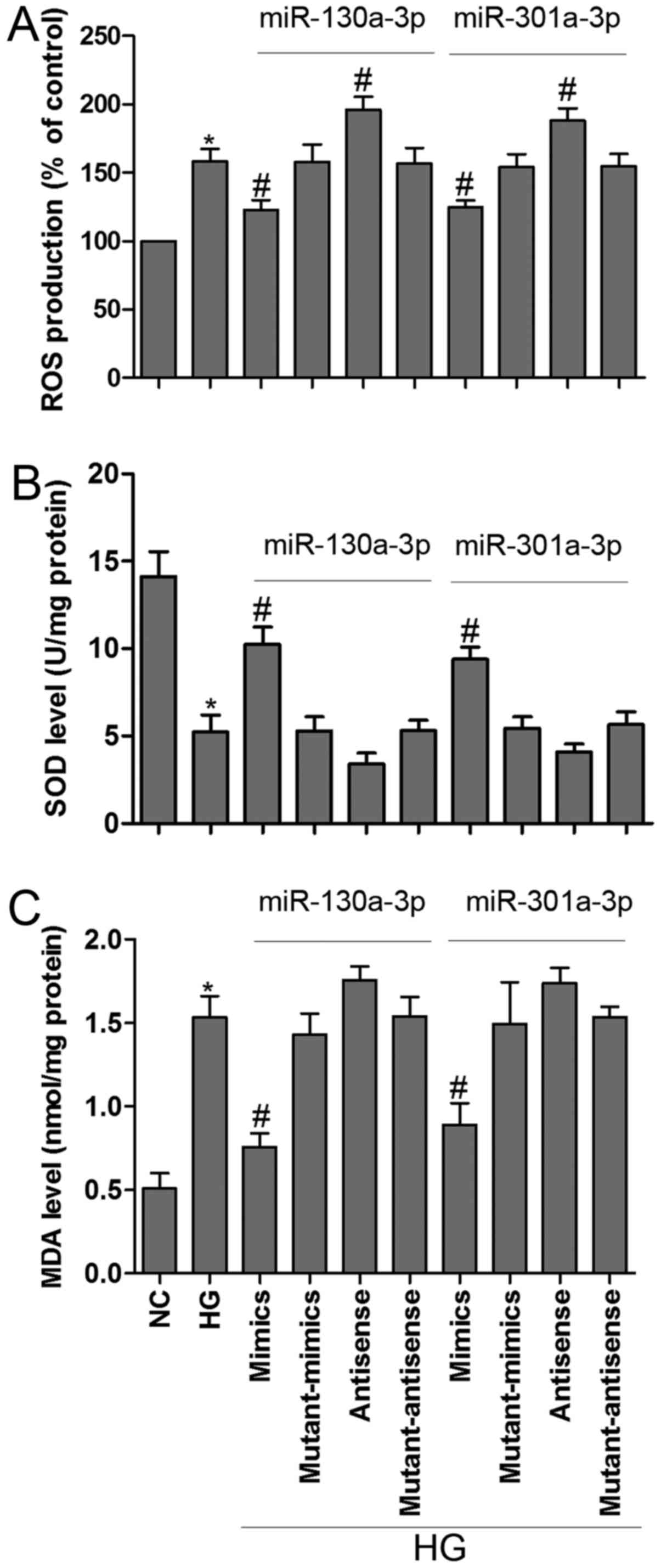
Oxidative stress occurs when ROS affect the balance
between oxidative pressure and antioxidant defense (23). SOD is an enzymatic scavenger of ROS,
which can combat the accumulation of ROS and limit oxidative injury
(24). In the present study, HG
induced ROS generation, these change was partially reversed by
miR-130a-3p or miR-301a-3p mimics transfection in MPC5 podocytes.
Moreover, inhibition of miR-130a-3p or miR-301a-3p could
significantly increased ROS generation as compared to corresponding
control group (Fig. 4A).
Intriguingly, exposure of podocytes to HG resulted in a remarkable
reduction in SOD activity and a dramatic increase in MDA level,
however, transfected with miR-130a-3p or miR-301a-3p mimics
prevented these changes in podocytes (Fig. 4B and C). MDA is a lipid peroxidation
product and is usually considered a reflection of cell injury
(25). The present results showed
that overexpressed miR-130a-3p or miR-301a-3p could ameliorate
HG-induced MPC5 podocytes metabolic disturbance.
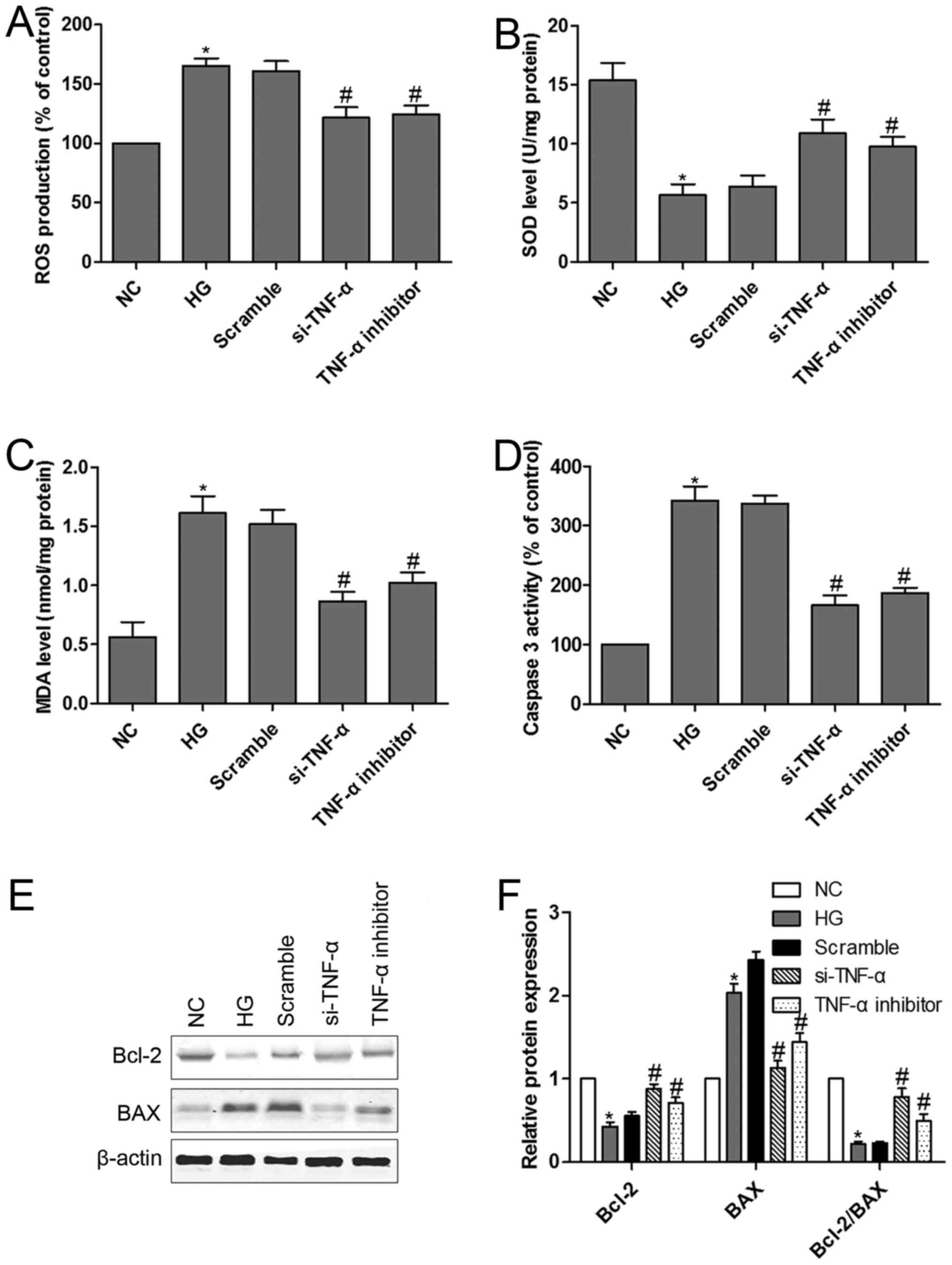
TNF-α loss-of-function alleviated
HG-induced MPC5 podocytes dysfunction and apoptosis
We have shown that downregulation of
miR-130a-3p/−301a-3p is accompanied by upregulation of TNF-α in
HG-treated MPC5 podocytes. TNF-α is a cognate target for
miR-130a-3p and miR-301a-3p. It is conceivable that TNF-α mediates
HG-induced MPC5 podocytes dysfunction. TNF-α si-RNA was designed to
confirm the hypothesis. We had confirmed that podocytes transfected
with si-TNF-α or treated with TNF-α inhibitor could significantly
reverse HG-induced the up-regulation of ROS (Fig. 5A). Moreover, si-TNF-α or TNF-α
inhibitor prevented HG-induced a remarkable reduction in SOD
activity and a dramatic increase in MDA levels in podocytes
(Fig. 5B and C). Furthermore, TNF-α
signaling in HG-induced podocytes apoptosis was investigated. We
examined caspase-3 activity and Bcl-2 and BAX protein expression in
podocytes with or without HG treatment. The results indicated that
HG induced caspase-3 activation, however, podocytes transfected
with si-TNF-α or treated with TNF-α inhibitor significantly
inhibited caspase-3 activity (Fig.
5D). Exposure of podocytes to HG resulted in a significant
decrease in Bcl-2 protein expression and Bcl-2/BAX ratio and a
significant increase in BAX protein expression (Fig. 5E and F). Interestingly, podocytes
transfected with si-TNF-α or treated with TNF-α inhibitor
effectively reversed HG-induced the down-regulation of Bcl-2
protein expression and Bcl-2/BAX ratio and the up-regulation of BAX
protein expression.
Discussion
This study demonstrated that miR-130a-3p/301a-3p was
a novel regulator of TNF-α in mouse podocytes. Results of our
bioinformatic analysis and luciferase reporter assay showed that
miR-130a-3p/301a-3p directly interacted with the 3′-UTR of TNF-α.
In MPC5 podocytes, the miR-130a-3p or miR-301a-3p mimic decreased
the TNF-α protein expression, which was rescued by the miR-130a-3p
or miR-301a-3p inhibitor. These results provide strong evidence
that TNF-α was a direct target gene of miR-130a-3p/301a-3p. Here we
showed forced expression of miR-130a-3p or miR-301a-3p via
transfection of its mimic in cultured podocytes resulted in the
down-regulation of ROS and MDA and the up-regulation of SOD in the
present of HG. Moreover, inhibition of TNF-α level also prevented a
remarkable reduction in SOD activity and a dramatic increase in ROS
and MDA levels in HG-treated podocytes. The combined results above
strongly suggested that overexpressed miR-130a-3p/301a-3p could
significantly ameliorate HG-induced MPC5 podocytes dysfunction, and
the underlying mechanism was mediated, at least partially, through
the suppression of TNF-α expression.
In our study, we found that miR-130a-3p and
miR-301a-3p shared the identical seed site of TNF-α. However, there
has been no relevant report about the functional similarity between
miR-130a-3p and miR-301a-3p in mammals. Our findings indicated that
miR-130a-3p and miR-301a-3p had played a similar role in HG-induced
MPC5 podocytes dysfunction. A recent study shows that miR-130a-3p
is aberrantly reduced in livers of a mouse model with nonalcoholic
fibrosing steatohepatitis, and the overexpression of miR-130a-3p
inhibits hepatic stellate cells (HSC) activation and proliferation
and promotes HSC apoptosis (26).
Moreover, miR-130a-3p has been proven regulation of liver
steatosis, glucose homeostasis and insulin sensitivity (10,21).
miR-301a-3p, as an oncogene, has been reported to be upregulated in
several malignant tumors (27,28).
However, relatively little is known about the role of miR-301a-3p
in HG-induced metabolic disorders. In our study, miR-130a-3p and
miR-301a-3p collectively target to TNF-α inhibition of HG-induced
podocytes dysfunction.
Inflammatory reaction is tightly regulated by
altering TNF-α (29). Numerous
studies have shown that TNF-α is implicated in the modulation of
HG-induced cell dysfunction, including uterine epithelial cells,
vascular smooth muscle cells (VSMCs) and primary human monocytes
(30–32). We also found that TNF-α was
upregulated in podocytes in the presence of HG, however, TNF-α
loss-of-function blocked HG-enhanced ROS and oxidative stress in
vitro. Previous study has shown that podocytes may play a
contributory role in the development of interstitial damage in IgA
nephropathy by amplifying the activation of tubular epithelial
cells with enhanced TNF-α synthesis after inflammatory changes of
human mesangial cell (1). Serum
levels of soluble TNF-α correlate with renal injury, and the
possible mechanism shows that soluble TNF-α activates NF-κB and
dose-dependently induces podocyte proliferation (33). Consistent with these reports, we also
observed the correlation between the up-regulation of TNF-α
expression and podocyte injury.
Numerous studies have shown that TNF-α is a
potential therapeutic target for chronic kidney disease (CKD) and
glomerulonephritis (34,35). Our data confirmed that TNF-α was a
target gene of miR-130a-3p and miR-301a-3p. We also observed an
inhibitory effect of si-TNF-α on HG-induced oxidative stress and
apoposis in podocytes, which could increase intrarenal
renin-angiotensin system (RAS) activity and reduce nitric oxide
(NO) availability to trigger glomerular disease (36). Because TNF-α is involved in
HG-induced podocytes dysfunction, our results suggest that
miR-130a-3p or miR-301a-3p is likely to be a novel biomarker or new
potent therapeutic target for podocytes injury-related renal
disease.
Taken together, the data obtained in our study
support the conclusion that miR-130a-3p/301a-3p targeting to TNF-α
inhibits HG-induced oxidative stress and apoposis in podocytes, and
suggest that a novel function for miR-130a-3p/301a-3p in podocytes
injury-related renal disease. Our results are also important in
providing new perspective for the understanding of the underlying
molecular mechanisms in podocytes dysfunction and potential
therapeutic target for glomerulonephritis.
Acknowledgements
This work was supported by the United Fund of the
Department of Science and Technology in Guizhou Province of China
(Grant no: LH-2016-7395).
References
|
1
|
Lai KN, Leung JC, Chan LY, Saleem MA,
Mathieson PW, Lai FM and Tang SC: Activation of podocytes by
mesangial-derived TNF-alpha: Glomerulo-podocytic communication in
IgA nephropathy. Am J Physiol Renal Physiol. 294:F945–F955. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Amico G and Bazzi C: Pathophysiology of
proteinuria. Kidney Int. 63:809–825. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang G, Zou B, Lv J, Li T, Huai G, Xiang
S, Lu S, Luo H, Zhang Y, Jin Y and Wang Y: Notoginsenoside R1
attenuates glucose-induced podocyte injury via the inhibition of
apoptosis and the activation of autophagy through the PI3K/Akt/mTOR
signaling pathway. Int J Mol Med. 39:559–568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verzola D, Gandolfo MT, Ferrario F,
Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L,
Lauria F, Miji M, et al: Apoptosis in the kidneys of patients with
type II diabetic nephropathy. Kidney Int. 72:1262–1272. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
White KE, Bilous RW, Marshall SM, El Nahas
M, Remuzzi G, Piras G, De Cosmo S and Viberti G: Podocyte number in
normotensive type 1 diabetic patients with albuminuria. Diabetes.
51:3083–3089. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang H, Wang Q and Li S: MicroRNA-218
promotes high glucose-induced apoptosis in podocytes by targeting
heme oxygenase-1. Biochem Biophys Res Commun. 471:582–588. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai H, Zhang Y, Yuan L, Wu J, Ma L and Shi
H: CTGF mediates high-glucose induced epithelial-mesenchymal
transition through activation of β-catenin in podocytes. Ren Fail.
38:1711–1716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin Y, Liu S, Ma Q, Xiao D and Chen L:
Berberine enhances the AMPK activation and autophagy and mitigates
high glucose-induced apoptosis of mouse podocytes. Eur J Pharmacol.
794:106–114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma L, Han C, Peng T, Li N, Zhang B, Zhen X
and Yang X: Ang-(1–7) inhibited mitochondrial fission in
high-glucose-induced podocytes by upregulation of miR-30a and
downregulation of Drp1 and p53. J Chin Med Assoc. 79:597–604. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao F, Yu J, Liu B, Guo Y, Li K, Deng J,
Zhang J, Wang C, Chen S, Du Y, et al: A novel function of microRNA
130a-3p in hepatic insulin sensitivity and liver steatosis.
Diabetes. 63:2631–2642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ofori JK, Salunkhe VA, Bagge A, Vishnu N,
Nagao M, Mulder H, Wollheim CB, Eliasson L and Esguerra JL:
Elevated miR-130a/miR130b/miR-152 expression reduces intracellular
ATP levels in the pancreatic beta cell. Sci Rep. 7:449862017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samandari N, Mirza AH, Nielsen LB, Kaur S,
Hougaard P, Fredheim S, Mortensen HB and Pociot F: Circulating
microRNA levels predict residual beta cell function and glycaemic
control in children with type 1 diabetes mellitus. Diabetologia.
60:354–363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panguluri SK, Tur J, Chapalamadugu KC,
Katnik C, Cuevas J and Tipparaju SM: MicroRNA-301a mediated
regulation of Kv4.2 in diabetes: Identification of key modulators.
PLoS One. 8:e605452013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Wan J, Hou X, Geng J, Li X and Bai
X: MicroRNA-27a promotes podocyte injury via PPARγ-mediated
β-catenin activation in diabetic nephropathy. Cell Death Dis.
8:e26582017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Song S and Luo H: Regulation of
podocyte lesions in diabetic nephropathy via miR-34a in the Notch
signaling pathway. Medicine. 95:e50502016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YQ, Wang XX, Yao XM, Zhang DL, Yang
XF, Tian SF and Wang NS: MicroRNA-195 promotes apoptosis in mouse
podocytes via enhanced caspase activity driven by BCL2
insufficiency. Am J Nephrol. 34:549–559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun J, Li ZP, Zhang RQ and Zhang HM:
Repression of miR-217 protects against high glucose-induced
podocyte injury and insulin resistance by restoring PTEN-mediated
autophagy pathway. Biochem Biophys Res Commun. 483:318–324. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin CL, Lee PH, Hsu YC, Ko JY, Chuang PC,
Huang YT, Wang SY, Wu SL, Chen YS, Chiang WC, et al: MicroRNA-29a
promotion of nephrin acetylation ameliorates hyperglycemia-induced
podocyte dysfunction. J Am Soc Nephrol. 25:1698–1709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosako H, Martin GS, Barrier M, Chen YA,
Ivanov IV and Mirkes PE: Gene and microRNA expression in
p53-deficient day 8.5 mouse embryos. Birth Defects Res A Clin Mol
Teratol. 85:546–555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Esguerra JL, Bolmeson C, Cilio CM and
Eliasson L: Differential glucose-regulation of microRNAs in
pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki
rat. PLoS One. 6:e186132011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun LN, Liu XC, Chen XJ, Guan GJ and Liu
G: Curcumin attenuates high glucose-induced podocyte apoptosis by
regulating functional connections between caveolin-1
phosphorylation and ROS. Acta Pharmacol Sin. 37:645–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun WH, Liu F, Chen Y and Zhu YC: Hydrogen
sulfide decreases the levels of ROS by inhibiting mitochondrial
complex IV and increasing SOD activities in cardiomyocytes under
ischemia/reperfusion. Biochem Biophys Res Commun. 421:164–169.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rahman MM, Muse AY, Khan DMIO, Ahmed IH,
Subhan N, Reza HM, Alam MA, Nahar L and Sarker SD: Apocynin
prevented inflammation and oxidative stress in carbon tetra
chloride induced hepatic dysfunction in rats. Biomed Pharmacother.
92:421–428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Du J, Niu X, Fu N, Wang R, Zhang
Y, Zhao S, Sun D and Nan Y: MiR-130a-3p attenuates activation and
induces apoptosis of hepatic stellate cells in nonalcoholic
fibrosing steatohepatitis by directly targeting TGFBR1 and TGFBR2.
Cell Death Dis. 8:e27922017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu Y, Gao W, Zhang C, Wen S, Huangfu H,
Kang J and Wang B: Hsa-miR-301a-3p acts as an oncogene in laryngeal
squamous cell carcinoma via target regulation of Smad4. J Cancer.
6:1260–1275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia X, Zhang K, Cen G, Jiang T, Cao J,
Huang K, Huang C, Zhao Q and Qiu Z: MicroRNA-301a-3p promotes
pancreatic cancer progression via negative regulation of SMAD4.
Oncotarget. 6:21046–21063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
West NR, Hegazy AN, Owens BMJ, Bullers SJ,
Linggi B, Buonocore S, Coccia M, Görtz D, This S, Stockenhuber K,
et al: Oncostatin M drives intestinal inflammation and predicts
response to tumor necrosis factor-neutralizing therapy in patients
with inflammatory bowel disease. Nat Med. 23:579–589. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pampfer S, Cordi S, Dutrieux C,
Vanderheyden I, Marchand C and De Hertogh R: Interleukin 1beta
mediates the effect of high D-glucose on the secretion of TNF-alpha
by mouse uterine epithelial cells. Cytokine. 11:500–509. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reddy AB, Ramana KV, Srivastava S,
Bhatnagar A and Srivastava SK: Aldose reductase regulates high
glucose-induced ectodomain shedding of tumor necrosis factor
(TNF)-alpha via protein kinase C-delta and TNF-alpha converting
enzyme in vascular smooth muscle cells. Endocrinology. 150:63–74.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonzalez Y, Herrera MT, Soldevila G,
Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K,
Guzmán-Beltrán S, Sada E and Torres M: High glucose concentrations
induce TNF-α production through the down-regulation of CD33 in
primary human monocytes. BMC Immunol. 13:192012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bruggeman LA, Drawz PE, Kahoud N, Lin K,
Barisoni L and Nelson PJ: TNFR2 interposes the proliferative and
NF-κB-mediated inflammatory response by podocytes to TNF-α. Lab
Invest. 91:413–425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khan SB, Cook HT, Bhangal G, Smith J, Tam
FW and Pusey CD: Antibody blockade of TNF-alpha reduces
inflammation and scarring in experimental crescentic
glomerulonephritis. Kidney Int. 67:1812–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Basturk T, Unsal A, Ulas T, Koc Y, Sakaci
T, Ahbap E and Borlu F: Effects of rosiglitazone treatment on
insulin resistance and TNF-alpha levels in patients with chronic
kidney disease: A prospective study. Eur Rev Med Pharmacol Sci.
16:1519–1524. 2012.PubMed/NCBI
|
|
36
|
Camici M, Carpi A, Cini G, Galetta F and
Abraham N: Podocyte dysfunction in aging-related
glomerulosclerosis. Front Biosci (Schol Ed). 3:995–1006. 2011.
View Article : Google Scholar : PubMed/NCBI
|