Introduction
Venous thromboembolism (VTE), which comprises
deep-vein thrombosis (DVT) or pulmonary embolism, is a common
disorder with a global annual incidence of 1–2 cases per 1,000
people (1,2). Studies of hospitalized and ambulatory
patients have demonstrated that patients with nephrotic syndrome
(NS) have an increased risk of 3–50% of thrombotic events (3–7).
Decreased antithrombin (AT)-III is one of the factors contributing
to hypercoagulability in patients with NS (3). The risk of VTE in patients with NS and
low AT-III is 3 to 7 times higher compared with the general
population (8). Standard treatment
for VTE is an initial course of heparin, followed by a vitamin K
antagonist (VKA) (9). This therapy
is effective but requires laboratory monitoring and dose
adjustments, which was inconvenient to outpatients. Meanwhile, the
anticoagulation of effect of heparin is dependent on adequate
circulating AT-III levels (10).
AT-III production and consumption constitute a dynamic process. In
the presence of reduced circulating levels of AT-III,
heparin-mediated thrombin inhibition is deficient, leading to
decreased heparin sensitivity (11).
This effect is called heparin resistance (HR) or heparin
tachyphylaxis (10,11). Reduced AT-III levels due to loss in
urine protein are common in patients with NS, with a reported
occurrence rate of 86.4% (12);
thus, the anticoagulation efficacy of heparin in these patients is
questionable. Rivaroxaban, an oral factor Xa inhibitor, may provide
a simple, fixed-dose regimen for treating DVT without laboratory
monitoring (13–17). Furthermore, it functions independent
of AT-III and may therefore be superior to heparin as a treatment
for patients with low AT-III levels. A previous study of
AT-III-deficient mice revealed that the clotting time prolongation
and antithrombotic effects of oral factor Xa inhibitors were not
affected by AT-III levels, whereas those of AT-dependent
anticoagulants (including fondaparinux, enoxaparin, and
unfractionated heparin) were attenuated (18). The aim of the present study was to
assess the efficacy and safety of rivaroxaban as a treatment for
VTE in patients with NS and low AT-III concentration and functional
activity as compared with low molecular weight heparin (LMWH)
treatment.
Materials and methods
Patients
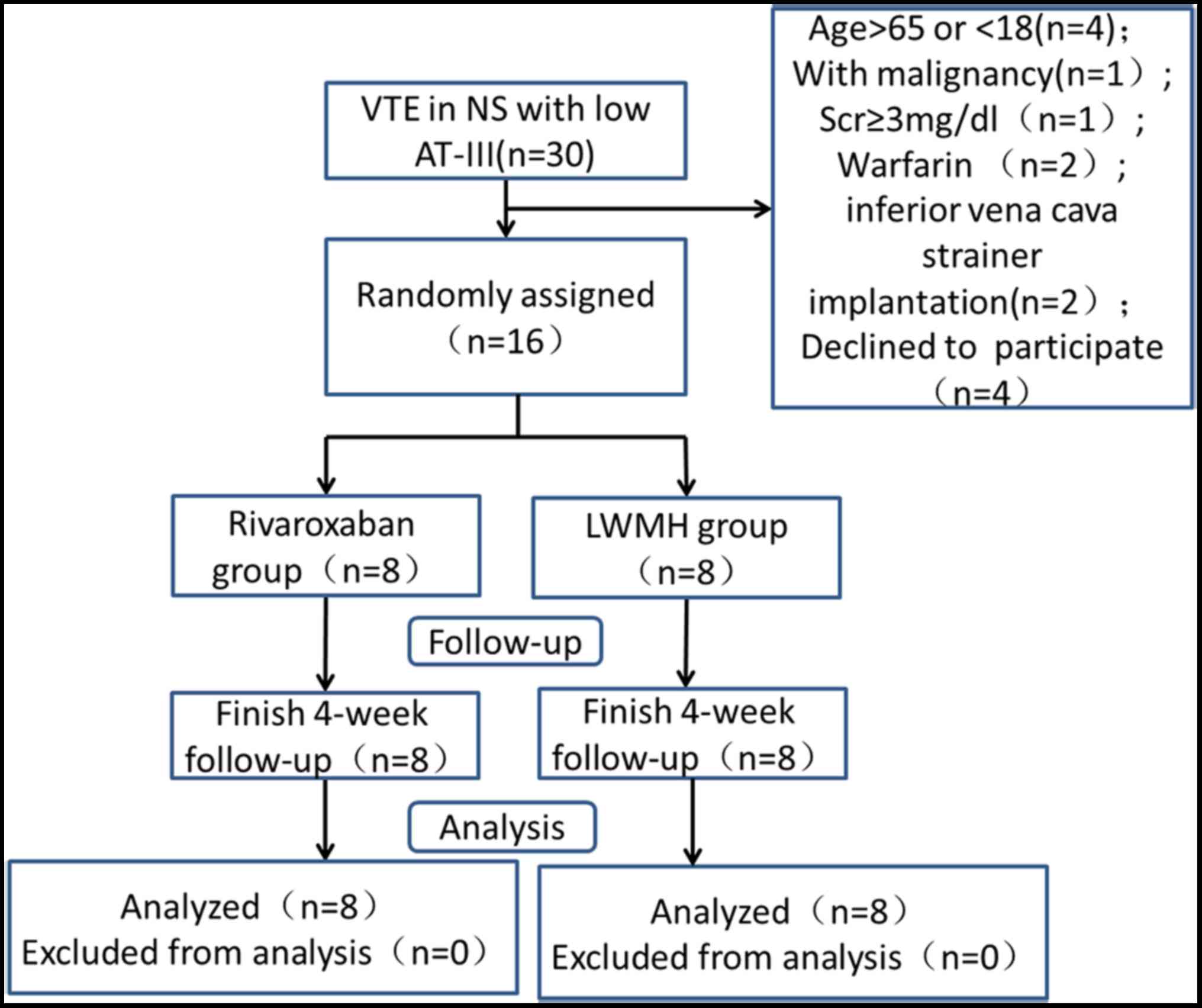
Between May 2010 and October 2015, a total of 16
patients were enrolled in the present study; each group had 8
patients. The patients enrolled in the present study fulfilled the
following inclusion criteria: Aged 18–65 year; diagnosed with NS;
proteinuria (>3.5 g/24 h); serum albumin (<25 g/l); serum
AT-III concentration <20 mg/dl and functional activity <70%;
and a diagnosis of DVT or PE using complete compression ultrasound
renal vein or pulmonary artery CT angiography. All patients provide
prior informed consent. The primary exclusion criteria were as
follows: Scr ≥3 mg/dl; requirement for thrombolytic therapy or
inferior vena cava strainer implantation; risk of cerebral
ischemia; intracerebral bleeding or gastrointestinal bleeding
within the previous 6 months; surgery within the previous 4 weeks;
an active peptic ulcer; a known bleeding disorder; prolonged
international normalized ratio or activated partial thromboplastin
time; platelet count <100×109 cells/l; treatment with
a VKA, unfractionated heparin, LMWH, antiplatelet agents, or potent
CYP3A4 inhibitors; impaired liver function (transaminase >2-fold
above the normal range); poor compliance; and simultaneous
enrollment in other clinical trials.
Study design and treatment
The present pilot study is a prospective,
active-controlled, open-label, randomized, clinical trial. The
present study was approved by the Ethics Committee of Jinling
Hospital (Nanjing, China) and adhered to the Declaration of
Helsinki and the principles outlined in the ‘Guidelines for Good
Clinical Practice’ at the International Conference on Harmonization
Tripartite Guideline (January 1997) (19).
Eligible patients were randomly assigned to the
rivaroxaban or LMWH groups. The patients in the rivaroxaban group
received 30 mg/day rivaroxaban (Bayer HealthCare Pharmaceuticals
LLC, Berlin, Germany) orally and patients in the LMWH group
received dalteparin (Pfizer, Inc., New York, NY, USA) 5000 U twice
daily via subcutaneous injection. Treatment was discontinued in the
two groups at the 2-week follow up if the thrombus had disappeared
and the patients achieved clinical remission.
Outcomes and follow-up
The primary endpoint was dissolution or a >90%
decrease in thrombus volume in 4 weeks. Secondary endpoints
included recurrent VTE, succumbing to thrombosis, volume increase
of existing thrombosis. Furthermore, changes of serum AT-III level
and safety of anticoagulation treatment were assessed. The
principal safety concern was clinically relevant bleeding, defined
as a composite of major or clinically relevant non-major
bleeding.
Patients were evaluated at the baseline and at weeks
2 and 4. Blood and urine samples were collected for laboratory
analysis. Blood coagulation using the CoaguChek XS system (Roche
Diagnostics GmbH, Mannheim Germany), AT-III concentration
(ACLTOP700; WerfenLife, Barcelona, Spain) and functional activity
using the Berichrom® Antithrombin III kit (Siemens
Healthineers, Erlangen, Germany), urine protein using biuret
colorimetry and clinical chemistry using a 7600 series automatic
analyzer (Hitachi, Ltd., Tokyo, Japan) were assessed. Serum AT-III
was tested using immune rate turbidimetry and AT-III functional
activity was tested via automated chromogenic assay as previously
described (20,21).
Complete compression ultrasound, renal vein and
pulmonary artery computed tomography (CT) angiography examinations
were performed at the baseline and during each visit. The size of
the thrombus was measured using Volume Software of Syngo MMWP
(VE32B) workstation of 64-slice dual source CT scanner (Siemens AG,
Munich, Germany) and the World Health Organization standard for
measuring gross tumor volume was used (22).
Definitions
Low serum AT-III level refers to an AT-III
concentration <20 mg/dl (23).
AT-III deficiency refers to an AT-III functional activity <70%
(24). Major bleeding is defined as
fatal bleeding involving a critical organ or requiring reoperation,
or clinically overt bleeding outside the surgical site associated
with a decrease in hemoglobin to ≥2 g/dl or requiring an infusion
of ≥2 units of blood. The efficacy of NS was definite complete
remission (CR) (proteinuria <0.4 g/24 h and serum albumin >35
g/l with normal SCr), partial remission (PR; ≥50% reduction in
proteinuria and urine protein <3.5 g/24 h, with normal or ≤25%
increase in SCr level from baseline) and no response (NR; not
reaching CR or PR definition).
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Normally distributed variables (serum albumin,
24 h urinary protein, AT-III concentration, AT-III activity, Tchol,
triglyceride, Scr, estimated glomerular filtration rate,
prothrombin time and activated partial thromboplastin time) were
expressed as the mean ± standard deviation and analyzed using the
Student's t-test. Non-parametric variables (age and time of
thrombotic events to NS) were expressed as the median values
(interquartile range) and compared using the Mann-Whitney U test.
Categorical variables were compared using Pearson χ2
test or Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Study patients
A flow diagram of the study is presented in Fig. 1. There was no family history of
thrombosis and all patients suffered from acute VTEs. No
significant differences were observed in the baseline
characteristics of the groups (Table
I).
 | Table I.Baseline clinical characteristics of
patients in both groups. |
Table I.
Baseline clinical characteristics of
patients in both groups.
| Characteristic | Rivaroxaban group
(n=8) | LWMH group (n=8) | P-value |
|---|
| Sex (M/F) | 6/2 | 7/1 | 0.5 |
| Age (years) | 35.0 (22.3,
47.5) | 21.0 (20.0,
43.8) | 0.579 |
| Definite causes of
renal disease |
|
|
|
| MCD | 5 | 2 |
|
| MN | 2 | 2 |
|
| FSGS | 0 | 2 |
|
| LN | 0 | 1 |
|
|
Unknown | 1 | 1 |
|
| Treatment for renal
disease |
|
|
|
| Pred | 4 | 4 |
|
|
Pred+CTX | 2 | 2 |
|
|
Pred+TW | 2 | 1 |
|
|
Pred+CsA | 0 | 1 |
|
| Time of thrombotic
events to NS onset (m) | 0.58 (0.25,
1.18) | 0.50 (0.31,
2.38) | 0.402 |
| Serum albumin
(g/l) | 18.76±1.90 |
20.07±3.56 | 0.374 |
| 24 h urinary protein
(g/24 h) | 13.36±5.9 |
8.62±3.13 | 0.065 |
| AT-III concentration
(mg/dl) |
14.6±4.8 |
16.7±3.6 | 0.337 |
| AT-III functional
Activity (%) |
43.6±21.5 |
48.6±17.7 | 0.408 |
| Tchol (mmol/l) |
11.53±2.18 | 12.37±1.7 | 0.978 |
| TG (mmol/l) |
3.45±1.98 |
3.47±1.37 | 0.507 |
| SCr (mg/dl) |
1.066±0.47 |
0.95±0.15 | 0.598 |
| eGFR [ml/(min. 1.73
m2)] |
99.02±31.45 |
106.64±24.65 | 0.863 |
| PT (sec) |
11.05±1.13 |
11.15±1.14 | 0.627 |
| APTT (sec) |
35.5±5.6 |
33.9±4.4 | 0.521 |
| Fibrin (mg/dl) |
481±49 |
532±121 | 0.288 |
Distribution of thrombosis
Thrombosis distribution is presented in Table II. Thrombus occurred in 3 sites for
3 patients, 2 sites for 1 patient and 1 site for 4 patients in the
rivaroxaban group. In the LWMH group, thrombus presented in 3 sites
for 1 patient, 2 sites for 3 patients, and 1 site for 4 patients. A
total of 5 patients in the rivaroxaban group and 6 patients in the
LWMH group had DVT.
 | Table II.Distribution of thrombosis in the
rivaroxaban and LWMH groups. |
Table II.
Distribution of thrombosis in the
rivaroxaban and LWMH groups.
| Position of
thrombus | Rivaroxaban group
(n=8) | LWMH group (n=8) |
|---|
| Renal vein | 4 | 6 |
| Bilateral renal
vein | 1 | 0 |
| Left renal vein | 2 | 4 |
| Right renal vein | 1 | 2 |
| Inferior cava
vein | 3 | 1 |
| Pulmonary artery | 8 | 6 |
Primary endpoint
The primary endpoint occurred in 7 patients from the
rivaroxaban group and 7 from the LWMH group at week 4. At week 2, 5
patients in the rivaroxaban group and 4 patients in the LWMH group
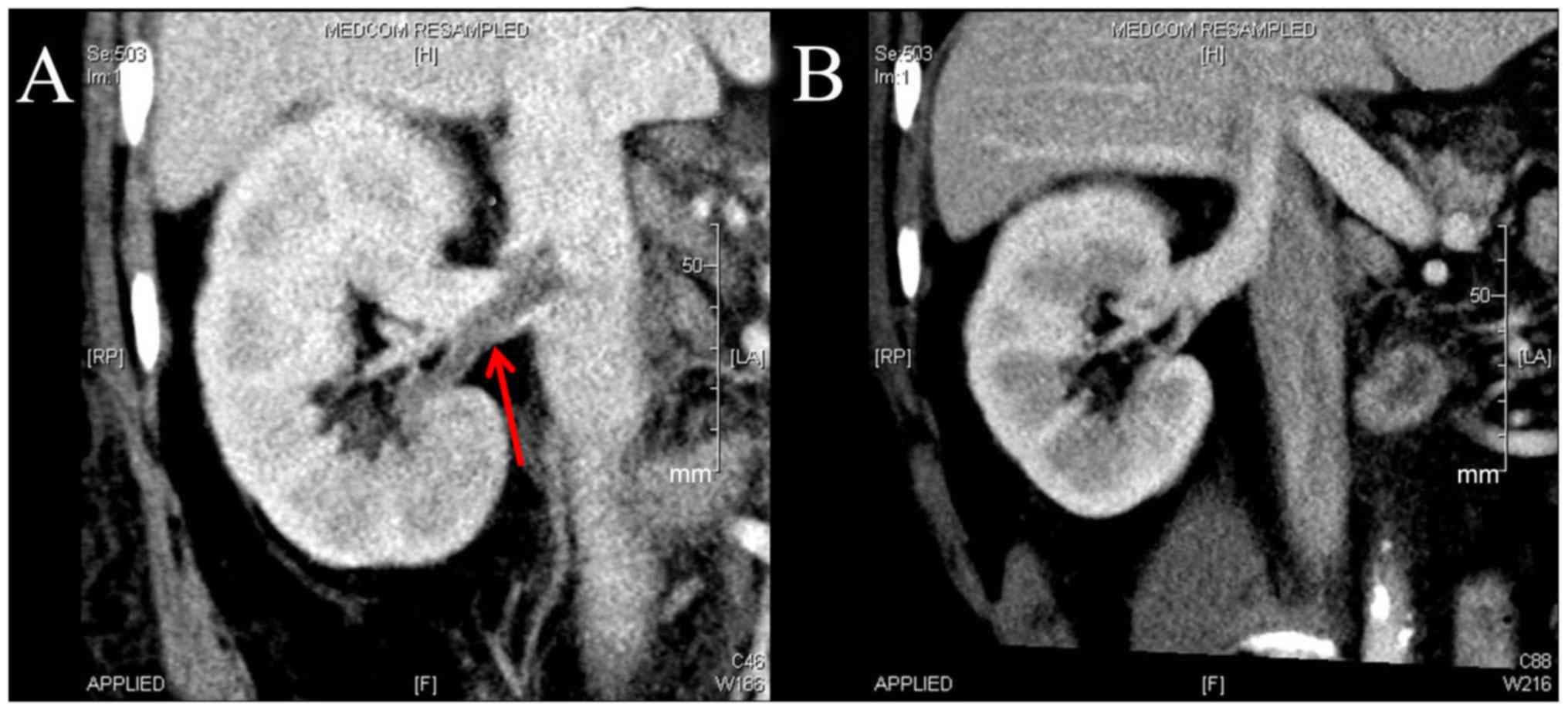
achieved the primary endpoint. Of the patients with DVT, 4 in the
rivaroxaban group and 4 in the LWMH group achieved the primary
endpoint at week 4 (Fig. 2). A total
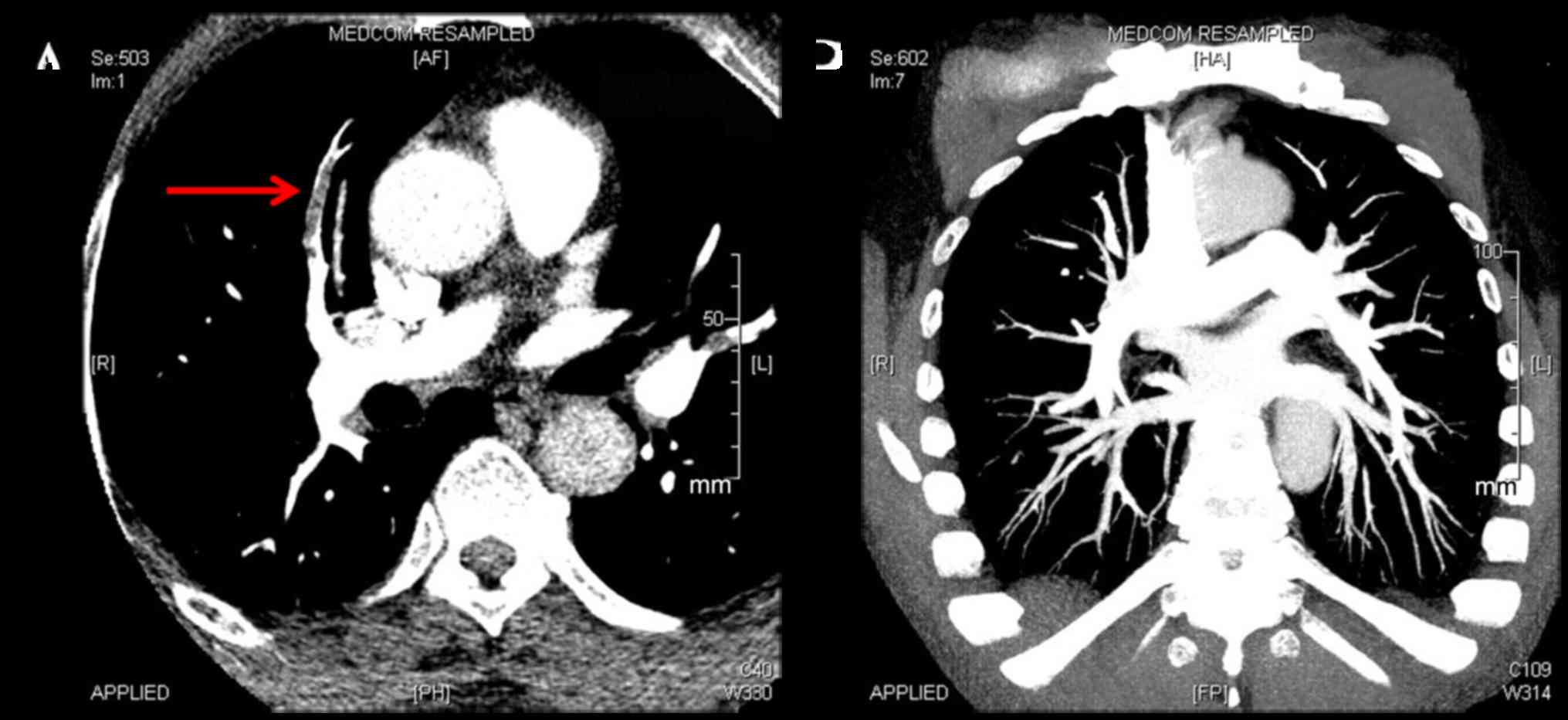
of 8 patients with PE in the rivaroxaban group achieved the primary
endpoint at week 4, whereas 5 of 6 in the LWMH group achieved the
primary endpoint (Fig. 3).
Serum AT-III changes and efficacy of
NS
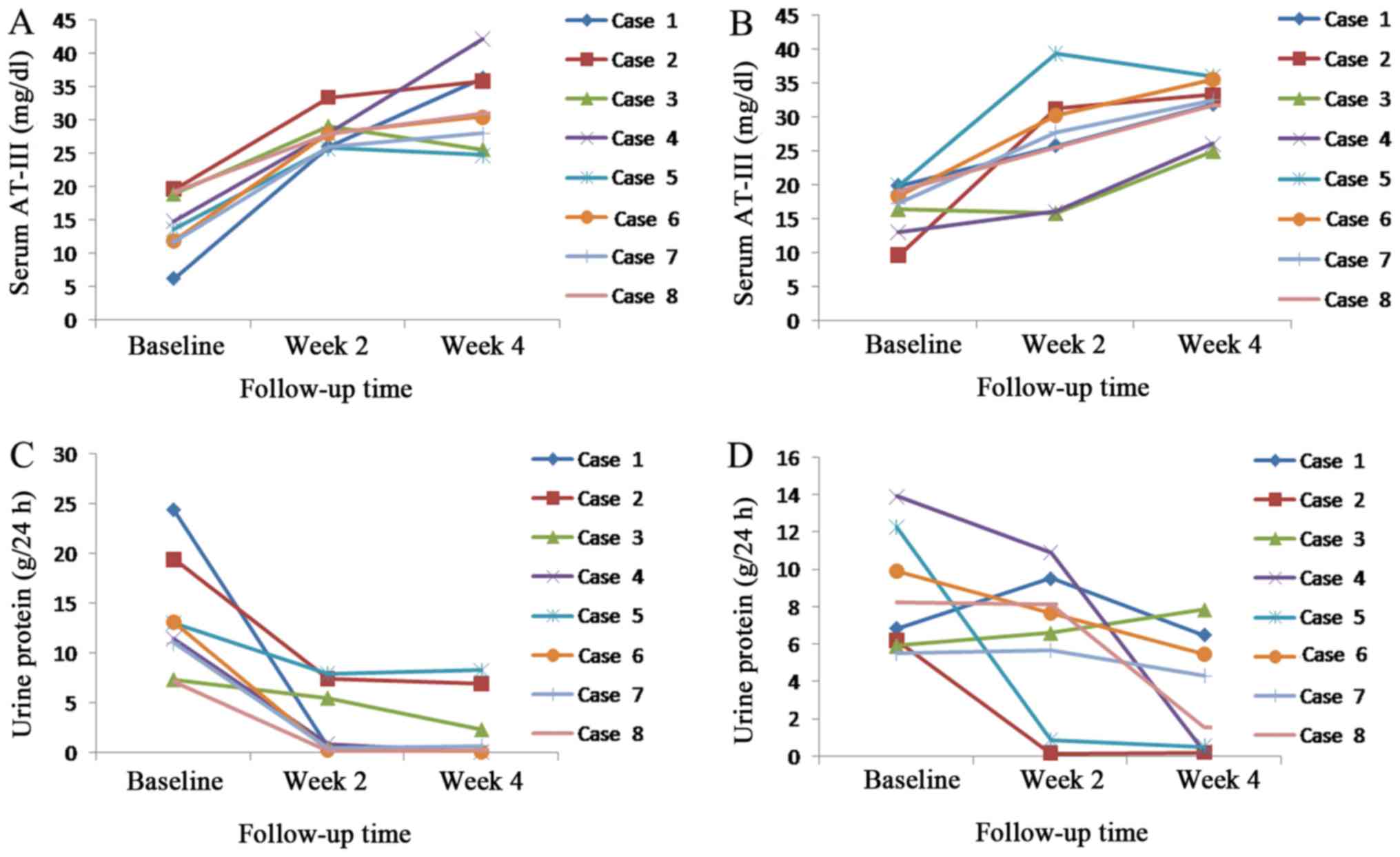
Urine protein decreased and serum AT-III
concentration rose with increased treatment time in the rivaroxaban
and LWMH groups (Fig. 4). Serum
AT-III concentration and functional activity returned to a normal
level at week 2 for all patients in the rivaroxaban group, whereas
in the LWMH group serum levels of AT-III returned to normal at week
2 for 6 patients at week 4 for 2 patients. A total of 5 patients
achieved remission (PR, 3; CR, 2) in the rivaroxaban group, as did
4 patients (PR, 2; CR, 2) in the LWMH group at week 4. The 2
patients in the LWMH group whose AT-III concentration and
functional activity remained low at week 2 did not achieve the
primary endpoint.
Adverse events
No major bleeding occurred in any group. Skin
ecchymosis occurred in 1 patient in the rivaroxaban group and 3 in
the LWMH group. Submucosal hemorrhage occurred in the oral cavity
in 1 patient in the rivaroxaban group. No patient in either group
had the combination of an alanine aminotransferase level >3× the
upper limit of the normal range or a bilirubin level >2× the
upper limit of the normal range.
Discussion
This present study demonstrated that rivaroxaban has
similar efficacy and safety as LWMH when used as a treatment for
VTE in patients with NS and low AT-III levels. No significant
differences in the primary endpoint and the occurrence of adverse
events were observed between the treatment groups.
Theoretically, as a direct Xa inhibitor and AT-III
independent agent, rivaroxaban should be superior to LWMH in
patients with NS and low AT-III levels. The results of the present
study, however, differ, which may be because the serum AT-III
concentration and functional activity returned to normal following
therapy, enhancing the efficacy of LWMH. The serum AT-III
concentration and functional activity returned to normal at week 2
in all patients treated with rivaroxaban and in 6 patients in the
LWMH group. The recovery of AT-III levels in both groups may be
attributed to the following factors: i) Patients achieved remission
in NS after therapy, which reduced the loss of AT-III from the
urine; ii) corticosteroids may elevate the serum AT-III levels by
promoting its synthesis (25).
The results of the present study demonstrate that
the anticoagulation efficacy during the first weeks of treatment
was similar for rivaroxaban and LWMH treatment. However, the number
of patients achieving the primary endpoint was slightly higher in
the rivaroxaban group at week 2. This observation suggests that
rivaroxaban was more effective than LWMH for the treatment of VTE
in patients with persistently low serum AT-III levels.
Additionally, at week 2, the two patients in the LWMH group with
low serum AT-III levels did not achieve the primary endpoint,
suggesting that the anticoagulation effect of LWMH decreased with
low serum AT-III levels. Thus, rivaroxaban may be suitable for the
treatment and prevention of VTE in patients with NS and low AT-III
levels.
In the present study, the tome from occurrence of
thrombotic events to NS onset ranged from 0.25 to 2.38 months.
Bellomo and Atkins (4) reported that
76.2% of thrombotic events occurred in the first 6 months following
the initial presentation of NS. Mahmoodi et al (6) reported that the incidence of VTE was
9.85% when calculated over the first 6 months of observation, a
9.7-fold increase over the mean annual incidence, with 0.9 years as
the median observation period until the first VTE was observed. Li
et al (7) reported that, in
patients with membranous nephropathy, venous thrombotic events
occurred in the first months (mean, 3.5 months) following the
initial presentation of NS. The risk of thrombotic events is
highest in the first months following NS diagnosis and the majority
of experts recommend that prophylactic anticoagulation be performed
in the first 6 months (6).
The present study has several limitations that
should be noted. Firstly, this was a prospective exploratory study
with a small sample size of 16 patients. Secondly, the serum AT-III
level in the majority of patients increased to normal during
treatment; therefore, AT-III was not persistently low. Thirdly
urine AT-III levels were not tested to verify that AT-III
deficiency was due to NS. However, AT-III function and levels were
low in all patients at the baseline and AT-III levels recovered
over time, which suggests acquired AT deficiency as opposed to
inherited AT deficiency. Acquired AT-III deficiency occurs as a
result of either decreased production (due to factors including
liver impairment), increased consumption [due to factors including
sepsis and diffuse intravascular coagulation (DIC)] or increased
renal loss, as in NS. No serious liver impairment, sepsis or DIC,
was observed in patients the present study, which suggests that NS
was the cause of low AT-III levels and functionality.
In conclusion, as an oral direct factor Xa
inhibitor, rivaroxaban has superior effects compared with
traditional anticoagulants, functions independently of serum AT-III
levels, has good compliance and does not require monitoring. The
results of the present study demonstrate that rivaroxaban (30
mg/day for 4 weeks) may provide an effective, safe, single-agent
approach for treating VTE in patients with NS and low AT-III
levels. Future research is required to explore rivaroxaban's
anticoagulation effect for treating VTE in patients with refractory
NS whose AT-III levels are persistently low.
Acknowledgements
The present study was supported by the Primary
Funding Source: National Key Technology R&D Program (grant nos.
2013BAI09B04 and 2015BAI12B05) and the Clinical Medical Research
Center Project of Jiangsu province (grant no. BL2012007).
References
|
1
|
Oger E: Incidence of venous
thromboembolism: A community-based study in Western France.
EPI-GETBP study group. Groupe d'Etude de la thrombose de bretagne
occidentale. Thromb Haemost. 83:657–660. 2000.PubMed/NCBI
|
|
2
|
Spencer FA, Emery C, Lessard D, Anderson
F, Emani S, Aragam J, Becker RC and Goldberg RJ: The worcester
venous thromboembolism study: A population-based study of the
clinical epidemiology of venous thromboembolism. J Gen Intern Med.
21:722–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llach F: Hypercoagulability, renal vein
thrombosis and other thrombotic complications of nephrotic
syndrome. Kidney Int. 28:429–439. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellomo R and Atkins RC: Membranous
nephropathy and thromboembolism: Is prophylactic anticoagulation
warranted? Nephron. 63:249–254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbour SH, Greenwald A, Djurdjev O, Levin
A, Hladunewich MA, Nachman PH, Hogan SL, Cattran DC and Reich HN:
Disease-specific risk of venous thromboembolic events is increased
in idiopathic glomerulonephritis. Kidney Int. 81:190–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahmoodi BK, ten Kate MK, Waanders F,
Veeger NJ, Brouwer JL, Vogt L, Navis G and van der Meer J: High
absolute risks and predictors of venous and arterial thromboembolic
events in patients with nephrotic syndrome: Results from a large
retrospective cohort study. Circulation. 117:224–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SJ, Guo JZ, Zuo K, Zhang J, Wu Y, Zhou
CS, Lu GM and Liu ZH: Thromboembolic complications in membranous
nephropathy patients with nephrotic syndrome-a prospective study.
Thromb Res. 130:501–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kauffman RH, Velgkamp JJ, Van Tilburg NH
and Van Es LA: Acuqired antithrombin III defiency and thrombosis in
the nephrotic syndrome. Am J Med. 65:607–613. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Büller HR, Agnelli GA, Hull RD, Hyers TM,
Prins MH and Raskob GE: Antithrombotic therapy for venous
thromboembolic disease: The seventh ACCP conference on
antithrombotic and thrombolytic therapy. Chest. 126 Suppl
3:401S–428S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bharadwaj J, Jayaraman C and Shrivastava
R: Heparin resistance. Lab Hematol. 9:125–131. 2003.PubMed/NCBI
|
|
11
|
Spiess BD: Treating heparin resistance
with antithrombin or fresh frozen plasma. Ann Thorac Surg.
85:2153–2160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saxena R, Batra VV and Singh ND:
Prothrombotic factors in nephritic syndrome. Indian J Pathol
Microbiol. 43:319–323. 2000.PubMed/NCBI
|
|
13
|
Kubitza D, Becka M, Voith B, Zuehlsdorf M
and WenSing G: Safety, pharmacodynamics, and pharmacokinetics of
single doses of BAY 59–7939, an oral, direct factor Xa inhibitor.
Clin Pharmacol Ther. 78:412–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eriksson BI, Kakkar AK, Turpie AG, Gent M,
Bandel TJ, Homering M, Misselwitz F and Lassen MR: Oral rivaroxaban
for the prevention of symptomatic venous thromboembolism after
elective hip and knee replacement. J Bone Joint Surg Br.
91:636–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turpie AG, Lassen MR, Davidson BL, Bauer
KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel
TJ, et al: Rivaroxaban versus enoxaparin for thromboprophylaxis
after total knee arthroplasty (RECORD4): A randomised trial.
Lancet. 373:1673–1680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
EINSTEIN Investigators, ; Bauersachs R,
Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing
AW, Misselwitz F, Prins MH, et al: Oral rivaroxaban for symptomatic
venous thromboembolism. N Engl J Med. 363:2499–2510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
EINSTEIN-PE Investigators, ; Büller HR,
Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J,
Verhamme P, Wells P, et al: Oral rivaroxaban for the treatment of
symptomatic pulmonary embolism. N Engl J Med. 366:1287–1297. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda T, Kamisato C, Honda Y, Matsushita
T, Kojima T, Furugohri T, Morishima Y and Shibano T: Impact of
antithrombin deficiency on efficacy of edoxaban and
antithrombin-dependent anticoagulants, fondaparinux, enoxaparin,
and heparin. Thromb Res. 131:540–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Switula D: Principles of good clinical
practice (GCP) in clinical research. Sci Eng Ethics. 6:71–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antovic J, Söderström J, Karlman B and
Blombäck M: Evaluation of a new immunoturbidimetric test (LIATEST
Antithrombin III) for determination of antithrombin antigen. Clin
Lab Haem. 23:313–316. 2001. View Article : Google Scholar
|
|
21
|
Ungerstedt JS, Antovic J, Blombäck M,
Bremme K and Johnsson H: Antithrombin antigen and activity in
patients with acquired antithrombin deficiency-is there a
difference? J Thromb Haemost. 2:838–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ak G, Metintas M, Metintas S, Yildirim H,
Ozkan R and Ozden H: Three-dimensional evaluation of chemotherapy
response in malignant pleural mesothelioma. Eur J Radiol.
74:130–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang X, Wang XF, Zhang LH, Chen ZH, Zhang
J, Zhang Y, Zeng CH and Liu ZH: The assay and clinical significance
of plasma antithrombin III in adult nephritic syndrome patients. J
Nephrol Dialy Transplant. 19:407–411. 2010.
|
|
24
|
Ranucci M: Antithrombin III. Key factor in
extracorporeal circulation. Minerva Anestesiol. 68:454–457.
2002.PubMed/NCBI
|
|
25
|
Meade TW, Dyer S, Howarth DJ, Imeson JD
and Stirling Y: Antithrombin III and procoagulant activity: Sex
differences and effects of the menopause. Br J Haematol. 74:77–81.
1990. View Article : Google Scholar : PubMed/NCBI
|