Introduction
Cardiac arrest is the world's leading cause of
fatality in heart disease (1).
Out-of-hospital cardiac arrests lead to 295,000 mortalities in the
USA, accompanied with 350,000 in Europe and 544,000 in China every
year (1–3). Despite efforts to improve the treatment
of cardiac arrest in recent years, survival to hospital discharge
is only 10.6% (1). Two-thirds of
patients with out-of-hospital cardiac arrest suffer mortality due
to neurological injury, which is sustained during the anoxic,
no-flow period of cardiac arrest or as a result of reperfusion
injury, even following a successful resuscitation (4).
During the last decade, laboratory and clinical
studies have demonstrated that targeted temperature management
(32–36°C) following cardiopulmonary resuscitation (CPR)
significantly improves neurological outcome (5,6). There
are a variety of cooling methods available for post-resuscitation
management, including pharmacologically-induced hypothermia, which
reduces the body temperature by regulating the temperature center
in the hypothalamus (7–9).
Cholecystokinin octapeptide (CCK8) is a type of
central and peripheral neurotransmitter, which induces
dose-dependent hypothermia when injected peripherally into rats and
mice (10,11). It was also reported to induce mild
hypothermia and improve myocardial and cerebral function in a rat
model of CPR (9). In addition, CCK8
is effective in counteracting progressive neuronal dysfunction and
damage, and inhibiting the systemic inflammatory response following
sepsis (12–14).
In the present study, previous experiments performed
in rodents were adapted for a large animal, porcine model of CPR,
which is considered more clinically relevant. The effect of CCK8 on
thermoregulation, myocardial function and neurological function was
examined in a porcine model of CPR. It was hypothesized that CCK8
would induce hypothermia and improve neurological outcomes after
resuscitation.
Materials and methods
Ethics statement
The present study was approved by the Animal Care
and Use Committee of the First Affiliated Hospital of Nanjing
Medical University (Nanjing, China). All animals received humane
care according to the National Research Council's 1996 Guide for
the Care and Use of Laboratory Animals (15).
Animal preparation
Bama miniature pigs were selected for use in the
present study and they were purchased from Shanghai Jiagan
Biological Technology Inc., (Shanghai, China). A total of 12 male
Bama miniature pigs at the age of 6 months, weighing 20–25 kg,
underwent overnight fasting except for free access to water. The
animal room was maintained at 19–24°C, with relative humidity
between 40 and 60% and a 12-h light/dark cycle. All animals were
anesthetized by an intramuscular injection of ketamine (20 mg/kg;
cat. no. K2753) and an ear vein injection of sodium pentobarbital
(30 mg/kg; cat. no. 1507002) (both Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Additional doses of sodium pentobarbital (8
mg/kg) were administered hourly to maintain anesthesia. The trachea
was orally intubated and the animals were mechanically ventilated
[tidal volume, 15 ml/kg; peak flow, 40 l/min; fraction of inspired
oxygen (FiO2), 0.21) with a volume-controlled SynoVent
E3 ventilator (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.,
Shenzhen, China). End-tidal PCO2 (ETCO2) was
monitored with a handheld ETCO2/SPO2 monitor
(PMSH-300; SunLife Science, Inc., Shanghai, China). Respiratory
frequency was adjusted to keep ETCO2 between 35 and 40
mmHg. A conventional lead II electrocardiogram (ECG) was monitored
continuously.
A fluid-filled 5F transducer-tipped catheter
(SPC-450S; Millar, Inc., Houston, TX, USA) was advanced through the
right femoral artery and into the thoracic aorta to monitor the
aortic pressure and collect blood samples. Another 7F pentalumen
thermodilution-tipped catheter (Abbott Pharmaceutical Co., Ltd.,
Lake Bluff, IL, USA) was advanced through the right femoral vein
and into the right atrium to measure the right atrial pressure and
core temperature. For the measurement of myocardial function,
including stroke volume (SV) and global ejection fraction (GEF), a
PiCCOplus monitor (Pulsion Medical Systems SE, Feldkirchen,
Germany) based on transpulmonary thermodilution was used. A 7F
central venous catheter was inserted into the right internal
jugular vein for the injection of iced saline. Another 4F
thermistor-tipped arterial catheter was inserted into the left
femoral artery. The arterial and central venous catheters were
connected to the PiCCO system for discontinuous monitoring of SV
and GEF. A 5F pacing catheter (EP Technologies, Inc., Sunnyvale,
CA, USA) was then advanced through the right external jugular vein
and into the right ventricle to induce ventricular fibrillation
(VF), as confirmed by characteristic pressure morphology and
fluoroscopy. The body temperature was maintained at 37.5±0.5°C with
a cooling/warming blanket (Shanghai Full-Ying Biomedical Technology
Co., Shanghai, China) prior to cardiac arrest.
Experimental procedures
The established porcine model of CPR was utilized as
previously described (16,17). A total of 15 min prior to inducing
VF, baseline data were recorded. VF was electrically induced with a
1-mA alternating current through a 5F pacing catheter delivered to
the right ventricle. Mechanical ventilation was stopped following
the onset of VF. After 10 min of untreated VF, precordial
compression was initiated with a mechanical chest compressor (Weil
MCC; SunLife Science, Inc.) and mechanical ventilation was
performed again (tidal volume, 15 ml/kg; peak flow, 40 l/min; FiO2,
21%). Mechanical compression was programmed to maintain at a rate
of 100 compressions/min and synchronized to keep a
compression/ventilation ratio of 30:2. The force of compression was
adjusted to reduce the anterior-posterior diameter of the chest by
25%. Following 2.5 min of CPR, 20 µg/kg epinephrine (Guangzhou
Baiyunshan Mingxing Pharmaceutical Co., Ltd., Guangzhou, China) was
injected via the femoral vein. After 5 min from the start of CPR,
defibrillation (150 J biphasic shock) was attempted with a Zoll
defibrillator (E-Series; ZOLL Medical Corporation, Chelmsford, MA,
USA). If restoration of spontaneous circulation (ROSC) was not
achieved, CPR was continued for a further 2 min followed by a
subsequent defibrillation attempt. Additional doses of epinephrine
(20 µg/kg) were injected at an interval of 3 min after the initial
administration. CPR was continued for a total of 15 min or until
ROSC. If an organized cardiac rhythm with mean aortic pressure
(MAP) of >50 mmHg persisted for ≥5 min, the animal was regarded
as ROSC (16,17).
At 5 min following resuscitation, the animals were
randomized and equally assigned into two groups (n=6/group); the
CCK8 group (44.4 µg/kg CCK in 20 ml saline) or the control group
(20 ml saline). Animals in the CCK8 group were continuously infused
with CCK8 (Cellmano Biotech Limited, Hefei, China) for 1 h at a
dose of 44.4 µg/kg/h at a rate of 20 ml/h. Saline was continuously
infused at the same rate and time interval in the control
group.
All animals were monitored for 4 h. The animals were
then brought out of anesthesia and the catheters, including the
endotracheal tube, were removed and any wounds were sutured. The
animals were returned to their cages and observed for an additional
20 h. Following this, all animals were euthanized by intravenous
injection of sodium pentobarbital (150 mg/kg). A necropsy was
performed for documentation of cerebral apoptosis.
Measurements
Hemodynamic data, ECG and blood temperatures were
continuously recorded using ECG monitoring equipment (BeneView T6;
Shenzhen Mindray Bio-Medical Electronics Co., Ltd.).
ETCO2 was monitored with the
ETCO2/SPO2 monitor. SV and GEF, as the
indexes of myocardial function, were discontinuously measured for 4
h following ROSC with the PiCCO system.
Venous blood was collected in EDTA-coated
Vacutainers (BD Biosciences, Franklin Lakes, NJ, USA) at baseline,
4, 12 and 24 h following ROSC. Using these blood samples, brain
injury markers, including neuron specific enzyme (NSE) (cat. no.
AE90705Po) and S100B protein (cat. no. AE90735Po; both Shanghai
Lianshuo Biological Technology Co., Ltd., Shanghai, China), and
inflammatory factors, including tumor necrosis factor (TNF)-α (cat.
no. MEXN-P0010) and interleukin (IL)-6 (cat. no. MEXN-P0019; both
Shanghai Meixuan Biological Science and Technology Ltd., Shanghai,
China), were measured using porcine ELISA kits.
At 24 h after ROSC, the neurologic function of the
pigs was evaluated using neurologic deficit scores (NDS) as
previously described (18). NDS
included the levels of respiratory pattern, motor and sensory
function, consciousness and behaviour. The scores from each item
were summed to yield a total score, ranging from 0 (no observed
neurological deficit) to 400 (brain death) (18). The NDS was examined by two
investigators blinded to the pig's treatment group.
Apoptosis in the cerebrum was detected using a
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labelling (TUNEL) assay. Tissue samples taken from the frontal
cortex of pigs 24 h after resuscitation were fixed in 4%
paraformaldehyde overnight at room temperature, and embedded in
paraffin and then cut into 6-µm-thick slices. TUNEL staining was
conducted using a commercially available kit (cat. no. 293-71501,
Wako Pure Chemical Industries, Ltd., Osaka, Japan) following the
manufacturer's protocol. Following deparaffinization and
rehydration, the tissue samples on the glass slices were digested
with proteinase solution at 37°C for 5 min. Following this, samples
were washed with PBS and treated with 100 µl TdT reaction solution
for 10 min in a moist chamber at 37°C. Samples were washed with PBS
and intrinsic peroxidase activity was eliminated following
treatment with 3% H2O2 for 5 min at room
temperature. The slides were washed with PBS, and covered with 100
µl POD-conjugated antibody solution for 10 min in a moist chamber
at 37°C. Samples were rinsing with PBS again, and the slides were
covered with 100 µl 3,3′-diaminobenzidine solution (3%; cat. no.
45-053-150038, GenWay Biotech, Inc., San Diego, CA, USA) for 5 min
at room temperature, and washed in distilled deionized water.
Finally, the slides were counterstained for 20 sec with
hematoxylin, dehydrated, and mounted with Softmount (cat. no.
192-16301, Wako Pure Chemical Industries, Ltd., Osaka, Japan). The
integrated optical density (IOD) of positive TUNEL staining from
four random high-power fields (magnification, ×100) was analyzed
with a light microscope (BX53, Olympus Corporation, Tokyo, Japan)
and Image-Pro Plus 5.0.1 software (Media Cybernetics, Inc.,
Rockville, MD, USA) by a pathologist blinded to the study.
Statistical analysis
All quantitative variables were reported as the mean
± standard deviation. Variation between two groups was compared
using a Student's two-tailed t-test. A Mann-Whitney U test was
performed when the normal distribution and homogeneity of variance
were not met. All statistical analyses were performed with SPSS
20.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline and resuscitation data
A total of 15 pigs were used in the present study,
of which 12 successfully completed the study and were included.
There were 3 pigs that failed to be resuscitated, meaning that 80%
of the animals survived. The baseline blood temperature,
hemodynamics, blood analytical measurements, number of shocks
required to achieve ROSC, as well as duration of CPR did not differ
significantly between the CCK8 group and the control group
(Table I).
 | Table I.Baseline characteristics of the pigs
in the control and CCK8 groups. |
Table I.
Baseline characteristics of the pigs
in the control and CCK8 groups.
|
| Group |
|---|
|
|
|
|---|
| Characteristic | Control (n=6) | CCK8 (n=6) |
|---|
| Body weight, kg |
24.2±1.6 |
23.8±1.1 |
| PaO2,
mmHg |
94.2±16.1 |
95.3±17.1 |
| PaCO2,
mmHg |
40.4±5.8 |
39.2±4.9 |
| pH |
7.5±0.1 |
7.5±0.1 |
| Temperature, °C |
37.4±0.3 |
37.4±0.5 |
| Heart rate, bpm |
102.0±8.9 |
113.4±31.0 |
| Mean aortic pressure,
mmHg |
112.3±17.3 |
130.8±12.3 |
| Right atrial blood
pressure, mmHg |
2.8±0.7 |
3.1±0.6 |
| End-tidal
CO2, mmHg |
38.1±1.8 |
37.6±2.0 |
| Defibrillations
(n) |
2.0±1.0 |
2.2±1.1 |
| Duration of
cardiopulmonary resuscitation, min |
5.0±0.0 |
5.0±0.0 |
Blood temperature, hemodynamics and
myocardial function
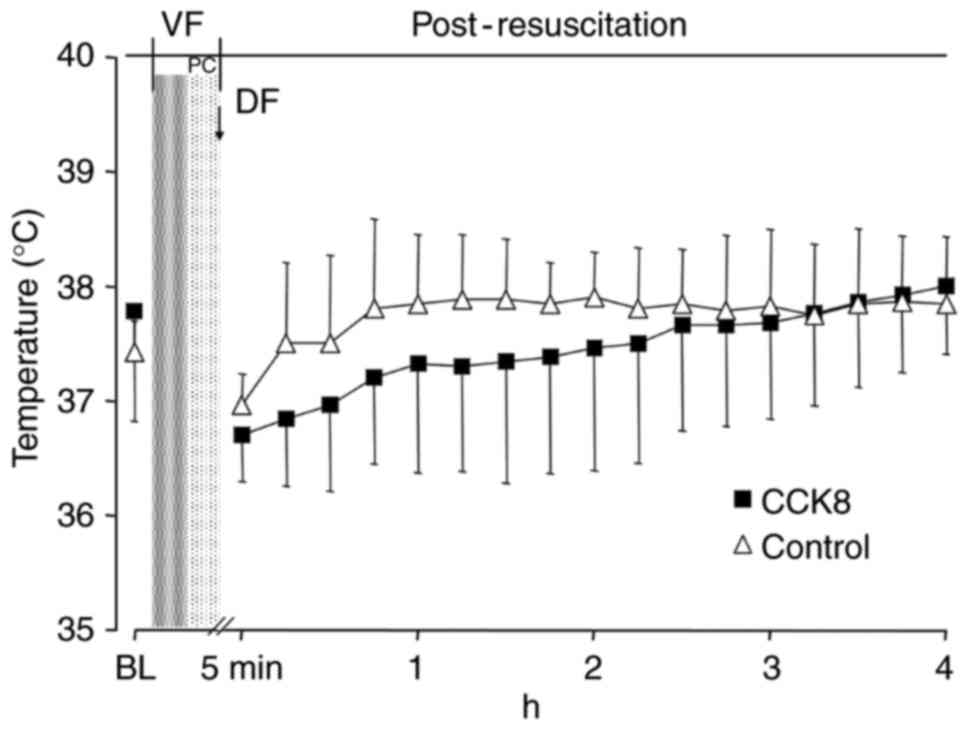
Following resuscitation, the blood temperature in
the CCK8 group was notably lower than that observed in the control
group in the first 2 h (Fig. 1).
However, there was no significant difference in the blood
temperature between the CCK8 group and the control group at any
time throughout the experiment (Fig.
1).
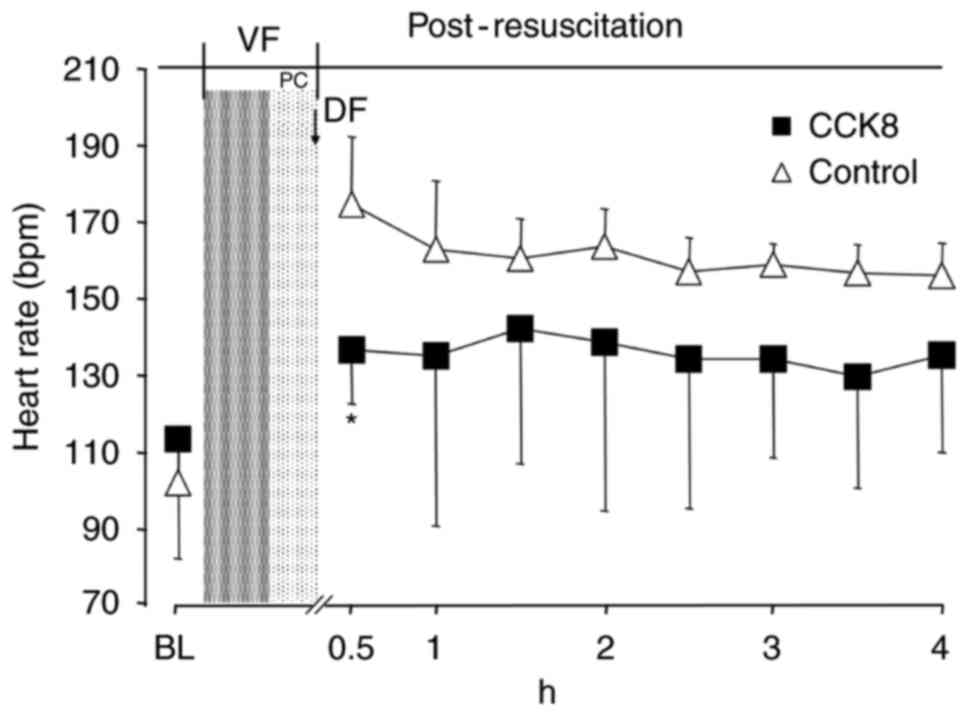
The heart rate in the CCK8 group was significantly
reduced in the first 30 min following ROSC compared with that of
the control group (P<0.05; Fig.
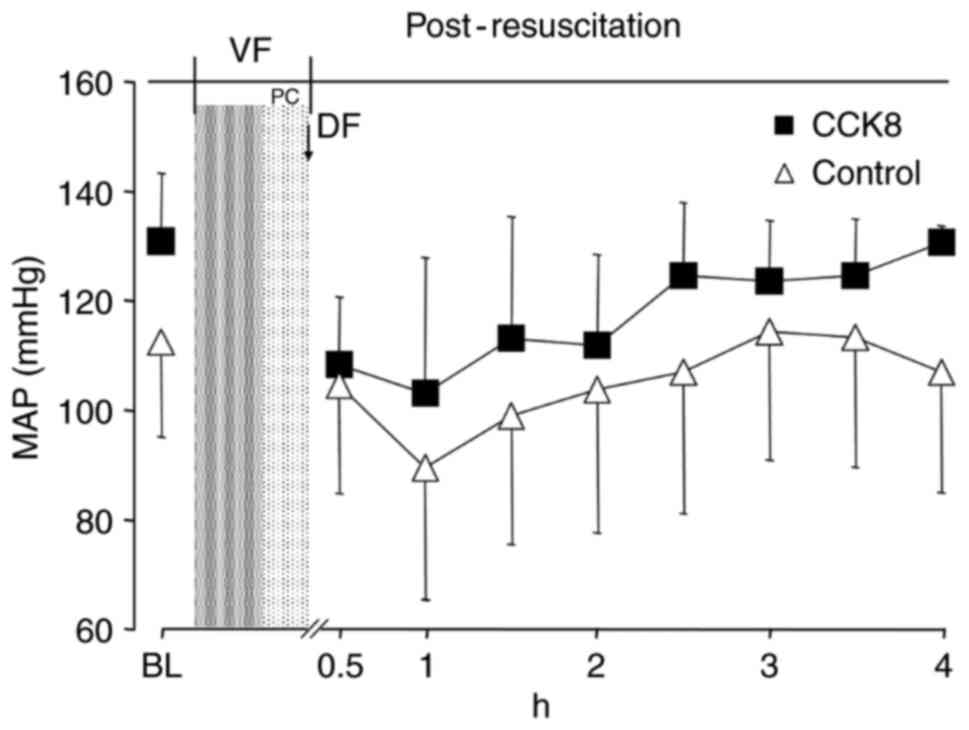
2). However, there was no significant difference in the MAP
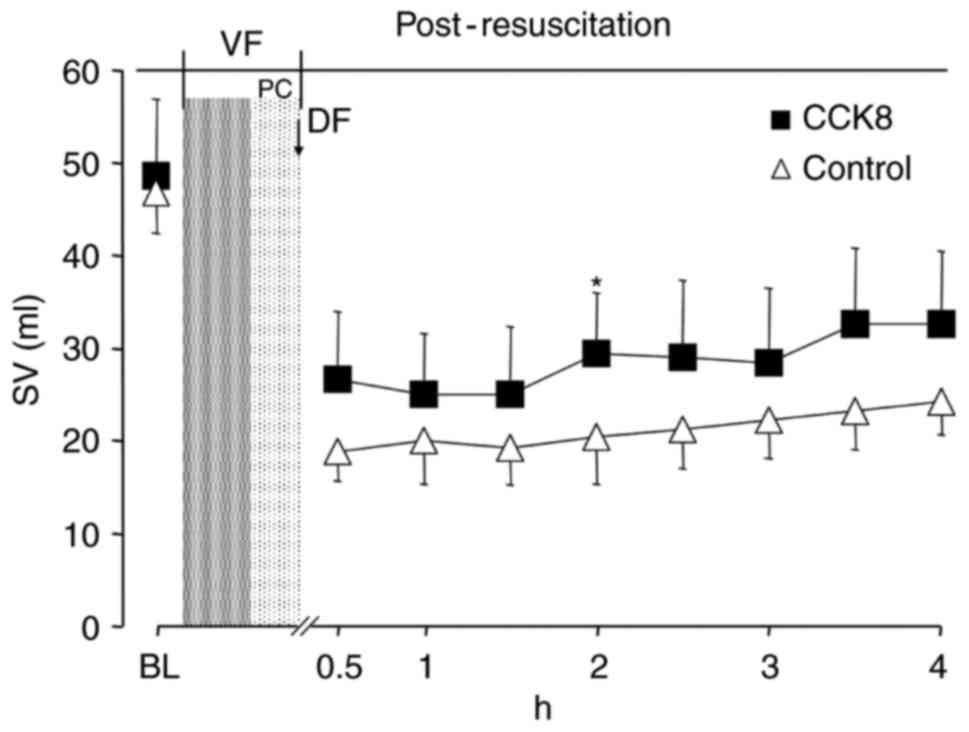
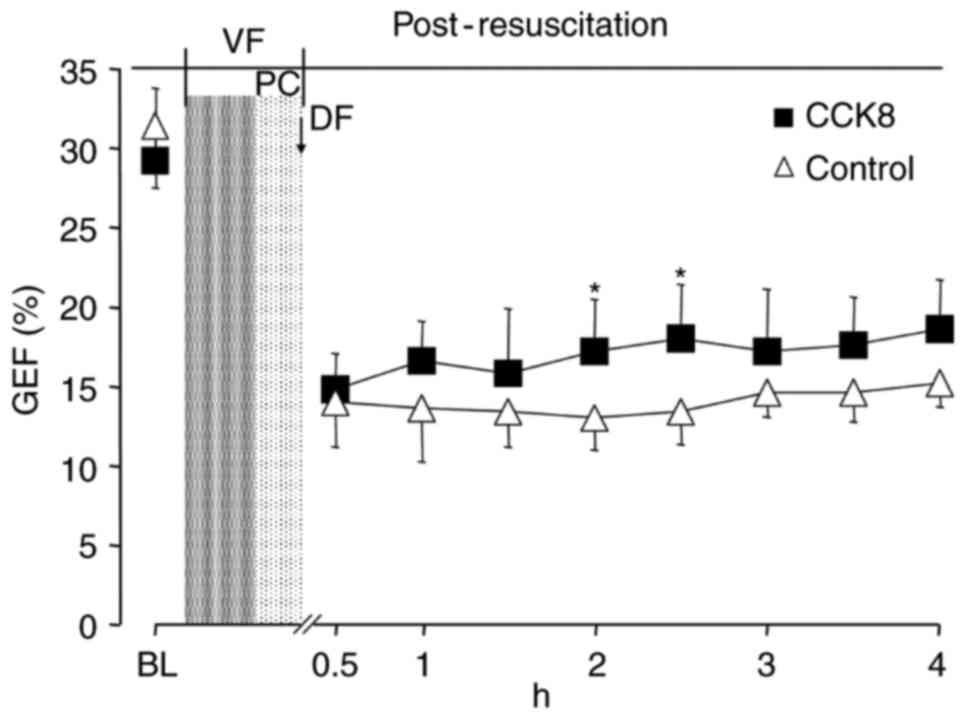
between the two groups at any time point (Fig. 3). The SV and GEF were significantly
increased in the CCK8 group compared with that observed in the
control group at 2 h after resuscitation (P<0.05; Figs. 4 and 5).
Brain injury and neurologic
function
The brain injury markers (NSE and S100B) were
significantly reduced in the CCK8 group compared with the control
group at 12 and 24 h after resuscitation (P<0.05; Table II).
 | Table II.Levels of brain injury markers in the
control and CCK8 groups. |
Table II.
Levels of brain injury markers in the
control and CCK8 groups.
|
| Time point, h |
|---|
|
|
|
|---|
| Brain injury
marker | Baseline | 4 | 12 | 24 |
|---|
| Neuron specific
enzyme, ng/ml |
|
|
|
|
| Control
(n=6) |
12.1±1.6 |
18.5±1.7 |
24.4±1.0 |
24.1±0.6 |
| CCK8
(n=6) |
13.2±2.0 |
16.8±0.6 |
20.3±0.7a |
20.9±0.9a |
| S100B (pg/ml) |
|
|
|
|
| Control
(n=6) |
720±185 |
1,441±21 |
1,504±53 |
1,415±36 |
| CCK8
(n=6) |
740±136 |
1,226±291 |
1,160±204a |
1,146±38a |
At 24 h after resuscitation, a significantly
improved NDS was observed in animals treated with CCK8 compared
with that observed in the control group (68±21 and 160±13,
respectively; P<0.05; data not shown).
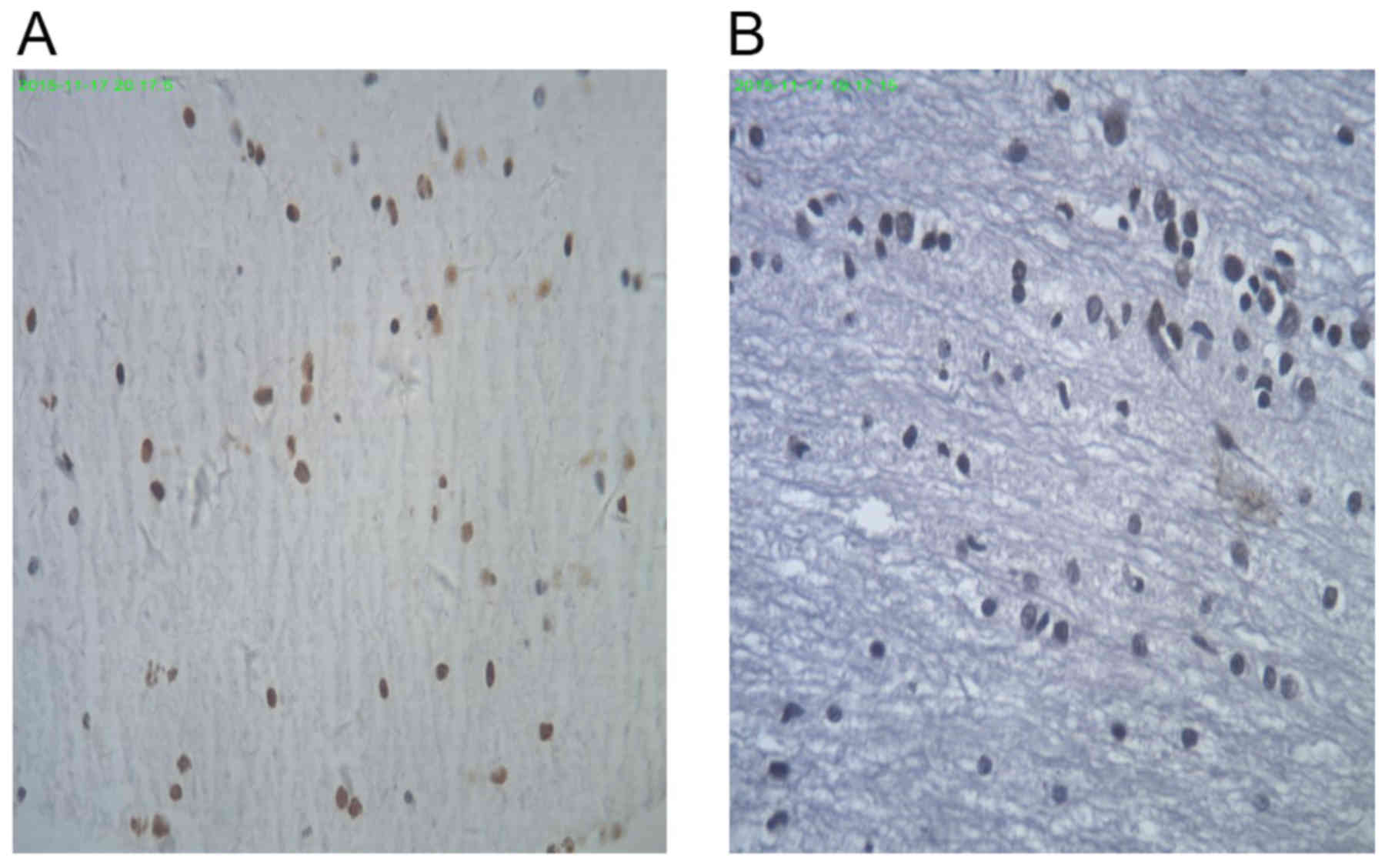
Broken nuclei in TUNEL-positive cells in the control
group were stained brown or yellow, which varied in size and shape
(Fig. 6A). TUNEL-negative cells in
the CCK8 group were stained blue with hematoxylin (Fig. 6B). There was a significantly lower
IOD in the CCK8 group than in the control group (3.1±1.3 and
5.4±3.3, respectively; P<0.05; data not shown).
Inflammatory response following
resuscitation
Compared with the control group, TNF-α and IL-6 were
significantly decreased in the CCK8 group at 4 and 8 h following
ROSC (P<0.05; Table III). IL-6
levels were also significantly lower in the CCK8 group than those
in the control group at 24 h (P<0.05).
 | Table III.Levels of cytokines in the control
and CCK8 groups. |
Table III.
Levels of cytokines in the control
and CCK8 groups.
|
| Time point, h |
|---|
|
|
|
|---|
| Cytokine | Baseline | 4 | 12 | 24 |
|---|
| Interleukin-6,
pg/ml |
|
|
|
|
| Control
(n=6) |
277±16 |
404±50 |
404±40 |
411±30 |
| CCK8
(n=6) |
261±5 |
303±14a |
321±20a |
317±48a |
| Tumor necrosis
factor-α, pg/ml |
|
|
|
|
| Control
(n=6) |
663±90 |
836±26 |
738±26 |
659±50 |
| CCK8
(n=6) |
638±84 |
762±21a |
667±26a |
610±23 |
Discussion
The results of the present study revealed that CCK8
did not successfully induce hypothermia; however, it did
significantly inhibit the inflammatory response and apoptosis, as
well as significantly improve the neurological outcomes in a
porcine model of CPR. The cardioprotective effect of CCK8 following
CPR was not observed in the present study. To the best of our
knowledge, the present study is the first to evaluate the effect of
CCK8 in a large mammalian model of CPR.
As a neurotransmitter or neuromodulator in the
central nervous system, CCK8 has been reported to induce
dose-dependent hypothermia when injected peripherally into a rat or
murine model of CPR (10,11). This is potentially because CCK8 was
involved in the activation of CCK-B receptors in the hypothalamus,
which led to a long latency period for the thermoregulatory
response (9,19). However, the findings of the present
study appear to be inconsistent with previous studies in rats and
mice. One possible explanation is that the lower surface
area-to-mass ratio in pigs compared with that in rats and mice
resulted in a decrease in heat loss at the same ambient
temperature, thus counteracting the effect of CCK8 on
thermoregulation. Previous studies have revealed that for the body
temperature to reach 33°C following CPR by rapid surface cooling,
it would take ~190 min in pigs but only 10 min in rats, which also
demonstrates the difference in heat loss between the two models
(20,21). Another possible reason is the
difference in the dose-effect association of CCK8 between pigs and
rats. Due to the discrepancies in the dose-effect association
between various species, serotonin and norepinephrine, which are
considered neurotransmitters associated with thermoregulation, may
lead to different or even opposite effects on body temperature
(22).
Although CCK8 was unsuccessful at inducing
hypothermia in the present porcine model of CPR, CCK8 directly
inhibited the inflammatory response and reduced apoptosis
independently of hypothermia, which resulted in an improved
neurological outcome following resuscitation. These results
demonstrate that CCK8 may be an anti-inflammatory factor with
therapeutic potential for the treatment of post-resuscitation
disease. Post-resuscitation disease is associated with an early
systemic inflammatory response, leading to an exacerbation of the
inflammatory balance, as observed in severe sepsis (23,24).
CCK8 has been indicated to have an anti-inflammatory effect in
several previous studies (25–27).
Although the underlying mechanisms require further investigation,
initial studies have demonstrated that CCK8 downregulated cluster
of differentiation (CD)80 and CD86 expression in dendritic cells,
suppressed co-stimulatory activity and immunoglobin G1 in
lipopolysaccharide (LPS)-activated B cells, decreased the secretion
of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, and
increased the production of anti-inflammatory cytokines, such as
IL-4, in LPS-activated macrophages and B cells (13,28–30).
The selection of the CCK8 dose in the present study
was based on the results of former experiments in rat models. A
previous study demonstrated that CCK8 injected peripherally led to
dose-dependent hypothermia at a dosage of 5–200 µg/kg in rats at an
ambient temperature of 21°C (19).
Weng et al (9) revealed that
CCK8 (200 µg/kg) injected intravenously within 1 min after CPR
induced and maintained hypothermia for 5 h and improved
post-resuscitation outcomes in a rat model of CPR. Therefore in the
present study, the rat doses of CCK8 (200 µg/kg) were converted to
equivalent doses in miniature pigs (44.4 µg/kg) according to the
body surface area (31).
There were certain limitations in the present study.
Firstly, the proposal of the study was to ascertain whether CCK8
would induce hypothermia and improve post-resuscitation outcomes in
a porcine model of CPR. Although hypothermia was not induced by
CCK8 at a dosage of 44.4 µg/kg, the effect of CCK8 on
thermoregulation in large mammals at various doses remains unclear.
Further study is required to investigate the dose-effect
association of CCK8 in a porcine model. Secondly, cell death
following cardiac arrest is a complex process postponed well beyond
the study period. Therefore, 24 h of observation may not be long
enough to evaluate neurological damage following resuscitation.
In conclusion, CCK8 at a dose of 44.4 µg/kg did not
induce hypothermia; however, it inhibited the inflammatory response
and significantly improved neurological outcomes in a porcine model
of CPR. The present findings therefore demonstrate that CCK8 may be
a further option for anti-inflammatory therapy after cardiac
arrest.
Acknowledgements
The present study was supported by the Zhejiang
Provincial Medical Technology Foundation (grant no.
2014KYB245).
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American Heart Association. Circulation.
131:e29–e322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atwood C, Eisenberg MS, Herlitz J and Rea
TD: Incidence of EMS-treated out-of-hospital cardiac arrest in
Europe. Resuscitation. 67:75–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hua W, Zhang LF, Wu YF, Liu XQ, Guo DS,
Zhou HL, Gou ZP, Zhao LC, Niu HX, Chen KP, et al: Incidence of
sudden cardiac death in China: Analysis of 4 regional populations.
J Am Coll Cardiol. 54:1110–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laver S, Farrow C, Turner D and Nolan J:
Mode of death after admission to an intensive care unit following
cardiac arrest. Intensive Care Med. 30:2126–2128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neumar RW, Shuster M, Callaway CW, Gent
LM, Atkins DL, Bhanji F, Brooks SC, de Caen AR, Donnino MW, Ferrer
JM, et al: Part 1: executive summary: 2015 American Heart
Association Guidelines Update for Cardiopulmonary Resuscitation and
Emergency Cardiovascular Care. Circulation. 132(18 Suppl 2):
S315–S367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nielsen N, Wetterslev J, Cronberg T,
Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J,
Kuiper M, et al: Targeted temperature management at 33°C versus
36°C after cardiac arrest. N Engl J Med. 369:2197–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun S, Tang W, Song F, Chung SP, Weng Y,
Yu T and Weil MH: Pharmacologically induced hypothermia with
cannabinoid receptor agonist WIN55, 212-2 after cardiopulmonary
resuscitation. Crit Care Med. 38:2282–2286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chung SP, Song FQ, Yu T, Weng Y, Sun S,
Weil MH and Tang W: Effect of therapeutic hypothermia vs δ-opioid
receptor agonist on post resuscitation myocardial function in a rat
model of CPR. Resuscitation. 82:350–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weng Y, Sun S, Song F, Phil Chung S, Park
J, Harry Weil M and Tang W: Cholecystokinin octapeptide induces
hypothermia and improves outcomes in a rat model of cardiopulmonary
resuscitation. Crit Care Med. 39:2407–2412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zetler G: Cholecystokinin octapeptide,
caerulein and caerulein analogues: Effects on thermoregulation in
the mouse. Neuropharmacology. 21:795–801. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katsuura G and Itoh S: Effect of
cholecystokinin octapeptide on body temperature in the rat. Jpn J
Physiol. 31:849–858. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tirassa P and Costa N: CCK-8 induces NGF
and BDNF synthesis and modulates TrkA and TrkB expression in the
rat hippocampus and septum: Effects on kindling development.
Neurochem Int. 50:130–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JG, Liu JX, Jia XX, Geng J, Yu F and
Cong B: Cholecystokinin octapeptide regulates the differentiation
and effector cytokine production of CD4(+) T cells in vitro. Int
Immunopharmacol. 20:307–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling YL, Huang SS, Wang LF, Zhang JL, Wan
M and Hao RL: [Cholecystokinin-octapeptide (CCK-8) reverses
experimental endotoxin shock. Sheng Li Xue Bao. 48:390–394.
1996.(In Chinese). PubMed/NCBI
|
|
15
|
Institute for Laboratory Animal Research,
. Guide for the Care and Use of Laboratory Animals. 8th. National
Research Council; Washington, DC: pp. 1072–1073S. 1996
|
|
16
|
Xu J, Hu X, Yang Z, Wu X, Bisera J, Sun S
and Tang W: Miniaturized mechanical chest compressor improves
calculated cerebral perfusion pressure without compromising
intracranial pressure during cardiopulmonary resuscitation in a
porcine model of cardiac arrest. Resuscitation. 85:683–688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Z, Tang D, Wu X, Hu X, Xu J, Qian J,
Yang M and Tang W: A tourniquet assisted cardiopulmonary
resuscitation augments myocardial perfusion in a porcine model of
cardiac arrest. Resuscitation. 86:49–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei
X, Zhang MY and Cui J: Mild hypothermia attenuates mitochondrial
oxidative stress by protecting respiratory enzymes and upregulating
MnSOD in a pig model of cardiac arrest. PLoS One. 7:e353132012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kapás L, Benedek G and Penke B:
Cholecystokinin interferes with the thermoregulatory effect of
exogenous and endogenous opioids. Neuropeptides. 14:85–92. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yannopoulos D, Zviman M, Castro V,
Kolandaivelu A, Ranjan R, Wilson RF and Halperin HR:
Intra-cardiopulmonary resuscitation hypothermia with and without
volume loading in an ischemic model of cardiac arrest. Circulation.
120:1426–1435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye S, Weng Y, Sun S, Chen W, Wu X, Li Z,
Weil MH and Tang W: Comparison of the durations of mild therapeutic
hypothermia on outcome after cardiopulmonary resuscitation in the
rat. Circulation. 125:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farkas M and Komáromi I: Central effects
of chemical transmitters on temperature regulation in the adult rat
and the newborn guinea pig. Int J Biometeorol. 15:316–320. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geppert A, Zorn G, Karth GD, Haumer M,
Gwechenberger M, Koller-Strametz J, Heinz G, Huber K and
Siostrzonek P: Soluble selectins and the systemic inflammatory
response syndrome after successful cardiopulmonary resuscitation.
Crit Care Med. 28:2360–2365. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamoto S, Shikata K, Miyasaka K, Okada
S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka
HU, et al: Cholecystokinin plays a novel protective role in
diabetic kidney through anti-inflammatory actions on macrophage:
Anti-inflammatory effect of cholecystokinin. Diabetes. 61:897–907.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luyer MD, Greve JW, Hadfoune M, Jacobs JA,
Dejong CH and Buurman WA: Nutritional stimulation of
cholecystokinin receptors inhibits inflammation via the vagus
nerve. J Exp Med. 202:1023–1029. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McDermott JR, Leslie FC, D'Amato M,
Thompson DG, Grencis RK and McLaughlin JT: Immune control of food
intake: Enteroendocrine cells are regulated by CD4+ T lymphocytes
during small intestinal inflammation. Gut. 55:492–497. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Ni Z, Cong B, Gao W, Xu S, Wang C,
Yao Y, Ma C and Ling Y: CCK-8 inhibits LPS-induced IL-1beta
production in pulmonary interstitial macrophages by modulating PKA,
p38, and NF-kappaB pathway. Shock. 27:678–686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JG, Cong B, Li QX, Chen HY, Qin J
and Fu LH: Cholecystokinin octapeptide regulates
lipopolysaccharide-activated B cells co-stimulatory molecule
expression and cytokines production in vitro. Immunopharmacol
Immunotoxicol. 33:157–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang JG, Cong B, Jia XX, Li H, Li QX, Ma
CL and Feng Y: Cholecystokinin octapeptide inhibits immunoglobulin
G1 production of lipopolysaccharide-activated B cells. Int
Immunopharmacol. 11:1685–1690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Center for Drug Evaluation and Research
(CDER), . Estimating the Maximum Safe. Starting Dose in Initial
Clinical Trials for Therapeutics in Adult Healthy. Volunteers. U.S.
Department of Health and Human Services. Food and Drug
Administration; Rockville, MD: pp. 6–7S. 2005
|