Introduction
Ovarian cancer presents as a complex cystic mass in
the pelvis and is the leading cause of mortality amongst all types
of gynecological cancer (1,2). No anatomical barrier exists to prevent
the widespread metastasis of ovarian cancer (3). Currently, the most effective therapy
for this disease is cytoreductive surgery alongside chemotherapy
regimens; these predominantly use platinum analogues with the
addition of a taxane and induce apoptosis to kill cancer cells
(3,4). However, the overall cure rate of
patients with ovarian cancer remains low at ~30% and the survival
rate at 5 years is 38%, meaning a more effective therapy is
urgently needed (3).
Ionizing radiation, including X-rays and
α-particles, is often used to treat a variety of types of cancer
(5–7). The radiation can induce cell death
through a variety of different mechanisms, including apoptosis,
autophagy, necrosis and accelerated senescence (7). The type of radiation used, the dosage
and the region of the body targeted all determine the type of cell
death that occurs. When treating cancer with irradiation, the
degree of carcinogenic risk and radioresistance must be taken into
account. To help improve understanding of this, it has been
reported that the combination of radiotherapy with a
radiosensitizing agent was being investigated (8).
Pseudolaric acid B (PAB) is present in a traditional
Chinese medicine named ‘Tu-Jin-Pi’, which is derived from
Pseudolarix kaempferi Gordon (9). Previous studies have demonstrated that
PAB can have a range of different pharmacological effects,
including antifungal, antimicrobial, antifertility and cytotoxic
effects (9–12). It has been reported that PAB
possesses antitumor activity against an ovarian cancer cell line by
inducing caspase-dependent apoptosis (13). However, few studies have focused on
investigating the potential combination of radiotherapy and
PAB.
The present study aimed to investigate the
anticancer activity of PAB, and the combination of PAB and
irradiation as a therapy against human ovarian cancer cells. First,
it was established that the combination of PAB with irradiation was
a potential treatment against ovarian cancer cells. Secondly, the
type of cell death taking place and the underlying mechanisms
causing it were investigated using the ovarian cancer cell line
SKOV-3.
Materials and methods
Reagents and chemicals
PAB was obtained from the National Institute for the
Control of Pharmaceutical and Biological Products (Beijing, China)
with a purity of >98%. PAB was dissolved in dimethyl sulfoxide
(DMSO) to make a stock solution, which was then diluted using
McCoy's 5A (modified) medium (Invitrogen, Thermo Fisher Scientific,
Inc., Waltham, MA, USA) to a final concentration of DMSO of
<0.05%, which was considered to have no detectable effects on
cell growth and viability. Trypsin, EDTA, PBS, monodansylcadaverine
(MDC), propidium iodide (PI) and Annexin V were all purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). McCoy's 5A
(modified) medium and fetal bovine serum (FBS) were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Primary antibodies
directed against microtubule-associated proteins 1/2 light chain 3
(LC3-I/II), autophagy protein 5 (ATG5), β-actin, phosphorylated
(p)-Ras, p-RAF proto-oncogene serine/threonine-protein kinase (Raf)
and extracellular signal-regulated kinase (ERK) were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Secondary
antibodies were also obtained from Cell Signaling Technology, Inc.
All other chemical reagents used in the study were of analytical
reagent grade.
Cells and culture
The human ovarian adenocarcinoma SKOV-3 and OVCAR-3
cell lines were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). SKOV-3 cells are resistant to tumor
necrosis factor-induced apoptosis, and to several cytotoxic drugs,
including diphtheria toxin, cisplatin and Adriamycin (14). The cells were cultured in Invitrogen
McCoy's 5A (modified) medium supplemented with 10% FBS at a
temperature of 37°C with 5% CO2. The cells were passaged
with 0.25% (w/v) trypsin-0.53 mM EDTA solution. The cells were
assayed in the logarithmic growth phase.
Radiation
Cells were cultured at a density of
1.5×105 cells/well in a 6-well plate and pretreated with
PAB at different concentrations (2.5–10 µM) in a humidified
atmosphere of 37°C with 5% CO2 for 24 h. Then, the cells
were irradiated with an X-ray source using a MD2 Linear Accelerator
(Primus; Siemens AG, Munich, Germany) at a dose of 0–10 Gy.
Cell viability assay
An MTT assay was performed to determine cell
viability. The SKOV-3 or OVCAR-3 cells were seeded into 96-well
plates at a density of 1×104 cells/well. The cells were
treated with PAB (2.5, 5 or 10 µM) for 24 h, by which time they had
attained 70–80% confluence. Then, the cells were irradiated at 4
Gy. Afterwards, 10 µl MTT solution (5 g/l) was added to each well
and the plates were incubated at 37°C with 5% CO2 for
another 2 h. The resulting crystals were dissolved in 150 µl DMSO
and the absorbance (A) of the wells was measured using a microplate
reader. The cell growth inhibitory ratio (%) was calculated as
follows: (A490,control -
A490,sample)/(A490,control -
A490,blank) × 100.
Clonogenic assay
Cells were seeded into 6-well plates at a density of
2×102 cells/well and cultured at 37°C in an atmosphere
with 5% CO2 for 12 h. The cells were then treated with
PAB (2.5–10 µM) and/or irradiated (2–10 Gy). A total of 2 weeks
later, the colonies were fixed with 4% paraformaldehyde for 20 min
at room temperature, and stained with 0.2% crystal violet for 10
min at room temperature. The surviving fraction (%) was calculated
as follows: (1 - Nirradiated colonies/Ncontrol
colonies) × 100. All data were fitted into the linear
quadratic model using Graphpad Prism software (version 5; GraphPad
Software, Inc., La Jolla, CA, USA).
Observation of morphological
changes
The cells were seeded in 6-well plate at a density
of 1.5×105 cells/well, cultured overnight, then
irradiated at a dose of 4 Gy following PAB treatment (2.5–10 µM).
The cellular morphology was subsequently observed using a
phase-contrast microscope (Olympus Corporation, Tokyo, Japan).
Cell autophagy assay
Cell autophagy was measured using Sigma-Aldrich MDC
staining assay (cat. no. D4008; Sigma-Aldrich; Merck KGaA)
according to the manufacturer's instructions. The cells were seeded
at a density of 2×106 cells/well in a 6-well plate. Once
the cells reached 70–80% confluence they were treated with
different doses of PAB (2.5–10 µM) and irradiated at a dose of 4
Gy. After 24 h the cells were washed, the staining buffer of 0.05
mM MDC in PBS was added and the plates were incubated at 37°C for
30 min. The samples were analyzed using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). Apoptosis ratios were
analyzed by CellQuest software (version 3; BD Biosciences).
Cell apoptosis assay
Cell apoptosis was measured using an Annexin V/PI
staining assay. The cells were seeded at a density of
2×106 cells in a 6-well plate, and when the cells
reached 80% confluence they were treated with different doses of
PAB (2.5–10 µM) and irradiated with 4 Gy. After 24 h the cells were
washed with PBS, collected and then suspended in a Sigma-Aldrich
Annexin V binding buffer (Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions. Annexin V and PI (Sigma-Aldrich;
Merck KGaA) were added to the sample and it was incubated for 30
min at 37°C. The cell apoptosis rate was analyzed using a flow
cytometer (BD Biosciences). Autophagy ratios were analyzed by
CellQuest software (version 3; BD Biosciences).
Western blot analysis
The cells were seeded into 6-well plate at a density
of 1.5×105 cell/well, cultured overnight and then
treated with 2.5–10 µM PAB and 4 Gy radiation. After incubation for
24 h, the cells were washed, collected and then lysed using
radioimmunoprecipitation assay lysis buffer containing a protease
inhibitor cocktail (cOmplete™, Mini, EDTA-free Protease Inhibitor
Cocktail; Sigma-Aldrich; Merck KGaA). Protein concentration was
determined using the bicinchoninic acid protein assay. Samples
contain 30 µg proteins were loaded into each lane of an 8–12%
SDS-PAGE gel. Electrophoresis was performed at 120 V for 1 h to
separate the proteins. The proteins were transferred onto Millipore
Immobilon®-P transfer membrane (EMD Millipore,
Billerica, MA, USA) over 2 h at 100 mA. The membrane was blocked
using 5% non-fat dry milk in PBS for 2 h. The membranes were then
probed with antibodies directed against LC3-I/II (cat. no. 3868,
1:1,000), ATG5 (cat. no. 12994, 1:1,000), p-Ras (cat. no. 3321,
1:1,000), p-Raf (cat. no. 9421, 1:1,000), p-ERK (cat. no. 4370,
1:1,000) and β-actin (cat. no. 4970, 1:1,000) overnight at 4°C. The
membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit secondary antibodies (cat. no. 7074, 1:1,000) for 1 h
at room temperature and the proteins were then detected using
visualized by electrochemiluminescence (Pierce™ ECL Plus Western
Blotting Substate, cat. no. 32132; Thermo Fisher Scientific, Inc.).
The films were scanned and quantified using ImageJ software
(version 1.43; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All results are expressed as the mean ± standard
deviation of at least three repeats. A one-way ANOVA followed by a
Dunnett's post hoc test was performed to determine the statistical
differences among groups using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered statistically
significant. Four Gy radiation treatment group was considered as
control group in all assays.
Results
PAB enhances the radiosensitivity of
ovarian cancer cells
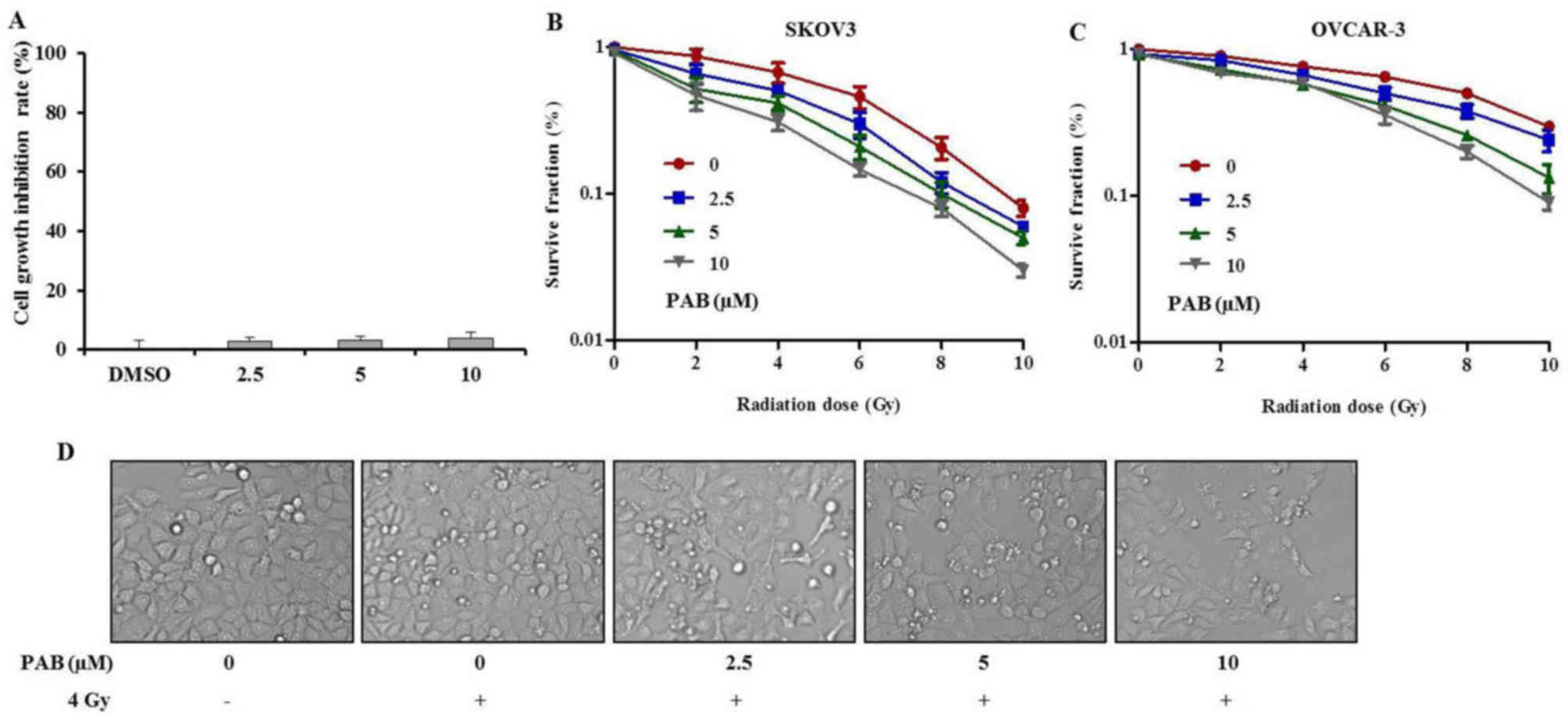
To examine the effects of PAB on SKOV-3 cells, an
MTT assay was performed (Fig. 1A).
Increasing doses of PAB were administered for 24 h to detect the
cytotoxicity of PAB. The results demonstrated that PAB had no
significant inhibitory effect on the growth of ovarian cells.
Following this, a clonogenic assay was performed to
investigate the effects of PAB on cell viability after irradiation.
The surviving fractions of SKOV-3 and OVCAR-3 cells decreased as
the doses of PAB and irradiation increased (Fig. 1B and C). Compared with OVCAR-3,
SKOV-3 cells were more sensitive to radiation. Thus, SKOV-3 cells
were used in subsequent assays. These results demonstrated that PAB
acted in synergy with the irradiation, further reducing the
surviving fraction of the cancer cells, when compared with
treatment with irradiation alone. In addition, the morphological
changes observed (Fig. 1D) were
consistent with the results of the cell viability and clonogenic
assays. These results indicate that PAB may be a potent
radiosensitizer and could have potential benefits in the treatment
ovarian cancer.
Autophagy is not involved in the
radiosensitizing effects of PAB on SKOV-3 cells
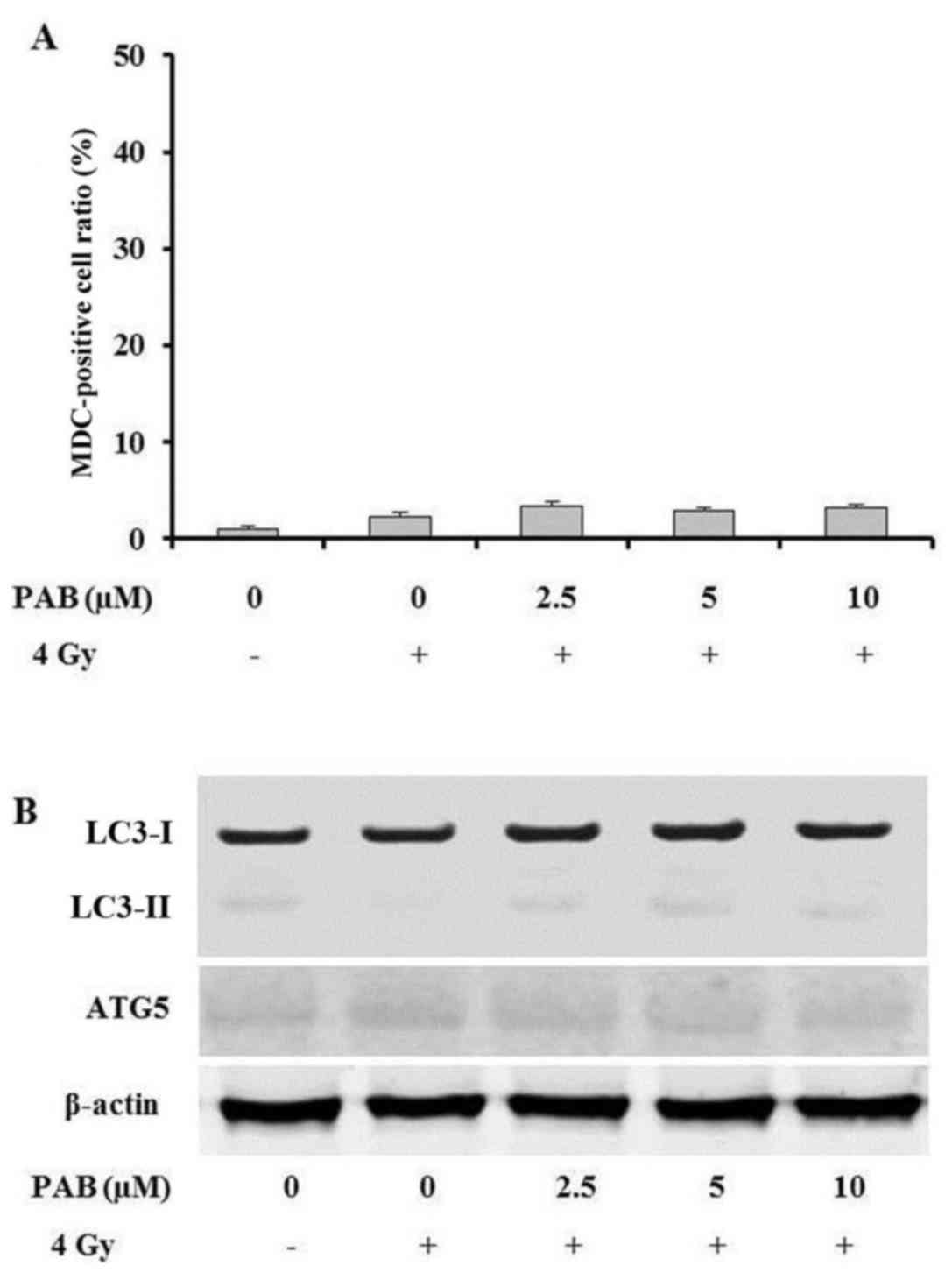
To help determine the type of cell death undergone
by SKOV-3 cells during combination treatment with PAB and
irradiation, an MDC staining assay was performed to detect whether
the cells underwent autophagy. The percentage ratio of MDC-positive
cells was measured by flow cytometry. The results revealed that
there were no notable changes in the level of autophagy following
treatment with PAB (Fig. 2A). In
order to confirm these findings, the expression of
autophagy-associated proteins was also investigated. The expression
of LC3-I, LC3-II and ATG5 exhibited no notable changes following
PAB treatment (Fig. 2B). These
results indicate that autophagy is not involved in the
radiosensitizing effects of PAB in SKOV-3 cells.
PAB dose-dependently increases the
irradiation-induced apoptosis of SKOV-3 cells
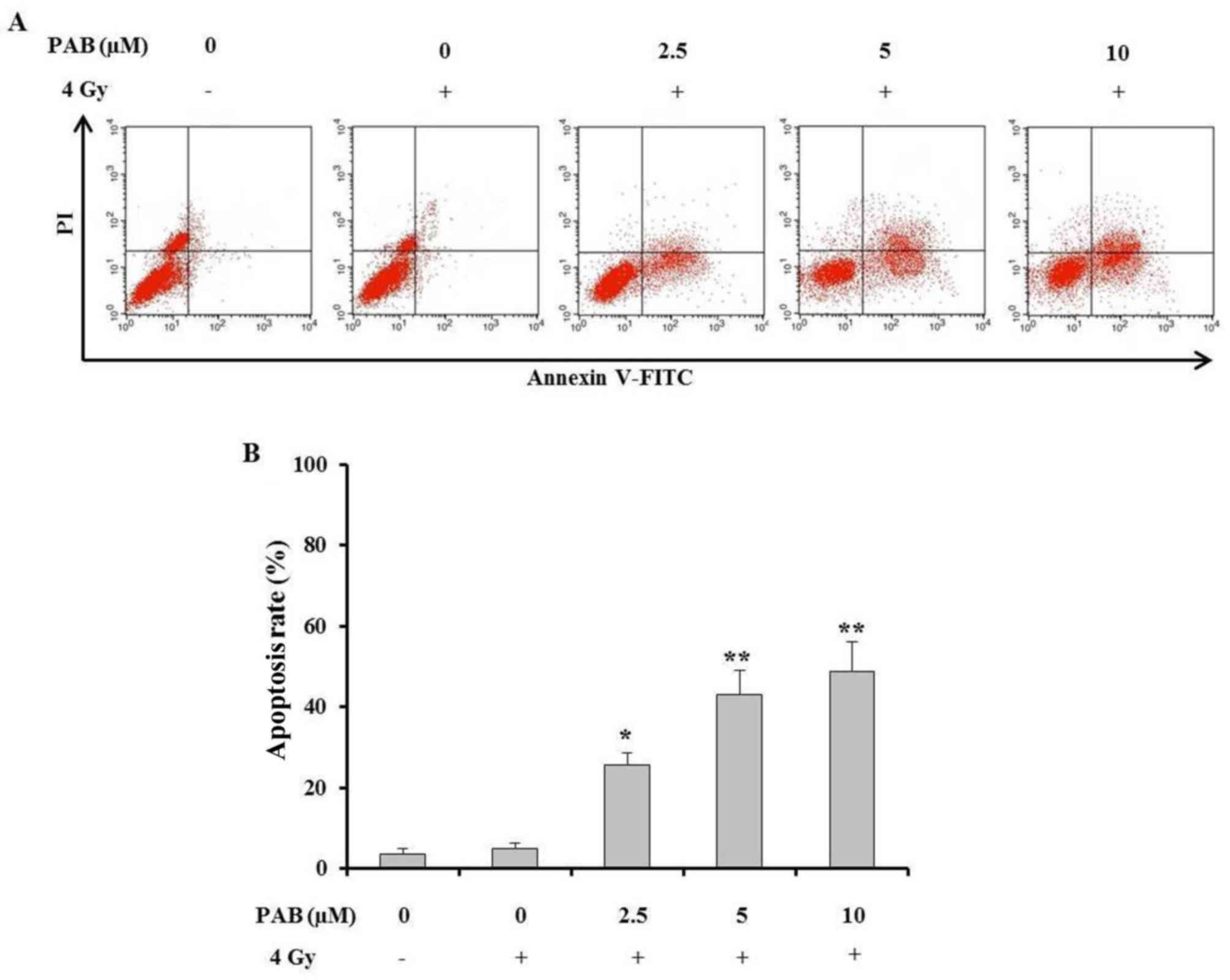
An Annexin V/PI staining assay was performed to
determine the percentage of SKOV-3 cell death after treatment with
a combination of PAB and irradiation (Fig. 3A). The results demonstrated that
concurrent treatment with PAB and irradiation significantly
increased the percentage of apoptotic SKOV-3 cells compared with
the positive and negative controls (Fig.
3B). This effect increased with an increasing PAB dose. These
results indicate that PAB dose-dependently enhances the
irradiation-induced apoptosis of SKOV-3 cells and is an effective
radiosensitizer.
PAB and irradiation combination
therapy inhibits the activation of the Ras-Raf-ERK signaling
pathway
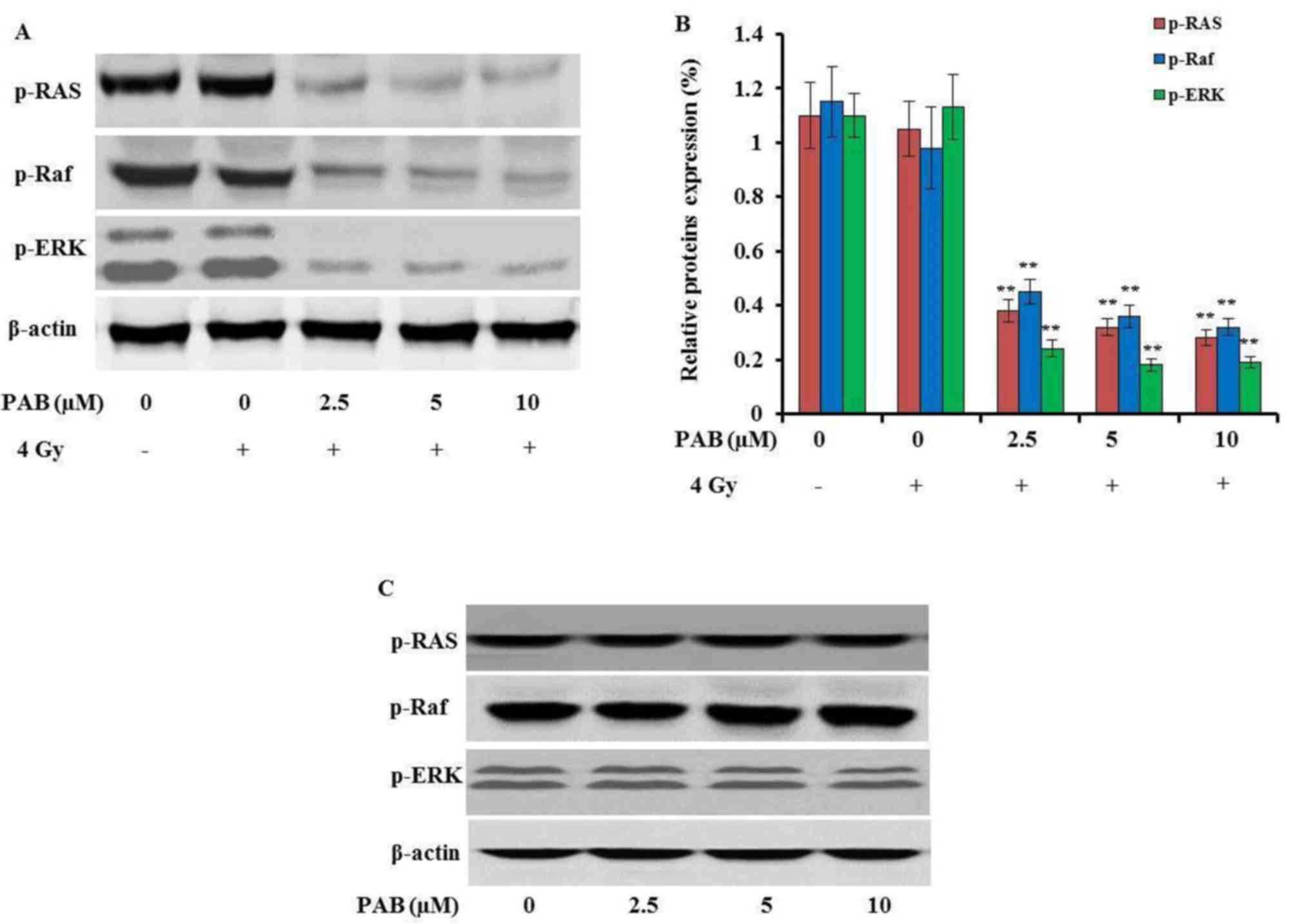
It has been reported that PAB could upregulate the
expression of ERK in MCF-7 cells (9). To detect the underlying molecular
mechanisms by which PAB radiosensitizes SKOV-3 cells, the effects
of PAB and irradiation on the Ras-Raf-ERK signaling pathway were
investigated. The expression of p-Ras, p-Raf and p-ERK was
dose-dependently decreased by treatment with PAB in combination
with irradiation (P<0.01; Fig. 4A and
B). In addition, the phosphorylation of these proteins was not
inhibited by treatment with either irradiation (Fig. 4A and B) or PAB (Fig. 4C) alone, which was consistent with
the apoptosis assay results. These results indicate that the
Ras-Raf-ERK signaling pathway is inhibited by the combination
treatment of PAB and irradiation, and that the disruption of this
pathway can induce apoptosis.
Discussion
Radiotherapy is a key constituent of ovarian cancer
treatment, which has been used for decades, with the primary
limitation being the radioresistance of tumor cells. Brown
(15) previously reported that
hypoxic conditions lead to resistance to radiotherapy, overcoming
this radioresistance would improve the efficacy of cancer radiation
therapy. In addition, several other studies have focused on
radioresistance of ovarian cancer (16,17). The
present study investigated the potential of PAB as a
radiosensitizer during the irradiation of SKOV-3 ovarian cancer
cells.
PAB has been demonstrated to exhibit antitumor
effects on a wide variety of tumor cells, including ovarian cancer
cells (9,13). However, there have been few studies
focused on the effects of PAB when administered alongside
radiotherapy. The present study aimed to determine the potential of
PAB as a radiosensitizer during the treatment of ovarian cancer, as
well as investigating the underlying mechanisms by which it is able
to have a radiosensitizing effect. The effectiveness of
radiotherapy as a single therapy and as a combined therapy with PAB
was studied by comparing the cell survival rates of the SKOV-3
ovarian cancer cell line when treated with each therapy type. In
addition, autophagy, apoptosis and the Ras-Raf-ERK signaling
pathway were studied in order to help determine the mechanism by
which PAB exerts a radiosensitizing effect. Yu et al
(13) reported that PAB could induce
HO-8910 and A2780 ovarian cancer cell apoptosis. However, in the
present study PAB had no apoptotic effect on the SKOV-3 cells. When
a combined treatment of radiotherapy and PAB was administered, the
surviving fraction of cells decreased compared to the group treated
with radiotherapy alone, indicating that PAB is a radiosensitizing
agent.
To determine the mechanisms of cell death induced by
the combination of PAB and irradiation, an autophagy assay was
completed using MDC, a fluorescent dye used to label autophagic
vacuoles. The results demonstrated no notable change in the number
of autophagic vacuoles. The expression of LC3-I, LC3-II and ATG5
was consistent with the MDC staining assay results, with no notable
changes seen. These results demonstrated that autophagy was not
affected by PAB. PAB treatment alone had no effect on SKOV-3 cell
growth, whereas irradiation treatment alone did not induce cell
autophagy either. Thus, it was hypothesized that the combination
treatment would induce apoptosis in SKOV-3 cells.
Previous studies have revealed that apoptosis is a
significant method of cell death during drug and irradiation
treatment (18,19). The increased induction of apoptosis
has been investigated using several different radiosensitizers,
including docetaxel (20),
oxaliplatin (21) and vinorelbine
(22). These previous results led to
the proposal that the radiosensitizing effect of PAB may be due to
it increasing the rate of apoptosis of SKOV-3 cells. The results of
the Annexin V/PI staining assay supported this theory,
demonstrating a significant increase in the rate of apoptosis
during treatment with PAB and irradiation in a dose-dependent
manner.
Raf proteins can be activated by the human
proto-oncogene Ras and regulated by the expression of the
downstream protein ERK (23).
Ovarian cancer is the result of multiple genetic and epigenetic
changes in patients. It has been reported that Ras is mutated in
>20% of cases of ovarian cancer (24) and that the Ras-Raf-ERK signaling
pathway is involved in the progression of ovarian cancer, thus it
represents a potential target for anticancer therapy (25). In the present study, combination
therapy with PAB and irradiation notably decreased the levels of
phosphorylated proteins in the Ras-Raf-ERK singling pathway when
compared with the irradiation treatment alone. Therefore, it was
hypothesized that decreasing the phosphorylation of proteins in the
Ras-Raf-ERK signaling pathway could also inhibit the progression of
ovarian cancer, which may due to enhanced apoptosis. In other
studies, it has been reported that irradiation enhanced the
interactions between Ras and Raf proteins, and the activation of
ERK (26–28). In the present study, treatment with
irradiation and PAB was demonstrated to lower the expression of
ERK, which could be due to the combination with PAB, but this
hypothesis requires further study to be confirmed.
Meanwhile, Kyula et al (29) reported that the Ras-Raf-ERK signaling
pathway not only serves a pivotal role in regulating cancer
progression, but is also involved in treatment resistance.
Currently, the main obstacle for radiotherapy is the degree of
radioresistance. In the present study, PAB treatment alone had no
effect on SKOV-3 cells, whereas combination therapy could inhibit
the Ras-Raf-ERK signaling pathway. This suggests that combination
therapy with PAB and irradiation not only inhibited the progression
of the ovarian cancer, but also reduced irradiation therapy
resistance by regulating the activity of the Ras-Raf-ERK signaling
pathway.
In conclusion, the combination of PAB with
irradiation increased the efficiency of radiation therapy in SKOV-3
cells by inhibiting the Ras-Raf-ERK signaling pathway and
increasing cell apoptosis. This indicates that the downregulation
of the Ras-Raf-ERK signaling pathway is not only involved in
inhibiting tumor progression, but also in reducing irradiation
resistance. However, this hypothesis requires further research to
be confirmed. The results of the present study indicate that PAB
has therapeutic potential as a novel radiosensitizer for the
treatment of ovarian cancer.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no.
ZR2014YL029).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goff BA, Mandel L, Muntz HG and Melancon
CH: Ovarian carcinoma diagnosis. Cancer. 89:2068–2075. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bookman MA, Brady MF, McGuire WP, Harper
PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De
Geest K, et al: Evaluation of new platinum-based treatment regimens
in advanced-stage ovarian cancer: A Phase III Trial of the
Gynecologic Cancer Intergroup. J Clin Oncol. 27:1419–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwarz R, Bruland O, Cassoni A, Schomberg
P and Bielack S: The role of radiotherapy in oseosarcoma. Cancer
Treat Res. 152:147–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang YC, Jiang G, Gao H, Liu HM and Liang
J: Influence of ionizing radiation on ovarian carcinoma SKOV-3
xenografts in nude mice under hypoxic conditions. Asian Pac J
Cancer Prev. 15:2353–2358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Pasqua AJ, Yuan H, Chung Y, Kim JK,
Huckle JE, Li C, Sadgrove M, Tran TH, Jay M and Lu X:
Neutron-activatable holmium-containing mesoporous silica
nanoparticles as a potential radionuclide therapeutic agent for
ovarian cancer. J Nucl Med. 54:111–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wakeford R: The cancer epidemiology of
radiation. Oncogene. 23:6404–6428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S, Onodera S and Ikejima T: Pseudolaric acid B induces
apoptosis, senescence, and mitotic arrest in human breast cancer
MCF-7. Acta Pharmacol Sin. 28:1975–1983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li E, Clark AM and Hufford CD: Antifungal
evaluation of pseudolaric acid B, a major constituent of
Pseudolarix kaempferi. J Nat Prod. 58:57–67. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang WC, Lu RF, Zhao SX and Gu ZP:
Comparison of early pregnancy-terminating effect and toxicity
between pseudolaric acids A and B. Zhongguo Yao Li Xue Bao.
9:445–448. 1988.(In Chinese). PubMed/NCBI
|
|
12
|
Wang WC, Lu RF, Zhao SX and Zhu YZ:
Antifertility effect of pseudolaric acid B. Zhongguo Yao Li Xue
Bao. 3:188–192. 1982.(In Chinese). PubMed/NCBI
|
|
13
|
Yu B, Yue DM, Shu LH, Li NJ and Wang JH:
Pseudolaric acid B induces caspase-dependent cell death in human
ovarian cancer cells. Oncol Rep. 31:849–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morimoto H, Yonehara S and Bonavida B:
Overcoming tumor necrosis factor and drug resistance of human tumor
cell lines by combination treatment with anti-Fas antibody and
drugs or toxins. Cancer Res. 53:2591–2596. 1993.PubMed/NCBI
|
|
15
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.PubMed/NCBI
|
|
16
|
Steffen AC, Gostring L, Tolmachev V, Palm
S, Stenerlow B and Carlsson J: Differences in radiosensitivity
between three HER2 overexpressing cell lines. Eur J Nucl Med Mol
Imaging. 35:1179–1191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akbarzadeh M, Nouri M, Banekohal MV,
Cheraghi O, Tajalli H, Movassaghpour A, Soltani S, Cheraghi H,
Feizy N, Montazersaheb S, et al: Effects of combination of
melatonin and laser irradiation on ovarian cancer cells and
endothelial lineage viability. Lasers Med Sci. 31:1565–1572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pauwels B, Vermorken JB, Wouters A, Ides
J, Van Laere S, Lambrechts HA J, Pattyn GG, Vermeulen K, Meijnders
P and Lardon F: The role of apoptotic cell death in the
radiosensitising effect of gemcitabine. Br J Cancer. 101:628–636.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mose S, Class R, Weber HW, Rahn A, Brady
LW and Bottcher HD: Radiation enhancement by gemcitabine-mediated
cell cycle modulations. Am J Clin Oncol. 26:60–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Creane M, Seymour CB, Colucci S and
Mothersill C: Radiobiological effects of docetaxel (Taxotere): A
potential radiation sensitizer. Int J Radiat Biol. 75:731–737.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hermann RM, Rave-Frank M and Pradier O:
Combining radiation with oxaliplatin: A review of experimental
results. Cancer Radiother. 12:61–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Boyer M, Rivory L, Hong A, Clarke
S, Stevens G and Fife K: Radiosensitization of vinorelbine and
gemcitabine in NCI-H460 non-small-cell lung cancer cells. Int J
Radiat Oncol Biol Phys. 58:353–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maurer G, Tarkowski B and Baccarini M: Raf
kinases in cancer-roles and therapeutic opportunities. Oncogene.
30:3477–3488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurman RJ and Shih IeM: Pathogenesis of
ovarian cancer: Lessons from morphology and molecular biology and
their clinical implications. Int J Gynecol Pathol. 27:151–160.
2008.PubMed/NCBI
|
|
25
|
Yap TA, Carden CP and Kaye SB: Beyond
chemotherapy: Targeted therapies in ovarian cancer. Nat Rev Cancer.
9:167–181. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasid U and Dritschilo A: RAF antisense
oligonucleotide as a tumor radiosensitizer. Oncogene. 22:5876–5884.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suy S, Anderson WB, Dent P, Chang E and
Kasid U: Association of Grb2 with Sos and Ras with Raf-1 upon gamma
irradiation of breast cancer cells. Oncogene. 15:53–61. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sylvain V, Lafarge S and Bignon YJ:
Molecular pathways involved in response to ionizing radiation of
ID-8 mouse ovarian cancer cells expressing exogenous full-length
Brca1 or truncated Brca1 mutant. Int J Oncol. 19:599–607.
2001.PubMed/NCBI
|
|
29
|
Kyula JN, Khan AA, Mansfield D,
Karapanagiotou EM, McLaughlin M, Roulstone V, Zaidi S, Pencavel T,
Touchefeu Y, Seth R, et al: Synergistic cytotoxicity of radiation
and oncolytic Lister strain vaccinia in (V600D/E)BRAF mutant
melanoma depends on JNK and TNF-α signaling. Oncogene.
33:1700–1712. 2014. View Article : Google Scholar : PubMed/NCBI
|