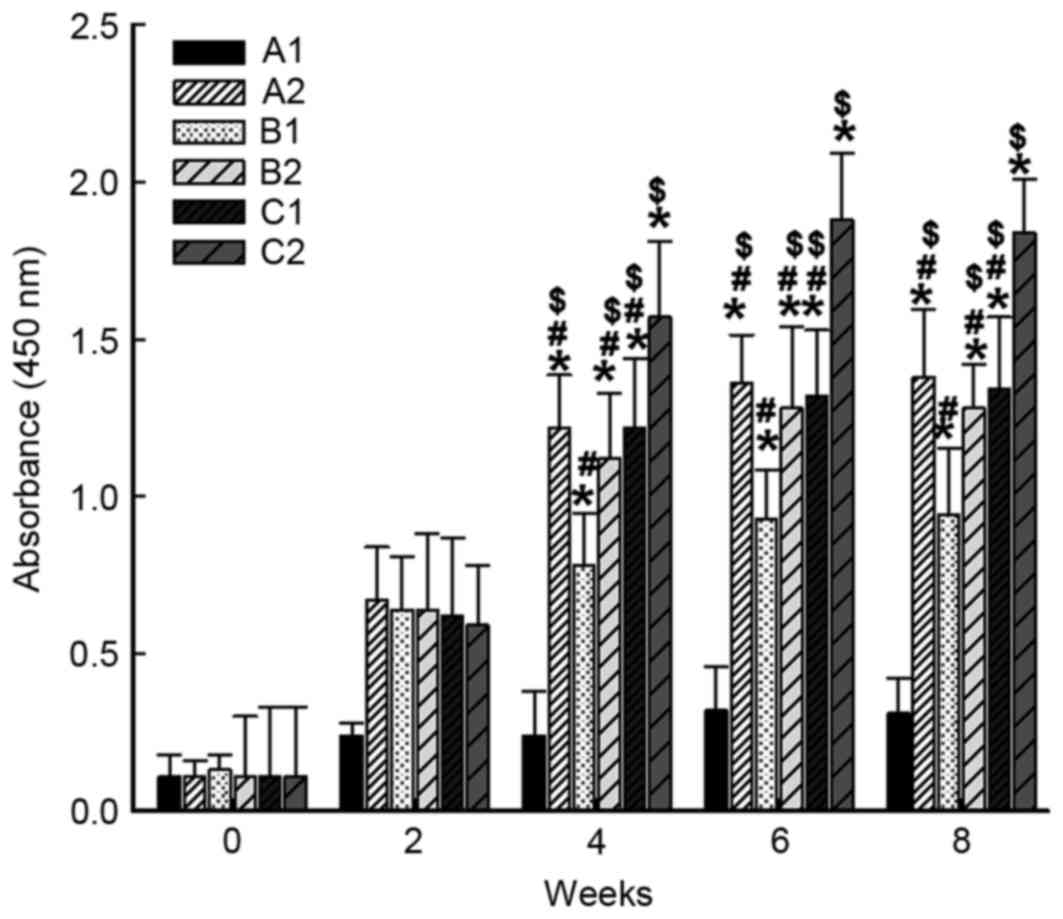
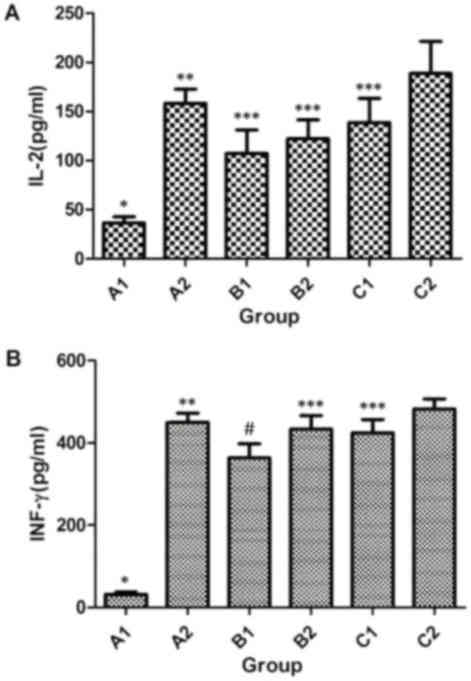
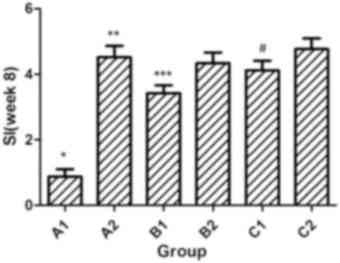
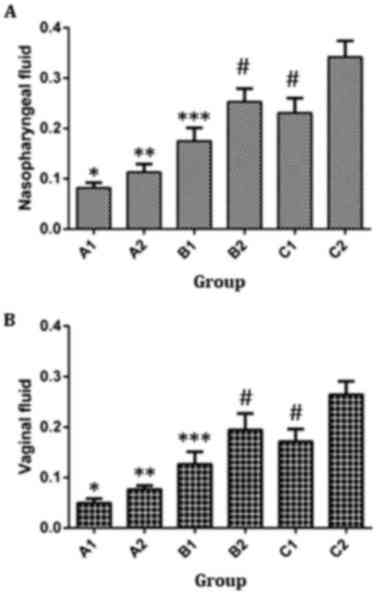
|
1
|
Shah BJ, Karia DR and Pawara CL: Syphilis:
Is it making resurgence? Indian J Sex Transm Dis. 36:178–181. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Zeng L, Ren X, Geng M, Li Z and Yu
H: Analysis of morbidity and mortality characteristics of the
notifiable diseases reported in China. Zhonghua Liu Xing Bing Xue
Za Zhi. 36:194–198. 2015.(In Chinese). PubMed/NCBI
|
|
3
|
Zheng N, Guo Y, Padmadas S, Wang B and Wu
Z: The increase of sexually transmitted infections calls for
simultaneous preventive intervention for more effectively
containing HIV epidemics in China. BJOG. 121:35–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiramatsu K, Serada S, Kobiyama K,
Nakagawa S, Morimoto A, Matsuzaki S, Ueda Y, Fujimoto M, Yoshino K,
Ishii KJ, et al: CpG oligodeoxynucleotides potentiate the antitumor
activity of anti-BST2 antibody. Cancer Sci. 106:1474–1478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li HT, Zhang TT, Chen ZG, Ye J, Liu H, Zou
XL, Wang YH and Yang HL: Intranasal administration of CpG
oligodeoxynucleotides reduces lower airway inflammation in a murine
model of combined allergic rhinitis and asthma syndrome. Int
Immunopharmacol. 28:390–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iho S, Maeyama J and Suzuki F: CpG
oligodeoxynucleotides as mucosal adjuvants. Hum Vaccin Immunother.
11:755–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai Y, Zhu Y, Harn DA, Wang X, Tang J,
Zhao S, Lu F and Guan X: DNA Vaccination by Electroporation and
Boosting with Recombinant Proteins Enhances the Efficacy of DNA
Vaccines for Schistosomiasis Japonica. Clin Vaccine Immunol.
16:1796–1803. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bae JY, Moon SH, Choi JA, Park JS, Hahn
BS, Kim KY, Kim B, Song JY, Kwon DH, Lee SC, et al: Recombinant DNA
and protein vaccines for foot-and-mouth disease induce humoral and
cellular immune responses in mice. Immune Netw. 9:265–273. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endmann A, Klünder K, Kapp K, Riede O,
Oswald D, Talman EG, Schroff M, Kleuss C, Ruiters MH and Juhls C:
Cationic lipid-formulated DNA vaccine against hepatitis B virus:
Immunogenicity of MIDGE-Th1 vectors encoding small and large
surface antigen in comparison to a licensed protein vaccine. PLoS
One. 9:e1017152014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nchinda G, Amadu D, Trumpfheller C,
Mizenina O, Uberla K and Steinman RM: Dendritic cell targeted HIV
gag protein vaccine provides help to a DNA vaccine including
mobilization of protective CD8+ T cells. Proc Natl Acad Sci USA.
107:pp. 4281–4286. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Wang S and Lu S: Pilot study on the
use of DNA priming immunization to Enhance Y. pestis LcrV-Specific
B cell responses elicited by a recombinant LcrV protein vaccine.
Vaccines (Basel). 2:36–48. 2014. View Article : Google Scholar
|
|
12
|
Zhao F, Wang S, Zhang X, Gu W, Yu J, Liu
S, Zeng T, Zhang Y and Wu Y: Protective efficacy of a Treponema
pallidum Gpd DNA vaccine vectored by chitosan nanoparticles and
fused with interleukin-2. Can J Microbiol. 58:117–123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao F, Liu S, Zhang X, Yu J, Zeng T, Gu
W, Cao X, Chen X and Wu Y: CpG adjuvant enhances the mucosal
immunogenicity and efficacy of a Treponema pallidum DNA vaccine in
rabbits. Hum Vaccin Immunother. 9:753–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Feijun, Zhang Xiaohong, Yu Jin, Liu
Shuangquan, Zhang Yuejun and Wu Yimou: Expression and
identification of Gpd-IL-2 recombinant protein of treponema
pallidum and its immuno-competence analysis. J Med Sci Central
South China. 40:131–135. 2012.(In Chinese).
|
|
15
|
Syphilis. Nurs Stand. 29:172015.
View Article : Google Scholar
|
|
16
|
Janier M, Hegyi V, Dupin N, Unemo M,
Tiplica GS, Potočnik M, French P and Patel R: 2014 European
guideline on the management of syphilis. J Eur Acad Dermatol
Venereol. 28:1581–1593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stamm LV and Drapp RL: A synthetic lymph
node containing inactivated Treponema pallidum cells elicits
strong, antigen-specifichumoral and cellular immune responses in
mice. Pathog Dis. 70:88–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khera AK, Afkhami S, Lai R, Jeyanathan M,
Zganiacz A, Mandur T, Hammill J, Damjanovic D and Xing Z: Role of B
cells in mucosal vaccine-induced protective CD8+ T cell immunity
against pulmonary tuberculosis. J Immunol. 195:2900–2907. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stary G, Olive A, Radovic-Moreno AF,
Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi
J, et al: VACCINES. A mucosal vaccine against Chlamydia trachomatis
generates two waves of protective memory T cells. Science.
348:aaa82052015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye T, Yue Y, Fan X, Dong C, Xu W and Xiong
S: M cell-targeting strategy facilitates mucosal immune response
and enhances protection against CVB3-induced viral myocarditis
elicited by chitosan-DNA vaccine. Vaccine. 32:4457–4465. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Umthong S, Buaklin A, Jacquet A, Sangjun
N, Kerdkaew R, Patarakul K and Palaga T: Immunogenicity of a DNA
and recombinant protein vaccine combining LipL32 and Loa22 for
leptospirosis using chitosan as a delivery system. J Microbiol
Biotechnol. 25:526–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Birk R, Aderhold C, Hörmann K, Wenzel A,
Kramer B, Eschenhagen T and Sommer JU: CpG-Oligodeoxynucleotides in
Chronic Rhinosinusitis Cell Culture. In Vivo. 30:47–52.
2016.PubMed/NCBI
|
|
23
|
Meng Z, Zhang X, Pei R, Zhang E, Kemper T,
Vollmer J, Davis HL, Glebe D, Gerlich W, Roggendorf M, et al:
Combination therapy including CpG oligodeoxynucleotides and
entecavir induces early viral response and enhanced inhibition of
viral replication in a woodchuck model of chronic hepadnaviral
infection. Antiviral Res. 125:14–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiang XX, Zhou XQ, Wang JX, Xie Q, Cai X,
Yu H and Zhou HJ: Effects of CpG-ODNs on phenotype and function of
monocyte-derived dendritic cells in chronic hepatitis B. World J
Gastroenterol. 17:4825–4830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lei J, Osen W, Gardyan A, Hotz-Wagenblatt
A, Wei G, Gissmann L, Eichmüller S and Löchelt M:
Replication-competent foamy virus vaccine vectors as novel epitope
scaffolds for immunotherapy. PLoS One. 10:e01384582015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
San R, Omán B, Gómez S, Irache JM and
Espuelas S: Co-encapsulated CpG oligodeoxynucleotides and ovalbumin
in PLGA microparticles; an in vitro and in vivo study. J Pharm
Pharm Sci. 17:541–553. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Cesare M, Sfondrini L, Pennati M, De
Marco C, Motta V, Tagliabue E, Deraco M, Balsari A and Zaffaroni N:
CpG-oligodeoxynucleotides exert remarkable antitumor activity
against diffuse malignant peritoneal mesothelioma orthotopic
xenografts. J Transl Med. 14:252016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Zheng M, Zhu XH, Li S, Ni J, Li
BL, Liu H, Gao F and Cai JM: Protective effect of
CpG-oligodeoxynucleotides against low- and high-LET irradiation.
Cell Physiol Biochem. 34:1663–1674. 2014. View Article : Google Scholar : PubMed/NCBI
|