Introduction
Oligodendrocytes (OLs) are major cells of the
central nervous system (CNS) and are critical for neuronal function
(1). CNS myelin abnormalities are
features of several neurological disorders (2), including white matter injury (3), multiple sclerosis (4) and neuromyelitis optica (5). The causes of demyelination typically
include immunological injury, excitotoxicity, viral infection,
dystrophia and oxidative stress (6).
At present, there are two methods used to treat demyelinating
diseases; the first is reducing demyelination and the second is
promoting remyelination (7). The
therapeutic approaches include transplanting OL progenitor cells
(OPCs) to protect endogenous OLs, or increasing the activity of
exogenous OPCs to reduce demyelination and promote remyelination
(8). Several studies have reported
that the survival rate of transplanted OPCs is low, that few OPCs
differentiate into mature OLs and that transplanting exogenous OPCs
can lead to host rejection (9).
Therefore, the aim of the present study was to explore a more
effective method of promoting myelination by protecting and
increasing the activity of endogenous OPCs.
Stimulating an endogenous regenerative response may
be a potential effective treatment for chronic demyelinating
diseases, including multiple sclerosis. Recently, Najm et al
(10) reported that miconazole
promotes OPC differentiation and induces remyelination in
demyelinating disease models via mitogen-activated protein kinase
(MEK)-dependent activation of extracellular signal-regulated kinase
(ERK)1/2. Their study investigated the miconazole-promoted
remyelination in a lysolecithin-induced demyelinating adult mouse
model. In the present study, whether miconazole also serves a
therapeutic role in premature infant cerebral white matter lesions
was investigated.
White matter damage (WMD) is the most common type of
cerebral lesion and cause of neural-developmental disorders,
including premature cognitive defects and cerebral palsy, in
premature infants (11). The
pathogenesis of WMD typically includes changes in cerebral blood
flow and cerebral tissue metabolism, which leads to a loss of OPCs
and mature OLs, causing cerebral white matter lesions (12). Ischemic hypoxia and infection are the
main causes of cerebral white matter lesions in premature infants
(13). At present, there is no
effective treatment for premature infants with hypoxia and ischemic
cerebral lesions. The aim of the present study was to explore the
effect of miconazole on myelin regeneration and formation in the
corpus callosum of rats with WMD.
Materials and methods
Experimental animals
Sprague-Dawley dams with litter were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd.
(Beijing, China). A total of 120 3-day-old Sprague Dawley rat pups
(62 males and 58 females, mean weight, 7–8 g) from 12 litters were
housed in a clean environment at 15–28°C with 45–44% humidity and a
12 h light/dark cycle, and provided with adequate food and water.
All experimental procedures were approved by the Animal Ethical and
Welfare Committee of the Navy General Hospital of People's
Liberation Army (Beijing, China).
Grouping and drug delivery
Experimental animals were randomly divided into four
groups (n=30/group): The sham surgery, model, 10 mg/kg miconazole
treatment and 40 mg/kg miconazole treatment groups. The treatment
groups were administered with intraperitoneal injections of 10 or
40 mg/kg/day miconazole (Sigma-Aldrich; Merck KGaA; Darmstadt,
Germany) at 4–8 days of age (the early treatment group consisting
of 10 rats from each group) or 5–11 days of age (the late treatment
group consisting of 10 rats from each group). The model group was
administered with intraperitoneal injections of dimethylsulfoxide
(DMSO) at the same concentration, whereas rats in the sham group
received no treatment.
Establishment of the WMD model
At 3 days old, the rats were anesthetized using
sodium pentobarbital (Shanghai Xinya Pharmaceutical, Co., Ltd.,
Shanghai, China in a sterile environment. The limbs were fixed in
the supine position on the operating table, the skin was sterilized
with iodine, and a midline incision was made at the neck to reveal
the right common carotid artery, internal jugular vein and vagus
nerve. The right common carotid artery was ligated and the skin
incision was subsequently sutured (14). In the sham group, only the right
common carotid artery was exposed. Rats were placed on a heating
mat at 37°C following the surgery for recovery, and subsequently
housed in an environment containing 6% oxygen and 94% nitrogen at
33°C to provide constant anoxia for 80 min before being returned to
their mother's cage.
Brain tissue sections
At either days 8 or days 12 post-surgery, rats (n=3
per group) were anesthetized by intraperitoneal injection with
pentobarbital sodium (50 mg/kg), the thoracic cavity was opened and
the right auricle was dissected. A total of ~100 ml saline followed
by ~100 ml paraformaldehyde were perfused into vascular system via
the left ventricle. The rats were then decapitated, and brain
tissue was harvested and fixed in 4% paraformaldehyde for 24 h at
4°C. Tissues were subsequently dehydrated in 30% sucrose solution
until the tissue blocks sank to the bottom. Serial sections of the
desiccated brain tissue (10-µm-thick) were then cut.
Hematoxylin and eosin (H&E)
staining
Brain tissue were fixed in 4% paraformaldehyde
solution at room temperature for 24 h, embedded in paraffin and cut
into continuous sections (5-µm-thick) starting from the anterior
fontanel. Sections were dehydrated in a graded series of alcohol,
washed in distilled water and stained with hematoxylin for 3 min at
room temperature. Sections were submerged in 1% hydrochloric acid
for 15 sec, washed and immersed in 0.5% eosin (alcoholic) solution
(cat no. G1001; Wuhan Servicebio Biotechnology Co., Ltd., Wuhan,
China) for 3–5 min at room temperature. Sections were subsequently
mounted and observed under an optical microscope at a magnification
of ×200.
Myelin basic protein (MBP)
immunohistochemical staining
Brain tissue sections were washed in PBS, digested
in 0.03% Triton X-100 and blocked with 5% sheep serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at
18–24°C. Tissues were incubated in anti-MBP antibodies (cat no.
ab62631; Abcam, Cambridge, UK; 1:200) at 4°C overnight, washed
three times with PBS and then incubated with goat anti mouse
secondary antibodies (1:500; cat no. 115-165-068; Jackson
ImmunoResearch Europe, Ltd., Newmarket, UK) for 2 h at room
temperature in the dark. The sections were washed again three times
with PBS, observed under a fluorescence microscope and images were
captured at a magnification of ×200. ImagePro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA) was used to convert
the fluorescent images into black and white images, and the
integrated optical density (IOD) value was measured.
Western blotting
At either days 8 or days 12 post-surgery, protein
was extracted from the corpus callosum of rats (n=3 per group)
using a Minute™ Total Protein Extraction kit (cat no.
SD-001/SN-002; Invent Biotechnologies, Co., Ltd, Beijing, China)
according to the manufacturer's protocols and quantified as
previously described (10). Samples
were denatured and equal amounts of protein (30 µg/lane) were
loaded, separated by 10% SDS-PAGE and transferred to a PVDF
membrane overnight. The membrane was blocked with 5% skim milk for
30 min at room temperature, incubated with anti-MBP antibodies
(1:1,000) for 12 h at 4°C and subsequently incubated with
horseradish peroxidase-conjugated goat anti mouse secondary
antibodies for 30 min at room temperature. Protein bands were
developed using enhanced chemiluminescence (cat no. 29050; Engreen
Co., Ltd, Beijing, China). ImageJ software 2.1.4.7 (National
Institutes of Health, Bethesda, MA, USA) was used to calculate the
expression of MBP relative to GAPDH (cat no. GB12002; Wuhan
Servicebio Biotechnology Co., Ltd, Wuhan, China) via densitometry.
The dilution of the anti-GAPDH antibody was 1:1,000.
Transmission electron microscopy
At either days 8 or days 12 post-surgery, sections
(1–3 mm thick) from the right corpus callosum of rats (n=3 per
group) were fixed in 2% glutaraldehyde at 4°C for 72 h and then in
1% osmic acid at 4°C for 2 h. The sections were dehydrated using a
series of 30–100% acetone and embedded in epoxy resin embedding
medium. Myelin cross-sectional slices (50-nm-thick) were obtained
using an ultramicrotome. Sections were observed under a
transmission electron microscope and images were captured for
further analysis. A total of 800 root axonal fibers were counted at
a magnification of ×15,000. Following image acquisition, axon and
myelin diameters were measured using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.) and the G-ratio value (axon
diameter/myelin diameter) was calculated as previously described
(15).
Neuroethology tests
The following neuroethology tests were performed in
a blinded manner at postnatal day 28.
Tail suspension test
A tail suspension test was performed as previously
reported (16), with minor
modifications. Rat forelimbs were allowed to grasp a glass rod
(diameter, 0.5 cm) at a height of 45 cm, and the time taken between
being placed on the rod and falling off was recorded and scored.
Scoring was as follows: <10 sec, 1 point; >10–30 sec, 2
points; >30–120 sec, 3 points; >120–300 sec, 4 points;
>300 sec, 5 points.
Slope test
Rats were placed with their heads at a 45° decline
and the time taken for rats to turn their heads up >135° was
recorded (17).
Field test
The bottom of a 30×30×30 cm square box was divided
into nine equal squares with chalk lines. Rats were placed in the
central square and their activity was observed for 30 min. Scoring
was performed as follows: 1 point for each instance where >50%
of the rat's body moved to an adjacent square and 1 point whenever
the rat was observed standing on hind legs.
Statistical analysis
Data are presented as the mean ± standard deviation
and statistical analysis was performed using SPSS 21.0 software
(IBM Corp., Armonk, NY, USA). One-way ANOVA followed by a post-hoc
Tukey's test was used to examine differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Successful establishment of the WMD
model
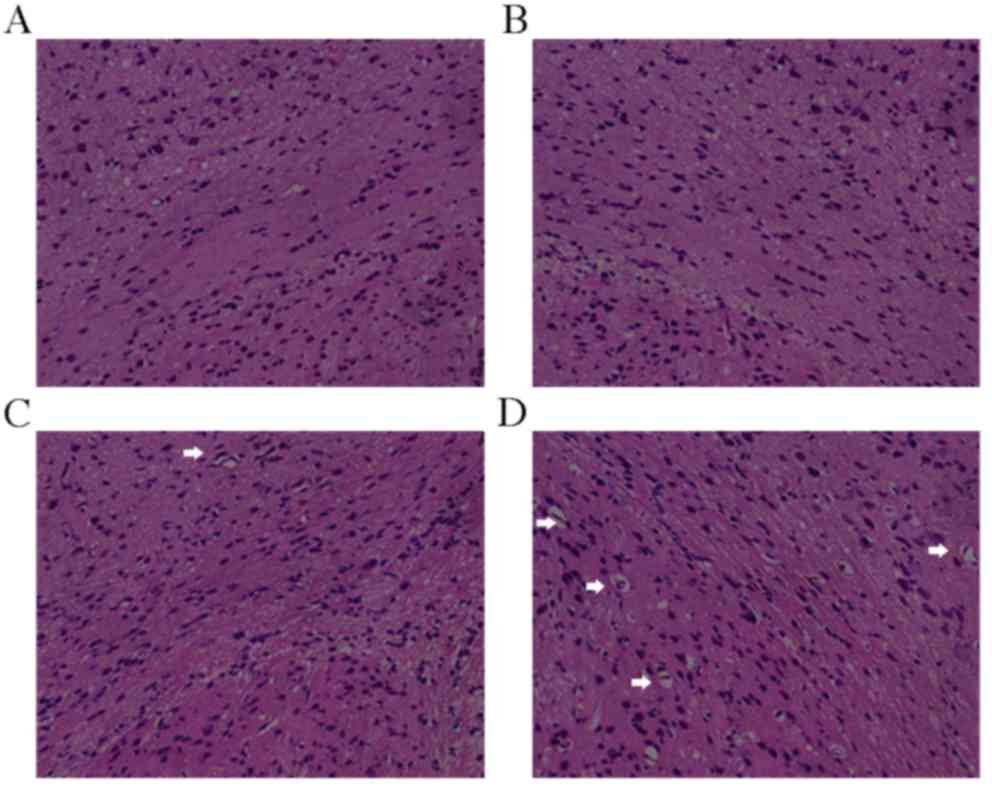
At 4 days post-surgery, H&E staining revealed
that the corpus callosum structure in rats from the sham group was
normal; cells had a dense and uniform distribution, the brain
tissue outline was clear, and no cell swelling, necrosis or
pathological changes were observed (Fig.
1A and B). Conversely, swelling, necrosis, nucleus pycnosis,
loose structure, fiber disorder, enlarged lateral ventricles,
hyperplastic inflammatory gliocytes, and active and selective white
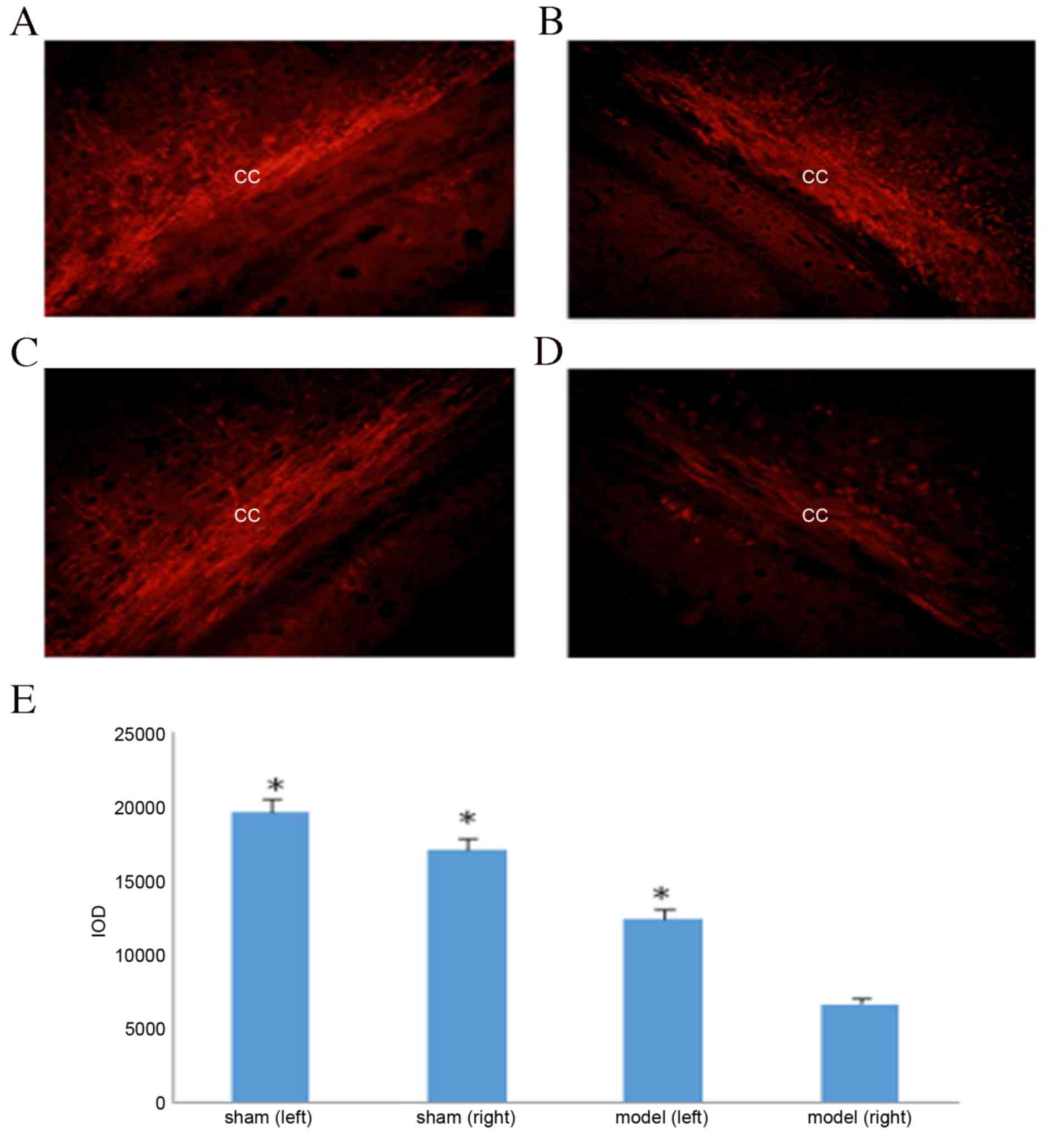
matter lesions were observed in the model group (Fig. 1C and D). MBP immunohistochemical
staining results demonstrated that the corpus callosum in the sham
group had no distinct myelin loss (Fig.
2A and B); however, significant myelin loss was observed in the
white matter outside the sac on the surgical side of the model
group compared with the non-surgical side and the sham group
(P<0.05; Fig. 2C-E). These
results indicate that the WMD model was successfully
established.
Miconazole treatment increases MBP
expression in the corpus callosum of rats with WMD
MBP immunohistochemical staining results revealed
significant myelin loss on the surgical side of the corpus callosum
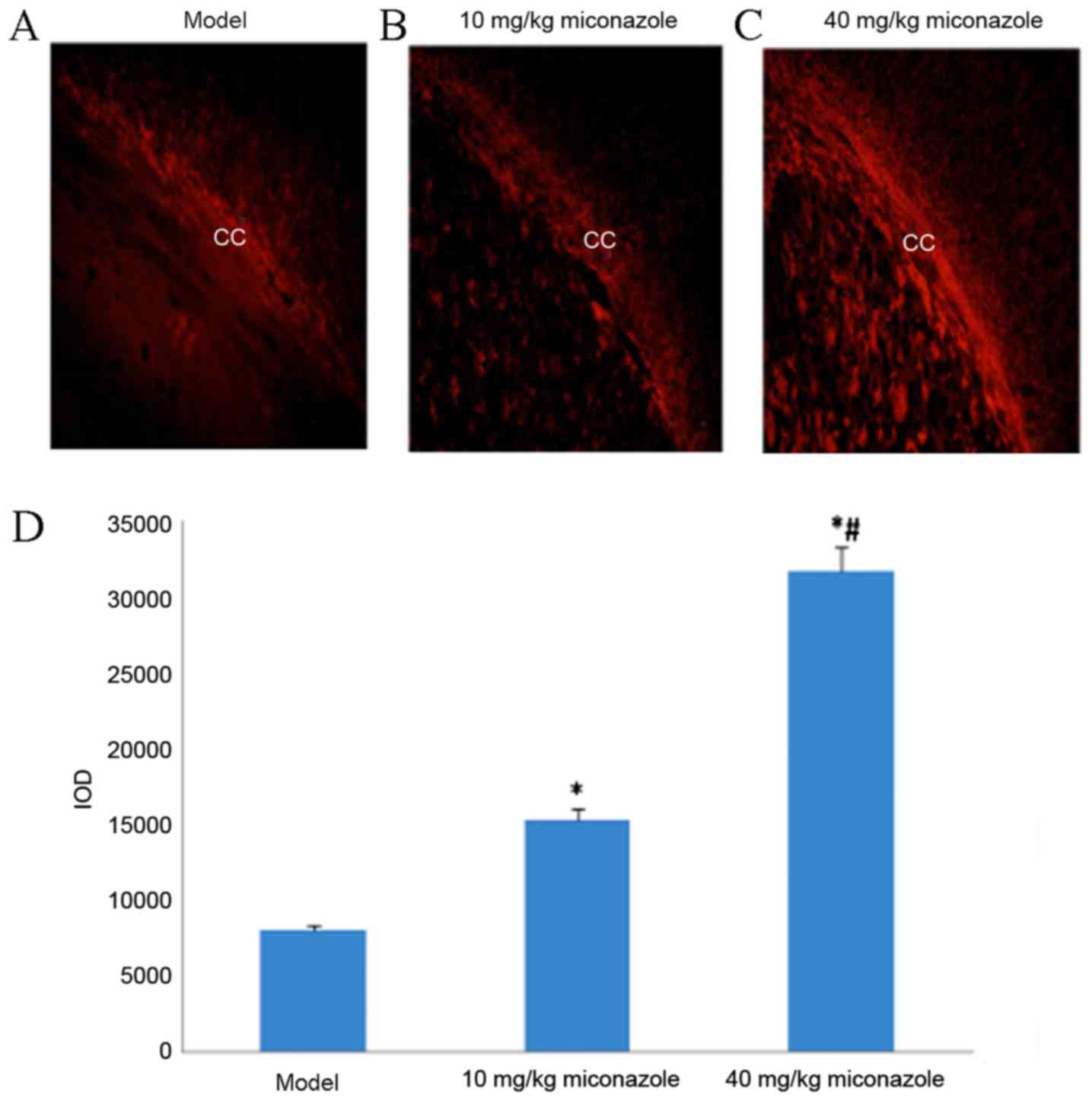
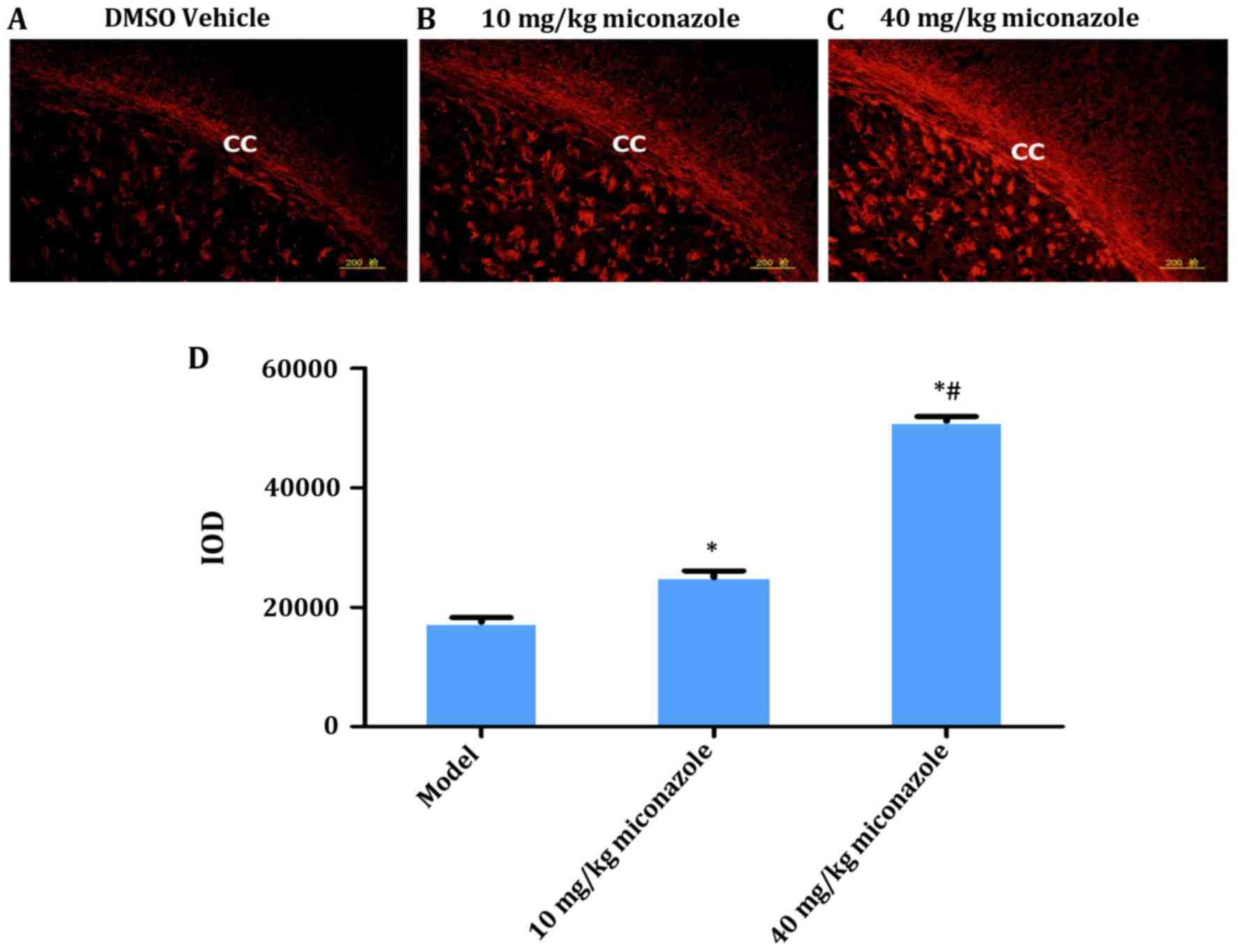
in the model control group (P<0.05; Fig. 2E). Rats treated with 10 or 40
mg/kg/day miconazole in the early (Fig.
3) and late (Fig. 4) treatment
groups demonstrated a significantly higher expression of MBP
compared with the model group (P<0.05). Furthermore, the IOD of
rats in the 40 mg/kg/day treatment group was significantly higher
compared with the rats treated with 10 mg/kg/day miconazole in the
early and late treatment groups (P<0.05).
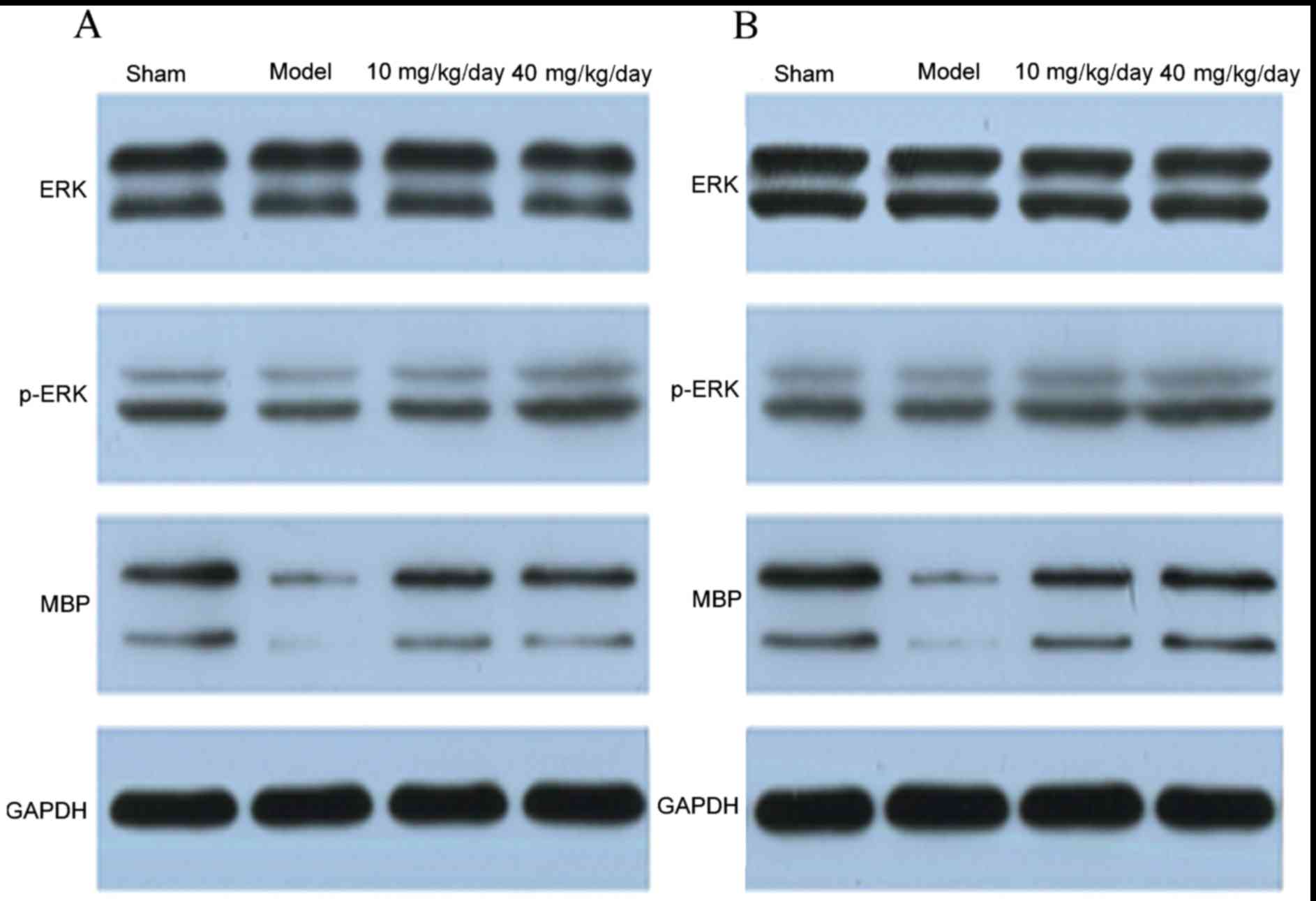
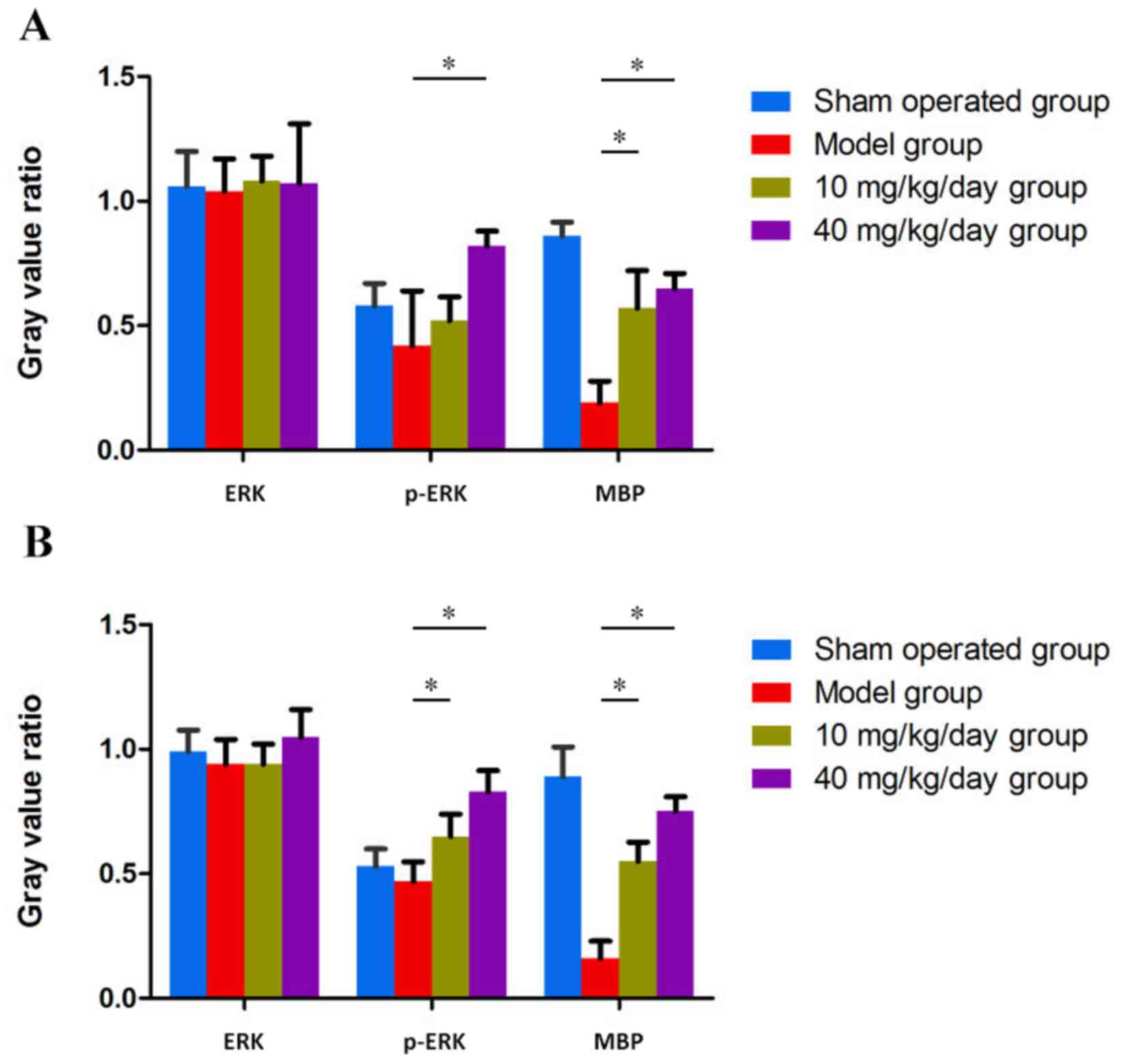
Miconazole treatment increases MBP
expression and enhances ERK1/2 activity in the corpus callosum of
rats with WMD
Western blotting results also revealed that the
protein expression of MBP was increased in the miconazole treatment
groups compared with the model group (P<0.05); however, no
significant differences were observed between the 40 and 10
mg/kg/day groups (Figs. 5 and
6). These results indicate that
miconazole is able to alter the protein expression of MBP in the
corpus callosum, which suggests that it exerts a protective role
during hypoxic ischemia. Furthermore, miconazole significantly
upregulated MBP expression and enhanced ERK1/2 activity, as
revealed by increased phosphorylated-ERK expression compared with
the model group.
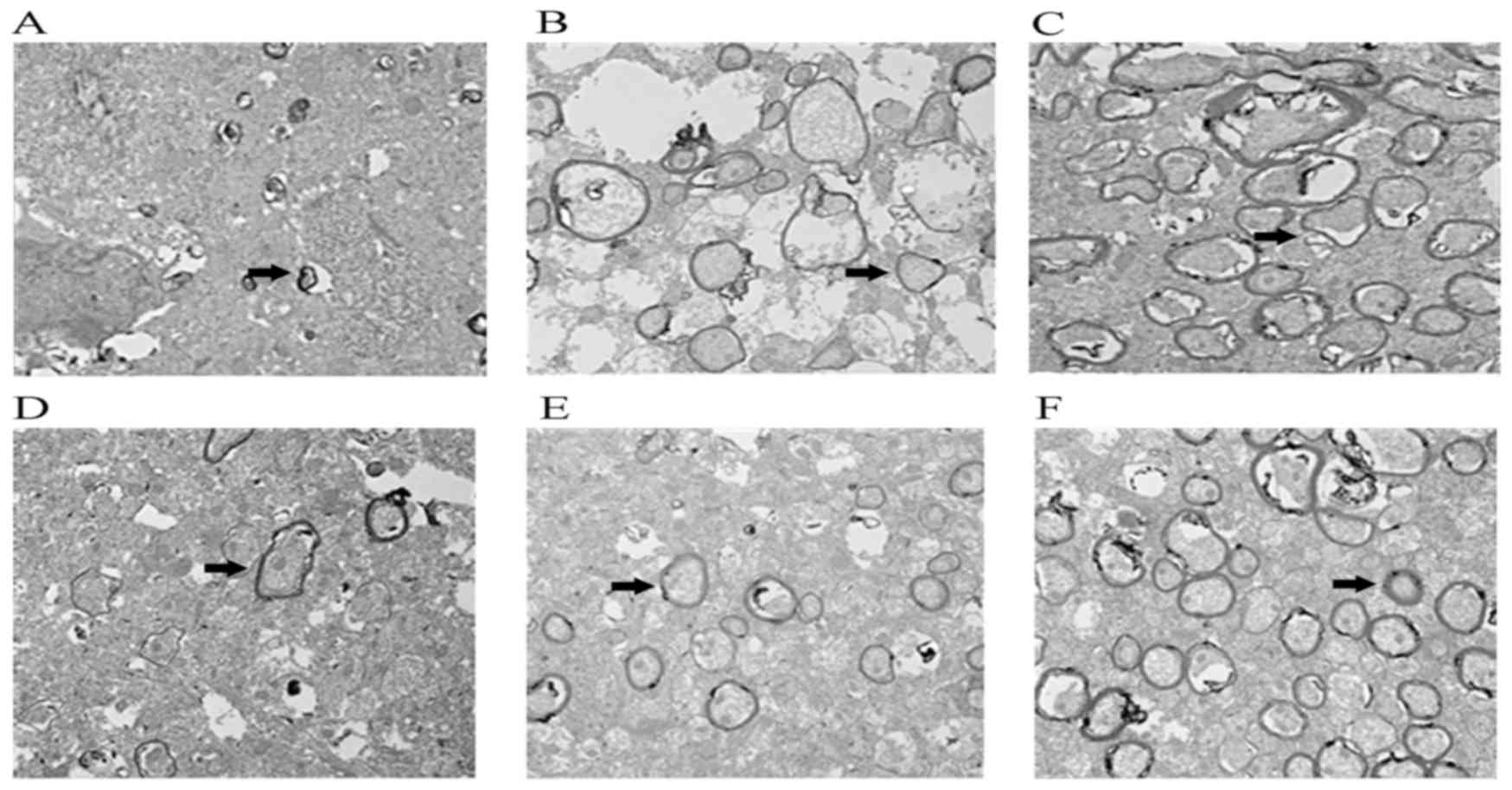
Miconazole treatment improves the
morphological features of WMD
Transmission electron microscopy results revealed
that, at either day 8 or day 12 post-surgery, little myelination
was present in the model group, nerve fibers were irregularly
shaped and arranged loosely, and myelin thickness was markedly
decreased (Fig. 7). Following
miconazole treatment, the quantity of myelin was markedly increased
compared with the control group, indicating that demyelination was
ameliorated and myelin thickness was improved. G-ratio analysis
demonstrated that the G-ratio in the control group was
significantly higher compared with the miconazole treatment groups,
while the 40 mg/kg miconazole treatment group G-ratio value was
lower compared with the 10 mg/kg treatment group (data not shown).
These results suggest that miconazole is able to improve cerebral
white matter injury in premature infants.
Miconazole treatment improves the
neurological function of rats with WMD
Neuroethology tests were performed to assess the
effect of miconazole treatment on the functioning of rats with WMD
(Table I). In the tail suspension
test, rats in the miconazole treatment groups demonstrated
flexibility, agility and accuracy of movement, and were able to
turn and crawl along the glass rod with a long suspension time.
Compared with the treatment groups, rats in the model group were
unresponsive with decreased autokinetic movement, flexibility and
stability. Furthermore, they exhibited shaky lower limbs and were
unable to stay on the rod for extended periods. Rats in the sham
group scored significantly higher in the suspension test compared
with the model and treatment groups (P<0.05). In the slope test,
rats in the sham group took a significantly shorter amount of time
to move compared with the model and treatment groups (P<0.05);
however, the times recorded for the treatment groups were
significantly shorter compared with the model group (P<0.05).
Lastly, in the field test the sham group scored significantly
higher compared with the model and treatment groups (P<0.05),
whereas the model group scored significantly lower compared with
the treatment groups (P<0.05) (Table
I). No significant differences were observed between the 10 and
40 mg/kg/day miconazole treatment groups for any of the
neuroethology tests.
 | Table I.Neuroethology test results. |
Table I.
Neuroethology test results.
| Group | Suspension test | Slope test (sec) | Field test |
|---|
| Sham operation | 3.6±0.97a–c | 3.2±0.92a–c |
13.4±1.43a–c |
| Model | 1.5±0.53b,c | 9.6±1.5b,c | 5.9±1.73b,c |
| 10 mg/kg
miconazole | 2.4±0.84c | 6.6±1.1c | 7.3±1.34c |
| 40 mg/kg
miconazole | 2.7±0.67 | 6.9±1.6 | 7.5±1.35 |
| P-value |
<0.05d |
<0.05e |
<0.05f |
Discussion
Cerebral white matter lesions in premature infants
may cause long periods of movement and cognitive dysfunction, which
is one of the main causes of cerebral palsy and mental retardation
in children (11). Infants with very
low birth weights (<1,500 g) have a high risk of premature
infant cerebral white matter lesions, with 10–15% developing
movement dysfunction, and 25–50% presenting with cognitive and
behavioral deficits or social dysfunction (18). This is the primary cause of nervous
system developmental disorders in premature infants and negatively
affects survival (18). At present,
there is no effective treatment for WMD (19). Cerebral OPC injury is the main cause
of WMD, resulting in myelin destruction and delayed remyelination
(20). In recent years, researchers
have performed exogenous OPC transplants in animal models in order
to verify whether the OPCs are able to survive, differentiate into
mature OLs and promote myelination (21). Webber et al (21) transplanted OPCs into portal vein
ligation model rats and reported that, 7 weeks later, the majority
of transplanted cells had survived and expressed OPC-specific
markers, including neural/glial antigen 2, O-antigen 4 and
OL-transcription factor 2, indicating that the transplanted OPCs
did not differentiate into mature OLs. Another study transplanted
OPCs induced from human neural stem cells into rat demyelination
models; at 8 weeks the majority of cells were alive, but <2%
expressed MBP, a specific marker of mature OLs (9). These results indicate that, although
transplanted OPCs are able to migrate to the injury and survive for
an extended period, the majority do not differentiate into mature
OLs. This is similar to previous observations of WMD; when
demyelinating lesions occur, endogenous OPCs migrate to the lesion
site, but few differentiate into mature OLs (22). It has been reported that the
microenvironment of the CNS is an important factor for neuron and
axonal regeneration (23); as such,
efforts have been made to improve the microenvironment when
attempting to identify effective targets for the promotion of
axonal regeneration (24).
The aim of the present study was to promote
endogenous OPC survival, differentiation and myelin formation in
order to treat WMD. Muscarine receptor antagonists, including
benztropine and clemastine, have previously been reported to
improve multiple sclerosis and promote myelin regeneration
(10). The results of the present
study demonstrated that miconazole, another member of the family
that has been approved by the US Food and Drug Administration as a
fungal infection treatment (10), is
also able to promote OPC differentiation in vitro.
Miconazole is also able to promote lysolecithin-induced
demyelinating rat endogenous OPC differentiation, where the number
of mature OLs is 13× that of the controls and axon myelination is
almost 12× (10). This indicates
that miconazole serves an important regulatory role in the early
stages of OPC differentiation. In the present study, it was
investigated whether miconazole was also able to promote endogenous
OPC differentiation in a rat model of infant WMD and whether it
induced myelin formation following ischemia or hypoxia.
The CNS of 7-day-old rats is the same as that of
term or near-term neonates, which has formed early myelin (25). Unilateral carotid artery ligation
combined with hypoxia induces brain lesions in the unilateral
cortex and hippocampal neurons, whereas it does not produce
significant white matter lesions. Rat cerebral white matter is
vulnerable 2–3 days after birth, at which point it consists mainly
of OPCs (26). As such, immature
3-day-old rats are ideal for studying premature cerebral white
matter injury (26). In the present
study, H&E staining and MBP immunohistochemical staining was
used to verify that the WMD model was successfully established
following carotid artery ligation. H&E staining revealed
swollen cells, dilated intercellular spaces, a disordered cellular
arrangement, tiny infarcts and white matter injuries of the corpus
callosum in the model group, and MBP immunohistochemical staining
revealed significant demyelination. These results indicate that the
animal model of hypoxic ischemic cerebral white matter injury was
successful.
The protective effect and underlying mechanisms of
miconazole on the myelin sheath in 3-day-old rats with cerebral
white matter injury were also investigated. The experimental
results provide a basis for the potential clinical use of
miconazole as a treatment for WMD. MBP immunohistochemical staining
and western blotting results revealed that MBP expression was
higher in the corpus callosum of miconazole-treated rats compared
with the model controls, and that treatment with 40 mg/kg was more
effective compared with 10 mg/kg. The results of transmission
electron microscopy further indicated that treatment with
miconazole ameliorates ischemia-induced demyelination and loss of
myelin sheath thickness. Miconazole may therefore be beneficial for
improving myelin dysplasia in premature infants with cerebral white
matter injury. In addition, the results of neurobehavioral tests
demonstrated that miconazole effectively improved ischemia-induced
neurobehavioral dysfunction in the immature rats.
Miconazole was also demonstrated to enhance the
activity of ERK. It has been reported that the ERK signaling
pathway serves a role in oligodendroglial and myelin development in
the CNS (27–32). Furthermore, Xie et al
(33) suggested that the suppression
of proto-oncogene tyrosine-protein kinase Fyn/MEK/ERK
phosphorylation inhibits the maturation of OPCs, leading to axonal
hypomyelination. In the present study, it was demonstrated that
miconazole significantly upregulated MBP expression and enhanced
ERK1/2 activity, as revealed by the increased expression of
phosphorylated ERK compared with the model group. Furthermore, the
present study demonstrated that the systemic delivery of miconazole
contributes to improved neurobehavioral functions. However,
miconazole is currently only approved for topical administration in
humans (34). Significant
optimization of dosing, delivery and chemical structure is required
to enhance the on-target pharmacology of miconazole in OPCs, and
diminish any potential off-target side effects. The ability of
miconazole to cross the blood-brain barrier raises the possibility
that these agents, or modified derivatives of them, may advance
into clinical trials for the treatment of WMD.
In conclusion, the present study provides evidence
that miconazole is able to significantly reduce
ischemia/anoxia-induced WMD via promoting myelination in a rat
model of ischemic brain injury. This protection is implemented via
the activation of ERK phosphorylation. The protective effect of
miconazole in the rat brain makes it an ideal candidate for further
investigation as a clinical treatment for WMD.
Acknowledgements
The present study was supported by the fund of The
National Key R&D Program of China (grant no. 2017YFA0104200),
which was given to Dr Zuo Luan.
References
|
1
|
Lopez Juarez A, He D and Richard Lu Q:
Oligodendrocyte progenitor programming and reprogramming: Toward
myelin regeneration. Brain Res. 1638:209–220. 2016. View Article : Google Scholar
|
|
2
|
Chew LJ and DeBoy CA: Pharmacological
approaches to intervention in hypomyelinating and demyelinating
white matter pathology. Neuropharmacology. 110:605–625. 2016.
View Article : Google Scholar
|
|
3
|
Ryan M, Ibrahim M and Parmar HA: Secondary
demyelination disorders and destruction of white matter. Radiol
Clin North Am. 52:337–354. 2014. View Article : Google Scholar
|
|
4
|
Bando Y, Nomura T, Bochimoto H, Murakami
K, Tanaka T, Watanabe T and Yoshida S: Abnormal morphology of
myelin and axon pathology in murine models of multiple sclerosis.
Neurochem Int. 81:16–27. 2015. View Article : Google Scholar
|
|
5
|
Jeong IH, Choi JY, Kim SH, Hyun JW, Joung
A, Lee J and Kim HJ: Comparison of myelin water fraction values in
periventricular white matter lesions between multiple sclerosis and
neuromyelitis optica spectrum disorder. Mult Scler. 22:1616–1620.
2016. View Article : Google Scholar
|
|
6
|
Counsell SJ, Rutherford MA, Cowan F and
Edwards AD: Magnetic resonance imaging of preterm brain injury.
Arch Dis Child FetalNeonatal Ed. 88:F269–F274. 2003. View Article : Google Scholar
|
|
7
|
Deverman BE and Patterson PH: Exogenous
leukemia inhibitory factor stimulates oligodendrocyte progenitor
cell proliferation and enhances hippocampal remyelination. J
Neurosci. 32:2100–2109. 2012. View Article : Google Scholar
|
|
8
|
Grulova I, Slovinska L, Blaško J, Devaux
S, Wisztorski M, Salzet M, Fournier I, Kryukov O, Cohen S and
Cizkova D: Delivery of alginate scaffold releasing two trophic
factors for spinal cord injury repair. Sci Rep. 5:137022015.
View Article : Google Scholar
|
|
9
|
Jiang S, Seng S, Avraham HK, Fu Y and
Avraham S: Process elongation of oligodendrocytes is promoted by
the Kelch-related protein MRP2/KLHL1. J Biol Chem. 282:12319–12329.
2007. View Article : Google Scholar
|
|
10
|
Najm FJ, Madhavan M, Zaremba A, Shick E,
Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, et
al: Drug-based modulation of endogenous stem cells promotes
functional remyelination in vivo. Nature. 522:216–220. 2015.
View Article : Google Scholar
|
|
11
|
Volpe JJ: Cerebral white matter injury of
the premature infant-more common than you think. Pediatrics.
112:176–180. 2003. View Article : Google Scholar
|
|
12
|
Erceg S, Ronaghi M, Oria M, Roselló MG,
Aragó MA, Lopez MG, Radojevic I, Moreno-Manzano V,
Rodríguez-Jiménez FJ, Bhattacharya SS, et al: Transplanted
oligodendrocytes and motoneuron progenitors generated from human
embryonic stem cells promote locomotor recovery after spinal cord
transection. Stem Cells. 28:1541–1549. 2010. View Article : Google Scholar
|
|
13
|
Welin AK, Sedin P, Lapatto R, Sultan B,
Hagberg H, Gressens P, Kjellmer I and Mallard C: Melatonin reduces
inflammation and cell death in white matter in the midgestation
fetal sheep following umbilical cord occlusion. Pediatr Res.
61:153–158. 2007. View Article : Google Scholar
|
|
14
|
Yuan QC, Jiang L, Zhu LH and Yu DF:
Impacts of erythropoietin on vascular endothelial growth factor
receptor 2 by the extracellular signal-regulated kinase signaling
pathway in a neonatal rat model of periventricular white matter
damage. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38: 217–221, 2016.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38: 217–221, 2016. 38: 217–221,
2016:217–221, 2016–221, 2016. 2016.(In Chinese).
|
|
15
|
Coetzee T, Fujita N, Dupree J, Shi R,
Blight A, Suzuki K, Suzuki K and Popko B: Myelination in the
absence of galactocerebroside and sulfatide: Normal structure with
abnormal function and regional instability. Cell. 86:209–219. 1996.
View Article : Google Scholar
|
|
16
|
Mineur YS, Belzung C and Crusio WE:
Effects of unpredictable chronic mild stress on anxiety and
depression-like behavior in mice. Behav Brain Res. 175:43–50. 2006.
View Article : Google Scholar
|
|
17
|
Breu M, Zhang J, Porambo M, Pletnikov MV,
Goeral K, Kakara M, Johnston MV and Fatemi A: Diffusion tensor
imaging abnormalities in the cerebral white matter correlate with
sex-dependent neurobehavioral deficits in adult mice with neonatal
ischemia. Dev Neurosci. 38:83–95. 2016. View Article : Google Scholar
|
|
18
|
Anderson PJ: Neuropsychological outcomes
of children born very preterm. Semin Fetal Neonatal Med. 19:90–96.
2014. View Article : Google Scholar
|
|
19
|
Barateiro A and Fernandes A: Temporal
oligodendrocyte lineage progression: In vitro models of
proliferation, differentiation and myelination. Biochim Biophys
Acta. 1843:1917–1929. 2014. View Article : Google Scholar
|
|
20
|
Nistor GI, Totoiu MO, Haque N, Carpenter
MK and Keirstead HS: Human embryonic stem cells differentiate into
oligodendrocytes in high purity and myelinate after spinal cord
transplantation. Glia. 49:385–396. 2005. View Article : Google Scholar
|
|
21
|
Webber DJ, van Blitterswijk M and Chandran
S: Neuroprotective effect of oligodendrocyte precursor cell
transplantation in a long-term model of periventricular
leukomalacia. Am J Pathol. 175:2332–2342. 2009. View Article : Google Scholar
|
|
22
|
Chong SY and Chan JR: Tapping into the
glial reservoir: Cells committed to remaining uncommitted. J Cell
Biol. 188:305–312. 2010. View Article : Google Scholar
|
|
23
|
Dooley D, Vidal P and Hendrix S:
Immunopharmacological intervention for successful neural stem cell
therapy: New perspectives in CNS neurogenesis and repair. Pharmacol
Ther. 141:21–31. 2014. View Article : Google Scholar
|
|
24
|
Zhao XH, Jin WL and Ju G: An in vitro
study on the involvement of LINGO-1 and Rho GTPases in Nogo-A
regulated differentiation of oligodendrocyte precursor cells. Mol
Cell Neurosci. 36:260–269. 2007. View Article : Google Scholar
|
|
25
|
Gard AL and Pfeiffer SE: Oligodendrocyte
progenitors isolated directly from developing telencephalon at a
specific phenotypic stage: Myelinogenic potential in a defined
environment. Development. 106:119–132. 1989.
|
|
26
|
Haynes RL, Folkerth RD, Keefe RJ, Sung I,
Swzeda LI, Rosenberg PA, Volpe JJ and Kinney HC: Nitrosative and
oxidative injury to premyelinating oligodendrocytes in
periventricular leukomalacia. J Neuropathol Exp Neurol. 62:441–450.
2003. View Article : Google Scholar
|
|
27
|
Jeffries MA, Urbanek K, Torres L, Wendell
SG, Rubio ME and Fyffe-Maricich SL: ERK1/2 activation in
preexisting oligodendrocytes of adult mice drives new myelin
synthesis and enhanced CNS function. J Neurosci. 36:9186–9200.
2016. View Article : Google Scholar
|
|
28
|
Xiao J, Ferner AH, Wong AW, Denham M,
Kilpatrick TJ and Murray SS: Extracellular signal-regulated kinase
1/2 signaling promotes oligodendrocyte myelination in vitro. J
Neurochem. 122:1167–1180. 2012. View Article : Google Scholar
|
|
29
|
Guardiola-Diaz HM, Ishii A and Bansal R:
Erk1/2 MAPK and mTOR signaling sequentially regulates progression
through distinct stages of oligodendrocyte differentiation. Glia.
60:476–486. 2012. View Article : Google Scholar
|
|
30
|
Gaesser JM and Fyffe-Maricich SL:
Intracellular signaling pathway regulation of myelination and
remyelination in the CNS. Exp Neurol. 283:501–511. 2016. View Article : Google Scholar
|
|
31
|
Ishii A, Furusho M, Dupree JL and Bansal
R: Role of ERK1/2 MAPK signaling in the maintenance of myelin and
axonal integrity in the adult CNS. J Neurosci. 34:16031–16045.
2014. View Article : Google Scholar
|
|
32
|
Fyffe-Maricich SL, Schott A, Karl M,
Krasno J and Miller RH: Signaling through ERK1/2 controls myelin
thickness during myelin repair in the adult central nervous system.
J Neurosci. 33:18402–18408. 2013. View Article : Google Scholar
|
|
33
|
Xie D, Shen F, He S, Chen M, Han Q, Fang
M, Zeng H, Chen C and Deng Y: IL-1β induces hypomyelination in the
periventricular white matter through inhibition of oligodendrocyte
progenitor cell maturation via FYN/MEK/ERK signaling pathway in
septic neonatal rats. Glia. 64:583–602. 2016. View Article : Google Scholar
|
|
34
|
Suzuki T, Hori N, Miyake T, Hori Y and
Mochizuki K: Keratitis caused by a rare fungus, Malassezia
restricta. Jpn J Ophthalmol. 51:292–294. 2007. View Article : Google Scholar
|