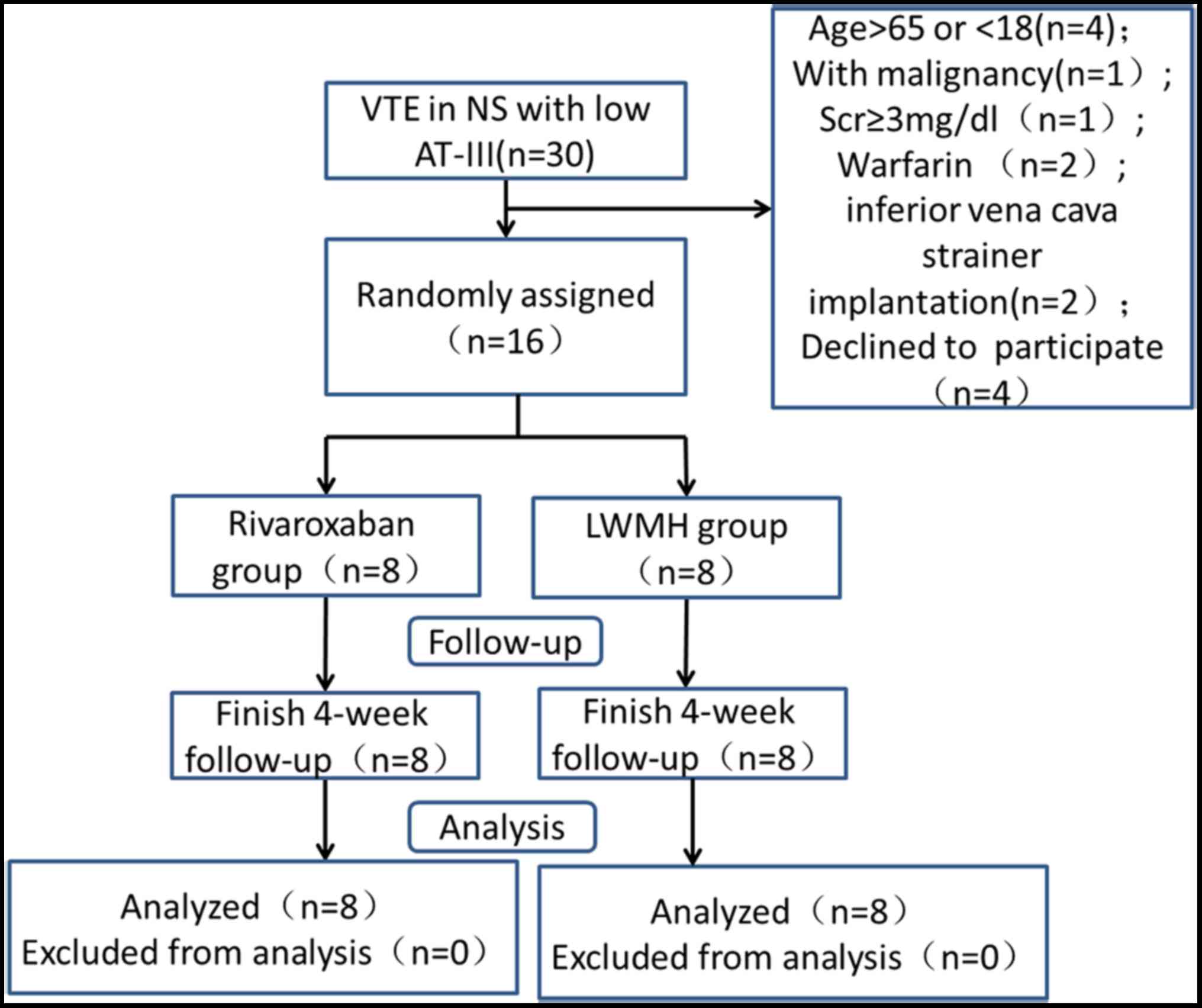
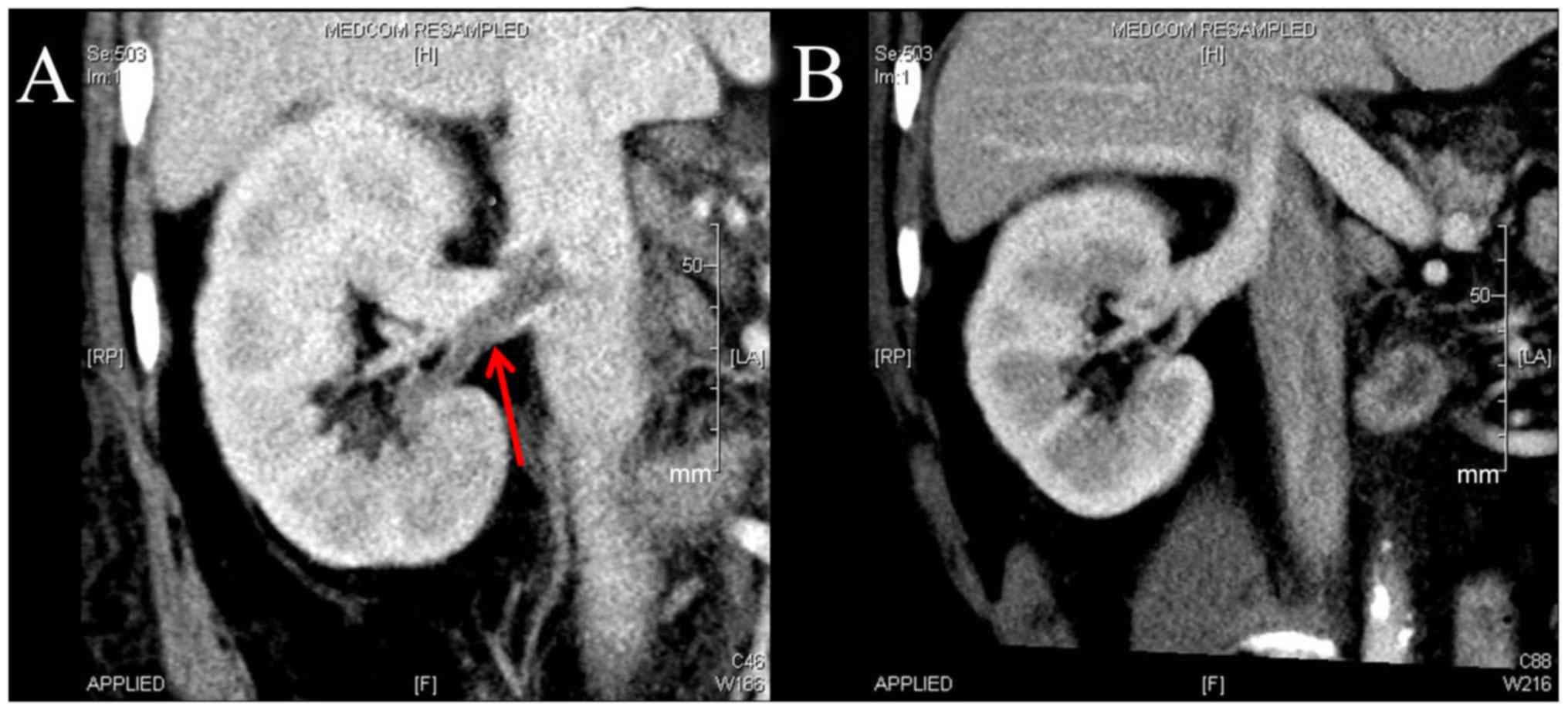
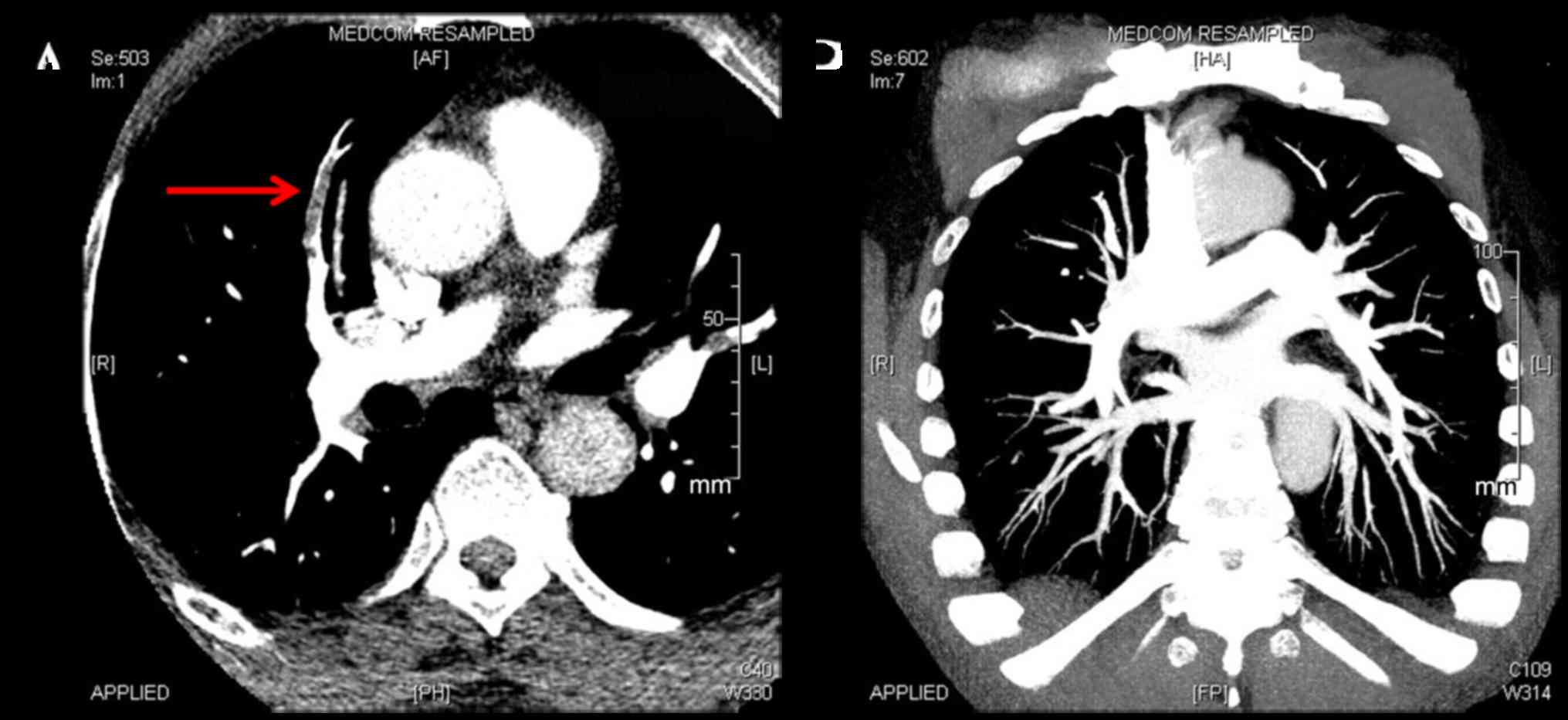
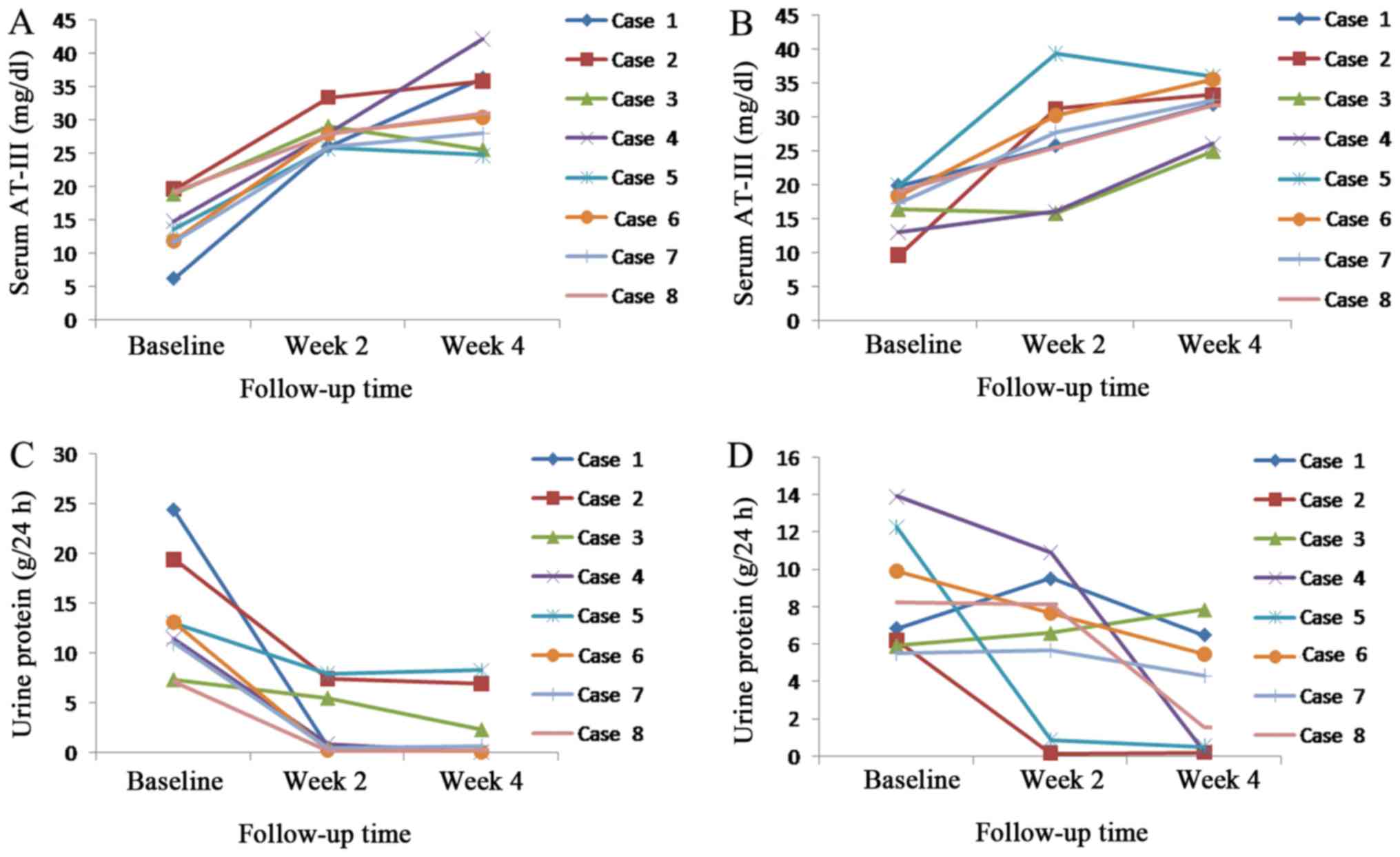
|
1
|
Oger E: Incidence of venous
thromboembolism: A community-based study in Western France.
EPI-GETBP study group. Groupe d'Etude de la thrombose de bretagne
occidentale. Thromb Haemost. 83:657–660. 2000.PubMed/NCBI
|
|
2
|
Spencer FA, Emery C, Lessard D, Anderson
F, Emani S, Aragam J, Becker RC and Goldberg RJ: The worcester
venous thromboembolism study: A population-based study of the
clinical epidemiology of venous thromboembolism. J Gen Intern Med.
21:722–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llach F: Hypercoagulability, renal vein
thrombosis and other thrombotic complications of nephrotic
syndrome. Kidney Int. 28:429–439. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellomo R and Atkins RC: Membranous
nephropathy and thromboembolism: Is prophylactic anticoagulation
warranted? Nephron. 63:249–254. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbour SH, Greenwald A, Djurdjev O, Levin
A, Hladunewich MA, Nachman PH, Hogan SL, Cattran DC and Reich HN:
Disease-specific risk of venous thromboembolic events is increased
in idiopathic glomerulonephritis. Kidney Int. 81:190–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahmoodi BK, ten Kate MK, Waanders F,
Veeger NJ, Brouwer JL, Vogt L, Navis G and van der Meer J: High
absolute risks and predictors of venous and arterial thromboembolic
events in patients with nephrotic syndrome: Results from a large
retrospective cohort study. Circulation. 117:224–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SJ, Guo JZ, Zuo K, Zhang J, Wu Y, Zhou
CS, Lu GM and Liu ZH: Thromboembolic complications in membranous
nephropathy patients with nephrotic syndrome-a prospective study.
Thromb Res. 130:501–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kauffman RH, Velgkamp JJ, Van Tilburg NH
and Van Es LA: Acuqired antithrombin III defiency and thrombosis in
the nephrotic syndrome. Am J Med. 65:607–613. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Büller HR, Agnelli GA, Hull RD, Hyers TM,
Prins MH and Raskob GE: Antithrombotic therapy for venous
thromboembolic disease: The seventh ACCP conference on
antithrombotic and thrombolytic therapy. Chest. 126 Suppl
3:401S–428S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bharadwaj J, Jayaraman C and Shrivastava
R: Heparin resistance. Lab Hematol. 9:125–131. 2003.PubMed/NCBI
|
|
11
|
Spiess BD: Treating heparin resistance
with antithrombin or fresh frozen plasma. Ann Thorac Surg.
85:2153–2160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saxena R, Batra VV and Singh ND:
Prothrombotic factors in nephritic syndrome. Indian J Pathol
Microbiol. 43:319–323. 2000.PubMed/NCBI
|
|
13
|
Kubitza D, Becka M, Voith B, Zuehlsdorf M
and WenSing G: Safety, pharmacodynamics, and pharmacokinetics of
single doses of BAY 59–7939, an oral, direct factor Xa inhibitor.
Clin Pharmacol Ther. 78:412–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eriksson BI, Kakkar AK, Turpie AG, Gent M,
Bandel TJ, Homering M, Misselwitz F and Lassen MR: Oral rivaroxaban
for the prevention of symptomatic venous thromboembolism after
elective hip and knee replacement. J Bone Joint Surg Br.
91:636–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turpie AG, Lassen MR, Davidson BL, Bauer
KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel
TJ, et al: Rivaroxaban versus enoxaparin for thromboprophylaxis
after total knee arthroplasty (RECORD4): A randomised trial.
Lancet. 373:1673–1680. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
EINSTEIN Investigators, ; Bauersachs R,
Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing
AW, Misselwitz F, Prins MH, et al: Oral rivaroxaban for symptomatic
venous thromboembolism. N Engl J Med. 363:2499–2510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
EINSTEIN-PE Investigators, ; Büller HR,
Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J,
Verhamme P, Wells P, et al: Oral rivaroxaban for the treatment of
symptomatic pulmonary embolism. N Engl J Med. 366:1287–1297. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fukuda T, Kamisato C, Honda Y, Matsushita
T, Kojima T, Furugohri T, Morishima Y and Shibano T: Impact of
antithrombin deficiency on efficacy of edoxaban and
antithrombin-dependent anticoagulants, fondaparinux, enoxaparin,
and heparin. Thromb Res. 131:540–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Switula D: Principles of good clinical
practice (GCP) in clinical research. Sci Eng Ethics. 6:71–77. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antovic J, Söderström J, Karlman B and
Blombäck M: Evaluation of a new immunoturbidimetric test (LIATEST
Antithrombin III) for determination of antithrombin antigen. Clin
Lab Haem. 23:313–316. 2001. View Article : Google Scholar
|
|
21
|
Ungerstedt JS, Antovic J, Blombäck M,
Bremme K and Johnsson H: Antithrombin antigen and activity in
patients with acquired antithrombin deficiency-is there a
difference? J Thromb Haemost. 2:838–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ak G, Metintas M, Metintas S, Yildirim H,
Ozkan R and Ozden H: Three-dimensional evaluation of chemotherapy
response in malignant pleural mesothelioma. Eur J Radiol.
74:130–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang X, Wang XF, Zhang LH, Chen ZH, Zhang
J, Zhang Y, Zeng CH and Liu ZH: The assay and clinical significance
of plasma antithrombin III in adult nephritic syndrome patients. J
Nephrol Dialy Transplant. 19:407–411. 2010.
|
|
24
|
Ranucci M: Antithrombin III. Key factor in
extracorporeal circulation. Minerva Anestesiol. 68:454–457.
2002.PubMed/NCBI
|
|
25
|
Meade TW, Dyer S, Howarth DJ, Imeson JD
and Stirling Y: Antithrombin III and procoagulant activity: Sex
differences and effects of the menopause. Br J Haematol. 74:77–81.
1990. View Article : Google Scholar : PubMed/NCBI
|