Introduction
Diabetes mellitus (DM) refers to a group of
metabolic disorders characterized by hyperglycemia, which have a
severe impact on public health. To date, the majority of studies
examining DM complications have focused on the pathogenesis and
management of diabetic cystopathy, one of the most common
complications of DM (1,2). Diabetic cystopathy is considered to be
a manifestation of polyneuropathy, involving sensory and autonomic
nerve fibers 1 (3). A previous study
reported that DM induces damage to the innervation of the urethra,
together with reduction of urethral relaxation during micturition
reflex (4). DM may also induce
impairment of urethral smooth muscle, which is closely associated
with urethral dysfunction. However, few studies have been conducted
to investigate the mechanism of urethral dysfunction in DM, so this
remains unclear.
Precise coordination between smooth muscle in the
urethra and bladder is crucial for the balance between urine
storage and bladder emptying (4).
Such coordination is largely dependent on the nitric oxide (NO)
released from nerve endings of parasympathetic nerves, which serves
as a major neurotransmitter (5,6). NO can
diffuse into the smooth musculature of the urethra, in which it
stimulates the synthesis of cyclic guanosine monophosphate (cGMP)
and finally induces smooth muscle cell relaxation (7).
Previous studies (8,9) have
suggested that diabetic patients are under a state of oxidative
stress characterized by a relative overload of oxidants. In these
patients, free oxygen radicals, especially superoxide
(O2−), are aberrantly accumulated in
vivo. The increased O2−could reduce the
bioavailability of NO via binding with NO rapidly to form
peroxynitrite (ONOO) (10). Thus, it
seems reasonable to assume that urethral dysfunction during reflex
micturition in DM would be associated with the decreased
bioavailability of NO and/or the over-production of
ONOO−.
Grape seed proanthocyanidin extract (GSPE), a
natural polyphenolic compound extracted from grape seeds, has
exhibited a broad spectrum of pharmacological and therapeutic
properties against oxidative stress (11). Previously, studies have been
performed to investigate the protective effects of GSPE in various
disorders induced by oxidative stress, such as diabetic nephropathy
(12), obesity (13) and cardiac disorders (14). For the protective effects of GSPE in
rats with diabetic nephropathy, the authors concluded that GSPE
could ameliorate the diabetic nephropathy through a reduction in
oxidative stress and an increase in renal antioxidant enzyme
(12). Nuclear factor erythroid
2-related factor 2 (Nrf2) is involved in regulating the cellular
antioxidative responses and redox status. In addition, previous
results have suggested that activation of the Nrf2 pathway serves a
critical function in myocyte differentiation as well as muscular
contractile and metabolic properties in a diabetic model of muscle
atrophy (15). The present study
aimed to investigate the antioxidant effects of GSPE in the
pathogenesis of urethral dysfunction in DM, in order to evaluate a
potential mechanism of its protection against urethral dysfunction.
The precise protective effects of GSPE were also investigated.
Materials and methods
Animals and drugs
A total of 108 female Wistar rats (8-weeks-old,
weighing 257.7±18.21 g) were purchased from the Animal Center of
Shandong University (license number: SCXX20090010; Jinan, China).
The rats were fed under a 12 h light/dark cycle and had free access
to standard rat chow and tap water in an environment at 24°C with
60% humidity. The rats were randomly divided into three groups
(Fig. 1): i) control group (n=36),
administered with 0.1 M citrate buffer (pH 4.5, 4 ml/kgc, at no.
C-0071; Shanghai Enzyme-linked Biotechnology, Shanghai, China) via
intraperitoneal injection, followed by intragastric injection of
0.9% normal saline (4 ml/kg) once a day for 8 weeks; ii) DM group
(n=36), administered with a single dose of 65 mg/kg streptozotocin
(STZ; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) dissolved in
0.1 M citrate buffer (pH 4.5) via intraperitoneal injection,
followed by intragastric injection of 0.9% normal saline (4 ml/kg)
once a day for 8 weeks; and iii) DM+GSPE group (n=36), which were
administered with a single dose of 65 mg/kg STZ via intraperitoneal
injection followed by intragastric injection of GSPE extracted from
European red grape seed (Cabernet sauvignon) once a day for
8 weeks (250 mg/kg, proanthocyanidin content >96%; Tianjin
Jianfeng Natural Product R&D Co., Ltd., Tianjin, China).
Subsequently, 10 rats selected randomly from each group (10/36)
were placed in metabolic cages to measure body weight, blood
glucose and urine volume. All experimental procedures were
compliant with the Guidelines for Laboratory Animal Use and Care
from the Chinese Center For Disease Control and Prevention and the
Rules for Medical Laboratory Animals from the Chinese Ministry of
Health, under protocol CAU-AEC-2013-073 (16) and were approved by the Ethical
Committee of Qilu Hospital of Shandong University (Jinan, China;
permit no. DWLL-20B-025). All surgeries (urodynamic testing and
isolation of urethra) were performed under pentobarbital anesthesia
(intraperitoneal), and rats were sacrificed after sample collection
by pentobarbital anesthesia (intraperitoneal) followed by cervical
dislocation. All efforts were made to minimize suffering.
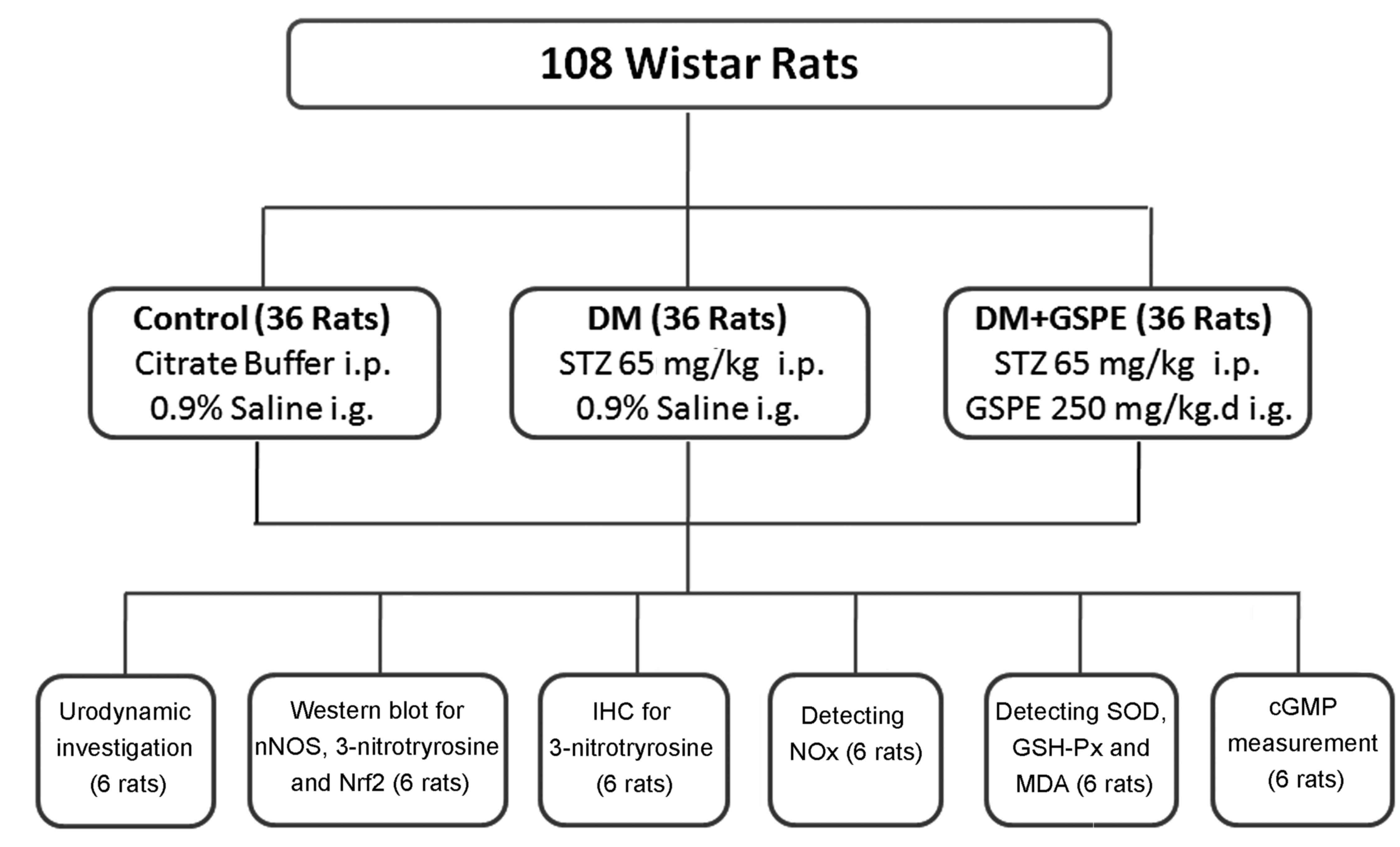 | Figure 1.Animal grouping and methods of
analysis. i.p., intraperitoneal injection; i.g., intragastric
administration; IHC, immunohistochemistry; STZ, streptozotocin;
GSPE, grape seed proanthocyanidin extract; nNOS, neural nitric
oxide synthase; Nrf2, nuclear factor erythroid 2-related factor 2;
NOx, total nitrate/nitrite; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; MDA, malondialdehyde; cGMP, cyclic
guanosine monophosphate. |
Simultaneous recordings of urethral
perfusion pressure (UPP) and intravesical pressure under
isovolumetric conditions
Simultaneous recordings of UPP and intravesical
pressure were monitored as previously described (17). Briefly, the rats (n=6 per group) were
anesthetized with 35 mg/kg pentobarbital via intraperitoneal
injection. Then, a midline abdominal incision was made to expose
the bladder and proximal urethra. The two ureters were tied
distally and cut to prevent urine filling in the bladder.
Subsequently, a bladder catheter and double lumen urethral catheter
were inserted through the bladder dome by separate cystostomy. Upon
fixing in the bladder neck, the double lumen urethral catheter
consisted of an outer cannula of PE-160 (160 µm) and an inner
cannula of PE-50 (50 µm) for infusing saline and recording UPP,
respectively. For the infusion of normal saline, the PE-160 cannula
was connected to a pump and the velocity of infusion was set at
0.075 ml per minute, in order to induce rhythmic isovolumetric
contractions. PowerLab V30 (Laborie Medical Technologies Inc.,
Beijing, China) was used to determine the intravesical pressure
under isovolumetric conditions, UPP nadir, baseline UPP and
intravesical pressure thresholds for inducing urethral relaxation
in each group.
Urethra tissue collection
Urethra tissue was collected and transferred to an
animal operating table. A 1.0 cm epidural sheath was catheterized
into the urethra as a support shaft to mark the urethra.
Afterwards, a midline abdominal incision was made to expose the
urethra. The urethra was distinguished along the sheath and was
removed for western blotting and immunohistochemistry. The isolated
tissues were stored at −80°C until analysis.
Western blot analysis
The expression of neural nitric oxide synthase
(nNOS), 3-nitrotyrosine and Nrf2 was determined using western
blotting. Urethra tissues were homogenized in lysis buffer (P0013B,
Beyotime Institute of Biotechnology, Haimen, China) containing
protease inhibitors. Protein concentration was determined using
commercial BCA kit (Beyotime Institute of Biotechnology). Protein
(40 µg) was separated on 10% SDS-PAGE gel and transferred to a 0.22
µm polyvinylidene difluoride membrane. The membranes were blocked
with 5% fat-free milk at 25°C for 1 h and incubated with primary
antibodies against nNOS (1:1,000; C12H1; Cell Signaling Technology,
Inc., Danvers, MA, USA), 3-nitrotyrosine (1:2,000; ab61392; Abcam,
Cambridge, UK) and Nrf2 (1:2,000; ab31163; Abcam) overnight at 4°C,
and then incubated with goat anti-rabbit secondary antibody
(1:4,000, cat no. sc2004; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 25°C for 1 h. The same membranes were probed with an
antibody against β-tubulin (1:400; cat no. AT809; Beyotime
Institute of Biotechnology) for loading control. The results were
visualized using an ECL chemiluminescence kit (cat no. P0018;
Beyotime Institute of Biotechnology) Finally, densitometric values
were quantified using a Kodak Image Station 2000 MM (Kodak,
Rochester, NY, USA).
Immunohistochemistry
Urethra tissue sections (3 µm) were fixed using 4%
paraformaldehyde for 24 h and embedded in paraffin at room
temperature. Upon dewaxing, the paraffin sections were immersed
into EDTA antigen retrieval solution (cat no. P0085; Beyotime
Institute of Biotechnology) at 92–98°C for 20 min, followed by 0.3%
hydrogen peroxide for 10 min to block endogenous peroxidase.
Afterwards, the sections were blocked using 5% bovine serum albumin
(cat no. ST023; Beyotime Institute of Biotechnology) for 30 min at
37°C, and incubated with rabbit monoclonal antibody against
3-nitrotyrosine (1:200; cat no. ab61392; Abcam) for 18 h at 4°C.
After washing with PBS, the sections were incubated with
peroxidase-conjugated secondary antibodies (1:200, cat no. sc-2345;
Santa Cruz Biotechnology, Inc.) at room temperature for 1 h.
Finally, the sections were stained using diaminobenzidine at room
temperature for 30 sec. Positive staining was visualized as a brown
reaction product in the cytoplasm. Histological analysis was
performed using a light microscope with a computerized imaging
system (Northern Eclipse 7.0; Empix Imaging, Inc., Mississauga, ON,
Canada) by two qualified observers blinded to the study. The degree
of staining was evaluated based on the intensity score, as
previously described (18), in which
a score of 0–3 represented absence of staining, weak, moderate and
strong staining, respectively. The proportional score was the
percentage of cells that stained positive in different cell groups
and was scored as: 0, no positivity; 1, <1–25%; 2, 26–50%; 3,
51–75%; 4, 76–100% positive staining.
Estimation of oxidative stress in the
urethra
Oxidative stress in the urethra was measured as
described previously (18).
Glutathione peroxidase (GSH-Px, cat no. S1058; Beyotime Institute
of Biotechnology), superoxide dismutase (SOD, cat no. S0109;
Beyotime Institute of Biotechnology) and malondialdehyde (MDA, cat
no. S1031, Beyotime Institute of Biotechnology) assays in urethra
tissues were conducted using ELISA kits and evaluated by
spectrophotometry, according to the manufacturer's protocol
(Beyotime Institute of Biotechnology). GSH-Px activity was measured
using the enzyme-catalyzed reaction product (reduced glutathione)
and the absorbance was recorded at a wavelength of 412 nm. The
measurement of SOD activity was based on the combination of
xanthine and xanthine oxidase at a wavelength of 550 nm. The MDA
level was detected using the thiobarbituric acid method with a
maximum absorbance at 532 nm (19).
Determination of nitrate/nitrite
Total nitrate/nitrite (NOx) levels, as
the final metabolites of NO, were measured in urethra samples using
an NOx colorimetric assay kit (cat no. S0023; Beyotime
Insititute of Biotechnology), according to the manufacturer's
protocol. The NOx production was determined with the use
of a Scientific Multiskan MK3 spectrophotometer (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 540 nm.
Measurement of urethral cGMP
content
Urethra tissues obtained from rats were used for the
urethral cGMP content determination. The concentration of cGMP was
evaluated using a cGMP-EIA kit purchased from Cayman Chemical
Company (cat no. 581021; Ann Arbor, MI, USA), according to the
manufacturer's protocol. The yellow-colored product formed with the
EIA is inversely proportional to the amount of cyclic nucleotide
present in the sample and was detected at 412 nm by a plate reader
(Victor2 1420; PerkinElmer, Inc., Waltham, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Experiments were performed at least in triplicate. Data were
analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). One-way
analysis of variance followed by a Tukey's post hoc test was used
for inter-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
GSPE exerts no obvious effects on body
weight, blood glucose or urine volume
A significant decrease in body weight was observed
in the DM group compared with the control (P<0.05; Table I). However, no significant difference
was observed in the DM + GSPE group compared with the DM group. The
concentration of blood glucose was significantly increased in the
DM group compared with the control group (P<0.05; Table I), but no significant difference in
blood glucose was observed in the DM + GSPE compared with the DM
group. The urine volume was significantly increased in the DM group
compared with the control group (P<0.05; Table I), but no significant difference in
urine volume was observed in the DM + GSPE compared with the DM
group.
 | Table I.Body weight, blood glucose and urine
volume. |
Table I.
Body weight, blood glucose and urine
volume.
| Group | Body weight
(g) | Blood glucose
(mg/dl) | 24-h urine volume
(ml) |
|---|
| Control (n=10) |
310.61±9.78 |
114.2±13.4 |
11.69±1.20 |
| DM group
(n=10) |
250.47±17.91a |
449.6±57.9a |
44.47±4.29a |
| DM+GSPE group
(n=10) |
254.42±19.32a |
443.3±47.0a |
42.74±4.03a |
GSPE ameliorates DM-induced
dysfunction in urethral relaxation
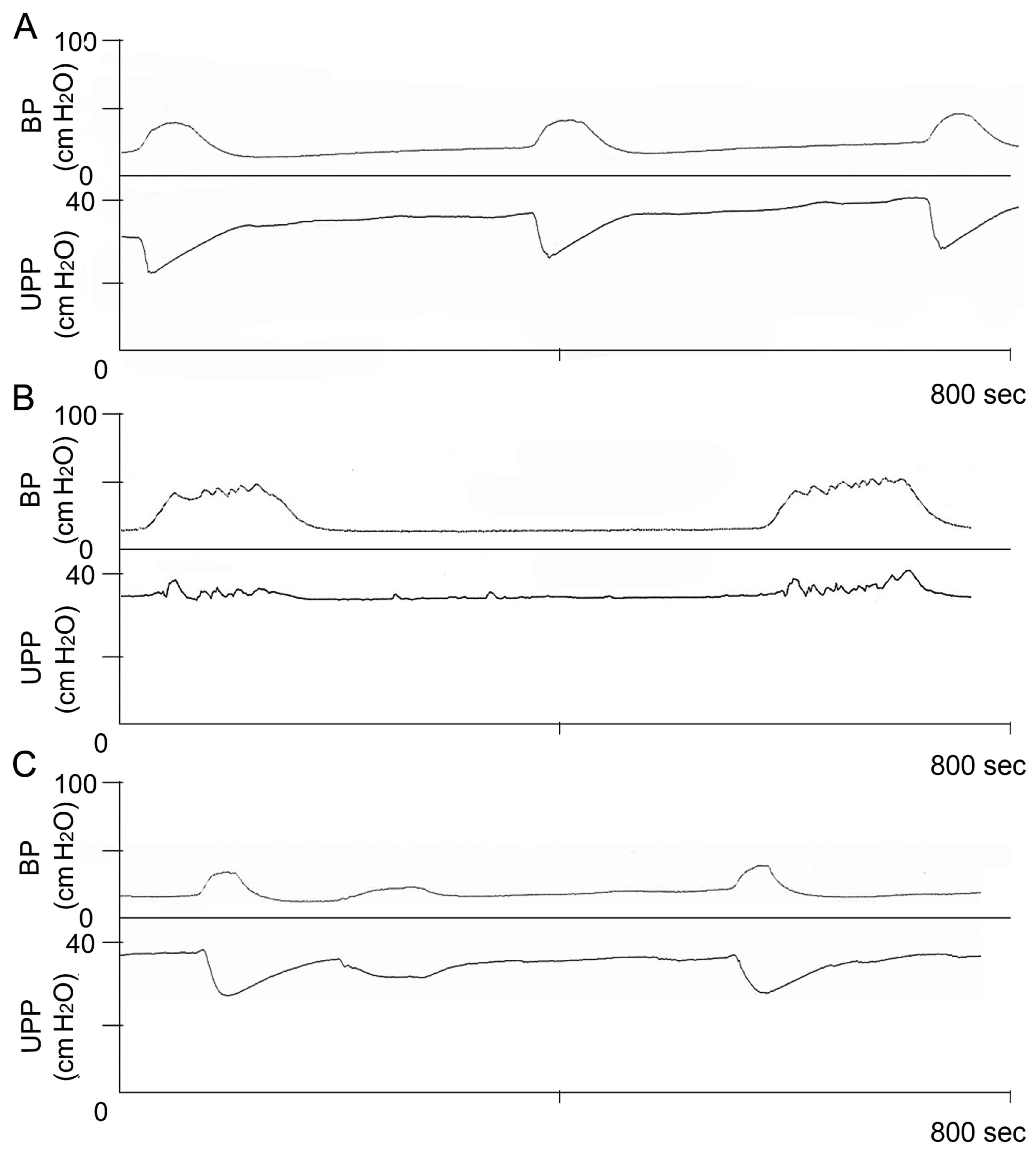
Baseline levels of UPP exhibited no obvious
difference among these groups (data not shown). Intravesical
pressure threshold for inducing urethral relaxation was
significantly higher in the DM group compared with the control
group. However, the threshold was significantly decreased in the DM
+ GSPE group compared with the DM group (P<0.05; Table II). Furthermore, the UPP nadir
during large amplitude bladder contractions was significantly
increased in the DM group compared with the control group. In the
DM + GSPE group, the UPP nadir was significantly decreased compared
with the DM group. These results indicated that DM induces
elevation of urethral pressure and dysfunction of urethral
relaxation, which subsequently resulted in urination disorder.
Whereas, GSPE ameliorated DM-induced dysfunction in urethral
relaxation (Fig. 2).
 | Table II.UPP and intravesical pressure. |
Table II.
UPP and intravesical pressure.
| Group | UPP nadir (cm
H2O) | Intravesical
pressure threshold for inducing urethral relaxation (cm
H2O) |
|---|
| Control (n=6) |
22.37±1.93a |
14.44±1.18a |
| DM group (n=6) |
36.24±2.66 |
24.24±1.15 |
| DM+GSPE group
(n=6) |
24.99±2.05a |
17.86±4.74a |
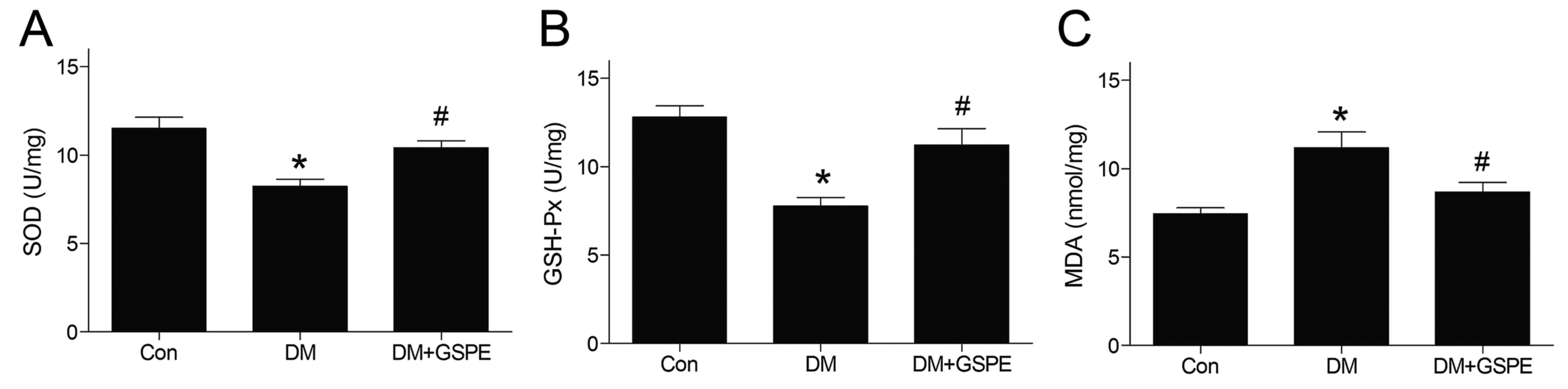
GSPE attenuates oxidative stress in
diabetic urethra
The activities of GSH-Px and SOD in urethra tissue
were significantly decreased in the DM group compared with the
control group (P<0.05; Fig. 3A and
B). However, GSPE administration significantly increased the
GSH-Px and SOD activities compared with the DM group (P<0.05;
Fig. 3A and B). The level of MDA in
the urethra was significantly increased in the DM group compared
with the control group (P<0.05; Fig.
3C). However, the level of MDA was decreased significantly
following the administration of GSPE (P<0.05; Fig. 3C).
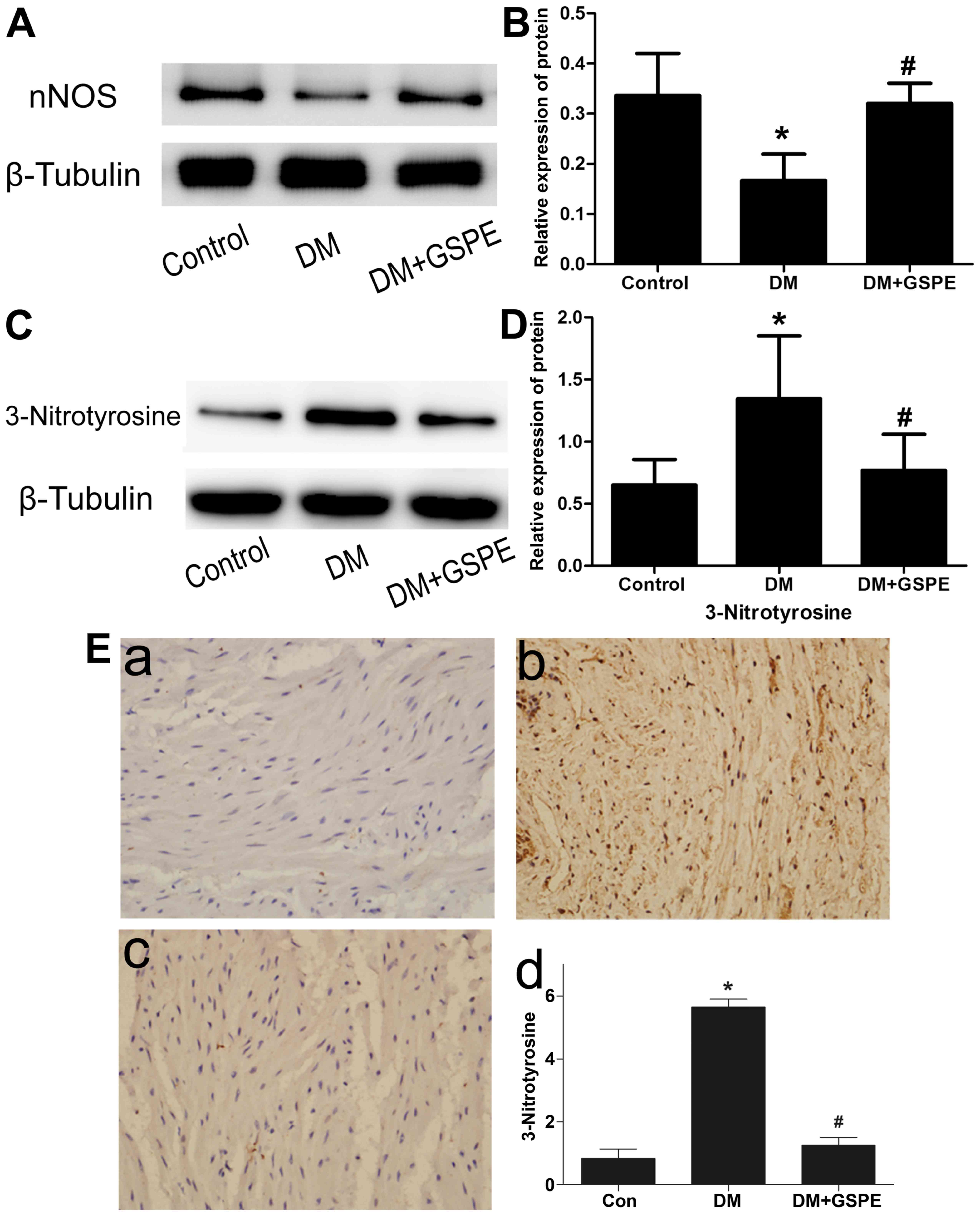
GSPE attenuates DM-induced dysfunction
in urethral relaxation via the NO-cGMP signaling pathway
To investigate how GSPE attenuated DM-induced
dysfunction in urethral relaxation, its role in the NO-cGMP
signaling pathway was evaluated. Western blotting was performed to
evaluate the expression of nNOS protein in each group. The results
indicated a significant decrease of nNOS expression in the DM group
compared with the control group (P<0.05; Fig. 4A and B). Compared with the DM group,
a significant increase in nNOS expression was observed in the DM +
GSPE group (P<0.05; Fig. 4A and
B). However, no significant difference in nNOS expression was
observed in the control group compared with the DM + GSPE group.
These results indicated that the protective effects of GSPE in
DM-induced urethral dysfunction are associated with the
upregulation of nNOS in vivo.
To analyze ONOO− formation in
vivo, western blotting and immunohistochemistry were performed
to determine the expression of 3-nitrotyrosine in the urethra.
Western blotting revealed that the expression of 3-nitrotyrosine in
the urethra was significantly increased in the DM group compared
with the control (P<0.05), and significantly decreased in the DM
+ GSPE group compared with the DM group (P<0.05; Fig. 4C and D). The immunohistochemistry
results indicated that the immunoreactivity of 3-nitrotyrosine was
low in the control group (Fig. 4E).
A significant increase in immunoreactivity of 3-nitrotyrosine was
observed in the DM group compared with the control group
(P<0.05; Fig. 4E), particularly
in the smooth muscle region. In the DM + GSPE group, a significant
decrease in the immunoreactivity of 3-nitrotyrosine was observed
compared with the DM group (P<0.05; Fig. 4E). These results suggested that GSPE
may attenuate the formation of ONOO− in vivo.
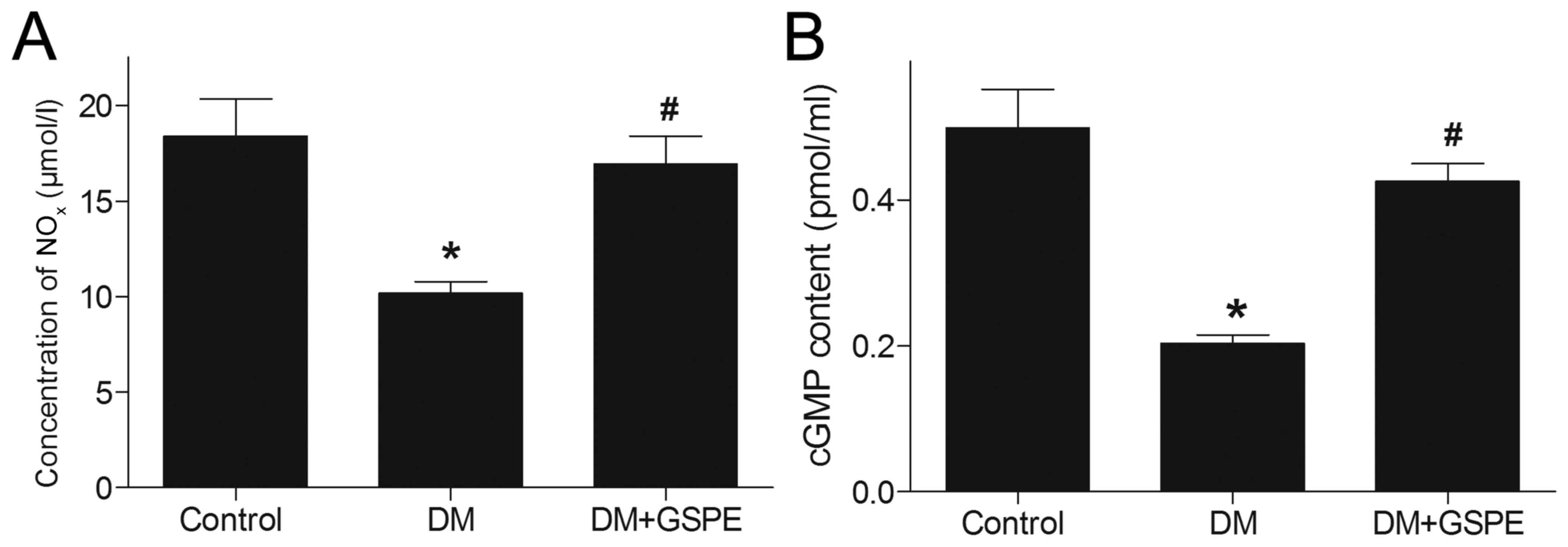
For the concentration of NOxN, DM induced
a significant decrease in NOx in vivo compared
with the control group (P<0.05; Fig.
5A). However, after administration of GSPE, NOx was
significantly increased compared with the DM group (P<0.05;
Fig. 5A). This suggested that GSPE
may upregulate the generation of NOx in vivo.
In the NO-cGMP signaling pathway, NO serves a key
function in the synthesis of cGMP, which is closely associated with
urethral relaxation during micturition reflex. In the present
study, a significant decrease in cGMP level was observed in the
urethra of DM rats compared with the control group (P<0.05;
Fig. 5B). By contrast, rats treated
with GSPE exhibited significantly increased cGMP levels in the
urethra compared with the DM group (P<0.05; Fig. 5B). This suggested that GSPE
contributes to the upregulation of cGMP in vivo.
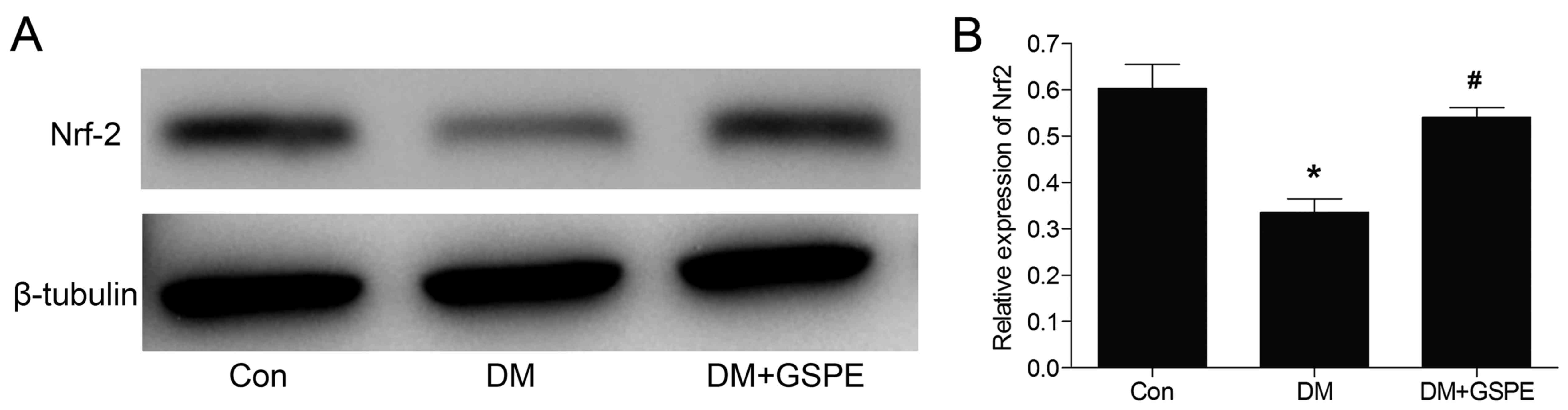
Protective effects of GSPE are
associated with activation of the Nrf2 pathway
The transcription factor Nrf2 is a vital mediator
involved in regulating cellular antioxidative responses. Activation
of the Nrf2 pathway leads to neuroprotective effects (20). In the present study, the effects of
GSPE on the expression of Nrf2 were investigated. Western blotting
(Fig. 6) results demonstrated that
the expression of Nrf2 was significantly decreased in the DM group
compared with the control (P<0.05). Nrf2 expression was
significantly increased following treatment with GSPE compared with
the DM group (P<0.05). These demonstrated that the protective
effects of GSPE were associated with the activation of the Nrf2
signaling pathway.
Discussion
DM is a major public health problem worldwide, which
causes severe complications and decreases quality of life for
sufferers. Although extensive studies have been conducted to
identify the potential mechanisms of urethral dysfunction, a common
complication in patients with DM (21,22), the
exact mechanism is still unclear. In the present study, the
antioxidant properties of GSPE were identified to be associated
with the repair of urethral dysfunction in DM rats.
Under in vivo conditions, NO is primarily
produced by three different NOS isoforms, including nNOS, inducible
NOS and endothelial NOS (23). nNOS
is specifically expressed in neurons of the nervous system, which
results in the generation of nNOS-derived NO. This serves as an
atypical neurotransmitter in the peripheral nervous system
(10). In addition, nNOS-derived NO
exhibits modulatory effects on parasympathetic nerves, which are
considered to serve a key function in urethral relaxation during
micturition reflex (6). The present
results demonstrated that nNOS production was significantly reduced
in the urethra of DM rats. However, in the GSPE group, the
production of nNOS was significantly elevated compared with the DM
group, indicating that GSPE-mediated protective effects on the
urethra may be associated with the elevation of nNOS.
Few studies have been conducted to investigate the
NO content in animals with urethral dysfunction. In a previous
study, NO production was reported to be increased in the urethra of
DM rabbits through the measurement of nicotinamide adenine
dinucleotide phosphate diaphorase, a marker of NOS activity
(24). However, in the present
study, the concentration of NO was determined directly in DM rats,
and the results indicated that the NO concentration was
significantly decreased in DM rats. Further studies are required in
order to explain these inconsistent results.
Previous reports have demonstrated that the cGMP
signaling pathway serves a critical function in the regulation of
micturition reflex (25,26). It is widely acknowledged that NO is
involved in the mediation of urethral relaxation as it can act on a
number of target enzymes and proteins, and is particularly
associated with the activation of soluble guanylyl cyclase and the
generation of cGMP (10,27). In the DM group of the present study,
the expression of cGMP was downregulated. However, in the DM + GSPE
group, the expression of cGMP was upregulated compared with the DM
group. These results suggested that GSPE may contribute to the
activity of the NO-cGMP signaling pathway.
O2− can combine with NO to
form ONOO− at a fast rate in DM (10). The generated ONOO− then
inactivates nNOS directly and results in a decrease of the
availability of free NO (10).
ONOO− may also damage NOS coupling through oxidization
of tetrahydrobiopterin to trihydrobiopterin radical, which results
in the accumulation of O2− rather than NO
(10). Furthermore, ONOO−
may induce toxicity due to its high reactivity and diffusibility
(28). Therefore, extensive studies
have been performed to reduce oxidative injury through preventing
the generation of ONOO (29). In the
present study, the effects of GSPE on oxidative stress were
investigated in diabetic urethropathy. The results indicated that
GSPE could protect urethral function by scavenging free oxygen
radicals and enhancing the activities of GSH-Px and SOD.
Interestingly, no notable effects were observed on weight loss,
hyperglycemia or polyuria in rats with administration of GSPE. On
this basis, it can be speculated that GSPE may exert protective
effects on the urethra rather than pancreatic beta cells.
The Nrf2 signaling pathway is known to exert
antioxidative effects on numerous cell types (30). Food polyphenols could activate the
Nrf2 signal pathway (31). In the
present study, the results indicated that administration of GSPE
could enhance the activity of the Nrf2 pathway in DM rats. In
addition, changes in the activity of SOD and GSH-Px corresponded
with changes in the Nrf2 pathway. Thus, the current findings
suggested that the protective effects of GSPE on the urethra of DM
rats may be associated with activation of the Nrf2 signaling
pathway.
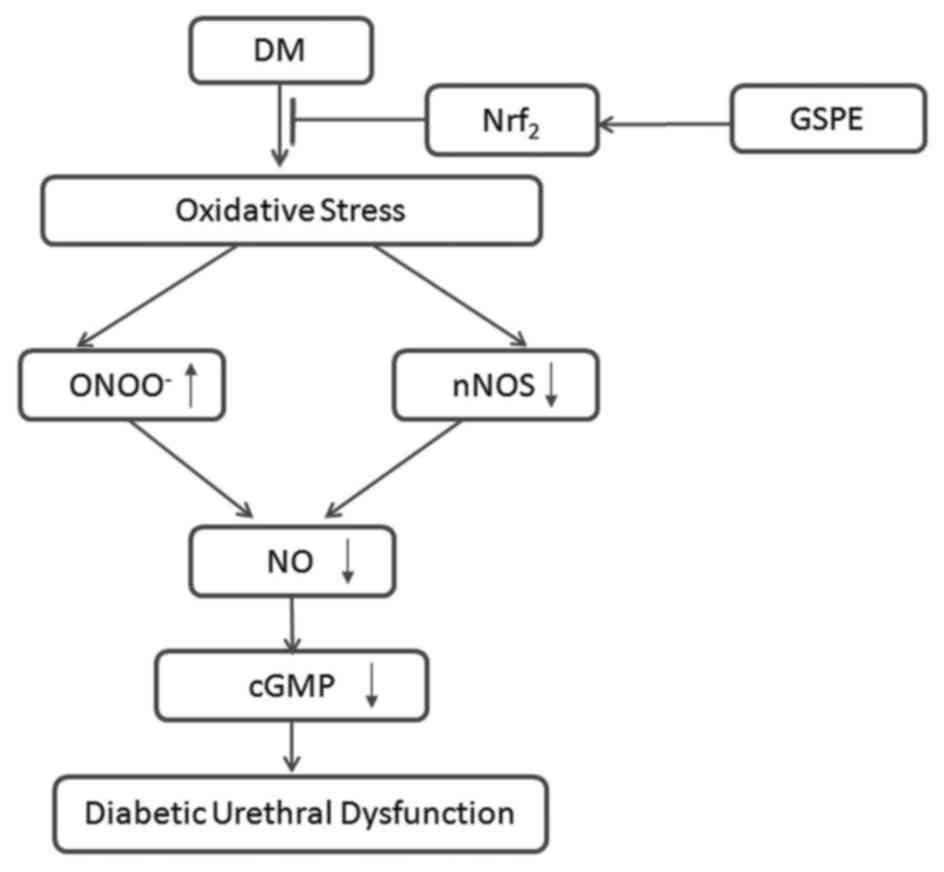
In summary, the present study demonstrated that GSPE
exerts protective effects against diabetic urethral dysfunction in
rats. A possible mechanism for this effect is proposed in Fig. 7. DM could induce oxidative stress,
followed by downregulation of nNOS and upregulation of
ONOO−, which synergically results in a decrease of NO
formation and bioavailability. Subsequently, this could induce the
downregulation of cGMP and cause urethral dysfunction. GSPE could
alleviate oxidative stress-induced injuries in DM rats through Nrf2
signaling pathways.
There are certain limitations of the present study.
GSPE is a compound rather than a single monomer. Although the
results indicate that GSPE exerts a protective effect on urethra
function by modulating the NO-cGMP signaling pathway, the exact
active component in GSPE could not be confirmed. Future studies
will focus on identifying the active component in GSPE, in order to
provide a basis for the research and development of drugs for
alleviating urethral dysfunction.
In conclusion, urethral relaxation during reflex
bladder contractions is impaired in DM rats. The relaxation may be
disturbed by oxidative stress, which would attenuate nNOS and NO
levels, finally resulting in a decrease of cGMP formation. GSPE
could reduce oxidative stress and protect urethra function by
preserving nNOS and NO levels, and contribute to the restoration of
urethral relaxation. GSPE treatment may attenuate DM-induced
oxidative damage by activating Nrf2.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470987 and
81170702), the Science and Technology Development Project of
Shandong Province (grant no. 2014GSF118054) and the Medical and
Health Science and Technology Development Project of Shandong
Province (grant no. 2016WS0019)
References
|
1
|
Yonekubo S, Tatemichi S, Maruyama K and
Kobayashi M: Alpha1A-adrenoceptor antagonist improves underactive
bladder associated with diabetic cystopathy via bladder blood flow
in rats. BMC Urol. 17:642017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong X, Song Q, Zhu J, Zhao J, Liu Q,
Zhang T, Long Z, Li J, Wu C, Wang Q, et al: Interaction of
Caveolin-3 and HCN is involved in the pathogenesis of diabetic
cystopathy. Sci Rep. 6:248442016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki K, Chanceller MB, Phelan MW,
Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC and
Yoshimura N: Diabetic cystopathy correlates with a long-term
decrease in nerve growth factor levels in the bladder and
lumbosacral dorsal root Ganglia. J Urol. 168:1259–1264. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Z, Dolber PC and Fraser MO: Diabetic
urethropathy compounds the effects of diabetic cystopathy. J Uorl.
178:2213–2219. 2007. View Article : Google Scholar
|
|
5
|
Bennett BC, Kruse MN, Roppolo JR, Flood
HD, Fraser M and de Groat WC: Neural control of urethral outlet
activity in vivo: Role of nitric oxide. J Urol. 153:2004–2009.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Persson K, Alm P, Uvelius B and Andersson
KE: Nitrergic and cholinergic innervation of the rat lower urinary
tract after pelvic ganglionectomy. Am J Physiol. 274:R389–R397.
1998.PubMed/NCBI
|
|
7
|
Warner TD, Mitchell JA, Sheng H and Murad
F: Effects of cyclic GMP on smooth muscle relaxation. Adv
Pharmacol. 26:171–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beshay E and Carrier S: Oxidative stress
plays a role in diabetes-induced bladder dysfunction in a rat
model. Urology. 64:1062–1067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szaleczky E, Prechl J, Feher J and Somogyi
A: Alternation in enzymatic antioxidant defence in diabetes
mellitus-a rational approach. Postgrad Med J. 75:8791999.
View Article : Google Scholar
|
|
10
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837,
837a-837d. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Dai X, Jiang Y, Zhang Z, Bao L, Li
Y, Zhang F, Ma X, Cai X, Jing L, et al: Grape seed proanthocyanidin
extracts alleviate oxidative stress and ER stress in skeletal
muscle of low-dose streptozotocin- and high-carbohydrate/high-fat
diet-induced diabetic rats. Mol Nutri Food Res. 57:365–369. 2013.
View Article : Google Scholar
|
|
12
|
Mansouri E, Panahi M, Ghaffari MA and
Ghorbani A: Effects of grape seed proanthocyanidin extract on
oxidative stress induced by diabetes in rat kidney. Iran Biomed J.
15:100–106. 2011.PubMed/NCBI
|
|
13
|
Casanova E, Baselga-Escudero L,
Ribas-Latre A, Cedó L, Arola-Arnal A, Pinent M, Bladé C, Arola L
and Salvadó MJ: Chronic intake of proanthocyanidins and
docosahexaenoic acid improves skeletal muscle oxidative capacity in
diet-obese rats. J Nutri Biochem. 25:1003–1010. 2014. View Article : Google Scholar
|
|
14
|
Peng N, Clark JT, Prasain J, Kim H, White
CR and Wyss JM: Antihypertensive and cognitive effects of grape
polyphenols in estrogen-depleted, female, spontaneously
hypertensive rats. Am J Physiol Regul Integr Comp Physiol.
289:R771–R775. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whitman SA, Long M, Wondrak GT, Zheng H
and Zhang DD: Nrf2 modulates contractile and metabolic properties
of skeletal muscle in streptozotocin-induced diabetic atrophy. Exp
Cell Res. 319:2673–2683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W, Zhu YH, Zhou D, Wu Q, Song D,
Dicksved J and Wang JF: Oral administration of a select mixture of
bacillus probiotics affects the gut Microbiota and goblet cell
function following Escherichia coli challenge in newly weaned pigs
of genotype muc4 that are supposed To Be Enterotoxigenic E. coli
F4ab/ac receptor negative. Appl Environ Microbiol.
83:pii:e02747–16. 2017. View Article : Google Scholar
|
|
17
|
Jung SY, Fraser MO, Ozawa H, Yokoyama O,
Yoshiyama M, De Groat WC and Chancellor MB: Urethral afferent nerve
activity affects the micturition reflex; implication for the
relationship between stress incontinence and detrusor instability.
J Urol. 162:204–212. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zik B, Altunbas K, Tutuncu S, Ozden O,
Ozquden Akkoc CG, Peker S and Sevimli A: Effects of capsaicin on
nitric oxide synthase isoforms in prepubertal rat ovary. Biotech
Histochem. 87:218–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang D, Li Y, Hou G, Wang P, Zhang J,
Laudon V and Shi B: Pygeum africanum: Effect on oxidative stress in
early diabetes-induced bladder. Int Urol Nephrol. 42:401–408. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, An C, Gao Y, Leak RK, Chen J and
Zhang F: Emerging roles of Nrf2 and phase II antioxidant enzymes in
neuroprotection. Proq Neurobiol. 100:30–47. 2013. View Article : Google Scholar
|
|
21
|
Torimoto K, Hirao Y, Matsuyoshi H, De
Groat WC, Chancellor MB and Yoshimura N: Alpha1-adrenergic
mechanism in diabetic urethral dysfunction in rats. J Urol.
173:1027–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masuda H, Tsujii T, Okuno T, Kihara K,
Goto M and Azuma H: Localization and role of nitric oxide synthase
and endogenous nitric oxide synthase inhibitors in the rabbit lower
urinary tract. J Urol. 167:2235–2240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Jamal J, Plaza C, Pineda SH, Chreifi
G, Jing Q, Cinelli MA, Silverman RB and Poulos TL: Structures of
human constitutive nitric oxide synthases. Acta Crystallogr D Biol
Crystallogr. 70:2667–2674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mumtaz FH, Sullivan ME, Thompson CS,
Dashwood MR, Naseem KM, Bruckdorfer KR, Mikhailidis DP and Morgan
RJ: Alterations in the nitric oxide synthase binding sites and
non-adrenergic, non-cholinergic mediated smooth muscle relaxation
in the diabetic rabbit bladder outlet: Possible relevance to the
pathogenesis of diabetic cystopathy. J Urol. 162:558–566. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warner T, Mitchell JA, Sheng H and Murad
F: Effects of cyclic GMP on smooth muscle relaxation. Adv
Pharmacol. 26:171–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Z, Wei RB, Hong Q, Cui SY and Chen
XM: Grape seed extract enhances eNOS expression and NO production
through regulating calcium-mediated AKT phosphorylation in
H2O2-treated endothelium. Cell Biol Int. 34:1055–1061. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caremel R, Oger-Roussel S, Behr-Roussel D,
Grise P and Giuliano FA: Nitric oxide/cyclic guanosine
monophosphate signalling mediates an inhibitory action on sensory
pathways of the micturition reflex in the rat. Eur Urol.
58:616–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marla SS, Lee J and Groves JT:
Peroxynitrite rapidly permeates phospholipid membranes. Proc Natl
Acad Sci USA. 94:pp. 14243–14248. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szabó C: The pathophysiological role of
peroxynitrite in shock, inflammation, and ischemia-reperfusion
injury. Shock. 6:79–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi M and Yamamoto M: Molecular
mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene
regulation. Antioxid Redox Signal. 7:385–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niture SK, Khatri R and Jaiswal AK:
Regulation of Nrf2-an update. Free Radic Biol Med. 66:36–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|