Introduction
Breast cancer is one of the most commonly diagnosed
cancers in women and accounts for ~30% of all new cancer diagnoses
in women (1). An estimate of 252,710
patients are expected to be newly diagnosed with breast cancer in
the US in 2017, and 40,610 patients will die from this disease
(1). Accumulated data have
demonstrated that certain molecular signaling pathways contribute
to the development and progression of breast carcinoma. For
instance, in breast tumors, constitutive activation of nuclear
factor (NF)-κB was reported to contribute to cellular
proliferation, angiogenesis and evasion of apoptosis (2). The expression of members of the Sonic
Hedgehog pathway was identified to be increased in MCF-7 breast
cancer mammospheres in comparison to that in MCF-7 cells cultured
as monolayers (3). Ibrahim et
al (4) reported that the Notch
and epidermal growth factor receptor signaling pathways are
involved in syndecan-1-mediated modulation of cancer stem cell
phenotypes of inflammatory breast cancer. In breast cancer, Wnt
signaling is constitutively activated by an autocrine mechanism. In
accordance with this, a study by Jang et al (5) reported that Wnt signaling is associated
with the maintenance of stem cell properties and that blockade of
Wnt/β-catenin signaling suppresses in vitro and in
vivo tumor formation and cellular migration.
S-phase kinase associated protein 2 (Skp2) has been
reported to regulate cellular senescence, cancer progression and
metastasis (6). It is an E3
ubiquitin ligase that belongs to the ubiquitin proteasome system
and has emerged as an important factor in tumorigenesis due to the
deregulated ubiquitination and proteolysis of its substrates
(7). Skp2 was reported to be an
oncoprotein and regulates the cell cycle, proliferation,
differentiation, apoptosis and metastasis of a variety of human
cancer types (7–9). In breast cancers, Skp2 has been
revealed to promote carcinogenesis and cancer progression (10–12),
rendering Skp2 a potential therapeutic target to combat breast
cancer.
4′,5,7-trihydroxyisoflavone (genistein) is a
biologically active small molecule that is highly abundant in soy
and soy products (13). This
important compound is well known to inhibit cancer progression. In
particular, genistein has emerged as an important inhibitor of
cancer metastasis (14). It has been
reported that genistein decreases cyclin B1 and induces p21,
leading to cell cycle arrest of breast carcinoma cells in G2/M
phase (15). Valachovicova et
al (16) reported that genistein
suppresses breast cancer cell adhesion and migration by inhibiting
the constitutively activated transcription factors NF-κB and
activator protein (AP)-1. It was also reported that genistein
treatment increased the expression of p21 and p16 in breast cancer
cells, and a genistein-rich diet inhibited the development of
breast cancer xenografts in mice (17). Due to its important role in
tumorigenesis, the potential of genistein as a promising
therapeutic inhibitor of metastasis is highlighted.
The present study assessed the effects of genistein
on cell proliferation, apoptotic cell death, cell cycle
distribution, migration and invasion of breast cancer cells. The
underling mechanisms of the antineoplastic activity of genistein in
breast cancer were also investigated. Specifically, it was explored
whether genistein exerted its anti-tumor effect in breast cancer
cells via inhibition of Skp2.
Materials and methods
Cell culture and reagents
The MDA-MB-231 and SKBR3 human breast cancer cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc.), penicillin (100 U/ml) and streptomycin
(100 U/ml) in a humidified atmosphere containing 5% CO2
at 37°C. Genistein, calcein-acetoxymethyl (AM) and MTT were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). An
Annexin V-FITC/PI Apoptosis Detection Kit, Transwell inserts and
Matrigel were purchased from BD Biosciences (Franklin Lakes, NJ,
USA). Primary antibody against tubulin (cat no. SC-5274) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Anti-p21 (cat no. 2946), anti-Skp2 (cat no. 4358), and anti-p27
(cat no. 2552) antibodies were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-mouse HRP-linked antibody
(cat no. 7076) and anti-rabbit HRP-linked antibody (cat no. 7074)
were purchased from Cell Signaling Technology, Inc.
MTT assay
Cells were seeded at 5×103 cells/well in
a 96-well plate and cultured overnight. Subsequently, the cells
were treated with different concentrations of genistein. After 48
and 72 h, 10 µl MTT solution (5 mg/ml) was added to each well,
followed by incubation for at 37°C for 4 h. Subsequently, the
supernatant was discarded and 100 µl dimethyl sulfoxide was added
to dissolve the MTT-formazan crystals. The cell viability was
evaluated by measuring the absorption of each well at 490 nm using
a microplate reader.
Analysis of cell apoptosis
Cells were seeded at 1×105 cells/well in
a six-well plate, cultured overnight and then treated with 20 or 40
µM genistein for 48 h. Cells were then harvested and washed with
PBS, resuspended in 500 µl binding buffer containing 5 µl propidium
iodide (PI) and 5 µl fluorescein isothiocyanate (FITC)-conjugated
Annexin V antibody in the dark for 15 min using a Dead Cell
Apoptosis kit with Annexin V FITC and PI (cat. no. V13242; Thermo
Fisher Scientific, Inc.). Genistein-induced breast cancer cell
apoptosis was analyzed using a FACSCalibur flow cytometer (BD
Biosciences).
Cell cycle analysis
Breast cancer cells in the exponential growth phase
cells were seeded in a 6-well plate at 2.5×105
cells/well. After incubation overnight, the cells were treated with
20 or 40 µM genistein for 48 h. Cells were collected, washed with
cold PBS and then fixed with ice-cold ethanol 70% (v/v), in which
they were kept at 4°C overnight. Prior to analysis, the cells were
washed with cold PBS, re-suspended at 1×106 cells/ml and
stained with PBS containing 0.1 mg/ml RNase I (Invitrogen; Thermo
Fisher Scientific, Inc.) and 50 mg/ml PI. After staining for 30 min
at room temperature in the dark, the cell cycle was determined
using a FACSCalibur flow cytometer (BD Biosciences).
Wound healing assay
Breast cancer cells were seeded and cultured in a
6-well plate. After the cells reached almost 100% confluency, the
cell monolayers were scraped with a pipette tip to generate linear
scratch wounds. The detached cells were rinsed off with PBS and
then supplemented with medium (without FBS) containing genistein.
Images of the wounds were captured at 0 and 16 h.
Transwell migration and invasion
assay
Breast cancer cells (1×104 cells/well)
were cultured in each of the upper chambers of Transwell without
Matrigel (for migration assay) or Matrigel-precoated inserts (for
invasion assay) in 200 µl serum-free DMEM with genistein. In the
lower chambers, 500 µl complete medium containing 10% FBS and the
same concentration of genistein was added. After 24 h of
incubation, the cells that had invaded through the pores and
attached to the bottom surface of the membrane were stained with
calcein-AM. Images of the stained invaded cells were captured under
a fluorescent microscope.
Western blot analysis
Cells were harvested and resuspended in protein
lysis buffer (Cell Signaling Technologies, Inc.). The protein
samples were quantified using a Bicinchoninic Acid Protein assay
kit (Thermo Fisher Scientific, Inc.) and heated for 5 min at 100°C.
Equal amounts of protein (30 µg) were then separated by 10%
SDS-PAGE. The decentralized proteins were transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA) and immunoblotted with the appropriate primary antibodies
(anti-Skp2, 1:1,000 dilution; anti-p21 and anti-p27, 1:500
dilution) at 4°C overnight. After washing with Tris-buffered saline
containing Tween-20, the membrane was probed with secondary
antibodies (anti-mouse HRP-linked antibody, 1:5,000; anti-rabbit
HRP-linked antibody, 1:5,000) and incubated at room temperature for
1 h. Enhanced chemiluminescence (Thermo Fisher Scientific, Inc.)
was then used to detect the expression of the proteins.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 4.0 (Graph Pad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance was performed followed by a Dunnett's test to
assess statistical significance. Values are expressed as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
Genistein suppresses breast cancer
cell proliferation
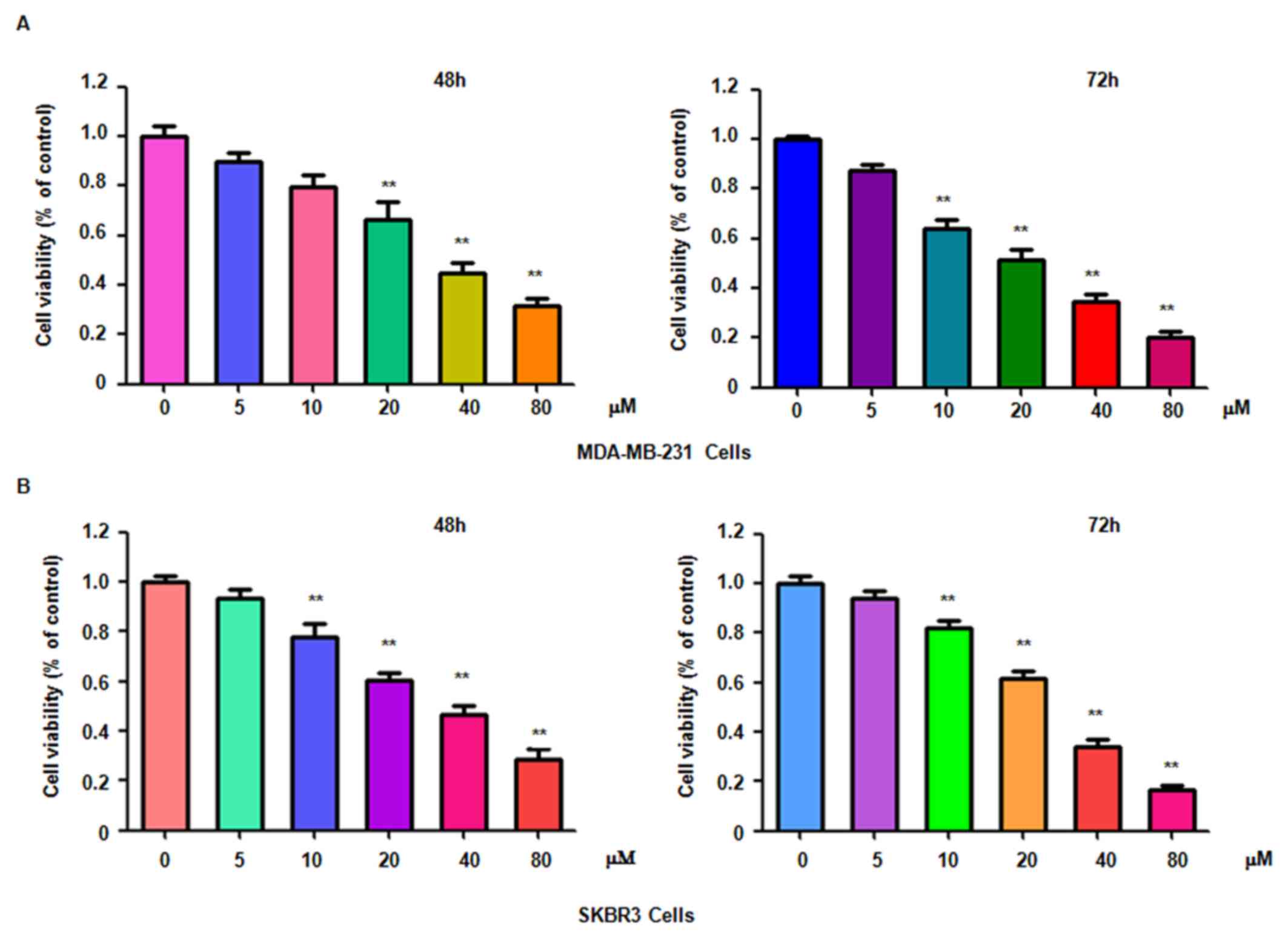
To determine whether genistein suppresses breast
cancer cell proliferation, MDA-MB-231 and SKBR3 cells treated with
different concentrations of genistein for 48 or 72 h were subjected
to an MTT assay. The results indicated that genistein significantly
suppressed cell proliferation in time- and dose-dependent manner
(Fig. 1). In particular, treatment
of each cell line with 20 and 40 µM genistein led ~40 and 60% cell
growth inhibition at 72 h, respectively. Therefore, genistein was
used at used at 20 and 40 µM concentrations in the subsequent
assays.
Genistein induces breast cancer cell
apoptosis
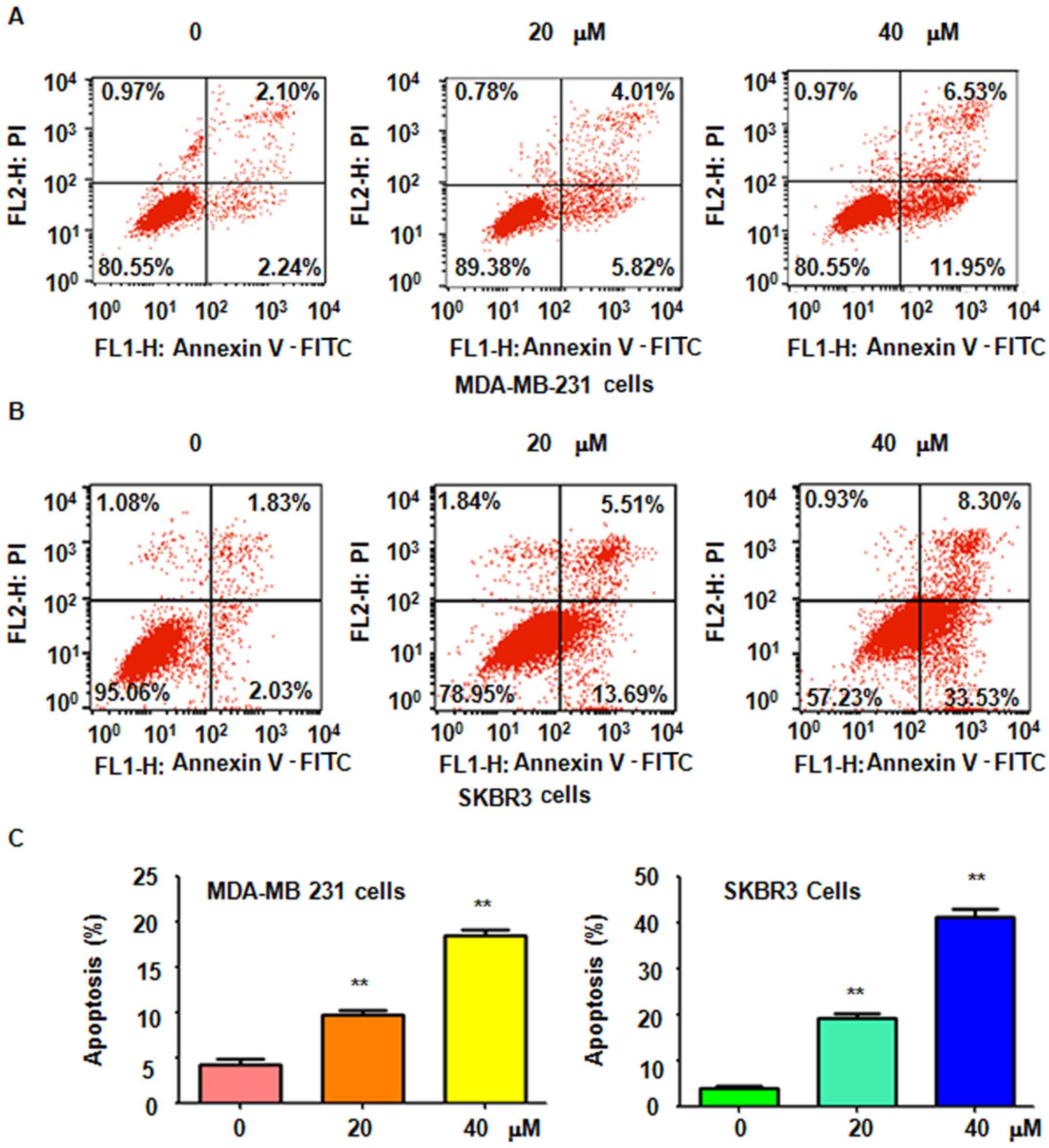
It was further determined whether genistein-induced
cell growth inhibition is due to induction of cell apoptosis. Thus,
after treatment with 20 and 40 µM genistein for 48 h, a
PI-FITC-Annexin V assay was performed to detect the rate of
apoptotic cell death in the two breast cell lines. It was revealed
that genistein treatment significantly induced cell apoptosis in a
dose-dependent manner (Fig. 2). The
results indicated that genistein stimulated apoptosis in breast
cancer cells, which was therefore partly responsible for the
observed inhibition of cell proliferation induced by genistein.
Genistein induces cell cycle arrest in
breast cancer cells
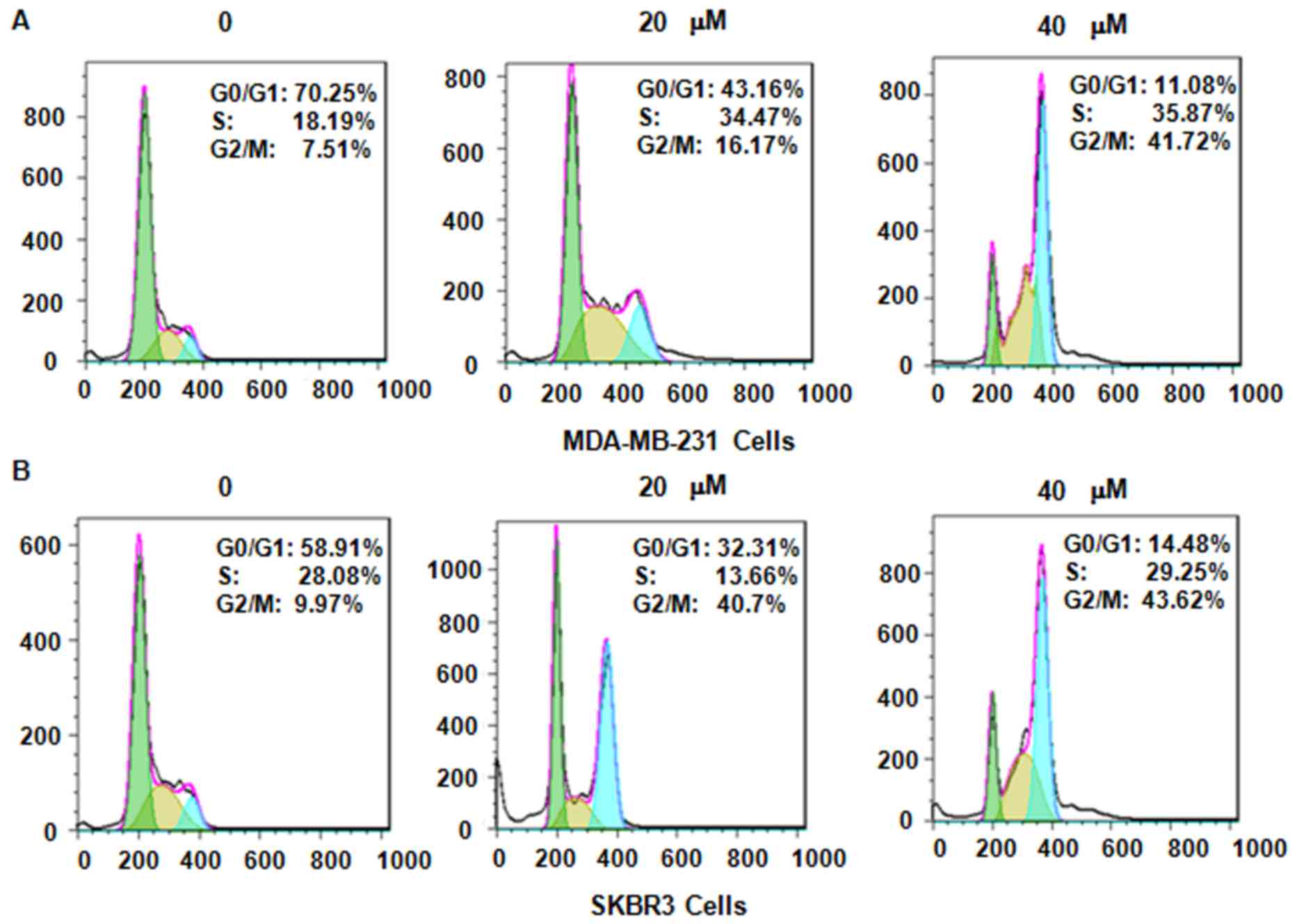
Next, it was assessed whether genistein modulates
cell cycle arrest in MDA-MB-231 and SKBR3 cells after treatment
with genistein. PI staining and flow cytometric analysis were
performed for each of the two breast cancer cell lines. It was
observed that genistein treatment caused cell cycle arrest in G2/M
phase in a dose-dependent manner (Fig.
3). In MDA-MB-231 cells, treatment with 0, 20 and 40 µM
genistein led to an increase in the G2/M phase population from 7.51
to 16.17 and 41.72%, respectively (Fig.
3A). A similar G2/M phase arrest was observed in SKBR3 cells
after genistein treatment (Fig. 3B).
These results demonstrate that genistein induced cell cycle arrest
in G2/M phase in the two breast cancer cell lines.
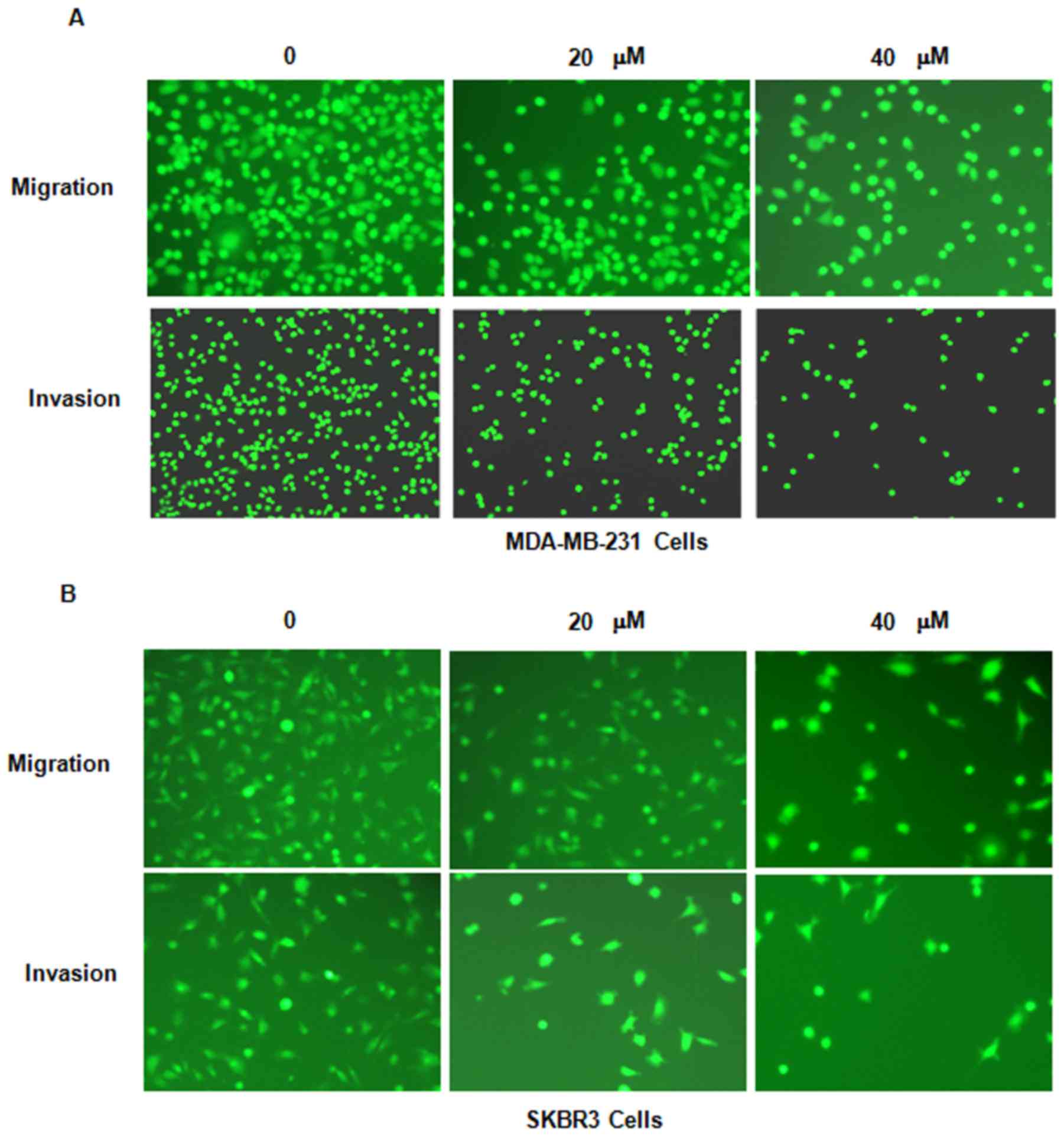
Genistein inhibits breast cancer cell
migration
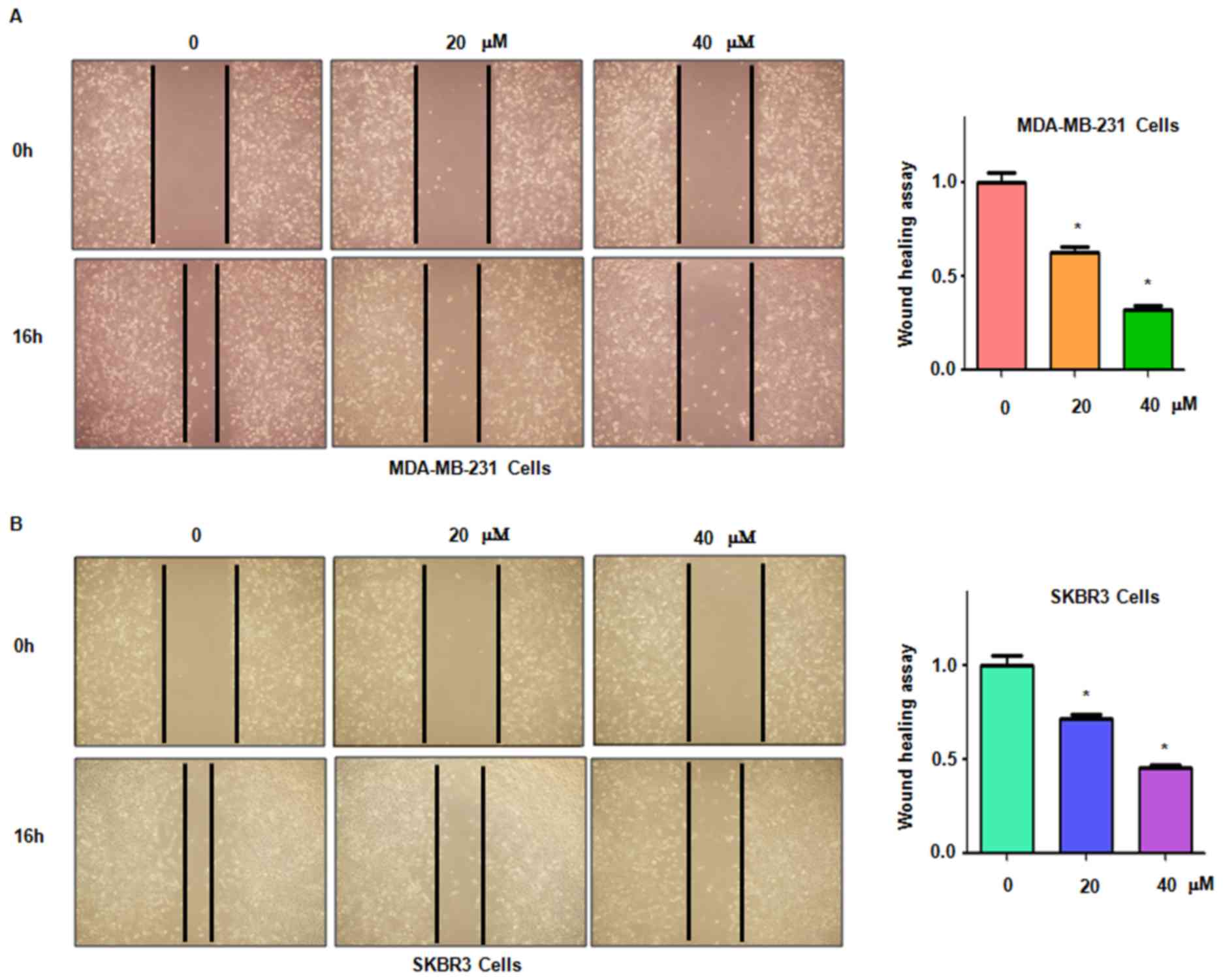
It was then assessed whether genistein inhibits
breast cancer cell migration. The MDA-MB-231 and SKBR3 cell lines
treated with genistein were subjected to a wound healing assay. The
results indicated that in each of the two cell lines, genistein
treatment significantly suppressed cell migratory activity in a
dose-dependent manner (P<0.05; Fig.
4). Consistent with this, the Transwell assay also indicated
that genistein inhibited cell migration in breast cancer cells
(Fig. 5).
Genistein inhibits breast cancer cell
invasion
In order to further assess whether genistein
inhibits the invasion potential of breast cancer cells, a Transwell
invasion assay was performed. The cells that had migrated through
the pores of the matrigel-coated membranes were significantly
decreased in each of the two genistein-treated breast cancer cell
lines (Fig. 5). This result
indicated that genistein reduces the invasive potential of breast
cancer cells.
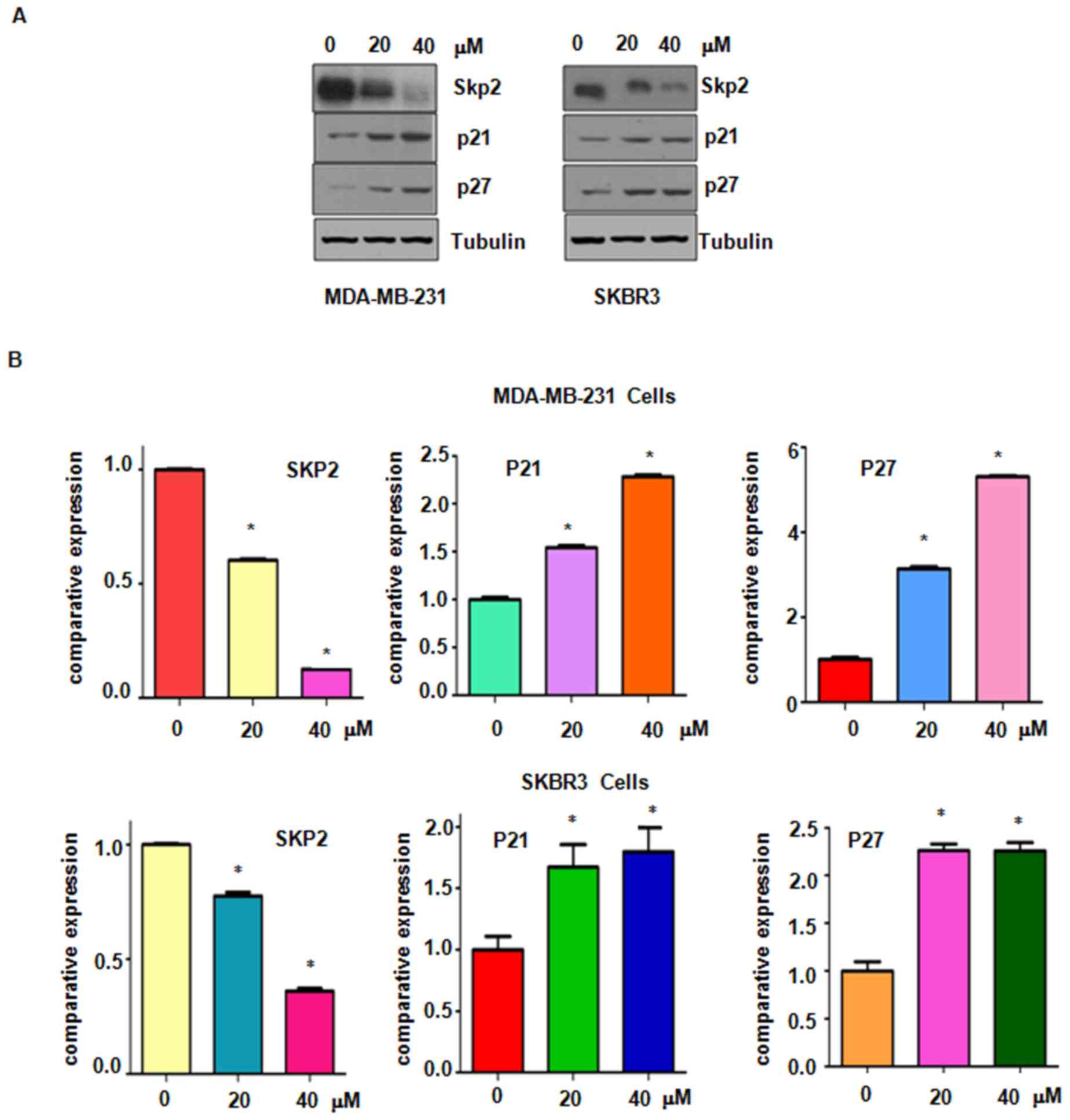
Genistein suppresses Skp2 expression
in breast cancer cells
Accumulated evidence has characterized Skp2 as an
oncoprotein in breast cancer and suppression of Skp2 may be a
promising target for the treatment of breast cancer (7). The present study explored whether
genistein suppresses Skp2 expression in breast cancer cells.
Western blot analysis results demonstrated a significantly
decreased expression of Skp2 in genistein-treated breasted cancer
cells (Fig. 6). Furthermore, the
protein levels of p21 and p27, two typical downstream targets of
Skp2, were increased after genistein treatment (Fig. 6). These results confirm that
genistein exerts its anti-tumor activity in breast cancer cells at
least partially by suppressing Skp2 expression.
Discussion
Breast cancer is one of the most lethal cancer types
affecting women and represents the second leading cause of
cancer-associated mortality in women (1). However, as the precise pathogenic
factors remain to be fully elucidated, the development of effective
measures of prevention and treatment methods for breast cancer
remain a significant challenge. Over the past decades, abundant
research has verified numerous natural products obtained from
dietary sources, which have potential chemopreventive and
anti-cancer effects (18). In
general, higher intake of soy products has been associated with
lower risk of breast cancer, particularly in Asian countries
(19). Genistein, a major active
component of soy isoflavones, is thought to be a potent
chemopreventive and therapeutic agent for breast cancer (20). However, it should be noted that it
has also been indicated that genistein is associated with breast
cancer development (20).
Accumulating evidence has suggested that genistein acts as a
multi-targeting antitumor agent in a variety of human cancer types
(21). Early studies have revealed
that genistein exerts its effects of phytoestrogen, tyrosine kinase
and topoisomerase inhibition by directly binding to the estrogen
receptor (22), receptor tyrosine
kinase (23) and topoisomerase
(24). With regard to its antitumor
function, genistein was identified to modulate numerous key
signaling molecules, including NF-κB, caspase-3, p38
mitogen-activated protein kinase and phosphoinositide 3 kinase/Akt
to induce growth inhibition and apoptosis (16,25,26).
Genistein also suppressed matrix metalloproteinase-9 transcription
by inhibiting the activity of AP-1 and NF-κB, which restrained the
invasiveness and metastatic potential of hepatocellular carcinoma
cells (26). Genistein was also
demonstrated to suppress cancerous inhibitor of protein phosphatase
2A, a newly identified oncogene frequently overexpressed in breast
cancer, which contributed to its growth inhibitory and
apoptosis-inducing effects (27). Li
et al (17) revealed that
genistein treatment significantly suppressed breast cancer cell
growth, increased the expression of the two crucial tumor inhibitor
genes p21 and p16, and decreased the two important tumor promoting
genes BMI1 and c-MYC. In addition, an in vivo experiment
suggested that the development of breast cancer xenografts in mice
was effectively inhibited by the intake of a genistein-rich diet
(17). It was suggested that
genistein represses early breast tumorigenesis by epigenetic
regulation of p21 and p16. Consistent with these results, the
present study observed that genistein significantly inhibited
breast cancer cell growth, migration and invasion, and induced
apoptosis in a dose-dependent manner. Two crucial tumor
suppressors, p21 and p27, were markedly upregulated in
genistein-treated breast cancer cells.
Available evidence suggests that Skp2 has important
roles in cell growth, apoptosis, invasion and metastasis, and is a
predictive factor for poor prognosis in human breast cancer
(12). Thus, inactivation of Skp2
may be a promising therapeutic strategy for breast cancer
treatment. Of note, several Skp2 inhibitors, including Compound A
and Compound 25, were reported to block Skp2 E3 ligase activity
(28,29). However, chemical inhibitors tend to
exhibit side effects during treatment, which are difficult to
overcome. Therefore, natural agents with innoxious properties and
the ability to inactivate Skp2 in human cancers are of great
interest. Several natural agents, including flavokawain A (30), chrysin (31), curcumin (32,33),
butylidenephthalide (34) and
rottlerin (35,36), have been identified to downregulate
Skp2 in various types of human cancer. The present study reported
on another natural product, genistein, which had a potent
inhibitory effect on Skp2 expression in breast cancer cells. The
results suggested that inactivation of Skp2 may, at least
partially, contribute to the anticancer effect of genistein.
Therefore, inactivation of Skp2 by genistein may be utilized for
the clinical treatment of breast cancer. However, it is necessary
to explore the precise molecular mechanisms of genistein-induced
inhibition of Skp2. More importantly, the anti-breast cancer
effects of genistein should be assessed in an animal model and a
clinical trial should be performed in the future.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu M, Sakamaki T, Casimiro MC, Willmarth
NE, Quong AA, Ju X, Ojeifo J, Jiao X, Yeow WS, Katiyar S, et al:
The canonical NF-kappaB pathway governs mammary tumorigenesis in
transgenic mice and tumor stem cell expansion. Cancer Res.
70:10464–10473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He M, Fu Y, Yan Y, Xiao Q, Wu H, Yao W,
Zhao H, Zhao L, Jiang Q, Yu Z, et al: The Hedgehog signalling
pathway mediates drug response of MCF-7 mammosphere cells in breast
cancer patients. Clin Sci (Lond). 129:809–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ibrahim SA, Gadalla R, El-Ghonaimy EA,
Samir O, Mohamed HT, Hassan H, Greve B, El-Shinawi M, Mohamed MM
and Götte M: Syndecan-1 is a novel molecular marker for triple
negative inflammatory breast cancer and modulates the cancer stem
cell phenotype via the IL-6/STAT3, Notch and EGFR signaling
pathways. Mol Cancer. 16:572017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang GB, Kim JY, Cho SD, Park KS, Jung JY,
Lee HY, Hong IS and Nam JS: Blockade of Wnt/b-catenin signaling
suppresses breast cancer metastasis by inhibiting CSC-like
phenotype. Sci Rep. 5:124652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang G, Chan CH, Gao Y and Lin HK: Novel
roles of Skp2 E3 ligase in cellular senescence, cancer progression
and metastasis. Chin J Cancer. 31:169–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan CH, Morrow JK, Zhang S and Lin HK:
Skp2: A dream target in the coming age of cancer therapy. Cell
Cycle. 13:679–680. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Cao L, Sun Z, Xu J, Tang L, Chen
W, Luo J, Yang F, Wang Y and Guan X: Skp2 is over-expressed in
breast cancer and promotes breast cancer cell proliferation. Cell
Cycle. 15:1344–1351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C, Nan H, Ma J, Jiang L, Guo Q, Han
L, Zhang Y, Nan K and Guo H: High Skp2/Low p57(Kip2) expression is
associated with poor prognosis in human breast carcinoma. Breast
Cancer (Auckl). 9 Suppl 1:S13–S21. 2015.
|
|
12
|
Wang Z, Fukushima H, Inuzuka H, Wan L, Liu
P, Gao D, Sarkar FH and Wei W: Skp2 is a promising therapeutic
target in breast cancer. Front Oncol. 1:2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Messina M, Nagata C and Wu AH: Estimated
Asian adult soy protein and isoflavone intakes. Nutr Cancer.
55:1–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pavese JM, Farmer RL and Bergan RC:
Inhibition of cancer cell invasion and metastasis by genistein.
Cancer Metastasis Rev. 29:465–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YH, Zhang L, Lee WH and Park KY:
Genistein-induced G2/M arrest is associated with the inhibition of
cyclin B1 and the induction of p21 in human breast carcinoma cells.
Int J Oncol. 13:391–396. 1998.PubMed/NCBI
|
|
16
|
Valachovicova T, Slivova V, Bergman H,
Shuherk J and Sliva D: Soy isoflavones suppress invasiveness of
breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent
and -independent pathways. Int J Oncol. 25:1389–1395.
2004.PubMed/NCBI
|
|
17
|
Li Y, Chen H, Hardy TM and Tollefsbol TO:
Epigenetic regulation of multiple tumor-related genes leads to
suppression of breast tumorigenesis by dietary genistein. PLoS One.
8:e543692013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonofiglio D, Giordano C, De Amicis F,
Lanzino M and Andò S: Natural products as promising antitumoral
agents in breast cancer: Mechanisms of action and molecular
targets. Mini Rev Med Chem. 16:596–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagata C, Mizoue T, Tanaka K, Tsuji I,
Tamakoshi A, Matsuo K, Wakai K, Inoue M, Tsugane S, Sasazuki S, et
al: Soy intake and breast cancer risk: an evaluation based on a
systematic review of epidemiologic evidence among the Japanese
population. Jpn J Clin Oncol. 44:282–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uifalean A, Schneider S, Ionescu C, Lalk M
and Iuga CA: Soy Isoflavones and breast cancer cell lines:
Molecular mechanisms and future perspectives. Molecules.
21:E132015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkar FH and Li Y: Mechanisms of cancer
chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev.
21:265–280. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang TT, Sathyamoorthy N and Phang JM:
Molecular effects of genistein on estrogen receptor mediated
pathways. Carcinogenesis. 17:271–275. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akiyama T, Ishida J, Nakagawa S, Ogawara
H, Watanabe S, Itoh N, Shibuya M and Fukami Y: Genistein, a
specific inhibitor of tyrosine-specific protein kinases. J Biol
Chem. 262:5592–5595. 1987.PubMed/NCBI
|
|
24
|
Corbett AH, Hong D and Osheroff N:
Exploiting mechanistic differences between drug classes to define
functional drug interaction domains on topoisomerase II. Evidence
that several diverse DNA cleavage-enhancing agents share a common
site of action on the enzyme. J Biol Chem. 268:14394–14398.
1993.PubMed/NCBI
|
|
25
|
Shafiee G, Saidijam M, Tavilani H,
Ghasemkhani N and Khodadadi I: Genistein induces apoptosis and
inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell
Med. 5:178–191. 2016.PubMed/NCBI
|
|
26
|
Wang SD, Chen BC, Kao ST, Liu CJ and Yeh
CC: Genistein inhibits tumor invasion by suppressing multiple
signal transduction pathways in human hepatocellular carcinoma
cells. BMC Complement Altern Med. 14:262014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Q, Zhao M, Parris AB, Xing Y and Yang
X: Genistein targets the cancerous inhibitor of PP2A to induce
growth inhibition and apoptosis in breast cancer cells. Int J
Oncol. 49:1203–1210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Xie W, Kuhn DJ, Voorhees PM,
Lopez-Girona A, Mendy D, Corral LG, Krenitsky VP, Xu W, Moutouh-de
Parseval L, et al: Targeting the p27 E3 ligase SCF(Skp2) results in
p27- and Skp2-mediated cell-cycle arrest and activation of
autophagy. Blood. 111:4690–4699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan CH, Morrow JK, Li CF, Gao Y, Jin G,
Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al: Pharmacological
inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem
cell traits and cancer progression. Cell. 154:556–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jandial DD, Krill LS, Chen L, Wu C, Ke Y,
Xie J, Hoang BH and Zi X: Induction of G2M arrest by flavokawain A,
a kava chalcone, increases the responsiveness of
her2-overexpressing breast cancer cells to herceptin. Molecules.
22:2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang C, Wei YX, Shen MC, Tu YH, Wang CC
and Huang HC: Chrysin, abundant in morinda citrifolia fruit
water-etoac extracts, combined with apigenin synergistically
induced apoptosis and inhibited migration in human breast and liver
cancer cells. J Agric Food Chem. 64:4235–4245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su J, Zhou X, Wang L, Yin X and Wang Z:
Curcumin inhibits cell growth and invasion and induces apoptosis
through down-regulation of Skp2 in pancreatic cancer cells. Am J
Cancer Res. 6:1949–1962. 2016.PubMed/NCBI
|
|
33
|
Feng S, Wang Y, Zhang R, Yang G, Liang Z,
Wang Z and Zhang G: Curcumin exerts its antitumor activity through
regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells.
Onco Targets Ther. 10:2377–2388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang MH, Lin SZ, Lin PC, Chiou TW, Harn
YW, Ho LI, Chan TM, Chou CW, Chuang CH, Su HL and Harn HJ: Brain
tumor senescence might be mediated by downregulation of S-phase
kinase-associated protein 2 via butylidenephthalide leading to
decreased cell viability. Tumour Biol. 35:4875–4884. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X,
Zhao Z, Zhou X, Li Y and Wang Z: Rottlerin exerts its anti-tumor
activity through inhibition of Skp2 in breast cancer cells.
Oncotarget. 7:66512–66524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X,
Zhou X and Wang Z: Rottlerin exhibits anti-cancer effect through
inactivation of S phase kinase-associated protein 2 in pancreatic
cancer cells. Am J Cancer Res. 6:2178–2191. 2016.PubMed/NCBI
|