Introduction
Bone marrow mesenchymal stem cells (BMSCs) are
multipotent cells, with a strong ability of division and
proliferation (1–3). At present, there have been great
advances in the research of BMSCs osteogenic differentiation
(4–6), and mainly have the following several
methods: i) Chemical drug induction (adding proper concentration of
dexamethasone, β-glycerophosphate and ascorbic acid in the culture
system), which are typically used to induce BMSCs osteogenic
differentiation; ii) inducted by cytokines and growth factors (such
as bone morphogenetic protein-2, transforming growth factor-β); and
iii) stimulated differentiation under physical methods (such as
mechanical strain, extracorporeal shockwaves, electromagnetic
fields) (7,8). However, the molecular mechanism of
BMSCs which were induced into osteoblasts with osteoinductive
medium remains to be fully elucidated. We believe that alteration
to gene expression by microRNAs plays an important role in the
osteoblast differentiation.
miRNAs are a class of small non-coding RNAs, about
21 nucleotides in length, which repress gene expression at the
post-transcriptional level by degrading their target mRNAs or
through translational repression (9,10).
miRNAs regulate cell proliferation, differentiation and apoptosis,
and control physiological changes, including growth and development
(9–11). Several miRNAs are also involved as
key modulators of bone formation or osteoblastic differentiation
(12–15).
In previous years, some miRNAs related to osteogenic
differentiation have been detected and identified in BMSC cells
(16,17). However, the differently expressed
miRNA in osteogenic differentiation of BMSCs has not been fully
studied, and the mechanism of miRNA regulating osteogenic
differentiation has not been fully understood, either. In the
proceess of osteogenic differentiation, we hypothesized that these
differently expressed miRNAs may act as a whole in regulating
specific cellular functions and pathways. In the present study,
mouse BMSCs were cultured with osteoinductive medium (18), which was performed to induce
osteoblastic differentiation. Subsequently, miRNA microarray and
RT-qPCR analyses were performed to identify the differentially
expressed miRNAs of BMSCs which were induced into osteoblasts in
vitro. Specific cellular functions and signal pathways related
to osteoblastic differentiation of these miRNAs were predicted
using bioinformatics analysis.
Materials and methods
Cell culture and application of
osteoinductive medium to cultured cells
BMSCs from C57BL/6 mice were purchased from the
Cyagen Biosciences, Inc. (Guangzhou, China). The culture were
maintained in α-MEM (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere containing 5%
CO2. Then, the medium was changed every 3 days. For all
experiments, BMSCs were seeded at the density of 2.5×104
cells/cm2 in the cell culture dishes and cultivated
until they reached 80% confluence (0 day). Then the cells were
induced with osteoinductive medium containing 100 nmol/l
dexamethasone, 50 µg⁄ml ascorbic acid and 10 mmol/l
β-glycerophosphate (Invitrogen; Thermo Fisher Scientific, Inc.) and
the medium was changed every 3 or 4 days. Control cultures were
incubated under the same conditions with normal medium (α-MEM
supplemented with 10% FBS and 1% penicillin-streptomycin).
ALP activity assay
Following induction of BMSCs with osteoinductive
medium, the BMSCs were lysed by brief sonication on ice in
radioimmunoprecipitation lysis buffer (CW Biotech, Beijing, China),
and the protein concentration of the cell lysates were measured
using the Bichinchoninic Acid Protein Assay kit (CW Biotech). The
activity of ALP in the lysates was measured with a ALP assay kit
(Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China) using a
p-nitrophenyl phosphate method, according to manufacturer's
instructions. A single unit of ALP activity represented 1 µmol
p-nitrophenyl phosphate hydrolyzed to p-nitrophenol/min, therefore
the ALP activity in the proteins was expressed in U/g protein.
ALP and alizarin red-S staining
Following cultured with osteoinductive medium, the
cells were washed with PBS and fixed with 4% paraformaldehyde for
20 min at 37°C. The fixed cells were soaked with the ALP staining
kit (Nanjing Jiancheng Biotechnology Co., Ltd.) and Alizarin red-S
staining kit (Nanjing Jiancheng Biotechnology Co., Ltd.) according
to the manufacturer's instructions, and were then observed under an
optical microscope.
Western blotting
After the BMSCs were induced with osteoinductive
medium for 21 days, extracts of the cells were harvested in lysis
buffer, protein contents of the cell lysates were quantified by BCA
Protein Assay kit (CW Biotech). Proteins were separated in 10%
sodium dodecyl sulfate-polyacrylamide gels, transferred to
nitrocellulose membranes blocked with 2.5% BSA in Tris-buffered
saline with 0.1% Tween-20, and incubated with primary antibodies,
respectively. Membranes were then incubated with secondary
antibodies conjugated with horseradish peroxidase (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) respectively, and
immunoreactive bands were detected using an enhanced
chemiluminescence detection reagent (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). β-actin in cell lysates was
used as a loading control, data were normalized against those of
corresponding optical density of β-actin.
miRNA microarray
miRNA microarray (Agilent Technologies, Inc., Santa
Clara, CA, USA) was used to detect the miRNA expression levels in
the BMSCs. The miRNA expression profiles of the BMSCs which were
induced with osteoinductive medium were compared with the control
group (cultured without osteoinductive medium). miRNAs were
enriched from total RNA extracted using a mirVana miRNA Isolation
Kit and labeled with a mirVana Array Labeling Kit (Ambion; Thermo
Fisher Scientific, Inc.). The labeled miRNAs were used for
hybridization on each miRNA microarray to determine the
differential expression of miRNAs. This procedure was repeated
twice. Target labeling, hybridization, imaging and data processing
were performed at Phalan Biotech (Beijing, China) according to the
protocols described by the manufacturer using the Mouse miRNA
OneArray Microarray (Agilent Technologies, Inc.) and Sanger miRBase
19. Data were acquired using Agilent Feature Extraction software
version 10.7 (Agilent Technologies, Inc.). Further data analyses
were performed using GeneSpring GX 10.0 (Agilent Technologies,
Inc.).
RT-qPCR
The expression levels of miRNAs were detected using
RT-qPCR. The primers for RT-qPCR of miRNAs were provided by
Guangzhou FulenGen Co., Ltd. (Guangzhou, China), the information of
the primers were listed in Table I.
Following cDNA synthesis using All-in-One™ miRNA
First-Strand cDNA Synthesis Kit (Guangzhou FulenGen), qPCR was
performed using All-in-One™ miRNA qPCR kit (Guangzhou
FulenGen) according to the manufacturer's instructions. The
reactions were incubated in a 96-well optical plate at 95°C for 10
min, followed by 40 cycles of 10 sec at 95°C, 20 sec at 60°C and 10
sec at 70°C (annealing and extension). Expression analysis was
performed in triplicate for each sample. The average value of six
reference genes were used as the normalization control (U6, U68,
U70, U49A, U65, U72). The miRNA expression levels were quantified
using an ABI Prism 7300 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.).
 | Table I.Information of primers used for miRNA
RT-qPCR.a |
Table I.
Information of primers used for miRNA
RT-qPCR.a
| Name | Mature_accession | Product_ID | Primer_ID |
|---|
| mmu-let-7c-5p | MIMAT0000523 | MmiRQP0006 | Mumq-0511 |
| mmu-miR-6338 | MIMAT0025081 | MmiRQP2840 | Mumq-0997 |
|
mmu-miR-181c-3p | MIMAT0017068 | MmiRQP3102 | Mumq-0998 |
| mmu-miR-690 | MIMAT0003469 | MmiRQP1118 | Mumq-0999 |
| mmu-miR-9-5p | MIMAT0000142 | MmiRQP0825 | Mumq-0167 |
|
mmu-miR-125b-2-3p | MIMAT0004529 | MmiRQP0877 | Mumq-0607 |
| mmu-miR-1b-5p | MIMAT0005835 | MmiRQP0878 | Mumq-0185 |
| mmu-miR-28b | MIMAT0019354 | MmiRQP2209 | Mumq-0903 |
| mmu-miR-361-3p | MIMAT0017075 | MmiRQP3108 | Mumq-1000 |
|
mmu-miR-1249-3p | MIMAT0010560 | MmiRQP0081 | Mumq-0633 |
| mmu-miR-3473c | MIMAT0020614 | MmiRQP2534 | Mumq-0917 |
| mmu-miR-3971 | MIMAT0019356 | MmiRQP2211 | Mumq-0905 |
| mmu-miR-6356 | MIMAT0025099 | MmiRQP2858 | Mumq-1001 |
|
mmu-miR-3072-3p | MIMAT0014853 | MmiRQP1644 | Mumq-0737 |
| mmu-miR-296-5p | MIMAT0000374 | MmiRQP0364 | Mumq-0077 |
| mmu-miR-223-3p | MIMAT0000665 | MmiRQP0342 | Mumq-0071 |
|
mmu-miR-129-2-3p | MIMAT0000544 | MmiRQP0139 | Mumq-0016 |
|
mmu-miR-129-1-3p | MIMAT0016994 | MmiRQP0136 | Mumq-1002 |
|
mmu-miR-3092-3p | MIMAT0014906 | MmiRQP1742 | Mumq-0832 |
|
mmu-miR-5132-3p | MIMAT0022988 | MmiRQP3535 | Mumq-1003 |
| mmu-miR-483-3p | MIMAT0003120 | MmiRQP1052 | Mumq-0710 |
Bioinformatics analysis
As miRNAs play their biological roles through
regulating target genes expression at the post-transcriptional
level, target genes of the miRNAs were predicted by online
softwares, including miRDB (http://www.mirdb.org/), MicroRNASeq (http://cm.jefferson. edu/rna22v2/), TargetScan
(http://wwwargetscan.org), and miRanda (http://www.microrna.org/). To identify the biological
functions of these genes, Gene Ontology (GO) (http://www.geneontology.org/) and Kyoto encyclopedia
of genes and genomes (KEGG) (http://www.genome.jp/) enrichment analysis were
performed, respectively. Molecular Functions, Biological Process
and Cellular Component of Gene Ontology were included. Search for
gene annotation information using Ontology Gene database
(http://www.geneontology.org/). According
to the gene GO annotation, statistical methods were applied to
calculate P-values, with P<0.01 as the significant threshold.
All genes in this species were used as background genes. The high
frequency notes with statistical significance were obtained and
normalized to the background, information of distribution and
significance of GO based gene sets were obtained. Based on the KEGG
database (http://www.genome.jp/), gene sets were
enriched in the biological pathway associated with osteoblastic
differentiation were selected.
Statistical analysis
To identify differentially expressed miRNAs among
the groups, Student's t-test was performed using SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA). The experiments were repeated in
triplicate. Statistical significance between the groups was
measured using Student's t-test; P<0.05 was considered to
indicate a statistically significant difference.
Results
Osteoinductive medium promoted
osteoblastic differentiation
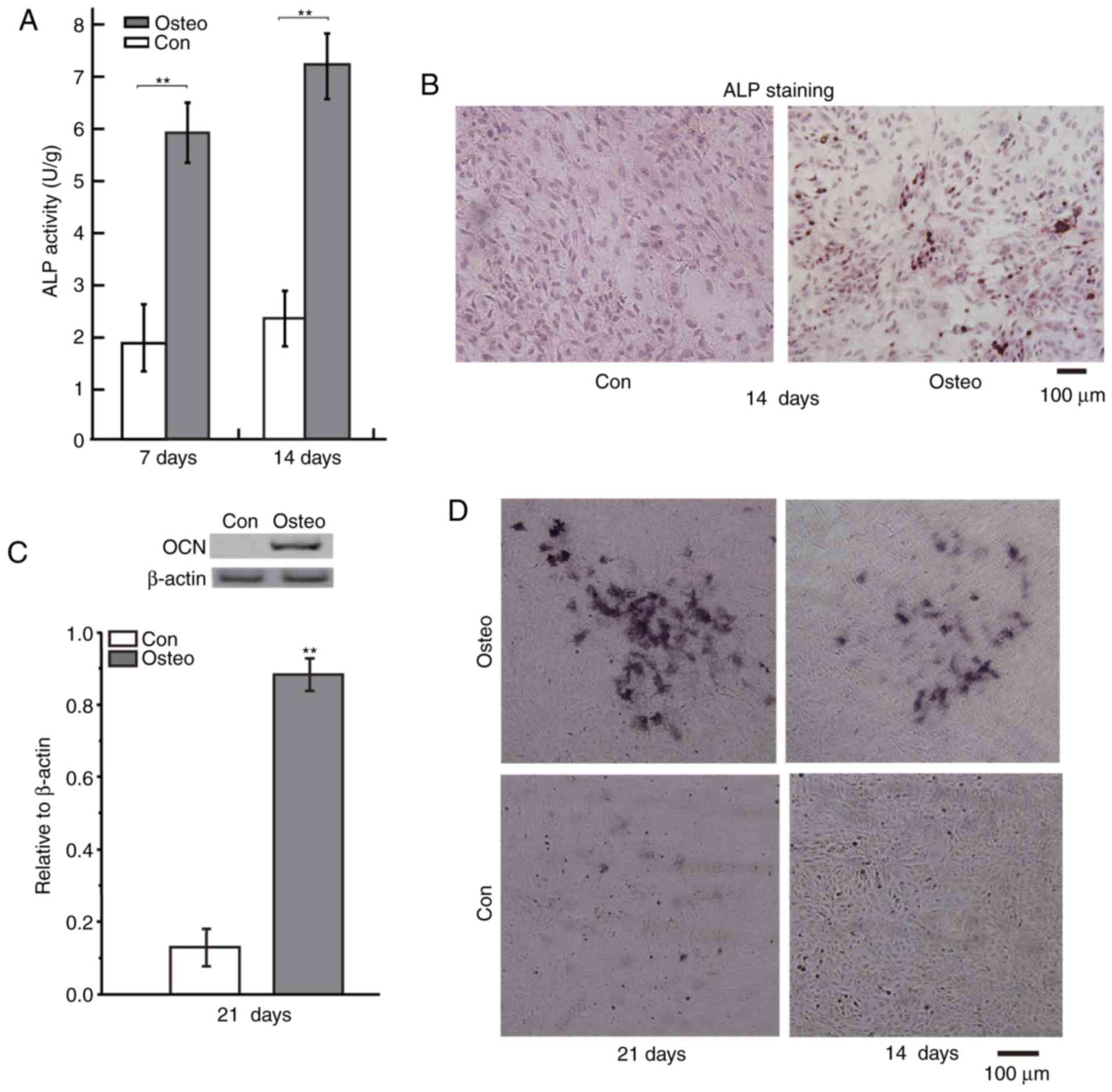
The effect of osteoinductive medium on the
differentiation of osteoblasts was investigated via measuring
various markers of osteoblastic differentiation, including ALP,
calcium deposits and OCN levels (Fig.
1). Following exposure of the BMSCs to osteoinductive medium,
the ALP activities in BMSCs which were induced for 7 days and 14
days were notably higher than that in control group (Fig. 1A). Protein levels of OCN were also
increased in BMSCs which were induced for 21 days (Fig. 1C). In addition, the images of ALP and
Alizarin red-S staining of BMSCs indicated that the osteoinductive
medium increased the levels of ALP and calcium deposits in the
BMSCs at indicated time (Fig. 1B and
D). ALP, OCN, and calcium deposits are all markers of
osteoblastic differentiation (19–22).
Therefore, the osteoinductive medium promoted osteoblastic
differentiation of the BMSCs.
Identification of four differently
expressed miRNAs in BMSCs which were induced with osteoinductive
medium
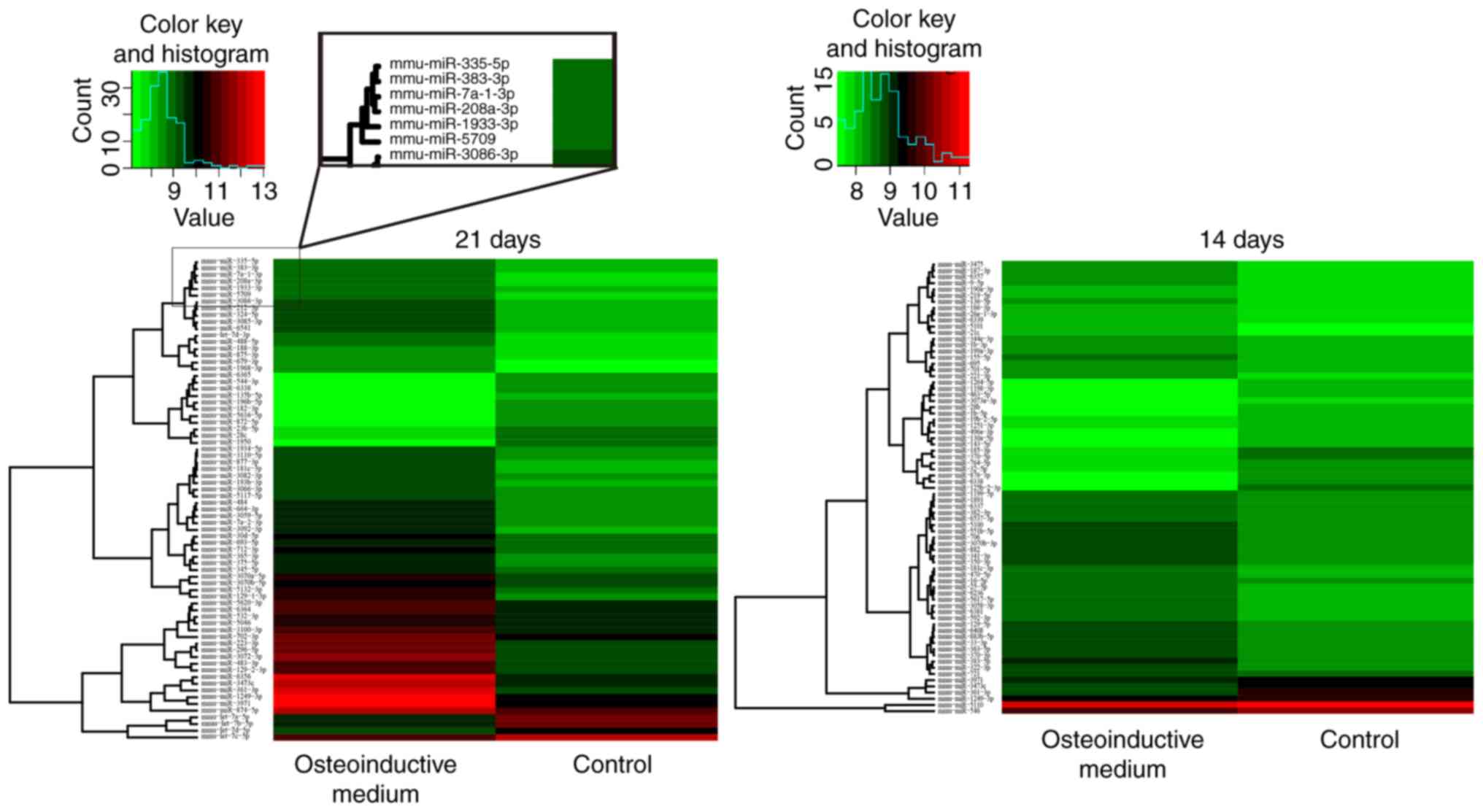
miRNA microarray was used to detect the miRNA
expression levels in BMSCs. The results revealed that the miRNAs
were deregulated in the induced group under the condition of
‘P<0.05 and fold-change >1.5’, compared with control group.
Among them, the expression levels of 107 miRNAs were higher and the
expression levels of 40 miRNAs were lower in the group induced with
osteoinductive medium compared with the non-induced control group
(Fig. 2, Table II).
 | Table II.miRNA microarray was used to detect
the miRNA expression levels in BMSCs. The expression levels of
miRNAs were upregulated or downregulated in the group induced with
osteoinductive medium compared with the non-induced control
group. |
Table II.
miRNA microarray was used to detect
the miRNA expression levels in BMSCs. The expression levels of
miRNAs were upregulated or downregulated in the group induced with
osteoinductive medium compared with the non-induced control
group.
| Comparison | Upregulated | Downregulated |
|---|
| 7 day | 1 | 1 |
| 14 day | 49 | 24 |
| 21 day | 57 | 15 |
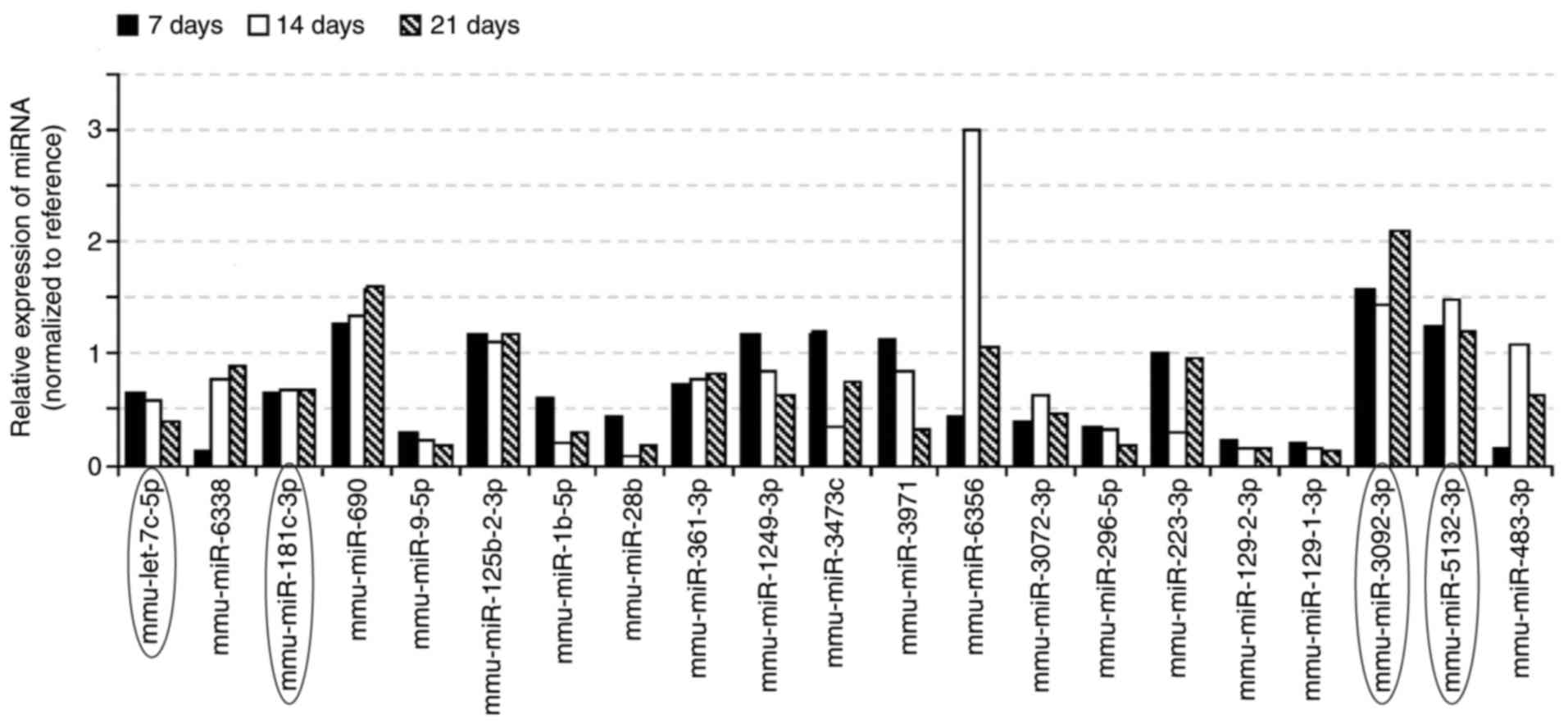
RT-qPCR for further validation
21 miRNAs were selected from the chip results (the
21 miRNAs were all expressed at 14 days and 21 days, amomg the 21
miRNAs, 2 miRNAs were both expressed at 7 days), and used RT-qPCR
for further validation. The results of RT-qPCR confirmed that the
expression levels of mmu-let-7c-5p, mmu-miR-181c-3p,
mmu-miR-3092-3p, and mmu-miR-5132-3p had the same expression trend
as the microarray in the osteoinductive medium groups (Fig. 3). In the four miRNAs, mmu-let-7c-5p
was differently expressed at 7, 14 and 21 days, which was testified
with both miRNA microarray and RT-qPCR. The other three miRNAs were
all differently expressed at 14 and 21 days (testified with both
miRNA microarray and RT-qPCR). Therefore, these four miRNAs were
considered to be responsive to the osteoinductive medium applied to
the BMSCs.
Target genes of mmu-let-7c-5p,
mmu-miR-181c-3p, mmu-miR-3092-3p and mmu-miR-5132-3p were
predicted
Four online softwares (miRanda, Clip-Seq,
TargetScan, and miRDB) were applied to predict target genes for the
four differentially expressed miRNAs. Prediction results of the
four software were further screened and sorted out in Table III (target genes predicted by any
three softwares mentioned above at the same time were regarded as a
candidate target gene of corresponding miRNA). As a result, 540
genes were predicted as putative target genes of the miRNAs respond
to osteoinductive medium.
 | Table III.Statistical table of the candidate
target genes of four miRNAs predicted by the four online softwares
(miRanda, Clip-Seq, TargetScan, and miRDB). |
Table III.
Statistical table of the candidate
target genes of four miRNAs predicted by the four online softwares
(miRanda, Clip-Seq, TargetScan, and miRDB).
| miRNA | Candidate target
genes |
|---|
| mmu-let-7c-5p | 373 |
|
mmu-miR-181c-3p | 30 |
|
mmu-miR-3092-3p |
8 |
|
mmu-miR-5132-3p | 129 |
Identification of the candidate target
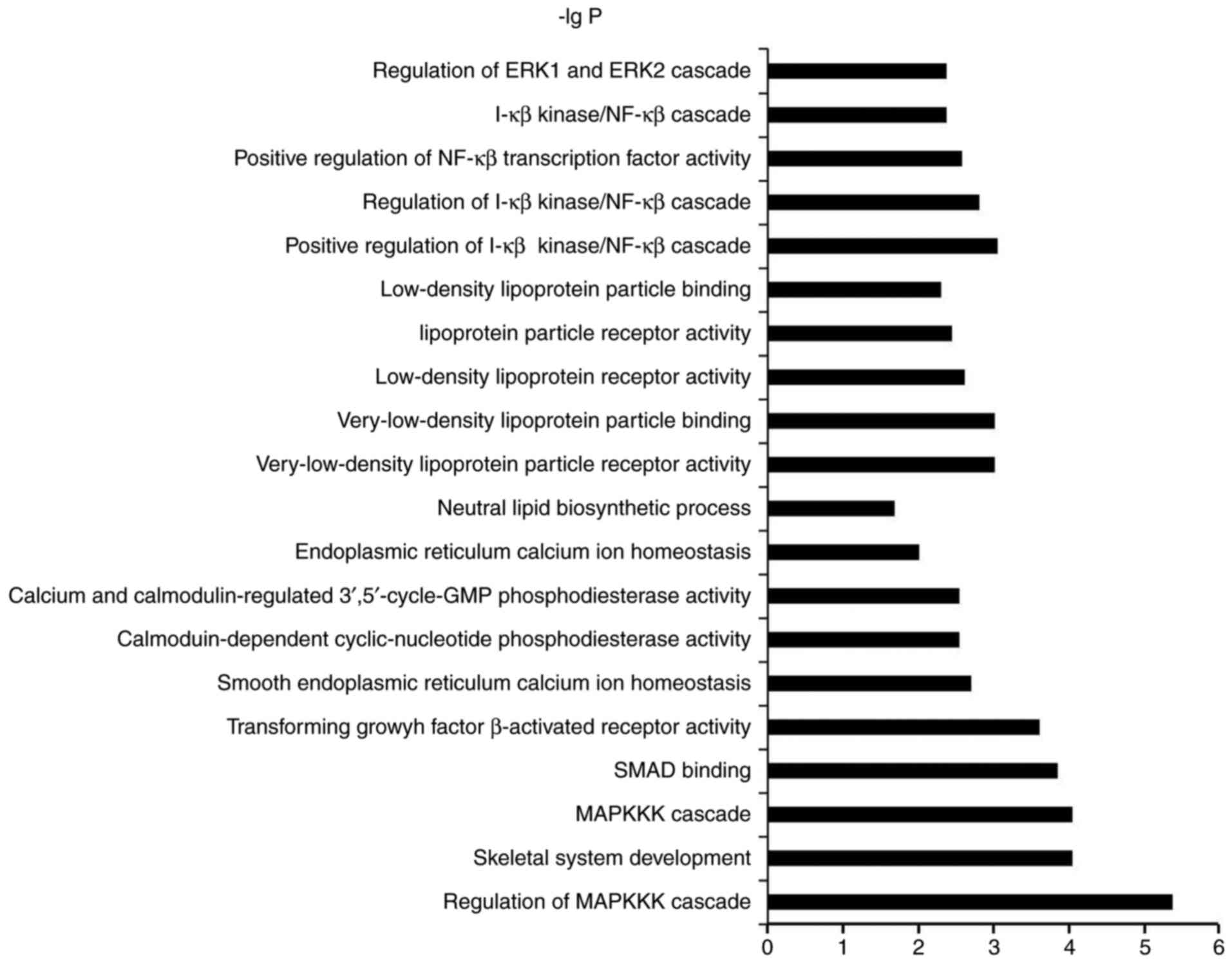
genes of miRNAs and GO/KEGG pathway enrichment analysis
To identify the biological functions of these genes,
GO and pathway enrichment analysis were performed, respectively.
All the GO/KEGG annotation results in pathways and molecular
functions related to osteogenic differentiation or bone formation
were searched to excavate functions related genes. As illustrated
in Fig. 4 and Table IV, the top 20 GOs sensitive to
osteoinductive medium were showed in Fig. 4. In the result of KEGG, three items
with minimum P-value were selected from the statistical results of
the data, showed in Table IV.
 | Table IV.Bioinformatics analysis the main
signaling pathways in BMSCs induced with osteoinductive medium. |
Table IV.
Bioinformatics analysis the main
signaling pathways in BMSCs induced with osteoinductive medium.
| miRNA | The KEGG signaling
pathway | Potential genes
(total number) | (Refs.) |
|---|
| mmu-let-7c-5p | MAPK signaling
pathway (mmu04010) | Casp3, Nlk, Dusp1,
Nras, Map3k3, Pla2g3, Map3k1, Rasgrp1, Tgfbr1, Map4k3, Map4k4 | (11) |
|
| TGF-β signaling
pathway (mmu04350) | E2f5, Acvr1c, Gdf6,
Tgfbr1, Thbs1 | (5) |
|
| Glycerophospholipid
metabolism (mmu00564) | Pla2g3, Etnk1,
Lpgat1, Pld3, Pla2g15 | (5) |
|
mmu-miR-181c-3p | Taste transduction
(mmu04742) | Pde1a | (1) |
|
| Pertussis
(mmu05133) | Sftpa1 | (1) |
|
| Adherens junction
(mmu04520) | Pvrl3 | (1) |
|
mmu-miR-3092-3p | Endocrine and other
factor-regulated calcium reabsorption (mmu04961) | Cltc | (1) |
|
| Synaptic vesicle
cycle (mmu04721) | Cltc | (1) |
|
| Bacterial invasion
of epithelial cells (mmu05100) | Cltc | (1) |
|
mmu-miR-5132-3p | Focal adhesion
(mmu04510) | Pak3, Thbs2, Igf1,
Braf, tga4, Vcl | (6) |
|
| Regulation of actin
cytoskeleton (mmu04810) | Pak3, Itgae, Itga4,
Braf, Vcl | (5) |
|
| Prion diseases
(mmu05020) | C8a, Ncam1 | (2) |
Discussion
The most basic problem in bone tissue engineering is
the selection of the seed cell, which is the main source of the
active components of tissue engineering. At present, BMSCs are
widely used in bone tissue engineering. It has the strong potential
of osteogenic differentiation, the material is convenient, the self
renewal ability is strong, and there is no immune rejection in
transplantation (23,24). Therefore, further study on the
mechanism of osteogenic differentiation of BMSCs will be helpful to
the clinical application of BMSCs in bone tissue engineering
(25).
In recent years, researches reported that miRNA
regulated osteogenesis of mesenchymal stem cells (16,26,27).
However, differentially expression profiles of miRNA in osteogenic
differentiation of BMSCs which were induced with osteoinductive
medium and the mechanism of miRNA regulating osteogenic
differentiation were not fully studied yet.
In the present study, the activity of ALP, protein
levels of OCN and deposit of calcium in the cell culture were all
increased, which confirmed that BMSCs were induced into osteoblast
with osteoinductive medium. In this study, at 7, 14 and 21 days,
the differently expressed miRNAs in BMSCs treated with
osteoinductive medium, were screened and testified. Four miRNAs
(mmu-let-7c-5p, mmu-miR-181c-3p, mmu-miR-3092-3p, and
mmu-miR-5132-3p) were all differently expressed in BMSCs treated
with osteoinductive medium for 14 and 21 days, which indicated that
those four miRNAs were responsive to osteoinductive medium.
Bioinformatics analysis (miRanda, Clip-Seq, TargetScan, miRDB) were
used to predict target genes of the four miRNAs, 540 genes were
predicted as putative target genes of the four miRNAs response to
osteoinductive medium.
According to the result of GO/KEGG analysis,
pathways associated with osteogenic differentiation or bone
formation were TGF-β pathway, MAPK pathway, PI-3K pathway and NF-κB
pathway, these signaling pathways had been proved to regulate
osteogenic differentiation (28–32). In
addition, the predicted target genes involved in calcium
metabolism, vitamin lipid metabolism and regulation of actin
cytoskeleton are likely to be related to osteogenic differentiation
or bone formation (33–35).
To screen for the important genes involved in
osteoblast differentiation of BMSCs, we searched potential gene
targets in the Table IV, and
several target genes [CASP3 (36),
NLK (37), MAP3K1 (38), ACVR1c (39), GDF6 (40), PDE1a (41), IGF1 (42)] correlated with osteoblast
differentiation, which have been reported. However, the other
target genes require further verification. In the future, we will
chose some target genes and signaling pathways which have high
possibility, and validate these target genes and signaling pathways
using western blot or qPCR, investigating the mechanism underlying
the involvement of the miRNAs in regulating osteogenic
differentiation.
In conclusion, the present study demonstrated that
the osteoinductive medium promoted osteoblastic differentiation of
BMSCs, and the four miRNAs (let-7c-5p, miR-181c-3p, miR-3092-3p and
miR-5132-3p) were identified as differentially expressed miRNAs in
the BMSCs, and were responsive to osteoinductive medium, these
miRNAs are potential regulators of osteoblastic differentiation and
the response of BMSCs to osteoinductive medium.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (nos. 11372351, 31660261, and
11432016) and the Natural Science Foundation of Guangxi (no.
2016GXNSFAA380322).
References
|
1
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W,
Patel N, et al: Adult bone marrow stromal cells differentiate into
neural cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedenstein AJ, Chailakhjan RK and
Lalykina KS: The development of fibroblast colonies in monolayer
cultures of guinea-pig bone marrow and spleen cells. Cell Tissue
Kinet. 3:393–403. 1970.PubMed/NCBI
|
|
4
|
Liu L, Liu M, Li R, Liu H, Du L, Chen H,
Zhang Y, Zhang S and Liu D: MicroRNA-503-5p inhibits
stretch-induced osteogenic differentiation and bone formation. Cell
Biol Int. 41:112–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong M, Jiao G, Liu H, Wu W, Li S, Wang Q,
Xu D, Li X, Liu H and Chen Y: Biological silicon stimulates
collagen type 1 and osteocalcin synthesis in human osteoblast-like
cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol Trace
Elem Res. 173:306–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao M, Chen J, Lin G, Li S, Wang L, Qin A,
Zhao Z, Ren L, Wang Y and Tang BZ: Long-term tracking of the
osteogenic differentiation of mouse BMSCs by aggregation-induced
emission nanoparticles. ACS Appl Mater Interfaces. 8:17878–17884.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heino TJ and Hentunen TA: Differentiation
of osteoblasts and osteocytes from mesenchymal stem cells. Curr
Stem Cell Res Ther. 3:131–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang C, Meng H, Wang X, Zhao C, Peng J and
Wang Y: Differentiation of bone marrow mesenchymal stem cells in
osteoblasts and adipocytes and its role in treatment of
osteoporosis. Med Sci Monit. 22:226–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y and Srivastava D: A developmental
view of microRNA function. Trends Biochem Sci. 32:189–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eskildsen T, Taipaleenmäki H, Stenvang J,
Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S and Kassem M:
MicroRNA-138 regulates osteogenic differentiation of human stromal
(mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA. 108:pp.
6139–6144. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Fan L, Liu S, Liu W, Zhang H, Zhou
T, Wu D, Yang P, Shen L, Chen J and Jin Y: The promotion of bone
regeneration through positive regulation of angiogenic-osteogenic
coupling using microRNA-26a. Biomaterials. 34:5048–5058. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim EJ, Kang IH, Lee JW, Jang WG and Koh
JT: MiR-433 mediates ERRγ-suppressed osteoblast differentiation via
direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci.
92:562–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang T, Wu Q, Zhou L, Mu S and Fu Q:
miR-106b-5p and miR-17-5p suppress osteogenic differentiation by
targeting Smad5 and inhibit bone formation. Exp Cell Res.
347:74–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y,
Gu P and Fan X: Effects of a miR-31, Runx2, and Satb2 regulatory
loop on the osteogenic differentiation of bone mesenchymal stem
cells. Stem Cells Dev. 22:2278–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuang W, Tan JL, Zhang HM, Duan JM, Wang
WJ and Li X: miR-146a down regulates the osteogenic differentiation
of murine bone marrow mesenchymal stem cells. Biomed Eng Clin Med.
15:413–417. 2011.(In Chinese).
|
|
18
|
Coelho MJ and Fernandes MH: Human bone
cell cultures in biocompatibility testing. Part II: Effect of
ascorbic acid, beta-glycerophosphate and dexamethasone on
osteoblastic differentiation. Biomaterials. 21:1095–1102. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Wang B, Li Y, Wang D, Lingling E,
Bai Y and Liu H: High glucose inhibits osteogenic differentiation
through the BMP signaling pathway in bone mesenchymal stem cells in
mice. EXCLI J. 12:584–597. 2013.PubMed/NCBI
|
|
20
|
Kim K, Dean D, Wallace J, Breithaupt R,
Mikos AG and Fisher JP: The influence of stereolithographic
scaffold architecture and composition on osteogenic signal
expression with rat bone marrow stromal cells. Biomaterials.
32:3750–3763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu H, Peng H, Wu Y, Zhang C, Cai Y, Xu G,
Li Q, Chen X, Ji J, Zhang Y and OuYang HW: The promotion of bone
regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by
effects on integrin-BMP/Smad signaling pathway in BMSCs.
Biomaterials. 34:4404–4417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Id Boufker H, Lagneaux L, Fayyad-Kazan H,
Badran B, Najar M, Wiedig M, Ghanem G, Laurent G, Body JJ and
Journé F: Role of farnesoid X receptor (FXR) in the process of
differentiation of bone marrow stromal cells into osteoblasts.
Bone. 49:1219–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gronthos S, Akintoye SO, Wang CY and Shi
S: Bone marrow stromal stem cells for tissue engineering.
Periodontol 2000. 41:188–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang SC, Chuang HL, Chen YR, Chen JK,
Chung HY, Lu YL, Lin HY, Tai CL and Lou J: Ex vivo gene therapy in
autologous bone marrow stromal stem cells for tissue-engineered
maxillofacial bone regeneration. Gene Ther. 10:2013–2019. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He H, Jazdzewski K, Li W, Liyanarachchi S,
Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al:
The role of microRNA genes in papillary thyroid carcinoma. Proc
Natl Acad Sci USA. 102:pp. 19075–19080. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C,
Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, et al: Altered MicroRNA
expression profile in exosomes during osteogenic differentiation of
human bone marrow-derived mesenchymal stem cells. PLoS One.
9:e1146272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Li H, Wang Y, Li T, Fan J, Xiao K,
Zhao RC and Weng X: microRNA-23a inhibits osteogenic
differentiation of human bone marrow-derived mesenchymal stem cells
by targeting LRP5. Int J Biochem Cell Biol. 72:55–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu DD, Zhang JC, Zhang Q, Wang SX and
Yang MS: TGF-β/BMP signaling pathway is involved in cerium-promoted
osteogenic differentiation of mesenchymal stem cells. J Cell
Biochem. 114:1105–1114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi C, Liu D, Fong CC, Zhang J and Yang M:
Gold nanoparticles promote osteogenic differentiation of
mesenchymal stem cells through p38 MAPK pathway. ACS Nano.
4:6439–6448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng ZL, Sharff KA, Tang N, Song WX, Luo
J, Luo X, Chen J, Bennett E, Reid R, Manning D, et al: Regulation
of osteogenic differentiation during skeletal development. Front
Biosci. 13:2001–2021. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Z, Jiang H, Dong K, Liu S, Zhou W,
Zhang J, Meng L, Rausch-Fan X and Xu X: Different concentrations of
glucose regulate proliferation and osteogenic differentiation of
osteoblasts via the PI3 kinase/Akt pathway. Implant Dent. 24:83–91.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hess K, Ushmorov A, Fiedler J, Brenner RE
and Wirth T: TNFalpha promotes osteogenic differentiation of human
mesenchymal stem cells by triggering the NF-kappaB signaling
pathway. Bone. 45:367–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura A, Dohi Y, Akahane M, Ohgushi H,
Nakajima H, Funaoka H and Takakura Y: Osteocalcin secretion as an
early marker of in vitro osteogenic differentiation of rat
mesenchymal stem cells. Tissue Eng Part C Methods. 15:169–180.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song I, Kim BS, Kim CS and Im GI: Effects
of BMP-2 and vitamin D3 on the osteogenic differentiation of
adipose stem cells. Biochem Biophys Res Commun. 408:126–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodríguez JP, González M, Ríos S and
Cambiazo V: Cytoskeletal organization of human mesenchymal stem
cells (MSC) changes during their osteogenic differentiation. J Cell
Biochem. 93:721–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
You L, Gu W, Chen L, Pan L, Chen J and
Peng Y: MiR-378 overexpression attenuates high glucose-suppressed
osteogenic differentiation through targeting CASP3 and activating
PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 7:7249–7261.
2014.PubMed/NCBI
|
|
37
|
Nifuji A, Ideno H, Ohyama Y, Takanabe R,
Araki R, Abe M, Noda M and Shibuya H: Nemo-like kinase (NLK)
expression in osteoblastic cells and suppression of osteoblastic
differentiation. Exp Cell Res. 316:1127–1136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suddason T and Gallagher E: A RING to rule
them all? Insights into the Map3k1 PHD motif provide a new
mechanistic understanding into the diverse roles of Map3k1. Cell
Death Differ. 22:540–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka K, Matsumoto E, Higashimaki Y,
Katagiri T, Sugimoto T, Seino S and Kaji H: Role of osteoglycin in
the linkage between muscle and bone. J Biol Chem. 287:11616–11628.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yeh LC, Tsai AD and Lee JC: Osteogenic
protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation
of the pluripotent mesenchymal cell line C2C12. J Cell Biochem.
87:292–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi HD, Noh WC, Park JW, Lee JM and Suh
JY: Analysis of gene expression during mineralization of cultured
human periodontal ligament cells. J Periodontal Implant Sci.
41:30–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saeed H, Qiu W, Li C, Flyvbjerg A,
Abdallah BM and Kassem M: Telomerase activity promotes osteoblast
differentiation by modulating IGF-signaling pathway.
Biogerontology. 16:733–745. 2015. View Article : Google Scholar : PubMed/NCBI
|