Introduction
In recent years, the worldwide morbidity of
traumatic brain injury has increased, and in North America alone,
traumatic brain injury is estimated to affect 1.7 million
individuals per year (1). As a
common disease of the central nervous system, the high mortality
and disability rates resulting from diffuse axonal injury (DAI) are
a great public health threat and financial burden for the families
of patients, as well as the society (2). Clinically, intracranial hemorrhage and
edema can be quickly acknowledged through computed tomography and
magnetic resonance imaging (3).
However, as the severity of injury and individual tolerance of
patients differ, demonstrations of vital signs are quite
distinctive in each case. Therefore, intracranial metabolic
abnormalities and the degree of injury should be evaluated on a
case-by-case basis, in order to offer individualized therapy and
reduce rates of mortality and disability (4).
Generally, DAI is accompanied by injury of the
endangium and destruction of the blood-brain barrier (BBB),
resulting in hypoxia-ischemia of injured brain tissues. Thus, this
injury results in DAI, which aggregates dysneuria (5). Subsequent to DAI, brain tissue regions
reconstruct the capillary network to recover the blood supply of
the ischemic cerebrum (6).
In recent years, various clinical observations and
animal experiments have suggested that berberine is an effective
treatment for diabetes mellitus type 2 (7). It can significantly regulate blood
sugar levels and improve impaired glucose tolerance. In addition,
it has preferable preventive and therapeutic effects against
diabetic complications, such as cardiovascular disease,
hypertension and hyperlipidemia (8).
As an isoquinoline derivative alkaloid, berberine exists in
Coptischinensis, golden cypress and the root of Chinese
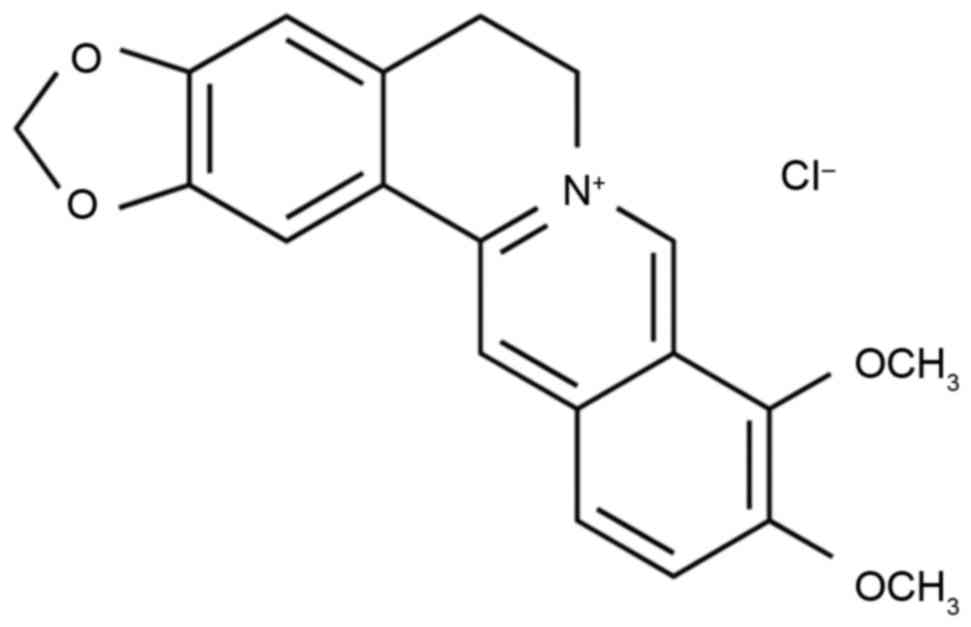
barberry (8). The chemical structure
of berberine is shown in Fig. 1. A
previous study observed that berberine has the pharmacological
functions of regulating blood sugar levels, reducing blood fat
content, reducing blood pressure and inhibiting aldose reductase
(9). These studies have indicated
that berberine may have a favorable clinical value and prospect as
a preventive therapy for diabetes (10,11). In
the present study, the neuroprotective effect of berberine against
learning and memory deficits in severe traumatic brain injury was
investigated.
Materials and methods
Animals and treatment groups
All procedures were approved by the Second
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China). Male Sprague-Dawley rats (10–11 weeks-old,
260–300 g, n=30) were housed in pairs with a 12/12 h light/dark
cycle at 22±2°C and 55±5% humidity, and standard rat chow and water
were provided ad libitum. All Sprague-Dawley rats were
randomly assigned to one of following three groups (n=10): Sham,
DAI model and berberine group. Rats of the berberine group were
orally gavaged with 200 mg/kg of body weight berberine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 4 weeks. Rats
of the sham and DAI model groups were orally gavaged with normal
saline. Rats of the sham group were anesthetized with an
intraperitoneal injection of 3% sodium pentobarbital (60
mg/kg).
DAI model
A DAI rat model was constructed as previously
described (12). Briefly, rats were
anesthetized with 3% sodium pentobarbital (60 mg/kg) by
intraperitoneal injection. The dorsal surface of the skull was
exposed using a midline incision, and a steel disc was fixed
centrally between the lambda and bregma regions using dental
cement. The laterally extended plate containing the foam bed was
restricted to a horizontal position. Next, the rat was placed at a
prone position and two belts were fixed at the trunk of the rat,
while a third belt was fixed at the head of the rat to maintain the
alignment between the center of rotation of the head and the
rotational bearing. A Plexiglas tube was positioned directly above
the cranium. Subsequently, three successive impacts were delivered
at 10-min intervals by dropping a 450 g weight on the cranium. At
24 h after establishment of the DAI models, the course of berberine
treatment was initiated for rats in the berberine group.
Learning and memory test
The learning and memory of rats was investigated by
a Morris water maze test. A circular tank (23±1°C) was used, which
was filled with water that was made opaque and included a variety
of extra-maze cues. Rats were habituated to the water and apparatus
prior to water maze testing for 5 min/day for 5 days. In the
spatial acquisition phase of the test, a platform (50×50×50 cm) was
submerged in the water with an extra-maze cue (90×90×40-cm square
container, 60-cm distance from the platform) for 10 min and their
movements were automatically tracked (SMART 3.0; Panlab, Barcelona,
Spain). A transparent lucite platform was submerged at 2 cm below
the water surface, which was remained at this position for all
spatial trials. All rats were subjected to 4 trials/day (1
trial/start position with the start positions being each corner of
a 3×2 cm2 parameter surrounding the platform) for 4
consecutive days. The rats were given 60 sec to locate the
submerged platform and remained there for 30 sec. The escape
latencies (in sec) and path lengths (in cm) were recorded. For each
rat, consecutive trials were initiated immediately after removal of
the rat from the platform. Following the spatial acquisition phase
of the trial, a daily 60-sec probe trial was used to evaluate how
well the rats had learned the location of the platform. The
swimming time was recorded inside a 1.8-m diameter pool in which
the platform was centrally located.
Measurement of tumor necrosis factor
(TNF-α), interleukin (IL)-1β and monocyte chemoattractant protein-1
(MCP-1) levels
After treatment with berberine, rats were
anesthetized with 30 mg/kg pentobarbital intraperitoneal injection
(i.p.; Sigma-Aldrich; Merck KGaA) and venous blood was collected
from the eye socket of each rat and centrifuged at 4,000 × g for 5
min at 4°C. According to the manufacturer's instruction of ELISA
kits (ExCell Bio, Shanghai, China), the serum was collected and
used to analyze the concentrations of TNF-α (ER006-48), IL-1β
(ER008-96) and MCP-1 (EM018-96).
Western blot assays
The protein levels of nuclear factor (NF)-κB,
Bcl-2-associated X protein (Bax), cytochrome c, p38
mitogen-activated protein kinase (p38 MAPK), activating
transcription factor 2 (ATF-2) and vascular endothelial growth
factor (VEGF) were determined by western blot analysis. Briefly,
rats were sacrificed by decollation after the final round of Morris
water maze tests under 30 mg/kg pentobarbital (i.p.) and
hippocampal tissue was immediately isolated. Protein was extracted
from the samples using radioimmunoprecipitation assay buffer
containing protease inhibitor (RIPA, Beyotime Institute of
Biotechnology, Haimen, China) at 4°C for 30 min, followed by
centrifugation at 12,000 × g for 10 min at 4°C. Protein
concentrations were determined using a BCA protein assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Subsequently,
50 ng total protein was fractionated by 10% SDS-polyacrylamide gel
electrophoresis and transferred to PVDF membranes (EMD Millipore,
Billerica, MA, USA). The membranes were then blocked at 37°C with
5% non-fat dry milk for 30 min and incubated with the appropriate
primary antibody, including anti-NF-κB (1:1,000; 8242), anti-Bax
(1:1,000; 5023), anti-cytochrome c (1:1,000; 11940), anti-p-p38
MAPK (1:2,000; 4511), anti-ATF-2 (1:2,000; 5112), anti-VEGF
(1:2,000; 9698) and anti-β-actin (1:2,000; 4970; all from Cell
Signaling Technology, Inc., Beverly, MA, USA) at 4°C for 12 h.
Membranes were subsequently incubated with secondary antibody
conjugated with horseradish peroxidase (sc-2357; 1:2,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at 37°C and
detected with enhanced chemiluminescence reagents (EMD Millipore).
The protein expression was scanned and quantified by densitometric
analysis using an image analyzer Quantity One System 3.0 (Bio-Rad
Laboratories, Inc., Richmond, CA, USA).
Statistical analysis
Data are presented as the means ± standard
deviation. Statistical analysis used the Student's t-test to assess
statistical differences. For all test, a difference with P<0.05
was considered as statistically significant.
Results
Neuroprotective effect of berberine
against learning and memory deficits in DAI
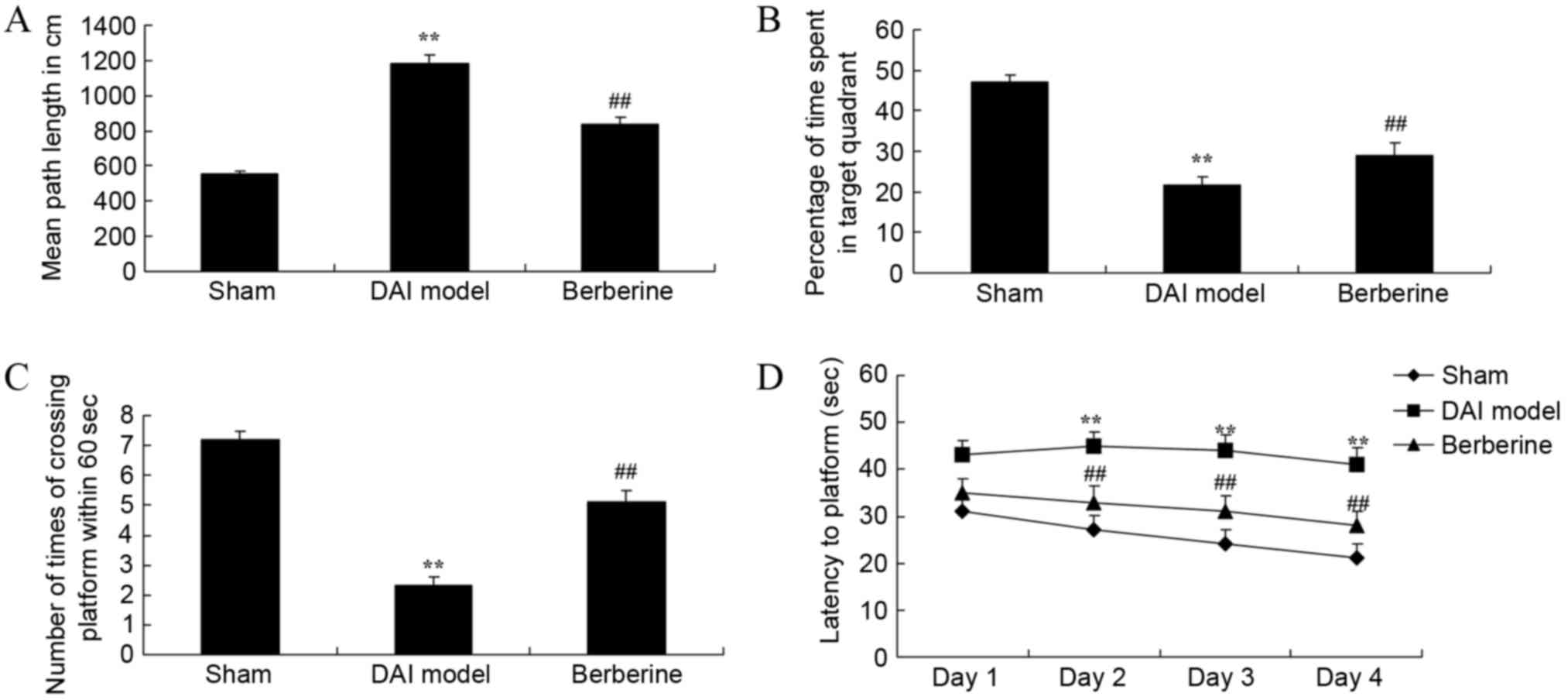
To examine the effect of berberine on the learning
and memory in DAI rats, behavioral tests were used. The results
demonstrated that the mean path length of the DAI model group was
higher compared with that of the sham group (Fig. 2A). Next, the percentage of time spent
in the target quadrant and the number of times of crossing the
platform within 60 sec were lower in DAI model group in comparison
with those of the sham group (Fig. 2B
and C). In addition, the time of latency to platform was also
higher in the DAI model group than in the sham group (Fig. 2D). However, treatment with berberine
significantly improved the results of the behavioral tests in DAI
rats, when compared with the untreated DAI model group (Fig. 2A-D).
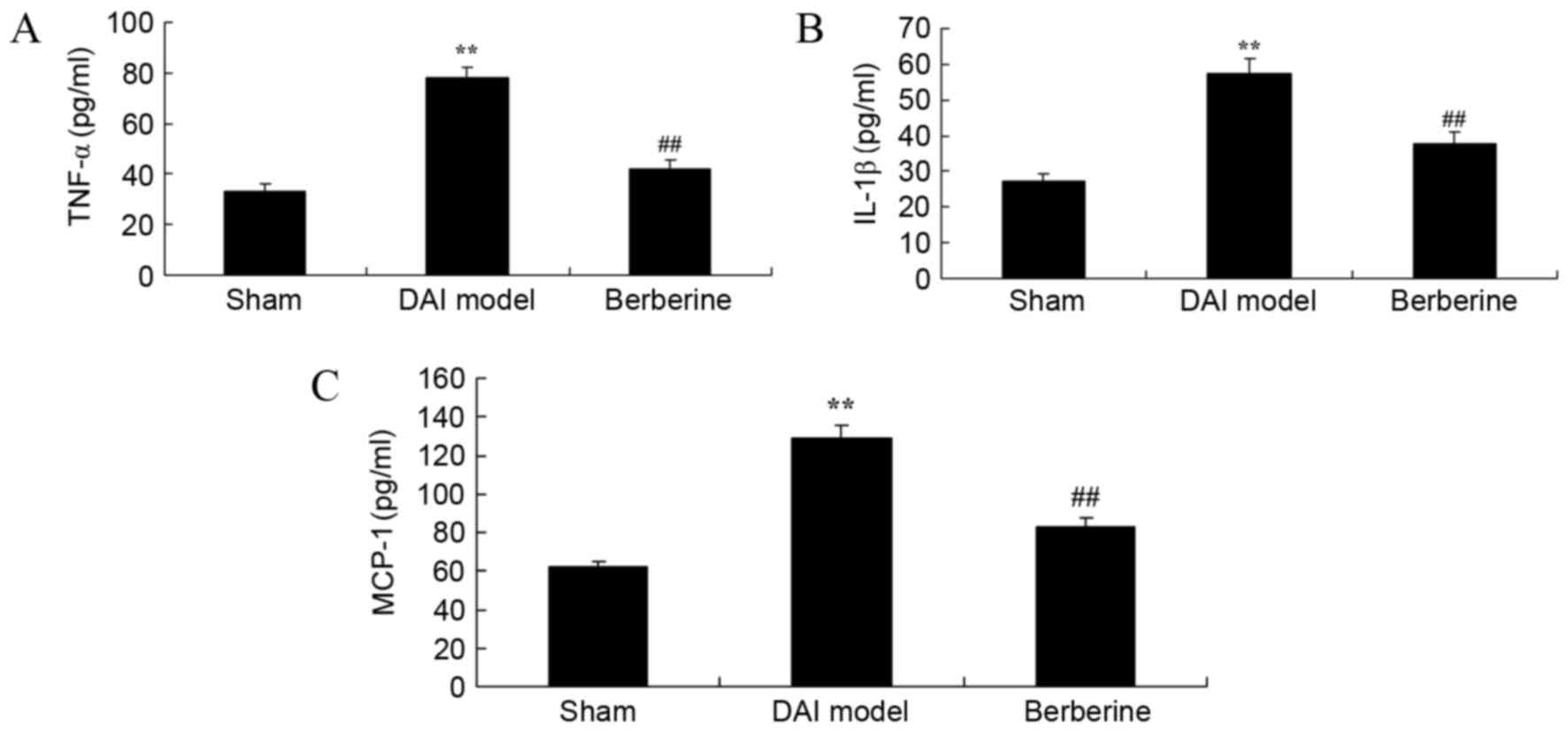
Effect of berberine on TNF-α, IL-1β
and MCP-1 levels in DAI
To study the anti-inflammatory effects of berberine
in DAI rats, the concentrations of TNF-α, IL-1β and MCP-1 were
surveyed using ELISA kits. The results revealed that the
concentrations of TNF-α, IL-1β and MCP-1 were significantly
increased in the DAI model group in comparison with those of the
sham group (Fig. 3). However,
treatment with berberine significantly reduced the levels of TNF-α,
IL-1β and MCP-1 in DAI rats, indicating an anti-inflammatory effect
(Fig. 3).
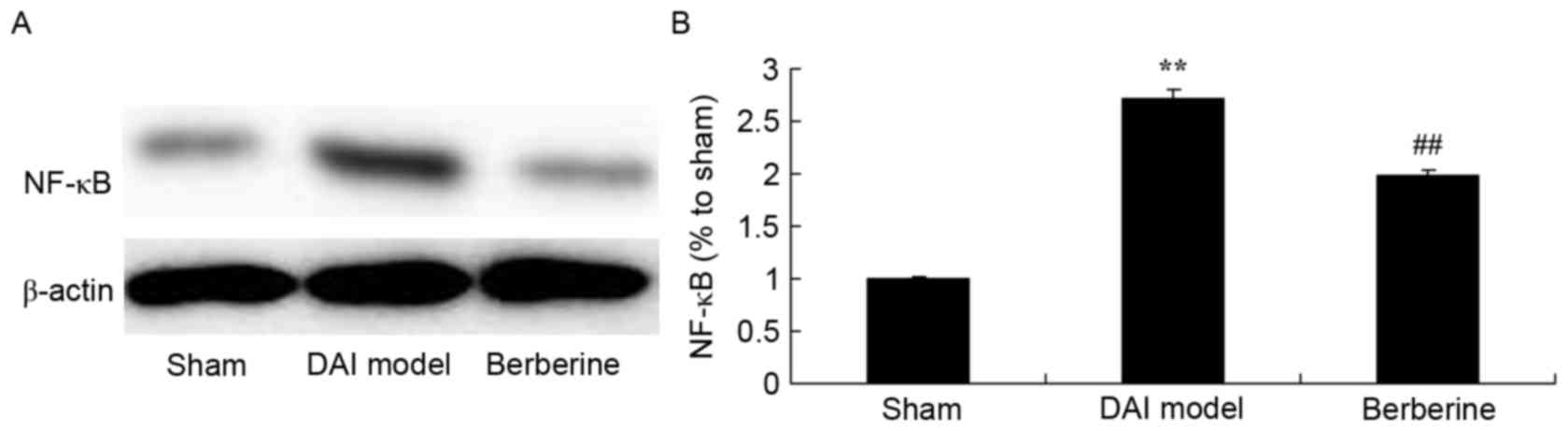
Neuroprotective effect of berberine
against NF-κB/p65 protein expression in DAI
To investigate the mechanism of berberine against
the inflammation induced by DAI, the NF-κB/p65 protein expression
was detected using western blot assay. The results demonstrated
that DAI significantly induced the NF-κB/p65 protein expression in
rats, compared with the sham group (Fig.
4). However, berberine pretreatment significantly suppressed
the protein expression of NF-κB/p65 in the DAI rats (Fig. 4).
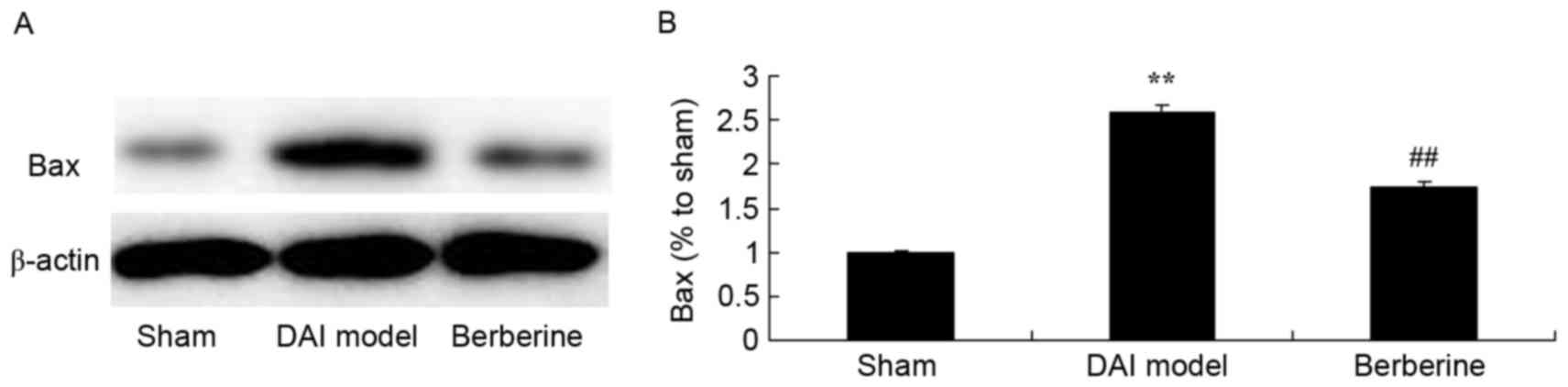
Neuroprotective effect of berberine against Bax
protein expression in DAI. To further investigate the molecular
mechanism of the action exerted by berberine against DAI, the Bax
protein expression was analyzed in the current study. As shown in
Fig. 5, a significant increase in
Bax protein expression induced by DAI was observed, when compared
with the sham group (Fig. 5).
Pretreatment with berberine, however, significantly suppressed Bax
protein expression in DAI rats (Fig.
5).
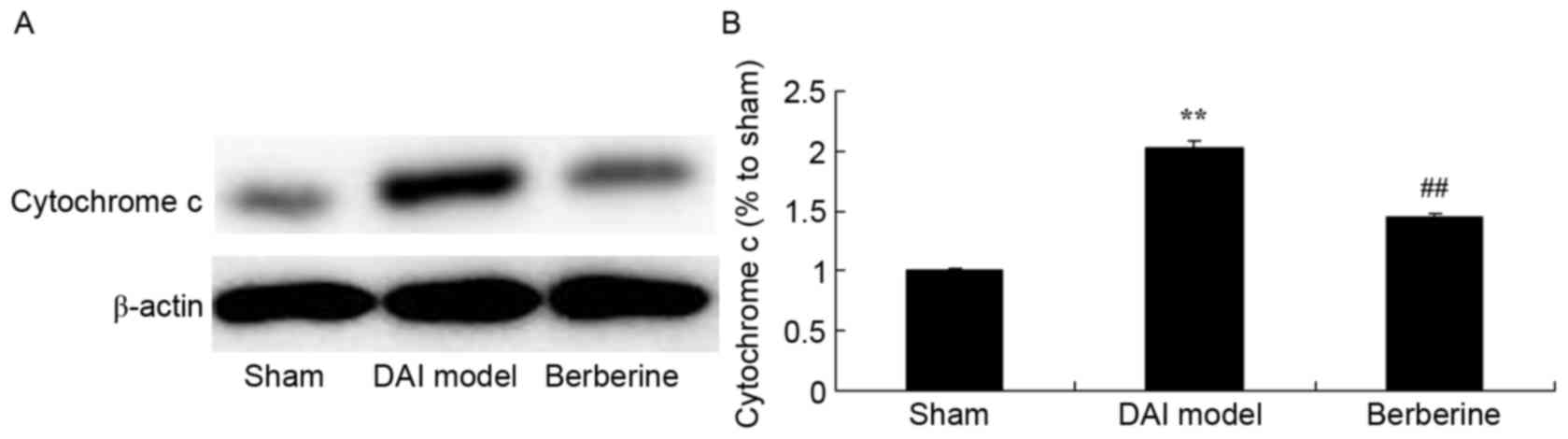
Neuroprotective effect of berberine against
cytochrome c protein expression in explore the molecular mechanism
of berberine against DAI, cytochrome c protein expression
was also analyzed. Fig. 6
demonstrates that there was a significant increase in cytochrome
c protein expression of DAI group rats, compared with the
sham group. Administration of berberine significantly weakened the
protein expression of cytochrome c in DAI rats (Fig. 6).
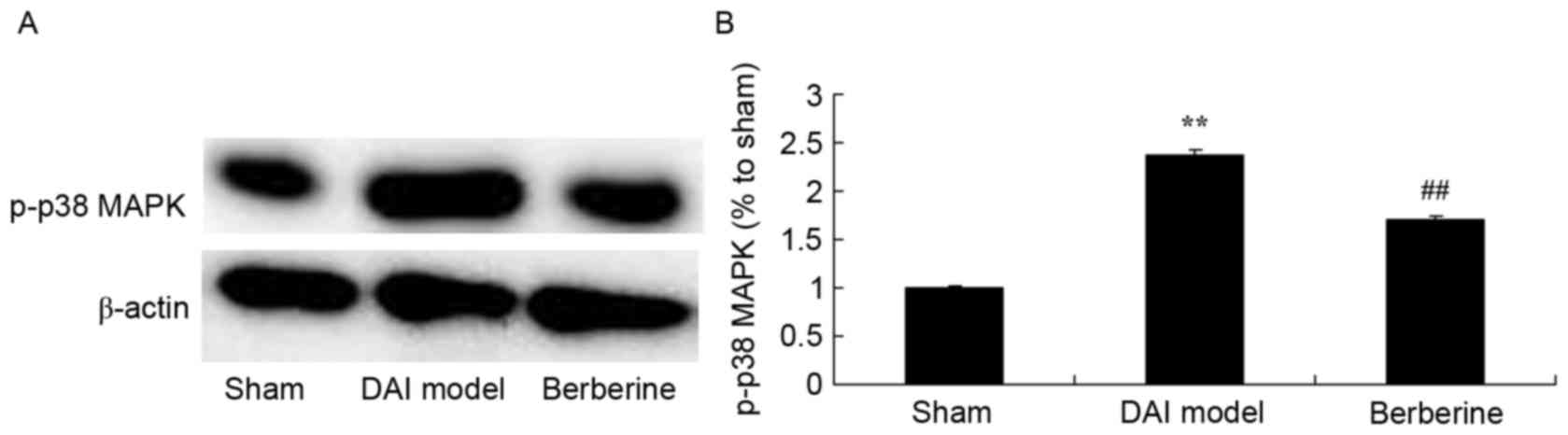
Neuroprotective effect of berberine against p38 MAPK
protein expression in further explore the molecular mechanism of
berberine against DAI, p38 MAPK was investigated as an important
pathway in the effect of berberine. The results of western blot
assays showed that p-p38 MAPK protein expression in the DAI model
group was higher than that of the sham group (Fig. 7). Treatment with berberine
significantly suppressed the protein expression of p-p38 MAPK in
DAI rats (Fig. 7).
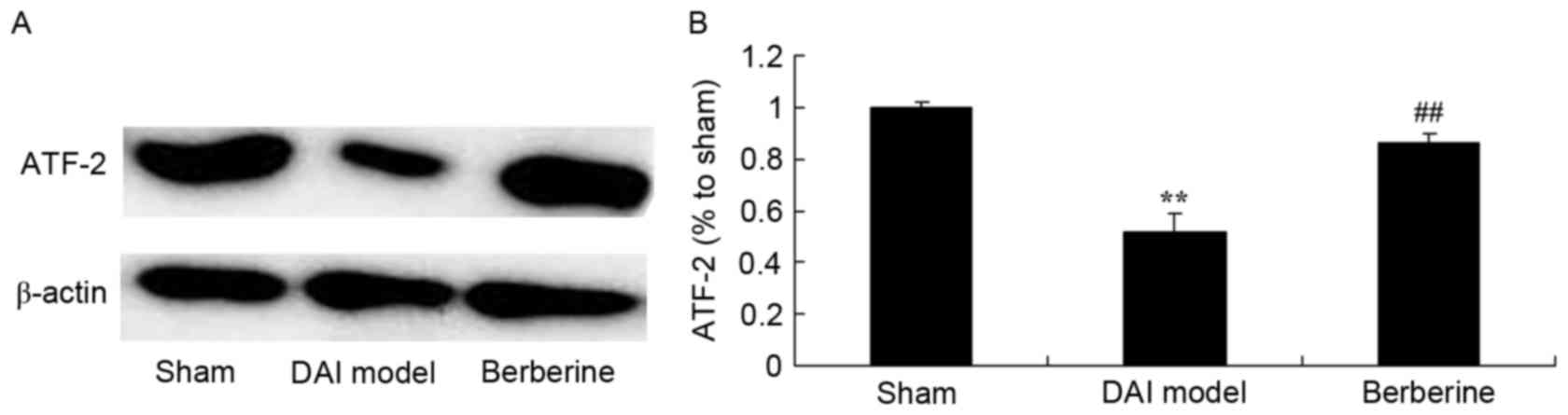
Neuroprotective effect of berberine
against reduced ATF-2 protein expression in DAI
To further study the effect of berberine on ATF-2
protein expression in DAI, western blot analysis was performed. As
shown in Fig. 8, the expression of
ATF-2 protein was significantly suppressed by DAI, as compared with
the sham group. However, berberine treatment significantly
recovered the protein expression of ATF-2 in the DAI rats (Fig. 8).
Neuroprotective effect of berberine
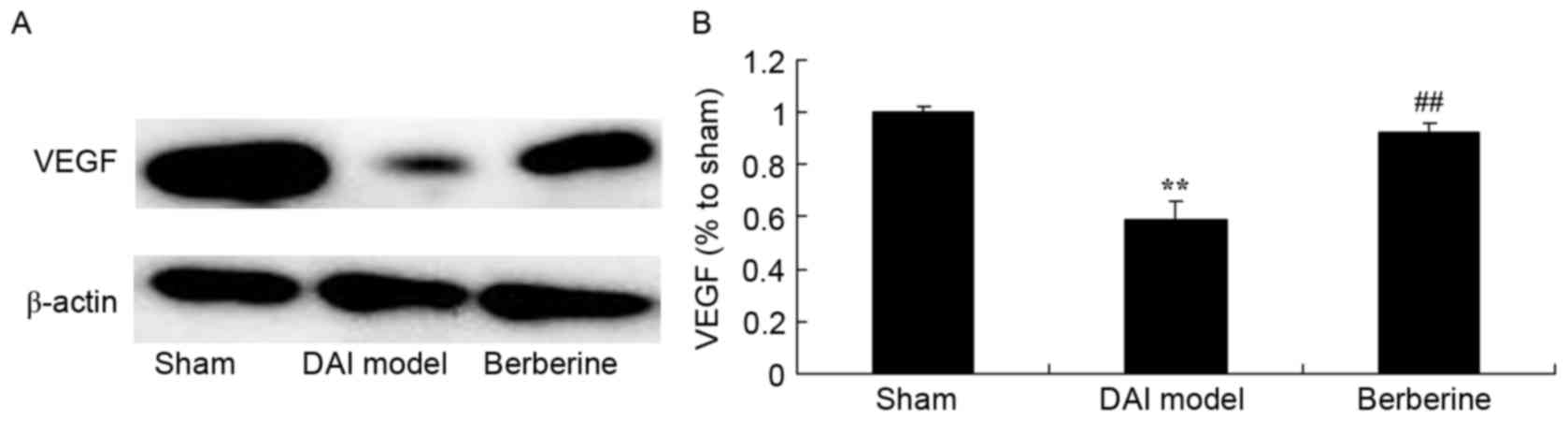
against reduced VEGF protein expression in DAI
The effect of berberine on VEGF protein expression
in DAI rats was further examined using western blot assay. Compared
with the sham group, the induction of DAI was found to markedly
suppress the expression of VEGF protein in the model group
(Fig. 9). Treatment with berberine
significantly recovered the protein expression of VEGF in DAI rats
(Fig. 9).
Discussion
The functions of extensive cerebral contusion and
laceration, and high intracranial pressure subsequent to DAI result
in increased production of pro-inflammatory cytokines, while the
inflammatory cascade reaction is initiated, which triggers
secondary cerebral lesion, cerebral metabolic changes and brain
death (13). It has been proven
that, after cerebral lesion, the concentrations of plasma TNF-α,
IL-6 in DAI and IL-1β are increased (14). Meanwhile, encephal edema,
hyperglycemia and subarachnoid hemorrhage further aggravate DAI and
destroy the BBB. This promotes the expression of biological effects
of pro-inflammatory mediators and inflammatory mediators, including
the increase of neutrophil granulocytes, fever and the increase of
endothelial permeability (15). The
present study demonstrated that treatment with berberine
significantly improved learning and memory in DAI rats. Certain
studies have suggested that berberine protects against brain
ischemia through regulatory effects on the Akt/glycogen synthase
kinase 3β and extracellular signal-regulated kinase 1/2 survival
and apoptotic signaling pathways, respectively (16,17).
Thus, the inhibitory effect of berberine may improve DAI-induced
learning and memory deficits in rats.
Monocytes, macrophages, endothelial cells and
contractile fiber cells express MCP-1 (18,19). The
major biological effects of MCP-1 are the chemotaxis of monocytes
and that it can act on lymphocytes and basophilic granulocytes,
while it has no biological effect on neutrophil granulocytes. MCP-1
receptor is a member of the g-protein coupled receptor super-family
(18). Following the combination of
MCP-1 and its targeted specific receptor, the receptor of MCP-1 is
activated, thus activating the phosphoinositide 3-kinase pathway
through g-protein coupling on the cytomembrane (20). Meanwhile, MCP-1 activation may
trigger the release of calcium ion in cytoplasm and induce the
activation of protein kinase C. Subsequent to DAI, injured cerebral
tissues produce various inflammatory chemokine factors. One of
these factors is MCP-1, which is a major inflammatory chemokine
factor that induces strong chemotaxis to monocytes/macrophages
(18). Monocytes/macrophages gather
in inflammatory response regions and participate in the occurrence
and progression of inflammatory response in DAI. Xu et al
(9) demonstrated that berberine
attenuated cigarette smoke-induced TNF-α, IL-1β and MCP-1
expression in mice. The present study showed that berberine
significantly reduced the concentrations of TNF-α and IL-1β, and
also reduced MCP-1 concentration, in DAI rats. These results
suggested that berberine had an anti-inflammation effect in DAI
rats.
NF-κB is a group of transcription factors of
eukaryotes, which is widely distributed in the nervous system. When
cells are stimulated, the phosphorylation and ubiquitylation are
initiated through a second messenger system. NF-κB and IκB are
activated and shifted into the karyon from the cytoplasm. It has
been observed that NF-κB was activated in rats with DAI at the
early stages of injury (21).
Activated NF-κB was identified in the cytoplasm and cell nucleus of
neurons after 24 h of injury. During the acute inflammatory
reaction process, NF-κB participates in the activation of
macrophages and hemamoeba, and controls the genetic expression of
proinflammatory factors (22).
Controlling this process would lead to the amplification of
inflammatory responses and tissue injuries (23). Chen et al reported that
berberine protects against neuronal damage via suppression of
inflammation and TLR4/MyD88/NF-κB signaling in traumatic brain
injury (11). In the present study,
berberine pretreatment was found to significantly suppress the
protein expression of NF-κB/p65 in DAI rats.
The activities of p38 MAPK are significantly
increased in microglial cells. Activated p38 MAPK is located in the
karyon or endochylema, which is possibly associated with the
functions and status of microglial cells. In addition, p38 MAPK
expression is correlated with the activities of NF-κB (24), thus, a p38 MAPK inhibitor may block
the transcription of NF-κB. Major biological functions after the
activation of p38 MAPK include generation and activation of various
inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8
(25). Macrophages at an ischemic
core region may present activated p38 MAPK, indicating that p38
MAPK may participate in the inflammatory responses when cerebral
ischemic injury occurs (26).
Activated p38 MAPK can enter into the cell nucleus or shift to
other regions (27), and may further
activate various transcription factors, including ATF-2/6, ATH-1/2,
ETS21, MAX, HSF21, myocyte enhancer binding factor-22, nuclear
transcription factor 2P, CHOP/GADD153, Elk-1 and SAP-1 (28). A study by Liu et al indicated
that berberine reduces fibronectin and collagen accumulation
through the p38 MAPK signaling pathway in rat glomerular mesangial
cells (29). The present study also
observed that berberinesignificantly suppressed the protein
expression levels of cytochrome c and p-p38 MAPK in DAI
rats.
VEGF is selectively distributed among the
endochylema and cell membrane of vascular endothelial cells, and is
highly expressed in the traumatic and tumor growth processes
(30). VEGF is known to be the
strongest antagonist, which can increase the permeability of
endothelial cells. It promotes angiogenesis and is a key regulatory
factor (24), while also promoting
the proliferation of endothelial cells. In addition, VEGF induces
endothelial cells to express proteolytic enzyme, interstitial
collagenase and tissue factors. The current study observed that
berberine activated ATF-2 and VEGF protein expression in DAI rats.
Similarly, Tsang et al demonstrated that berberine
suppressed the growth and development of lung metastases through
HIF-1α/VEGF signaling in hepatocellular carcinoma (10).
In conclusion, the present study confirmed that
berberine exerted a neuroprotective effect in DAI rats by improving
learning and memory, and suppressing inflammation. This
neuroprotective action resulted from inhibition of NF-κB/p65,
cytochrome c and p-p38 MAPK levels, as well as activation of
the ATF-2 and VEGF signaling pathway. Thus, these findings suggest
that berberine may be a potential drug in the prevention of DAI in
clinical practice.
Acknowledgements
The current study was supported by the Ningbo
Committee of Science and Technology (grant nos. 2011A610041 and
2014C50089) and the Ningbo Social Development Research Project
(grant nos. 2013A05 and 2014A03).
References
|
1
|
Vuletic S, Bell KR, Jain S, Bush N, Temkin
N, Fann JR, Stanfill KE, Dikmen S, Brockway JA, He F, et al:
Telephone problem-solving treatment improves sleep quality in
service members with combat-related mild traumatic brain injury:
Results from a randomized clinical trial. J Head Trauma Rehabil.
31:147–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakashima T, Nakayama N, Miwa K, Okumura
A, Soeda A and Iwama T: Focal brain glucose hypometabolism in
patients with neuropsychologic deficits after diffuse axonal
injury. AJNR Am J Neuroradiol. 28:236–242. 2007.PubMed/NCBI
|
|
3
|
Meythaler JM, Brunner RC, Johnson A and
Novack TA: Amantadine to improve neurorecovery in traumatic brain
injury-associated diffuse axonal injury: A pilot double-blind
randomized trial. J Head Trauma Rehabil. 17:300–313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suehiro E, Fujisawa H, Koizumi H, Nomura
S, Kajiwara K, Fujii M and Suzuki M: Significance of differences
between brain temperature and core temperature (δ T) during mild
hypothermia in patients with diffuse axonal injury. Neurol Med Chir
(Tokyo). 51:551–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Y and Wen L: Inflammatory response
following diffuse axonal injury. Int J Med Sci. 10:515–521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su E and Bell M: Diffuse axonal
injuryLaskowitz D and Grant G: Source Translational Research in
Traumatic Brain Injury. Boca Raton (FL): CRC Press/Taylor and
Francis Group. Front Neurosci; 2016
|
|
7
|
Dai P, Wang J, Lin L, Zhang Y and Wang Z:
Renoprotective effects of berberine as adjuvant therapy for
hypertensive patients with type 2 diabetes mellitus: Evaluation via
biochemical markers and color Doppler ultrasonography. Exp Ther
Med. 10:869–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed T, Gilani AU, Abdollahi M, Daglia M,
Nabavi SF and Nabavi SM: Berberine and neurodegeneration: A review
of literature. Pharmacol Rep. 67:970–979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu D, Wan C, Wang T, Tian P, Li D, Wu Y,
Fan S, Chen L, Shen Y and Wen F: Berberine attenuates cigarette
smoke-induced airway inflammation and mucus hypersecretion in mice.
Int J Clin Exp Med. 8:8641–8647. 2015.PubMed/NCBI
|
|
10
|
Tsang CM, Cheung KC, Cheung YC, Man K, Lui
VW, Tsao SW and Feng Y: Berberine suppresses Id-1 expression and
inhibits the growth and development of lung metastases in
hepatocellular carcinoma. Biochim Biophys Acta. 1852:541–551. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CC, Hung TH, Lee CY, Wang LF, Wu CH,
Ke CH and Chen SF: Berberine protects against neuronal damage via
suppression of glia-mediated inflammation in traumatic brain
injury. PLoS One. 9:e1156942014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HC, Duan ZX, Wu FF, Xie L, Zhang H
and Ma YB: A new rat model for diffuse axonal injury using a
combination of linear acceleration and angular acceleration. J
Neurotrauma. 27:707–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moein P, Abbasi Fard S, Asnaashari A,
Baratian H, Barekatain M, Tavakoli N and Moein H: The effect of
Boswellia Serrata on neurorecovery following diffuse axonal injury.
Brain Inj. 27:1454–1460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mofid B, Soltani Z, Khaksari M, Shahrokhi
N, Nakhaee N, Karamouzian S, Ahmadinejad M, Maiel M and Khazaeli P:
What are the progesterone-induced changes of the outcome and the
serum markers of injury, oxidant activity and inflammation in
diffuse axonal injury patients? Int Immunopharmacol. 32:103–110.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang SH, Kim SH, Kim OR, Byun WM, Kim MS,
Seo JP and Chang MC: Cingulum injury in patients with diffuse
axonal injury: A diffusion tensor imaging study. Neurosci Lett.
543:47–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen M, Tan M, Jing M, Liu A, Liu Q, Wen
S, Chen Z, Chao X, He X, Ramassamy C, et al: Berberine protects
homocysteic acid-induced HT-22 cell death: Involvement of Akt
pathway. Metab Brain Dis. 30:137–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simões Pires EN, Frozza RL, Hoppe JB,
Menezes Bde M and Salbego CG: Berberine was neuroprotective against
an in vitro model of brain ischemia: Survival and apoptosis
pathways involved. Brain Res. 1557:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rancan M, Otto VI, Hans VH, Gerlach I,
Jork R, Trentz O, Kossmann T and Morganti-Kossmann MC: Upregulation
of ICAM-1 and MCP-1 but not of MIP-2 and sensorimotor deficit in
response to traumatic axonal injury in rats. J Neurosci Res.
63:438–446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Zhang Y, Dai D and Xu Z: Expression
of NF-κB, MCP-1 and MMP-9 in a cerebral aneurysm rabbit model. Can
J Neurol Sci. 41:200–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haskins M, Jones TE, Lu Q and Bareiss SK:
Early alterations in blood and brain RANTES and MCP-1 expression
and the effect of exercise frequency in the 3xTg-AD mouse model of
Alzheimer's disease. Neurosci Lett. 610:165–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guedes RP, Csizmadia E, Moll HP, Ma A,
Ferran C and da Silva CG: A20 deficiency causes spontaneous
neuroinflammation in mice. J Neuroinflammation. 11:1222014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu YY, Huang M, Dong XQ, Xu QP, Yu WH and
Zhang ZY: Ginkgolide B reduces neuronal cell apoptosis in the
hemorrhagic rat brain: Possible involvement of Toll-like receptor
4/nuclear factor-kappa B pathway. J Ethnopharmacol. 137:1462–1468.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fei XJ, Zhu LL, Xia LM, Peng WB and Wang
Q: Acanthopanax senticosus attenuates inflammation in
lipopolysaccharide-induced acute lung injury by inhibiting the
NF-κB pathway. Genet Mol Res. 13:10537–10544. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berger S, Dyugovskaya L, Polyakov A and
Lavie L: Short-term fibronectin treatment induces endothelial-like
and angiogenic properties in monocyte-derived immature dendritic
cells: Involvement of intracellular VEGF and MAPK regulation. Eur J
Cell Biol. 91:640–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tovar-Y-Romo LB and Tapia R: VEGF protects
spinal motor neurons against chronic excitotoxic degeneration in
vivo by activation of PI3-K pathway and inhibition of p38MAPK. J
Neurochem. 115:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura H, Mikami D, Kamiyama K, Sugimoto
H, Kasuno K, Takahashi N, Yoshida H and Iwano M: Telmisartan, a
possible PPAR-δ agonist, reduces TNF-α-stimulated VEGF-C production
by inhibiting the p38MAPK/HSP27 pathway in human proximal renal
tubular cells. Biochem Biophys Res Commun. 454:320–327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang AY: Pro-life role for c-Jun
N-terminal kinase and p38 mitogen-activated protein kinase at
rostral ventrolateral medulla in experimental brain stem death. J
Biomed Sci. 19:962012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yosimichi G, Nakanishi T, Nishida T,
Hattori T, Takano-Yamamoto T and Takigawa M: CTGF/Hcs24 induces
chondrocyte differentiation through a p38 mitogen-activated protein
kinase (p38MAPK), and proliferation through a p44/42
MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem.
268:6058–6065. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu W, Tang F, Deng Y, Li X, Lan T, Zhang
X, Huang H and Liu P: Berberine reduces fibronectin and collagen
accumulation in rat glomerular mesangial cells cultured under high
glucose condition. Mol Cell Biochem. 325:99–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawamura H, Li X, Goishi K, van Meeteren
LA, Jakobsson L, Cébe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer
K, Kjellén L, et al: Neuropilin-1 in regulation of VEGF-induced
activation of p38MAPK and endothelial cell organization. Blood.
112:3638–3649. 2008. View Article : Google Scholar : PubMed/NCBI
|