Introduction
Bone cancer is a disease of cells that occur in the
skeleton and presents via aberrant growth and migration (1,2).
Osteosarcoma is a typical systemic malignant disease, which
predominantly leads to characteristic symptoms of bone and joint
pain and fatigue in patients (3,4). In
recent years, novel strategies have been proposed; however, the
overall survival for patients with osteosarcoma has remained
limited due to the stubborn resistance of osteosarcoma cells to
these strategies (5,6). The resistance of osteosarcoma cells to
apoptosis contributes to the growth and invasion of tumor cells
(7–9). Apoptotic resistance has become the
greatest challenge in cancer therapy due to the fierce resistance
of tumor cells via various mechanisms (10,11).
Furthermore, although the emergence of adjuvant and neoadjuvant
chemotherapy has improved the survival rate of patients with
osteosarcoma, the morbidity and mortality rates of patients with
osteosarcoma are steadily increasing (12). Hence, elucidating the underlying
mechanism of apoptotic resistance is urgently required in order to
identify novel efficacious target therapies that may improve the
overall survival rate of patients with osteosarcoma.
The superfamily of tripartite motif-containing
(TRIM) proteins, which includes >60 types of TRIM proteins, is
evolutionarily conserved with a highly conserved order of the
domains in the Ring, B-box, Coiled-Coil motif (13). A large number of reports have
suggested that TRIM proteins may be novel markers for human cancer
metastasis, including gastric cancer, liver cancer and colorectal
cancer (14). Recently, TRIM-14 was
identified as an important member of the TRIM family of proteins;
TRIM-14 promotes growth, invasiveness and resistance to
cisplatin-induced apoptosis (15).
In addition, increased expression of TRIM-14 has been identified in
monkey lymphomas caused by human immunodeficiency virus and Simian
immunodeficiency virus (16).
TRIM-14 gene expression has a mediator role in the immune response
and is associated with the transcription of various genes involved
in innate immunity by regulating nuclear factor (NF)-κB signaling
pathways (15). Therefore, these
reports suggest that TRIM-14 may be a potential target for the
treatment of human cancer via the regulation of the NF-κB signaling
pathway.
Aberrant activation of NF-κB has been observed in
various types of human cancer (17,18).
Previous reports have indicated that poor survival of bone cancer
patients is associated with aberrantly activation of NF-κB nuclear
translocation factors (19,20). Indicators of NF-κB activation,
including inhibitor of nuclear factor-κB kinase subunit β (Ikkβ),
p65 and NF-κB inhibitor α (IkBα), also exhibited increased activity
in clinical specimens of bone cancer tissues (21). In addition, the relationship between
the ubiquitin-proteasome system and activation of NF-κB has been
studied in human cancer cells, which demonstrated that NF-κB
activation may stimulate the ubiquitin-proteasome system (22). Furthermore, previous investigations
have demonstrated that the NF-κB pathway is involved in apoptosis
resistance induced by chemotherapy and enhances tumor cell
survival, invasion and angiogenesis (23). Nevertheless, exploring novel
molecules that regulate aberrant activation of the NF-κB signaling
pathway may be beneficial for the treatment of clinical
osteosarcoma.
The present study investigated TRIM-14 expression in
osteosarcoma cells and studied the efficacy of targeted therapy for
TRIM-14 on osteosarcoma growth and aggressiveness, in vitro
and in vivo. The findings demonstrated that antibody
targeting of TRIM-14 significantly inhibited the invasive phenotype
via the inactivation of the NF-κB pathway through inhibited MMP-9
expression levels in U-2OS-bearing xenograft mice. The results of
the present study provide new evidence that targeted therapy for
TRIM-14 may contribute to the inhibition of osteosarcoma
progression, suggesting that TRIM-14 may represent a potential
target for the treatment of patients with osteosarcoma.
Materials and methods
Ethics statement
This preclinical work was performed according to the
recommendations in the Guide for the Care and Use of Laboratory
Animals of The First Hospital of Hebei Medical University
(Shijiazhuang, China). All experimental protocols and animals were
approved by Committee on the Ethics of Animal Experiments Defence
Research. All surgery and euthanasia were made to minimize
suffering.
Cells and reagents
Osteosarcoma cell line U-2OS and human normal
osteoblast MC3T3-E1 cells were purchased from American Type Culture
Collection (Manassas, VA, USA). U-2OS cells were cultured in
Dulbecco's modified Eagle medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.).
MC3T3-E1 cells were cultured in RPMI-1640 (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) medium supplemented with 10% fetal calf
serum (FCS; Sigma-Aldrich; Merck KGaA). All cells were cultured at
37°C in a humidified atmosphere containing 5% CO2.
MTT assay
A total of 1×103 U-2OS cells
(1×103) were incubated with Chanti-TRIM (10–160 mg/ml)
or PBS (Control) in 96-well plates for 24, 48 or 72 h in
triplicate. Subsequently, 20 µl MTT solution (5 mg/ml;
Sigma-Aldrich; Merck KGaA) was added to cells and incubated for 2 h
at 37°C. The entire medium was removed and 100 µl of DMSO was added
into the wells to solubilize the crystals. Mitochondrial activity
was assessed by measuring the optical density at 570 nm with a
light microscope.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
In order to investigate the expression levels of
matrix metalloproteinase-9 (MMP-9), cyclin D1 gene (CCND1) and
NF-κB target genes, including B-cell lymphoma-extra-large (BcL-XL),
vascular endothelial growth factor (VEGF)-C and Myc proto-oncogene
protein (c-Myc), total RNA (2 µg) from U-2OS and MC3T3-E1 cells was
extracted using an RNeasy Mini kit (Qiagen Sciences, Inc.,
Gaithersburg, MD, USA). Total RNA (2 µg) was used to synthesize
cDNA with the SuperScript II First-strand Synthesis system
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The PCR comprised of the following
thermocycling conditions: Initial denaturation at 96°C for 1 min,
45 amplification cycles consisting of denaturation at 95°C for 30
sec, primer annealing at 66°C for 45 sec and then 54°C for 50 sec,
and applicant extension at 72°C for 60 sec. Gene expression levels
were measured by RT-qPCR. All the forward and reverse primers
(Table I) were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.). All mRNA levels were
quantified using Power SYBR Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Relative mRNA expression levels
were calculated by 2−ΔΔCq (24). Results were analyzed in triplicate
according to the ΔΔCq method and were presented as n-fold relative
to the control.
 | Table I.Sequences of primers were used in
this study. |
Table I.
Sequences of primers were used in
this study.
|
| Sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Reverse | Forward |
|---|
| c-Myc |
GAAATGTCCTGAGCAATCACCT |
TGAGGCAGTTTACATTATGGCT |
| BcL-XL |
CACCATGTCTCAGAGCAACCGGGAGCTGGTGGTT |
TGGTCATTTCCGACTGAAGAGTGAGCCCAG |
| VEGF-C |
TGCATTCACATTGTGCTGCTGTAG |
GCAGATTATGCGGATCAAACC |
| TRIM-14 |
CACCATGGCGTCTCCCAGTGGGAA |
TCACTTATCGGAACTCCTGCGC |
| β-actin |
CGGAGTCAACGGATTTGGTC |
AGCCTTCTCCATGGTCGTGA |
ELISA
Affinity of Chanti-TRIM with TRIM-14 was analyzed
using a commercial ELISA kit (cat. no. E5020h; Beijing Huaxia Ocean
Technology Co., Ltd., Beijing, China). Operational procedures were
performed as outlined by the manufacturer's instructions. Results
were assessed via an ELISA reader system (Bio-Rad Laboratories,
Inc.).
Tissue specimens
Osteosarcoma tissues from 12 patients with stage
І-IV cancer (n=5) were sliced into 4-µm-thick sections and
paraffin-embedded to achieve osteosarcoma sections. Clinical
staging (І-IV) of the patients was categorized as previously
reported (25). Osteosarcoma
sections were prepared to analyze the expression of TRIM-14.
Osteosarcoma sections were collected from 12 patients admitted to
The First Hospital of Hebei Medical University between February
2001 and October 2015. The analysis of these clinical osteosarcoma
specimens was approved by the Institutional Review Board of The
First Hospital of Hebei Medical University and written informed
consent was obtained from all patients.
Construction of Chanti-TRIM
Single chain variable fragments of the mouse
anti-human TRIM-14 antibody (cat. no. ab50941; dilution 1:1,000;
Abcam, Cambridge, MA, USA) were cloned and linked with the pET-27b
vector (pET-27b-TRIM-14; Takara Biotechnology Co., Ltd., Dalian,
China). The constant domain heavy chain and light chain of the
mouse anti-human TRIM-14 antibody were inserted into the
pET-27b-TRIM-14 vector (pET-27b-Chanti-TRIM). Vector of
pET-27b-Chanti-TRIM was transfected into the E. coli Rossetta (DE3;
Merck KGaA, Darmstadt, Germany) using electrotransformation and
induced by isopropyl β-D-1-thiogalactopyranoside (Sigma-Aldrich;
Merck KGaA) at a concentration of 0.5 mM and a wavelength of 600
nm. Cells were harvested, disrupted, and dissolved in PBS. Protein
was purified by gel filtration chromatography (26) and termed Chanti-TRIM.
Apoptosis analysis
U-2OS cells were cultured until 90% confluence. A
total of 1×106 Cells were subsequently incubated with
Chanti-TRIM (80.0 mg/ml) for 12 h at 37°C. Cells were washed three
times using PBS and treated with cisplatin (4.0 mg/ml) for 12 h at
37°C. Subsequently, cells were trypsinized and underwent apoptosis
analysis using an annexin V-fluorescein isothiocyanate and
propidium iodide kit (BD Biosciences, Franklin Lakes, NJ, USA).
Results were analyzed using a FACScan flow cytometer (BD
Biosciences).
Cell migration and invasion
assays
U-2OS cells were cultured in DMEM medium for 48 h.
PBS or Chanti-TRIM-treated (80.0 mg/ml) cells were suspended as a
density of 1×105 in 500 µl serum-free DMEM for 24 h at
37°C. U-2OS cells were then inserted into the tops of BD BioCoat
Matrigel Invasion Chambers (BD Biosciences) according to the
manufacturer's instructions. U-2OS cells were incubated with
Chanti-TRIM or PBS for 72 h at 37°C using a Matrigel Migration
Chamber (BD Biosciences) to analyze the migration of tumors cells.
For the invasion assay, a Matrigel Invasion Chamber (BD
Biosciences) was used to instead of a Matrigel Migration Chamber.
U-2OS tumor cell invasion and migration was measured using a
stain-field microscope.
MMP-9 overexpression
A total of 1×106 U-2OS cells were
cultured in DMEM in a 6-well plate until 90% confluence. The media
was then removed from culture plate and the cells were washed with
PBS three time. U-2OS cells were transfected with plentivirus-MMP-9
(100 pmol; Invitrogen; Thermo Fisher Scientific, Inc.) using
Lipofectamine 2000 (Sigma-Aldrich; Merck KGaA) according to
manufacturer's protocol. A total of 48 h after transfection,
subsequent experimentations were performed in MMP-9-overexpressed
U-2OS cells.
Western blot analysis
TRIM protein expression levels in U-2OS and MC3T3-E1
cells were analyzed via western blotting. U-2OS cells were treated
by PBS or Chanti-TRIM, homogenized in lysate buffer containing
protease-inhibitor and subsequently centrifuged at 5,000 × g for 10
min (4°C). The supernatant of the mixture was used to analyze the
target protein. To detect the target protein, transmembrane
proteins were extracted via a transmembrane protein extraction kit
(Qiagen Sciences, Inc.) according to the manufacturer's
instructions. Proteins were separated by 12% SDS-PAGE as previously
described (27). For western
blotting, primary antibodies: TRIM-14 (cat. no. ab50941), MMP-9
(cat. no. ab38898), CCND1 (cat. no. ab134175) and β-actin (cat. no.
ab8226) (all 1:1,000; Abcam) were added after blocking (5% skimmed
milk) for 1 h at 37°C. Following washing three times with PBS, the
membrane was incubated with HRP-conjugated IgG mAb secondary
antibodies (1:5,000; cat. no. PV-6001; OriGene Technologies, Inc.,
Beijing, China) for 24 h at 4°C. Finally, protein bands were
visualized using Advansta WesternBright enhanced chemiluminescent
HRP substrate (Menlo Park, CA, USA).
Animal experiments
To further evaluate the therapeutic efficacy of
Chanti-TRIM on osteosarcoma growth, a murine xenograft model of
osteosarcoma was established. A total of 68 female specific
pathogen-free BALB/c nude mice were purchased from Orient Bio Inc.,
(Seoul, Korea). All mice were free to access food and water, and
were housed under an artificial 12-h light-dark cycle. In total,
1×105 U-2OS cells were subcutaneously injected into the
backs of the BALB/c nude mice. Mice bearing osteosarcoma were
randomly divided into two groups (n=10 per group) and subsequently
received treatment with Chanti-TRIM (10 mg/kg) or PBS. Treatments
for tumor-bearing mice were initiated when tumor diameters reached
5 to 7 mm on day 5 after tumor inoculation. Full details of the
procedures have been outlined in a previous report (28). Treatments were administered seven
times with 2-day intervals. Tumor diameters were recorded once
every 2 days and tumor volumes were calculated using the following
formula: Tumor volume = 0.52 × smallest diameter2 ×
largest diameter.
Immunohistochemical staining
Osteosarcoma sections from experimental mice were
analyzed via an avidin-biotin-peroxidase technique.
Paraffin-embedded tumor tissue sections were prepared and epitope
retrieval was performed for further analysis. Paraffin sections
were subjected to hydrogen peroxide (3%) for 10–15 min, and
subsequently blocked by a regular blocking solution for 10–15 min
at 37°C. Finally, the sections were incubated with anti-p65 (cat.
no. ab16502; Abcam), anti-IKKβ (cat. no. IMG-129A; Novus
Biologicals, LLC, Littleton, CO, USA) and anti-IkBα (cat. no. 9242;
Cell Signaling Technology, Inc., Danvers, MA, USA) (all 1:1,000) at
4°C for 12 h. To analyze TRIM-14 expression, tumor sections were
stained with DAPI for 60 min at 37°C and incubated with
anti-TRIM-14 after washing with PBS three times for 60 min at 37°C.
All sections were washed three times with PBS and incubated with
peroxidase-labeled antibodies (1:5,000; PV-6013; OriGene
Technologies, Inc.) at 37°C for 60 min. From the sections, six
random fields of view were observed under a light microscope.
Histological assay
Tumor sections (4-µm-thick) from experimental mice
were prepared and fixed in 4% paraformaldehyde. Tumor sections then
were embedded in paraffin and stained with hematoxylin and eosin
(Sigma-Aldrich; Merck KGaA) for 60 min at 37°C. Total numbers of
TUNEL-positive cells were counted in 6 randomly views to calculate
the apoptotic tumor cells.
Statistical analysis
Statistical tests for data analysis included
Fisher's exact test, log-rank test, Chi-square test, and two-tailed
Student's t-test. Multivariate statistical analysis was performed
using a Cox regression model. Statistical analyses were performed
using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Data were
present as the mean and standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
TRIM-14 expression levels are
upregulated in osteosarcoma cells and clinical tumors
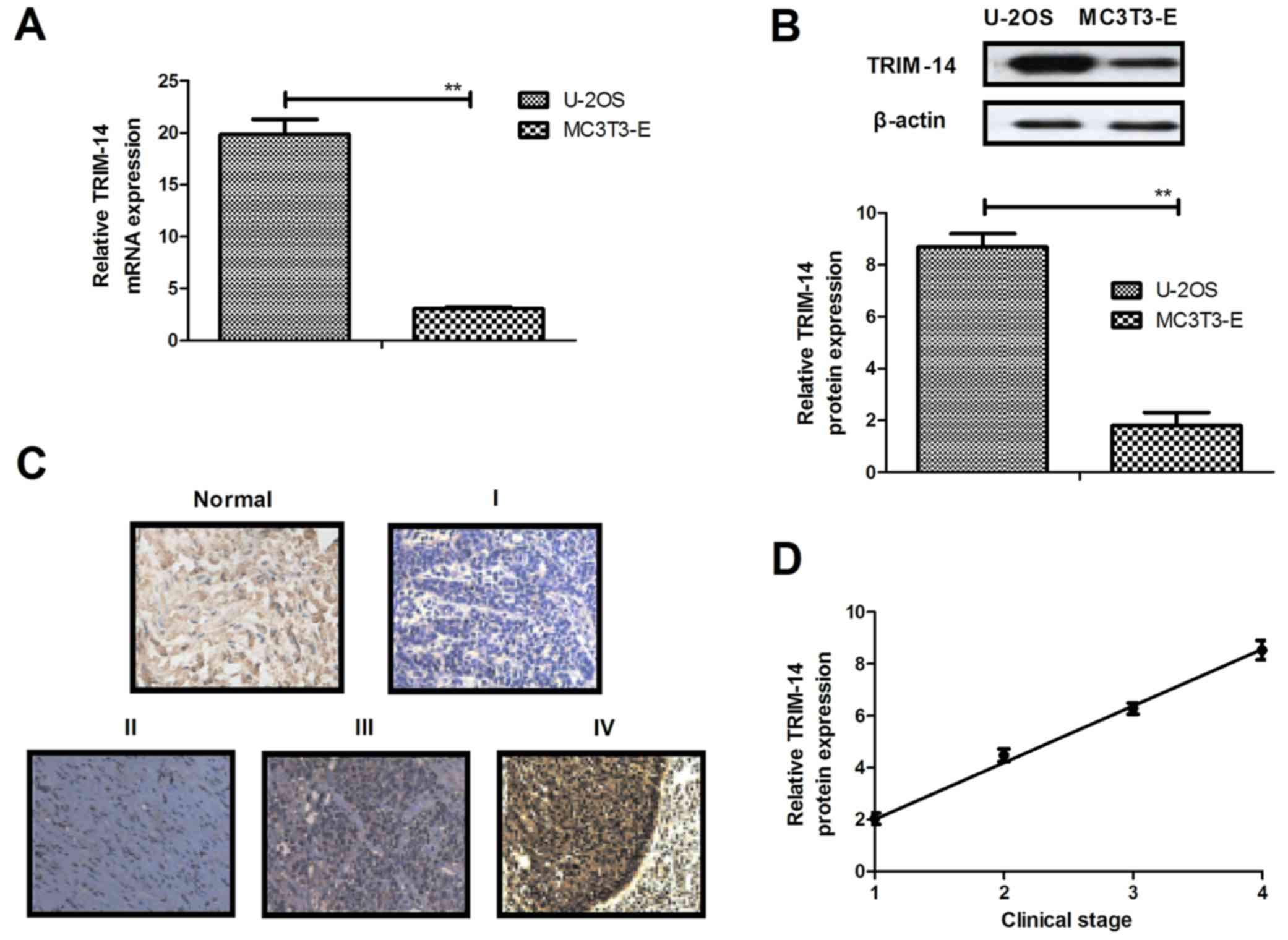
Expression levels of TRIM-14 were investigated in
osteosarcoma cells and clinical tumor tissues. As shown in Fig. 1A and B, respectively, TRIM-14 mRNA
and protein expression levels were significantly upregulated in
U-2OS cells. TRIM-14 expression levels were relatively increased in
osteosarcoma tissues when compared to normal adjacent tissues
(Fig. 1C). Furthermore, TRIM-14
expression levels were positively related with the clinical stage
of the patients with osteosarcoma (Fig.
1D). These results showed that TRIM-14 is overexpressed in
osteosarcoma cells and tumor tissues, suggesting TRIM-14 may be a
potential target for the treatment of osteosarcoma.
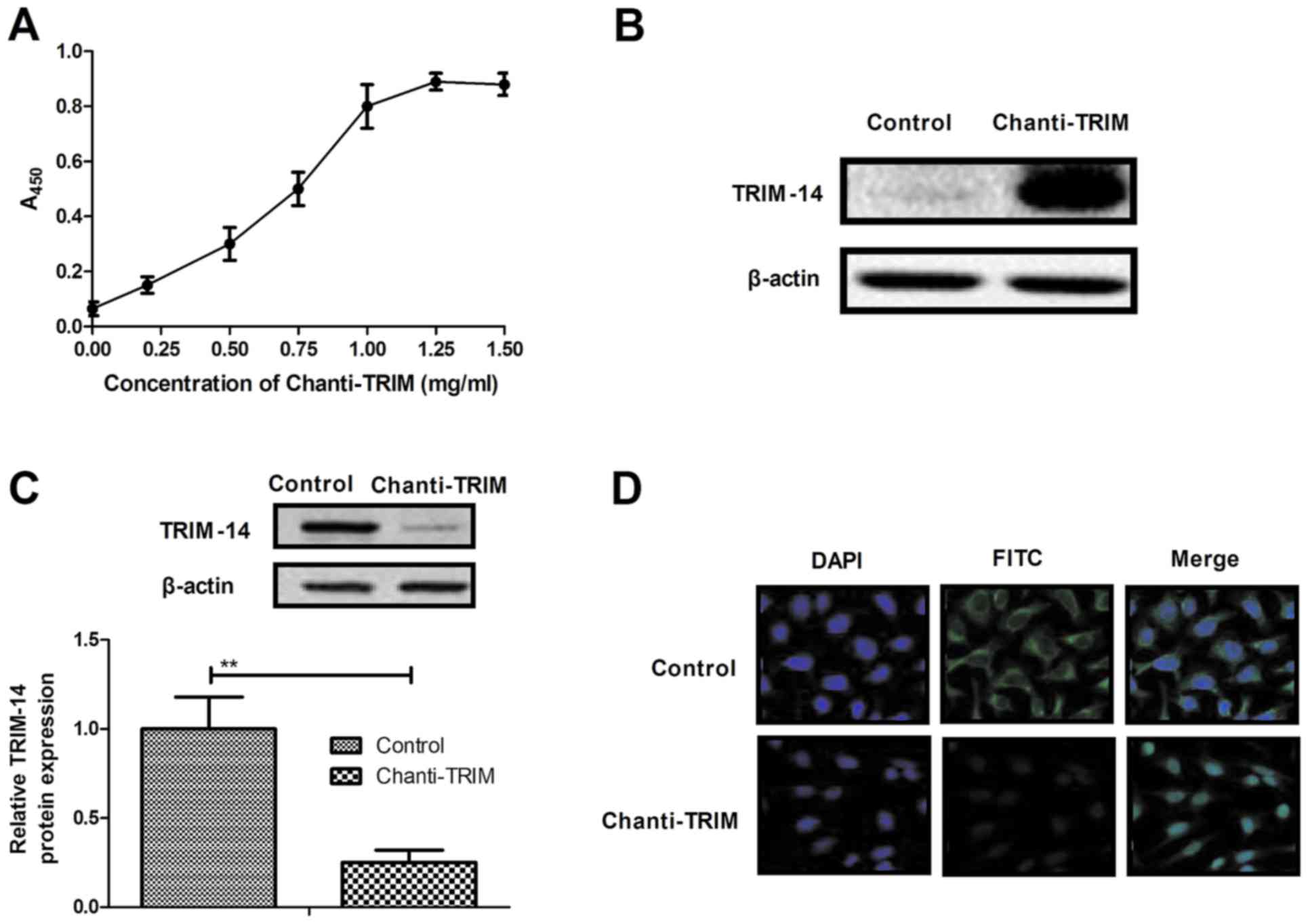
Construction of an antibody targeting
TRIM-14 and analysis of its characteristic β-actin
In order to study the efficacy of TRIM-14 on
osteosarcoma cells, a chimeric antibody targeting TRIM linked with
cell-penetrating peptide (Chanti-TRIM) was constructed. The
affinity of Chanti-TRIM for TRIM-14 was analyzed and presented high
affinity binding with TRIM, as determined by ELISA (Fig. 2A). Western blot analysis also showed
that Chanti-TRIM was able to specially bind with TRIM-14 (Fig. 2B). In addition, TRIM-14 expression
levels in U-2OS cells were analyzed after treatment with
Chanti-TRIM. As illustrated in Fig.
2C, TRIM-14 expression levels were significantly decreased by
Chanti-TRIM treatment. Furthermore, immunofluorescence staining
assay showed that Chanti-TRIM inhibited TRIM-14 expression in U-2OS
cells, as determined by fluorescence intensity (Fig. 2D). These findings suggest that
Chanti-TRIM is able to specially bind with TRIM-14 to neutralize
TRIM-14 expression in U-2OS cells.
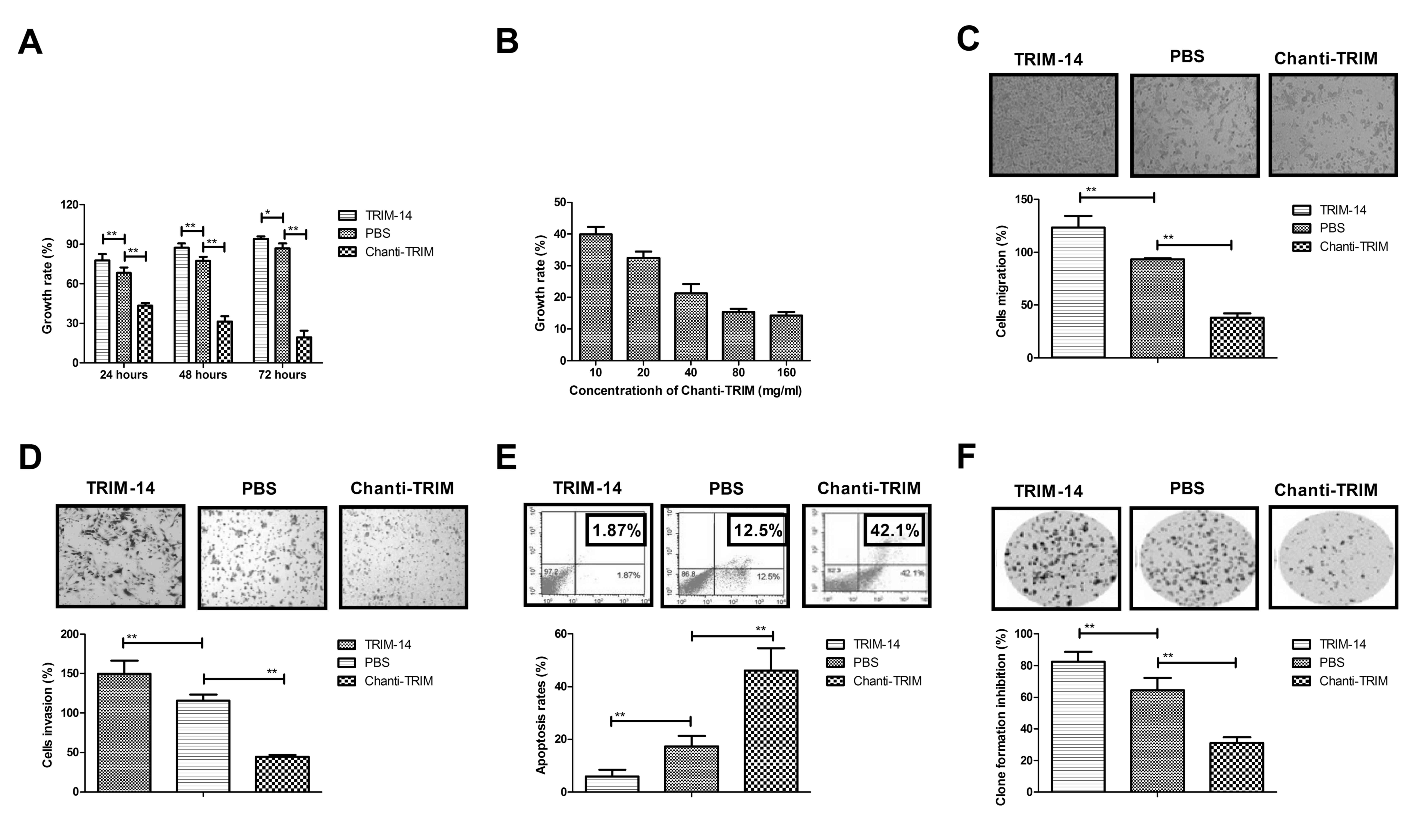
Chanti-TRIM inhibits growth and
aggressiveness and promotes apoptosis in osteosarcoma cells
We further analyzed the efficacy of Chanti-TRIM on
the growth, aggressiveness and apoptosis of osteosarcoma cells. The
results in showed that Chanti-TRIM significantly inhibited the
growth of U-2OS cells compared with the control cells (Fig. 3A). These inhibitory effects were
demonstrated to be dose-dependent (10, 20, 40, 80 and 160 mg/ml
Chanti-TRIM; Fig. 3B). It was also
observed that TRIM-14 enhanced migration, whereas Chanti-TRIM
treatment significantly suppressed U-2OS cells growth migration
compared with the control group (Fig.
3C). In addition, the results showed that the invasion of U-2OS
cells was significantly inhibited by Chanti-TRIM treatment compared
with the TRIM-14-treated cells; whereas TRIM-14 significantly
promoted the invasion of U-2OS cells compared with the control
(Fig. 3D). Furthermore, the results
of apoptosis analysis indicated that Chanti-TRIM significantly
promoted the apoptosis of osteosarcoma cells induced by cisplatin
when compared with the control group; whereas TRIM-14 significantly
promoted apoptotic resistance in U-2OS cells induced by cisplatin
when compared with the control group (Fig. 3E). Formation of U-20S colonies was
significantly promoted by TRIM-14 and was significantly inhibited
by Chanti-TRIM, as compared with the control (Fig. 3F). These results suggest that
Chanti-TRIM not only inhibits the growth and aggressiveness of
osteosarcoma cells, but also promotes apoptosis induced by
cisplatin.
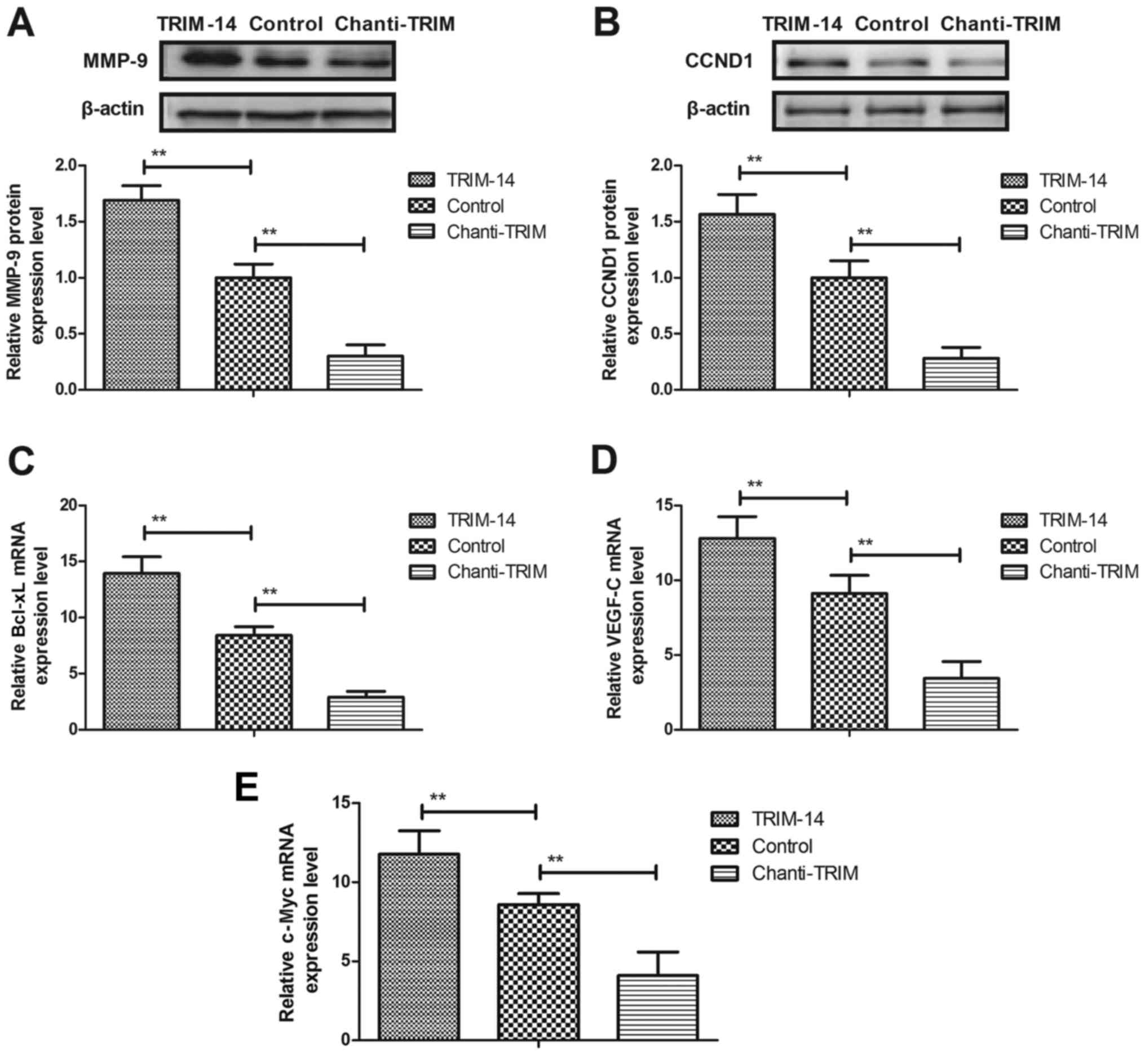
Chanti-TRIM regulates the growth of
osteosarcoma cells through the NF-κB signaling pathway
To elucidate the mechanisms underlying
TRIM-14-mediated osteosarcoma progression, the NF-κB signaling
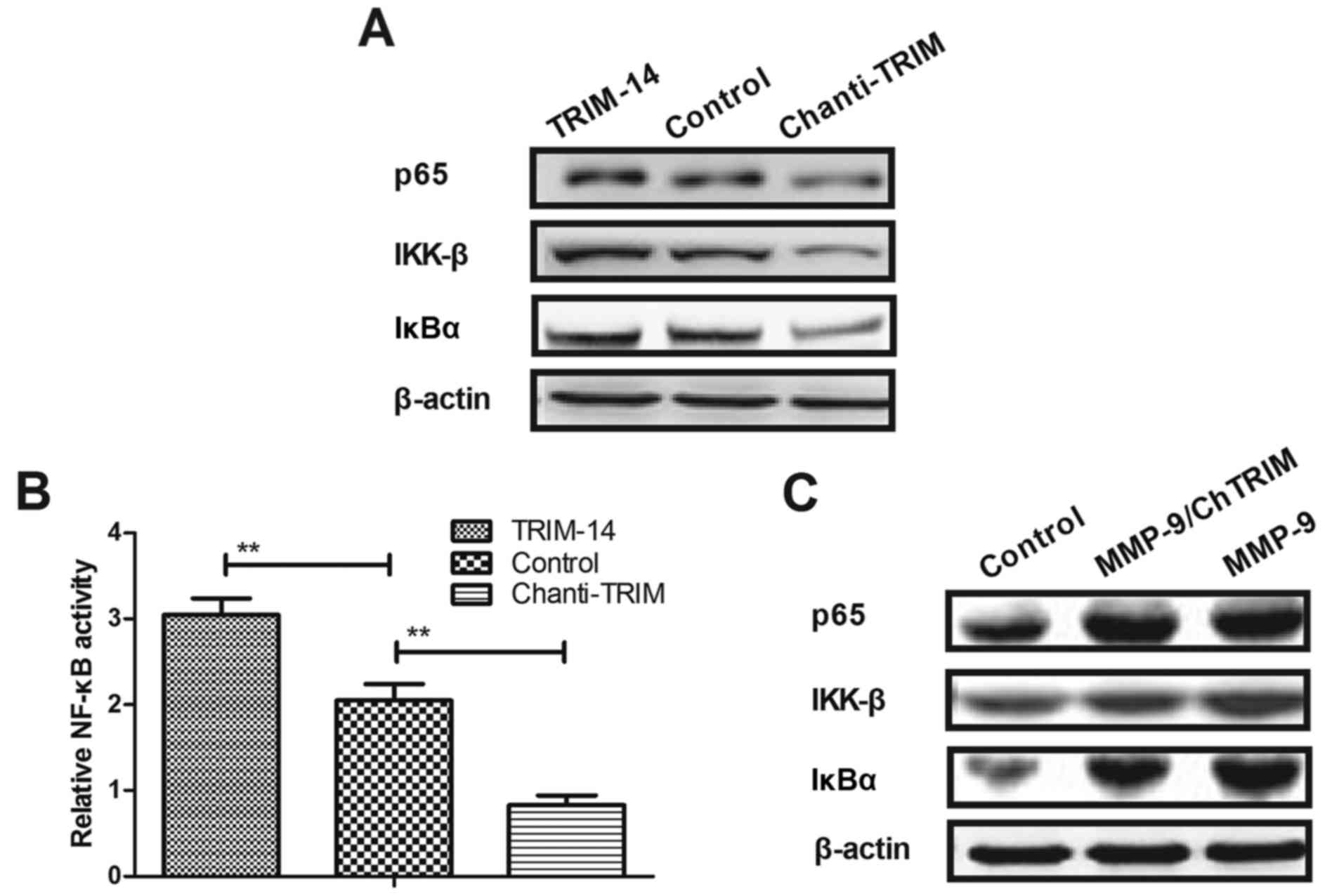
pathway was investigated in U-2OS cells. The results in Fig. 4A and B show that TRIM-14 treatment
significantly increased MMP-9 and CCND1 expression levels, whereas
Chanti-TRIM significantly downregulated MMP-9 and CCND1 expression
in U-2OS cells, as compared with the control group. Further
analysis indicated that Chanti-TRIM inhibited the expression of
NF-κB target genes, including BcL-XL, VEGF-C and c-Myc, in U-2OS
cells (Fig. 4C-E). Western blotting
assays demonstrated that the protein expression levels of p65,
IKK-β and IκBα were markedly decreased in U-2OS cells after
treatment with Chanti-TRIM, whereas TRIM increased expression
levels of p65, IKK-β and IκBα in U-2OS cells (Fig. 5A). Furthermore, MMP-9 overexpression
abrogated Chanti-TRIM-mediated (MMP-9/ChTRIM) inhibitory effects on
NF-κB activity and expression levels in U-2OS cells (Fig. 5B and C). These results indicated that
Chanti-TRIM may be able to inhibit the aggressive phenotype in
osteosarcoma cells via the MMP-9-induced NF-κB signaling
pathway.
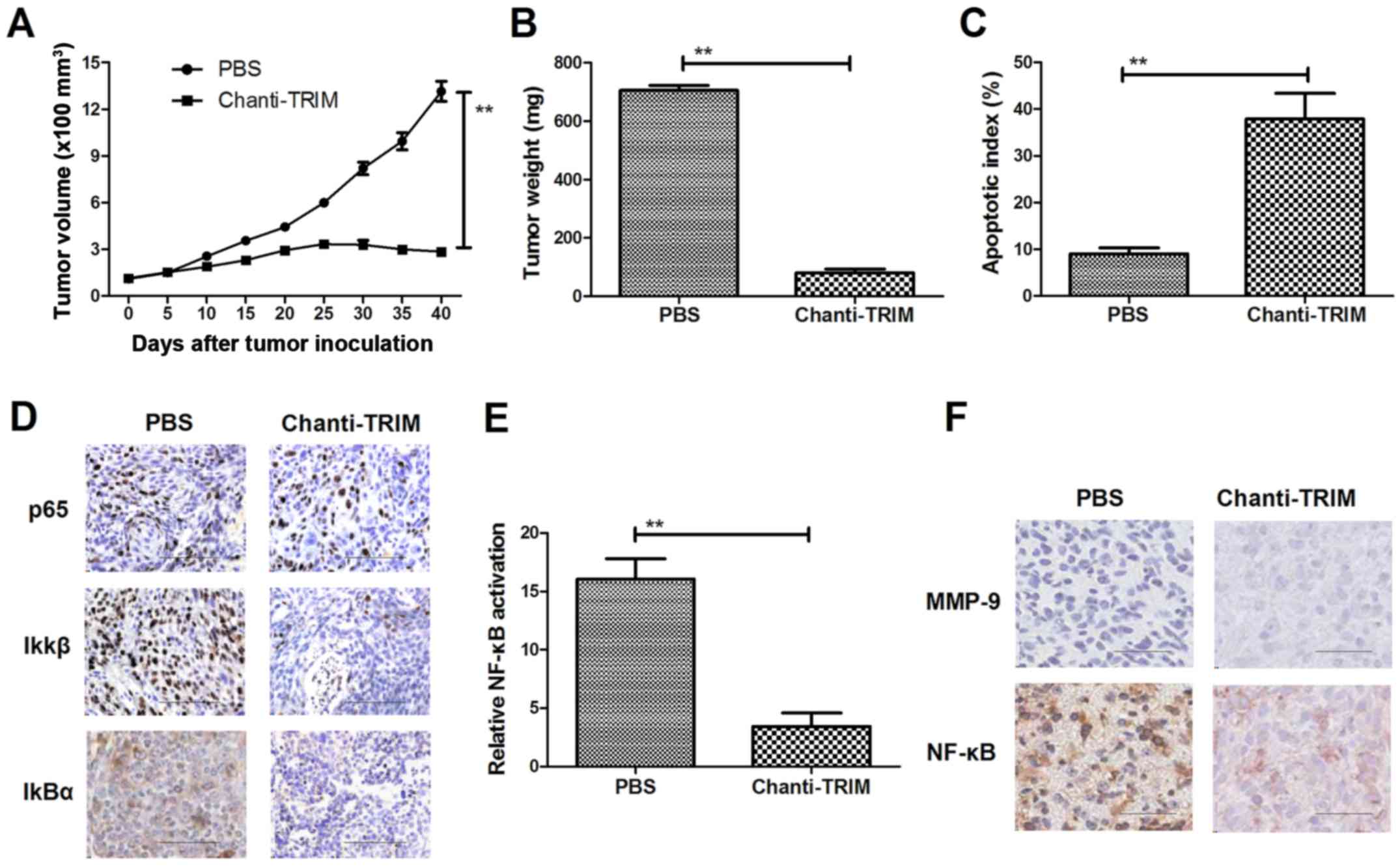
Chanti-TRIM inhibits osteosarcoma
growth in U-2OS-bearing xenograft mice
To further evaluate the therapeutic efficacy of
Chanti-TRIM on osteosarcoma growth, a murine xenograft model of
osteosarcoma was established and treated with Chanti-TRIM or PBS
(control). As shown in Fig. 6A and
B, tumor growth and tumor weight were significantly reduced in
the Chanti-TRIM treated mice, as compared with the control.
Histological analysis revealed that apoptotic bodies were increased
in the tumors of mice treated with Chanti-TRIM (Fig. 6C). NF-κB nuclear translocation
factors, p65, Ikβ and IkBα, were decreased in tumors treated with
Chanti-TRIM (Fig. 6D). Furthermore,
NF-κB luciferase activity in tumors was significantly inhibited by
treatment with Chanti-TRIM (Fig.
6E). MMP-9 and NF-κB expression levels were downregulated after
treatment with Chanti-TRIM (Fig.
6F). These results indicate that Chanti-TRIM inhibited
osteosarcoma growth in vivo, suggesting that Chanti-TRIM may
be a potential anti-cancer agent for osteosarcoma.
Discussion
In the present study, the efficacy a targeted
strategy of TRIM-14 osteosarcoma suppression was investigated in
osteosarcoma cells and osteosarcoma-bearing xenograft mice.
Previous studies have suggested that TRIM-14 overexpression may
induce an aggressive phenotype in cancer progression through
regulation of the NF-κB signaling pathway (29). Therefore, understanding the role of
TRIM-14 is necessary for tumor research and treatment in human
tumorigenesis and metastasis. The present study design involved
constructing a chimeric antibody target for TRIM-14 and
investigating its anti-cancer efficacy in vitro and in
vivo. The findings indicated that Chanti-TRIM decreased the
growth, migration and invasion of osteosarcoma cells by inhibiting
the MMP-induced NF-κB signaling pathway; whereas overexpression of
TRIM-14 promoted the growth, migration and invasion of osteosarcoma
cells. The results demonstrated that overexpression of TRIM-14
increased cisplatin-induced apoptosis resistance by activating the
NF-κB signal pathway. Notably, Chanti-TRIM-treated tumors in
xenograft mice were significantly inhibited, as determined via
reduced tumor volumes compared with the control group. These
results indicate that TRIM-14 may be a potential molecular target
and Chanti-TRIM may be a potential anti-cancer agent through the
inhibition of the MMP-9-induced NF-κB pathway for osteosarcoma
therapy.
To date, inducing apoptosis in tumor cells is the
most efficient clinical regiment for the treatment of patients with
cancer (30,31). Resistance to apoptosis is the
greatest obstacle to the treatment of human cancer (32,33).
Decreasing the apoptosis-resistance of cancer cells and tumors
tissues may improve the clinical treatment outcomes of patients
with osteosarcoma who have undergone oncotherapy and other
comprehensive treatments (34,35). In
recent years, TRIM-14 was identified as an oncogene that promotes
tumor growth, aggressiveness and tumor angiogenesis; however,
knockdown of TRIM-14 expression can significantly inhibit tumor
growth, migration, invasion and tumor angiogenesis in human
colorectal cancer cells (36). The
results of the present study demonstrated that Chanti-TRIM
treatment not only inhibits growth, but also enhances the apoptosis
of osteosarcoma cells induced by cisplatin. Notably, previous
findings have shown that TRIM-14 overexpression promotes cancer
cell proliferation and predicts poor survival in patients with
colorectal cancer, which is consistent with the present findings
(37). The findings of the present
study suggest that Chanti-TRIM is able to neutralize TRIM-14
expression, which can lead to opposite outcomes by upregulating
MMP-9 through the activation of the NF-κB signaling pathway.
Notably, different signaling pathways that promote
the aggressiveness of osteosarcoma have been associated with the
modulation of MMP-9 transcription (38,39).
NF-κB transcription factors may induce the expression and
activation of MMP-9 by interacting with binding sites, and may
consequently promote tumor progression (40,41). The
results of this study suggest that TRIM-14 may induce MMP-9
expression and promote NF-κB activity, whereas Chanti-TRIM-mediated
blocking of the activity of NF-κB may significantly downregulate
the expression of TRIM-14 and prevent MMP-9 activity in the NF-κB
pathway.
The results of the present study indicate that
Chanti-TRIM was able to downregulate MMP-9 expression by inhibiting
the NF-κB signaling pathway in U-2OS cells. Previous studies have
demonstrated that targeting CCND1 suppresses osteosarcoma cell
metastasis (42–44). In addition, BcL-XL, VEGF-C, and c-Myc
are overexpressed in osteosarcoma cells, which are associated with
the apoptosis, growth and aggressiveness of malignant osteosarcoma
(45,46). Furthermore, Yu et al (47) have suggested that downregulation of
the NF-κB signaling pathway is capable of inhibiting cell invasion
and the migration ability of human osteosarcoma in vitro.
The findings of the present study indicate that Chanti-TRIM
suppresses the expression levels of BcL-XL VEGF-C and c-Myc, which
contributes to inhibiting the aggressive phenotype in osteosarcoma
cells.
In conclusion, the findings of the present study
indicated that TRIM-14 is overexpressed in bone cancer cells and
clinical bone cancer tissues. This research suggested that
inhibition of TRIM-14 expression by Chanti-TRIM treatment markedly
suppressed the growth, aggressiveness, metastasis and
apoptosis-resistance in osteosarcoma via MMP-9-induced NF-κB
signaling. According to the molecular and therapeutic study of
Chanti-TRIM, TRIM may be a potential target for the treatment of
patients with osteosarcoma.
References
|
1
|
Vijayamurugan N and Bakhshi S: Review of
management issues in relapsed osteosarcoma. Expert Rev Anticancer
Ther. 14:151–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Liu Z, Jing P, Shao L, Chen L, He
X and Gong W: Effects of murine double minute 2 polymorphisms on
the risk and survival of osteosarcoma: A systemic review and
meta-analysis. Tumour Biol. 35:1649–1652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeyama I: Review of bone tumor. Iryo.
24(Suppl): S2271970.(In Japanese).
|
|
4
|
Sanchez-Pareja A, Larousserie F,
Boudabbous S, Beaulieu JY, Mach N, Saiji E and Rougemont AL: Giant
cell tumor of bone with pseudosarcomatous changes leading to
premature denosumab therapy interruption: A case report with review
of the literature. Int J Surg Pathol. 24:366–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dell'Amore A, Asadi N, Caroli G, Dolci G,
Bini A and Stella F: Recurrent primary cardiac osteosarcoma: A case
report and literature review. Gen Thorac Cardiovasc Surg.
62:175–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farcas N, Arzi B and Verstraete FJ: Oral
and maxillofacial osteosarcoma in dogs: A review. Vet Comp Oncol.
12:169–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Zhao RH, Tseng KF, Li KP, Lu ZG,
Liu Y, Han K, Gan ZH, Lin SC, Hu HY and Min DL: Sirolimus induces
apoptosis and reverses multidrug resistance in human osteosarcoma
cells in vitro via increasing microRNA-34b expression. Acta
Pharmacol Sin. 37:519–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao H, Peng C, Ruan G, Zhou J, Li Y and
Hai Y: Adenovirus-delivered PDCD5 counteracts adriamycin resistance
of osteosarcoma cells through enhancing apoptosis and inhibiting
Pgp. Int J Clin Exp Med. 7:5429–5436. 2014.PubMed/NCBI
|
|
9
|
Tsai HC, Huang CY, Su HL and Tang CH: CCN2
enhances resistance to cisplatin-mediating cell apoptosis in human
osteosarcoma. PLoS One. 9:e901592014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Locklin RM, Federici E, Espina B, Hulley
PA, Russell RG and Edwards CM: Selective targeting of death
receptor 5 circumvents resistance of MG-63 osteosarcoma cells to
TRAIL-induced apoptosis. Mol Cancer Ther. 6:3219–3228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vourvouhaki E, Carvalho C and Aguiar P:
Model for Osteosarcoma-9 as a potent factor in cell survival and
resistance to apoptosis. Phys Rev E Stat Nonlin Soft Matter Phys.
76:0119262007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozato K, Shin DM, Chang TH and Morse HC
III: TRIM family proteins and their emerging roles in innate
immunity. Nat Rev Immunol. 8:849–860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang DX, Li K, Liu B, Zhu ZM, Xu XW, Zhao
SH, Yerle M and Fan B: Chromosomal localization, spatio-temporal
distribution and polymorphism of the porcine tripartite
motif-containing 55 (TRIM55) gene. Cytogenet Genome Res.
114:93B2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nenasheva VV, Kovaleva GV, Uryvaev LV,
Ionova KS, Dedova AV, Vorkunova GK, Chernyshenko SV, Khaidarova NV
and Tarantul VZ: Enhanced expression of trim14 gene suppressed
Sindbis virus reproduction and modulated the transcription of a
large number of genes of innate immunity. Immunol Res. 62:255–262.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimsa MW, Strzalka-Mrozik B, Kimsa MC,
Mazurek U, Kruszniewska-Rajs C, Gola J, Adamska J and Twardoch M:
Differential expression of tripartite motif-containing family in
normal human dermal fibroblasts in response to porcine endogenous
retrovirus infection. Folia Biol (Praha). 60:144–151.
2014.PubMed/NCBI
|
|
17
|
Hassanzadeh P: Colorectal cancer and NF-κB
signaling pathway. Gastroenterol Hepatol Bed Bench. 4:127–132.
2011.PubMed/NCBI
|
|
18
|
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng
Y, Lv J, Song Z, Li Y, Ji L, et al: Shikonin suppresses tumor
growth and synergizes with gemcitabine in a pancreatic cancer
xenograft model: Involvement of NF-κB signaling pathway. Biochem
Pharmacol. 88:322–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elghonaimy EA, Ibrahim SA, Youns A,
Hussein Z, Nouh MA, El-Mamlouk T, El-Shinawi M and Mostafa Mohamed
M: Secretome of tumor-associated leukocytes augment
epithelial-mesenchymal transition in positive lymph node breast
cancer patients via activation of EGFR/Tyr845 and NF-κB/p65
signaling pathway. Tumour Biol. 37:12441–12453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
El-Ghonaimy EA, Ibrahim SA, Youns A,
Hussein Z, Nouh MA, El-Mamlouk T, El-Shinawi M and Mohamed MM:
Erratum to: Secretome of tumor-associated leukocytes augment
epithelial-mesenchymal transition in positive lymph node breast
cancer patients via activation of EGFR/Tyr845 and NF-kB/p65
signaling pathway. Tumour Biol. 37:143332016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY,
Luo QF, Shu Y, Liu ZL and Huang SH: Knockdown of Aurora-B inhibits
osteosarcoma cell invasion and migration via modulating
PI3K/Akt/NF-kappaB signaling pathway. Int J Clin Exp Pathol.
7:3984–3991. 2014.PubMed/NCBI
|
|
22
|
Kravtsova-Ivantsiv Y and Ciechanover A:
The ubiquitin-proteasome system and activation of NF-kappaB:
Involvement of the ubiquitin ligase KPC1 in p105 processing and
tumor suppression. Mol Cell Oncol. 2:e10545522015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jamshidi M, Fagerholm R, Khan S, Aittomäki
K, Czene K, Darabi H, Li J, Andrulis IL, Chang-Claude J, Devilee P,
et al: SNP-SNP interaction analysis of NF-κB signaling pathway on
breast cancer survival. Oncotarget. 6:37979–37994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeon DG, Song WS, Cho WH, Kong CB and Cho
SH: Proximal tumor location and fluid-fluid levels on MRI predict
resistance to chemotherapy in stage IIB osteosarcoma. Clin Orthop
Relat Res. 472:1911–1920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hagel L: Gel-filtration chromatography.
Curr Protoc Protein Sci. 8:Unit8.32001.PubMed/NCBI
|
|
27
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai FL, Yu YH, Tian H, Ren GP, Wang H,
Zhou B, Han XH, Yu QZ and Li DS: Genetically engineered Newcastle
disease virus expressing interleukin-2 and TNF-related
apoptosis-inducing ligand for cancer therapy. Cancer Biol Ther.
15:1226–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su X, Wang J, Chen W, Li Z, Fu X and Yang
A: Overexpression of TRIM14 promotes tongue squamous cell carcinoma
aggressiveness by activating the NF-κB signaling pathway.
Oncotarget. 7:9939–9950. 2016.PubMed/NCBI
|
|
30
|
Rivoltini L, Chiodoni C, Squarcina P,
Tortoreto M, Villa A, Vergani B, Bürdek M, Botti L, Arioli I, Cova
A, et al: TNF-related apoptosis-inducing ligand (TRAIL)-armed
exosomes deliver proapoptotic signals to tumor site. Clin Cancer
Res. 22:3499–3512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai HF and Hsu PN: Modulation of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated
apoptosis by Helicobacter pylori in immune pathogenesis of gastric
mucosal damage. J Microbiol Immunol Infect. 50:4–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chinchar E, Makey KL, Gibson J, Chen F,
Cole SA, Megason GC, Vijayakumar S, Miele L and Gu JW: Sunitinib
significantly suppresses the proliferation, migration, apoptosis
resistance, tumor angiogenesis and growth of triple-negative breast
cancers but increases breast cancer stem cells. Vasc Cell.
6:122014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guidicelli G, Chaigne-Delalande B,
Dilhuydy MS, Pinson B, Mahfouf W, Pasquet JM, Mahon FX, Pourquier
P, Moreau JF and Legembre P: The necrotic signal induced by
mycophenolic acid overcomes apoptosis-resistance in tumor cells.
PLoS One. 4:e54932009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han J, Tian R, Yong B, Luo C, Tan P, Shen
J and Peng T: Gas6/Axl mediates tumor cell apoptosis, migration and
invasion and predicts the clinical outcome of osteosarcoma
patients. Biochem Biophys Res Commun. 435:493–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kushlinskii NE, Solov'ev YN, Babkina IV,
Abbasova SG, Kostanyan IA, Lipkin VM and Trapeznikov NN: Leptin and
apoptosis inhibitor soluble Fas antigen in the serum of patients
with osteosarcoma and neuroectodermal bone tumors. Bull Exp Biol
Med. 129:496–498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Zhu J, Dong M, Yu H, Dai X and Li
K: Knockdown of tripartite motif containing 24 by lentivirus
suppresses cell growth and induces apoptosis in human colorectal
cancer cells. Oncol Res. 22:39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jiang T, Tang HM, Lu S, Yan DW, Yang YX
and Peng ZH: Up-regulation of tripartite motif-containing 29
promotes cancer cell proliferation and predicts poor survival in
colorectal cancer. Med Oncol. 30:7152013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han J, Yong B, Luo C, Tan P, Peng T and
Shen J: High serum alkaline phosphatase cooperating with MMP-9
predicts metastasis and poor prognosis in patients with primary
osteosarcoma in Southern China. World J Surg Oncol. 10:372012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SM, Lee H, Park YS, Lee Y and Seo SW:
ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J
Orthop Res. 30:1040–1044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning L, Ma H, Jiang Z, Chen L, Li L, Chen
Q and Qi H: Curcumol suppresses breast cancer cell metastasis by
inhibiting MMP-9 via JNK1/2 and Akt-dependent NF-κB signaling
pathways. Integr Cancer Ther. 15:216–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim JM, Noh EM, Kim HR, Kim MS, Song HK,
Lee M, Yang SH, Lee GS, Moon HC, Kwon KB and Lee YR: Suppression of
TPA-induced cancer cell invasion by Peucedanum japonicum Thunb.
extract through the inhibition of PKCα/NF-κB-dependent MMP-9
expression in MCF-7 cells. Int J Mol Med. 37:108–114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han K, Chen X, Bian N, Ma B, Yang T, Cai
C, Fan Q, Zhou Y and Zhao TB: MicroRNA profiling identifies MiR-195
suppresses osteosarcoma cell metastasis by targeting CCND1.
Oncotarget. 6:8875–8889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma
YL, Ji ZW, Li XX, Han K, Gao J, et al: miR-15a and miR-16-1
downregulate CCND1 and induce apoptosis and cell cycle arrest in
osteosarcoma. Oncol Rep. 28:1764–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
He N and Zhang Z: Baicalein suppresses the
viability of MG-63 osteosarcoma cells through inhibiting c-MYC
expression via Wnt signaling pathway. Mol Cell Biochem.
405:187–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weiss KR, Cooper GM, Jadlowiec JA, McGough
RL III and Huard J: VEGF and BMP expression in mouse osteosarcoma
cells. Clin Orthop Relat Res. 450:111–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Z, Zheng Y, Zhu R, Zhu Y, Yao W, Liu
W and Gao X: The ERK/eIF4F/Bcl-XL pathway mediates SGP-2 induced
osteosarcoma cells apoptosis in vitro and in vivo.
Cancer Lett. 352:203–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu X, Wang Q, Zhou X, Fu C, Cheng M, Guo
R, Liu H, Zhang B and Dai M: Celastrol negatively regulates cell
invasion and migration ability of human osteosarcoma via
downregulation of the PI3K/Akt/NF-kappaB signaling pathway in
vitro. Oncol Lett. 12:3423–3428. 2016. View Article : Google Scholar : PubMed/NCBI
|