Introduction
Infectious mononucleosis (IM) is a common disease
associated with Epstein-Barr virus (EBV) infection and ~5 cases
occur per 1,000 people every year (1,2) The
results of a survey conducted in Beijing indicated that the
incidence of IM peaks among preschool children and young people
aged 15–24 years old (3). Diagnosis
of IM is generally based on identifying the triad of clinical
manifestations of fever, pharyngitis and lymphadenopathy in
patients, as well as a positive result following a serological test
for EBV (1,2). However, patients with IM also exhibit
other symptoms, including headache, fatigue, exanthema,
hepatomegaly and splenomegaly (4).
The majority of patients with IM recover without complications and
return to their normal activities ~2 months following disease onset
(1,2). However, severe complications, including
upper-airway obstruction, hemolytic anemia, thrombocytopenia,
hepatitis, myocarditis, splenic rupture, neurological and
hematological complications may occur in certain cases (1,2). The
treatment for IM is generally supportive and only alleviates
symptoms, as EBV cannot be cured; these treatments include glucose
and sodium chloride intravenous infusion, reduced glutathione
intravenous infusion, pantoprazole sodium injection, and oral
loxoprofen and bicyclol tablets intake (1,2).
Abnormality of hepatic function is commonly encountered in IM;
however, it has been demonstrated for many years that increases in
alanine aminotransferase (ALT), aspartate aminotransferase (AST)
and γ-glutamyl transferase (GGT) occur more frequently in IM than
bilirubin abnormalities (1–12). However, the underlying reasons for
these elevations remain unknown. The results of previous studies
demonstrated that >80% of patients with IM presented with
abnormal transferase levels, particularly ALT, but jaundice was
uncommon (1,2,13). In
addition, Hu et al (14)
indicated that the presence of atypical lymphocytes and
transaminase may be regarded as a diagnostic marker of IM.
The aim of the current study was to analyze the
associations between hepatic function parameters and IM in a
comprehensive manner. The aim was to identify whether abnormal
hepatic function could be indicative of IM and whether there were
any significant differences between hepatic function parameters
between males and females.
Patients and methods
Patients
Between December 2014 and December 2015, 95 patients
(mean age, 39.36 years; 47 males and 48 females) with a confirmed
diagnosis of IM were enrolled in the Department of Infectious
Diseases, Tianjin Medical University General Hospital (Tianjin,
China). Patients with pre-existing liver diseases, including viral
hepatitis, fatty liver disease, alcoholic liver disease and
medication-induced liver damage, were excluded from the current
study. During the same period, a normal control cohort consisting
of 95 healthy participants (mean age, 39.45 years; 47 males and 48
females) was recruited from the Health Management Department,
Tianjin Medical General University. None of the healthy
participants had been diagnosed with any diseases and had attended
Tianjin Medical General University for an annual health checkup.
The ethical, methodological and protocol aspects of the current
investigation were approved by the Institutional Review Board of
Tianjin Medical University General Hospital and written informed
consent was provided by all participants.
Parameter measurements
Following admittance of patients with IM to the
Department of Infectious Diseases, blood tests and anthropometric
measurements were conducted. For participants in the control group,
blood tests and anthropometric measurements were completed during
their visit to the hospital. Physical examinations included
measurements of body height (BH) and body weight (BW).
Subsequently, body mass index (BMI; kg/m2) was
calculated using the following formula: BW/(BH2).
Fasting blood tests were performed following venipuncture and the
following serological parameters were measured: White blood cell
(WBC), red blood cell (RBC), hemoglobin (Hg) and platelet (PLT)
levels. A total of 3 ml of whole blood samples were collected and
analyzed using a hemocytometer analyzer (Sysmex Corporation, Kobe,
Japan). ALT, AST, GGT, total bilirubin (TB), blood urea nitrogen
(BUN) and creatinine (Cr) levels were measured using the Hitachi
Model 7170 analyzer (Hitachi Ltd., Tokyo, Japan). Levels of
immunoglobulin (Ig)M and IgG antibodies against specific EBV
antigens were measured by ELISA using three commercial kits (viral
capsid antigen antibody IgM, cat. no. E170606AK; viral capsid
antigen antibody IgG, cat. no. E170706BO; viral nuclear antigen
antibody IgG, cat. no. E170706BR) supplied by (Euroimmun;
Medizinische Labordiagnostika AG, Lübeck, Germany). Ultrasonography
on neck lymph nodes and abdominal organs was performed the using
PHILIPS HD11 XE (Philips, Amsterdam, The Netherlands).
Diagnostic criteria
IM was generally diagnosed based on clinical
presentations of a fever, pharyngitis, lymphadenopathy, the
presence of atypical lymphocytes on a peripheral blood smear and a
positive heterophile antibody test. The majority of the included
patients referred to Tianjin Medical University General Hospital
(Tianjin, China) were diagnosed with IM in local hospitals prior to
recruitment. In order to establish definite IM diagnosis,
serological testing for the identification of antibodies against
specific EBV antigens was required by the aforementioned ELISA kits
(1,2).
Statistical analysis
All data for males and females were analyzed
separately and presented as either the mean ± standard deviation or
the median (lower quartile, upper quartile). Differences of
parameters between groups were measured using an independent
samples T test or a Mann-Whitney test. Pearson's bivariate
correlation analysis was performed between hepatic function indices
and other variables. Receiver operating characteristic (ROC) curves
were constructed and diagnostic efficacies were determined using
optimal sensitivity and specificity values calculated from hepatic
function indices. To compare ROC curves, a previously described
protocol was used (15). By
stratifying data with quartiles of hepatic function indices, the
odds ratio (OR) for IM with a 95% confidence interval (CI) was
calculated using binary logistic regression models. OR adjustments
were completed by using age and BMI as covariates in a logistic
regression model. The Statistical Package for Social Sciences (SPSS
v17.0, Chicago, IL, USA) was used for statistical analysis and
P<0.05 was determined to indicate a significant difference.
Results
Patient clinicopathological
characteristics
Parameters were compared in males and females
separately (Tables I and II). In males, WBC, ALT, AST and GGT were
significantly higher in patients with IM than in healthy controls
(Table I). However, RBC, Hg and TB
were significantly lower in males with IM compared with controls.
In females, ALT, AST and GGT were significantly higher in females
with IM than in controls (Table
II). However, RBC, Hg, TB, BUN and Cr were significantly lower
in females with IM compared with controls.
 | Table I.Parameter characteristics in
males. |
Table I.
Parameter characteristics in
males.
| Parameters | Patients with
IM | Controls | T value/Z
value |
|---|
| Case no. | 47 | 47 |
|
| Age (years) | 37.40±16.83 | 37.68±16.83 | −0.080 |
| BMI (kg/m2) | 23.58±3.02 | 24.74±3.50 | −1.725 |
| WBC (×109/l) | 7.17±3.81 | 5.93±1.49 | 2.085a |
| RBC (×1012/l) | 4.46±0.51 | 5.19±0.38 | −7.870b |
| Hg (g/l) | 133.19±14.59 | 155.15±10.21 | −8.453b |
| PLT (×109/l) | 208.47±80.43 | 214.81±46.92 | −0.467 |
| ALT (U/l) | 50.00 (22.00,
107.00) | 19.00 (14.00,
27.00) | 4.411b |
| AST (U/l) | 31.00 (19.00,
63.00) | 18.00 (14.00,
22.00) | 4.216a |
| GGT (U/l) | 57.00 (25.00,
123.00) | 20.00 (16.00,
29.00) | 5.164b |
| TB (µmol/l) | 9.50 (7.10,
11.90) | 11.70 (9.90,
17.90) | 2.934b |
| BUN (mmol/l) | 4.10±1.74 | 4.56±1.22 | −1.496 |
| Cr (µmol/l) | 76.30±28.92 | 83.34±12.75 | −1.528 |
 | Table II.Parameter characteristics in
females. |
Table II.
Parameter characteristics in
females.
| Parameters | Patients with
IM | Controls | T value |
|---|
| Case no. | 48 | 48 |
|
| Age (years) | 41.27±17.54 | 41.19±17.35 | 0.023 |
| BMI (kg/m2) | 23.52±3.81 | 22.60±2.94 | 1.314 |
| WBC (×109/l) | 6.29±3.62 | 5.17±1.54 | 1.975 |
| RBC (×1012/l) | 3.93±0.43 | 4.44±0.27 | −6.954a |
| Hg (g/l) | 113.54±13.34 | 130.73±8.49 | −7.530a |
| PLT (×109/l) | 235.65±90.10 | 226.15±50.15 | 0.638 |
| ALT (U/l) | 20.00 (12.00,
68.50) | 13.00 (9.25,
15.75) | 3.342a |
| AST (U/l) | 21.50 (16.00,
49.00) | 15.50 (13.00,
18.75) | 4.096a |
| GGT (U/l) | 25.50 (13.00,
49.50) | 11.00 (10.00,
16.75) | 4.101a |
| TB (µmol/l) | 6.10 (4.90,
7.55) | 9.70 (7.65,
12.28) | 4.379a |
| BUN (mmol/l) | 2.97±1.05 | 4.15±1.15 | −5.242a |
| Cr (µmol/l) | 52.13±9.46 | 60.77±9.59 | −4.446a |
Correlations between key
variables
Correlation coefficients between hepatic function
indices and other parameters were calculated in patients with IM
(Table III). In males, ALT was
positively correlated with WBC, AST and GGT, whereas in females,
ALT was positively correlated with AST and GGT. AST was positively
correlated with WBC, ALT and GGT in males and positively correlated
with ALT and GGT in females. GGT was positively correlated with
WBC, ALT and AST in males and positively correlated with age, BMI,
WBC, ALT and AST in females. In males, TB only exhibited a positive
correlation with Hg; however in females, TB was positively
correlated with RBC, Hg and Cr.
 | Table III.Pearson bivariate correlations in
males and females. |
Table III.
Pearson bivariate correlations in
males and females.
|
| Correlation
coefficients in males | Correlation
coefficients in females |
|---|
|
|
|
|
|---|
| Parameters | ALT | AST | GGT | TB | ALT | AST | GGT | TB |
|---|
| Age | −0.242a | −0.175 | −0.041 | −0.072 | −0.132 | −0.079 | 0.232a | 0.007 |
| BMI | 0.017 | 0.008 | 0.047 | −0.011 | −0.005 | 0.043 | 0.355b | 0.122 |
| WBC | 0.453b | 0.216a | 0.427b | 0.090 | 0.129 | 0.115 | 0.232a | −0.097 |
| RBC | −0.129 | −0.097 | −0.307b | 0.167 | −0.228a | −0.232a | −0.099 | 0.243a |
| Hg | −0.166 | −0.152 | −0.291b | 0.235a | −0.134 | −0.149 | −0.047 | 0.337b |
| PLT | −0.086 | −0.132 | −0.032 | 0.088 | −0.291b | −0.306b | 0.058 | −0.258a |
| ALT | – | 0.850b | 0.672b | 0.002 | – | 0.942b | 0.403b | 0.001 |
| AST | 0.850b | – | 0.534b | −0.036 | 0.942b | – | 0.426b | −0.003 |
| GGT | 0.672b | 0.534b | – | 0.080 | 0.403b | 0.426b | – | 0.161 |
| TB | 0.002 | −0.036 | 0.080 | – | 0.001 | −0.003 | 0.161 | – |
| BUN | −0.237a | −0.209a | −0.031 | −0.048 | −0.205a | −0.240a | −0.021 | 0.195 |
| Cr | −0.082 | −0.075 | 0.131 | 0.127 | −0.084 | −0.101 | 0.028 | 0.284b |
Diagnostic or predicative values of
hepatic function indices for IM
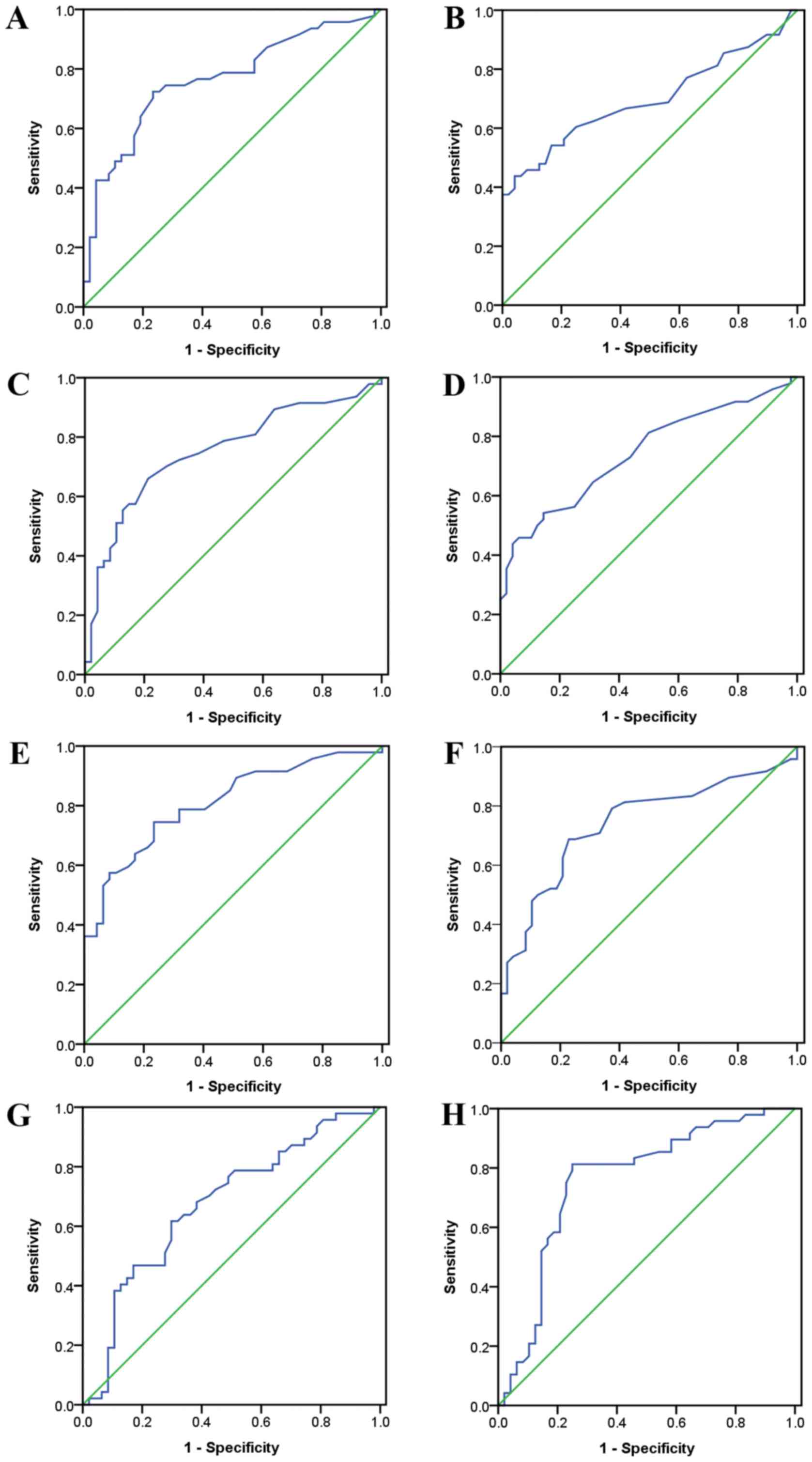
From ROC analyses, all hepatic function indices
exhibited significant diagnostic or predicative values for IM
(Fig. 1 and Table IV). If ALT, AST and GGT
concentrations were higher than the cut-off values of 27.5, 21.5
and 29.5 U/l in males, diagnostic accuracies of IM were 74.468,
71.277 and 75.532%, respectively. In females, the cut-off values
for ALT, AST and GGT were 15.5, 17.5 and 18.0 U/l and diagnostic
accuracies of IM were 67.708, 66.667 and 72.917%, respectively. If
TB concentrations were lower than the cut-off values of 10.70
µmol/l in males and 7.45 µmol/l in females, diagnostic accuracies
were 64.89 and 78.125%, respectively. Comparison of ROC curves in
males and females did not identify any evident differences.
 | Table IV.ROC indices of hepatic parameters for
diagnosing or predicting IM. |
Table IV.
ROC indices of hepatic parameters for
diagnosing or predicting IM.
| A, Male |
|---|
|
|---|
| Parameters | AUC | Cut-off value | Sensitivity
(%) | Specificity
(%) | Accuracy (%) | PPV (%) | NPV (%) |
|---|
| ALT | 0.764a | 27.5 U/l | 72.300 | 76.600 | 74.468 | 75.556 | 73.469 |
| AST | 0.752a | 21.5 U/l | 70.200 | 72.300 | 71.277 | 71.739 | 70.833 |
| GGT | 0.809a | 29.5 U/l | 74.500 | 76.600 | 75.532 | 76.087 | 75.000 |
| TB | 0.676a | 10.70 µmol/l | 63.800 | 66.000 | 64.894 | 64.583 | 65.217 |
|
| B,
Female |
|
|
Parameters | AUC | Cut-off
value | Sensitivity
(%) | Specificity
(%) | Accuracy
(%) | PPV (%) | NPV (%) |
|
| ALT | 0.698a | 15.5 U/l | 60.400 | 75.000 | 67.708 | 70.732 | 65.455 |
| AST | 0.742a | 17.5 U/l | 64.600 | 68.700 | 66.667 | 67.391 | 66.000 |
| GGT | 0.742a | 18.0 U/l | 68.800 | 77.100 | 72.917 | 75.000 | 71.154 |
| TB | 0.759a | 7.45 µmol/l | 81.300 | 75.000 | 78.125 | 80.000 | 76.471 |
Risks of IM in different hepatic
function status
Binary logistic regression models were utilized to
calculate the risks of IM in the males and females (Table V). For transferases, crude OR
calculations were performed using the lowest quartiles as
references. High transferase levels significantly increased IM
risk, particularly in males. Following adjustments using age and
BMI as covariates, males with high levels of transferases exhibited
a much greater susceptibility to IM. Crude ORs in ALT quartile 4
were 21.667 and 10.111 for males and females, respectively; however
adjusted ORs were 38.054 and 9.882, respectively (all P<0.01).
For TB, crude OR calculations were performed using the highest
quartile as a reference. Lower TB levels were deemed to
significantly increase the risk of IM in males and females.
Adjusted OR calculations used age and BMI as covariates. Notably,
females exhibited a higher risk of developing IM when they
exhibited low serum TB. Crude ORs in quartile 1 were 8.229 and
8.257 for males and females, respectively; however, adjusted ORs
were 8.883 and 10.048, respectively (all P<0.01).
 | Table V.IM risk according to liver parameter
quartiles in the two sexes. |
Table V.
IM risk according to liver parameter
quartiles in the two sexes.
|
| Males | Females |
|---|
|
|
|
|
|---|
|
| Parameter
values | Crude OR
(CI)c | Adjusted OR
(CI)d | Parameter
values | Crude OR
(CI)c | Adjusted OR
(CI)d |
|---|
| ALT (U/l) |
|
|
|
|
|
|
| ALT
Quartile 1 | ALT≤15.00
(reference) |
|
| ALT≤11.00
(reference) |
|
|
| ALT
Quartile 2 |
15.00<ALT≤25.00 | 1.238
(0.310–4.947) | 1.279
(0.306–5.341) |
11.00<ALT≤14.00 | 0.674
(0.196–2.320) | 0.679
(0.196–2.351) |
| ALT
Quartile 3 |
25.00<ALT≤63.00 | 4.875
(1.230–19.319)a | 7.149
(1.635–31.265)b |
14.00<ALT≤27.50 | 0.935
(0.299–2.920) | 0.933
(0.281–3.103) |
| ALT
Quartile 4 | ALT>63.00 | 21.667
(4.154–113.020)b | 38.054
(6.241–232.038)b | ALT>27.50 | 10.111
(2.306–44.348)b | 9.882
(2.076–47.038)b |
| AST (U/l) |
|
|
|
|
|
|
| AST
Quartile 1 | AST≤17.00
(reference) |
|
| AST≤14.00
(reference) |
|
|
| AST
Quartile 2 |
17.00<AST≤21.00 | 1.813
(0.487–6.758) | 2.517
(0.639–9.909) |
14.00<AST≤17.00 | 1.486
(0.386–5.722) | 1.462
(0.378–5.663) |
| AST
Quartile 3 |
21.00<AST≤38.25 | 4.636
(1.309–16.423)a | 6.948
(1.783–27.072)b | 17.00<
AST≤26.00 | 1.878
(0.536–6.581) | 1.924
(0.545–6.793) |
| AST
Quartile 4 | AST>38.25 | 16.150
(3.718–70.142)b | 26.313
(5.384–128.603)b | AST>26.00 | 19.067
(3.899–93.227)b | 20.642
(3.936–108.262)b |
| GGT (U/l) |
|
|
|
|
|
|
| GGT
Quartile 1 | GGT≤18.00
(reference) |
|
| GGT≤10.00
(reference) |
|
|
| GGT
Quartile 2 |
18.00<GGT≤28.00 | 1.684
(0.427–6.642) | 3.074
(0.694–13.610) |
10.00<GGT≤16.00 | 0.943
(0.253–3.509) | 0.977
(0.260–3.668) |
| GGT
Quartile 3 |
28.00<GGT≤63.25 | 6.667
(1.690–26.298)b | 20.922
(3.910–111.945)b |
16.00<GGT≤34.75 | 3.000
(0.807–11.147) | 3.030
(0.797–11.513) |
| GGT
Quartile 4 | GGT>63.25 | 26.667
(5.199–136.777)b | 79.281
(11.149–563.762)b | GGT>34.75 | 8.360
(1.971–35.461)b | 9.093
(1.927–42.903)b |
| TB (µmol/l) |
|
|
|
|
|
|
| TB
Quartile 1 | TB≤8.250 | 8.229
(2.175–31.132)b | 8.883
(2.287–34.502)b | TB≤5.700 | 8.257
(2.184–31.222)b | 10.048
(2.482–40.682)b |
| TB
Quartile 2 |
8.250<TB≤10.550 | 5.040
(1.400–18.140)a | 5.220
(1.402–19.436)a |
5.700<TB≤7.600 | 9.229
(2.463–34.583)b | 10.457
(2.633–41.535)b |
| TB
Quartile 3 |
10.550<TB≤15.575 | 3.600
(1.007–12.865)a | 3.839
(1.041–14.158)a |
7.600<TB≤11.650 | 0.578
(0.155–2.151) | 0.586
(0.153–2.249) |
| TB
Quartile 4 | TB>15.575
(reference) |
|
| TB>11.650
(reference) |
|
|
Discussion
Worldwide, >95% of adults are infected with EBV
and in industrialized countries, it is estimated that ~50% of the
population experiences primary EBV infection <5 years old
(1). Most people infected with EBV
are either asymptomatic or exhibit mild symptoms; however, some
will develop IM. Mild to moderate elevations of liver enzymes occur
in 80–90% of patients with IM, however jaundice only occurs in 5%
(1–3,13).
Furthermore, hepatic failure in patients with IM has been reported
only occasionally (16–18). The three most clinically relevant
transferases are ALT, AST and GGT, which are the primary indices of
liver function (19). Transaminases
transfer amino and keto groups between amino acids and keto acids
(19). ALT catalyzes this
interconversion between L-alanine and α-ketoglutarate on one side
and pyruvate and L-glutamate on the other. AST catalyzes this
interconversion between aspartate and α-ketoglutarate on one side
and oxaloacetate and glutamate on the other (19). GGT belongs to the subclass of
amino-acyltransferases, which transfer acyl groups between amino
acids and peptides (19). GGT
catalyzes the transfer from a 5-L-glutamyl-peptide and an amino
acid on one side to a peptide and a 5-L-glutamyl amino acid on the
other (19). Analysis of liver
biopsies taken from patients with IM identified marked periportal
lymphocytic infiltration, marked Kupffer cell activity and
considerable acidophilic degeneration of individual liver cells
(20), which may explain why
transferase levels are increased in patients with IM. By contrast,
patients with infectious hepatitis exhibit necrosis and
inflammatory exudates (21). The
results of the current study indicate that ALT, AST and GGT levels
are significantly increased in males and females with IM compared
with controls, indicating that transferase levels may be used to
diagnose IM or determine IM risk. High transferase levels
significantly increased the risk of IM, particularly in males.
Bilrubin is a by-product of heme degradation
(22). The enzyme heme-oxygenase
catalyzes heme and results in the formation of three products:
Biliverdin, ferrous iron and carbon monoxide. Biliverdin is reduced
to bilirubin by biliverdin reductase, bilirubin then binds to
albumin and circulates in the blood. Bilirubin is then neutralized
following its encounter with hepatocytes (22). The metabolic pathway that converts
biliverdin, a soluble and nontoxic heme product, into bilirubin,
which is insoluble and potentially toxic, has been evolutionarily
conserved (22). It has therefore
been hypothesized that bilirubin may serve an important
physiological role. Previous studies have demonstrated that,
although bilirubin is cytotoxic at high concentrations, at
physiological concentrations bilirubin is able to scavenge free
radicals and has powerful immunosuppressive effects (23–25).
Indeed, the antioxidant effect of bilirubin is ~20 times more
potent than that of Vitamin E, a known scavenger of free radicals
(26).
Low serum bilirubin was identified as a strong,
independent risk factor for coronary disease in 1994 (27) and the inverse association between
bilirubin levels and coronary artery disease has been confirmed
(28,29). Targeting heme-oxygenase and
regulating biliverdin reductase may be novel methods of treating
coronary artery disease and are currently under investigation
(28,29). Niacin induces heme-oxygenase, which
may partially explain its vascular protective properties (30). Bilirubin affects the immune system at
physiological concentrations (23);
for example, bilirubin inhibits the complement cascade by
interrupting the binding of the C1 complex to the complement
antibody (31). Bilirubin may also
inhibit the cell-surface expression of major histocompatibility
complex II molecules on antigen-presenting cells (32). Furthermore, following entry into a
target cell, bilirubin may engage in the widespread inhibition of
protein kinases (33). In various
inflammatory and immunological disorders, including multiple
sclerosis (34), Crohn's disease
(35) and systemic lupus
erythematosus (36), bilirubin may
be depleted following completion of its functions.
One of the objectives of the current study was to
investigate the association between IM and bilirubin. It has been
demonstrated that oxidative stress serves a crucial role in the
pathogenesis of various infections, including the hepatitis virus
(37), respiratory viruses (38), human immunodeficiency virus (39), influenza virus (40), Staphylococcus aureus (41), Helicobacter pylori (42) and mycoplasma (43). It is hypothesized that an infection
due to EBV may also cause oxidative stress, leading to the marked
depletion of antioxidants, such as bilirubin. This may be the
underlying mechanism explaining the decrease in bilirubin observed
in patients with IM in the current study. Furthermore, the present
study indicates that females with low bilirubin levels are more
susceptible to developing IM than males with low bilirubin levels.
Males generally exhibit much higher serum bilirubin than females
(44) and this was confirmed in the
current study. Higher bilirubin levels may confer more antioxidant
protection in males, therefore females may be more susceptible to
the oxidative stress caused by bilirubin depletion. Consequently,
decreased bilirubin may be more predictive of IM infection in
females than males.
In the present study, there were differences between
males and females among indices other than liver function
parameters. Cr and BUN levels, as well as WBC levels, were
significantly higher in males than in females. Previous studies
have demonstrated that Cr levels are equal in children; however
during adolescence, Cr levels increase more in males than females
(45,46). In adulthood, females have lower serum
Cr values than males with similar renal function (47). Cr and BUN induce the same effects in
males and females (48–53). The results of studies investigating
WBC levels are inconsistent; certain studies indicate that WBC
levels are higher in males (54,55),
whereas others indicate that WBC levels are higher in females
(56,57). However, major influential factors
include age and sex hormone levels; indeed, it has been suggested
that WBC levels may change in females at different stages of the
menstrual cycle (54). In addition,
liver function parameters are associated with age, body fat, renal
function and blood cells (48–53) and
correlations between these key variables were identified in the
current study.
There were several limitations of the current study.
Firstly, the cross-sectional design of the current investigation
meant that no causality could be determined. A prospective study
should therefore be performed in the future. Secondly, a limited
number of patients with IM and controls were evaluated and they
were only recruited from a single center. More participants should
be recruited from multiple centers in order to verify the results
of the current study. Thirdly, due to budget limitations of the
current study, measurements of serum parameters were based on a
single determination and markers of inflammation or activities of
antioxidants were not assessed. Finally, the administration of
bilirubin as a possible adjuvant therapy should be investigated in
the future to validate the results of the current study.
In conclusion, the results of the current study
identified a positive association between transferases and IM and a
negative association between TB and IM, suggesting that there is a
progressive decrease of antioxidant reserves during IM. High
transferase levels were suggestive of IM, particularly in males,
whereas low TB was suggestive of IM, particularly in females.
Acknowledgements
The present study was supported by the National Key
Clinical Specialty Project; the Tianjin Medical University General
Hospital New Century Excellent Talent Program; the Young and
Middle-aged Innovative Talent Training Program from Tianjin
Education Committee; and the Talent Fostering Program (the 131
Project) from Tianjin Education Committee, Tianjin Human Resources
and Social Security Bureau. The present study was also supported by
China National Natural Science Foundation (grant no. 81571709), the
Key Project of Tianjin Science and Technology Committee Foundation
(grant no. 16JCZDJC34300); and the Tianjin Science and Technology
Committee Foundation (grant nos. 11ZCGYSY05700, 12ZCZDSY20400 and
13ZCZDSY20200).
Glossary
Abbreviations
Abbreviations:
|
IM
|
infectious mononucleosis
|
|
EBV
|
Epstein-Barr virus
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
GGT
|
γ-glutamyl transferase
|
|
BH
|
body height
|
|
BW
|
body weight
|
|
BMI
|
body mass index
|
|
WBC
|
white blood cell
|
|
RBC
|
red blood cell
|
|
Hg
|
hemoglobin
|
|
PLT
|
platelet
|
|
TB
|
total bilirubin
|
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
ROC
|
receiver operating characteristic
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Luzuriaga K and Sullivan JL: Infectious
mononucleosis. N Engl J Med. 362:1993–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vouloumanou EK, Rafailidis PI and Falagas
ME: Current diagnosis and management of infectious mononucleosis.
Curr Opin Hematol. 19:14–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Li J, Ren YY and Zhao H: The
levels of liver enzymes and atypical lymphocytes are higher in
youth patients with infectious mononucleosis than in preschool
children. Clin Mol Hepatol. 19:382–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Topp SK, Rosenfeldt V, Vestergaard H,
Christiansen CB and Von Linstow ML: Clinical characteristics and
laboratory findings in Danish children hospitalized with primary
Epstein-Barr virus infection. Infect Dis (Lond). 47:908–914. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohn C and Lidman BI: Hepatitis without
jaundice in infectious mononucleosis. J Clin Invest. 25:145–151.
1946. View Article : Google Scholar
|
|
6
|
Rosalki SB, Jones TG and Verney AF:
Transaminase and liver-function studies in infectious
mononucleosis. Br Med J. 1:929–932. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horwitz CA, Burke MD, Grimes P and Tombers
J: Hepatic function in mononucleosis induced by Epstein-Barr virus
and cytomegalovirus. Clin Chem. 26:243–246. 1980.PubMed/NCBI
|
|
8
|
Yang SI, Geong JH and Kim JY: Clinical
characteristics of primary Epstein Barr virus hepatitis with
elevation of alkaline phosphatase and γ-glutamyltransferase in
children. Yonsei Med J. 55:107–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uluğ M, Celen MK, Ayaz C, Geyik MF and
Hoşoğlu S: Acute hepatitis: A rare complication of Epstein-Barr
virus (EBV) infection. J Infect Dev Ctries. 4:668–673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zenda T, Itoh Y, Takayama Y, Masunaga T,
Asaka S, Oiwake H, Shinozaki K and Takeda R: Significant liver
injury with dual positive IgM antibody to Epstein-Barr virus and
cytomegalovirus as a puzzling initial manifestation of infectious
mononucleosis. Intern Med. 43:340–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baron DN, Bell JL and Dunnet WN:
Biochemical studies on hepatic involvement in infectious
mononucleosis. J Clin Pathol. 18:209–211. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans AS: Liver involvement in infectious
mononucleosis. J Clin Invest. 27:106–110. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Straus SE, Cohen JI, Tosato G and Meier J:
NIH conference. Epstein-Barr virus infections: Biology,
pathogenesis, and management. Ann Intern Med. 118:45–58. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu L, Yang J, Cui T, Xing H and Cai P:
Diagnosis of infectious mononucleosis by combined detection of
atypical lymphocytes and transaminase. J Huazhong Univ Sci
Technolog Med Sci. 26:384–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ainley NJ: A fatal case of infectious
mononucleosis with extensive zonal necrosis of the liver. Ulster
Med J. 18:219–224. 1949.PubMed/NCBI
|
|
17
|
Harries JT and Ferguson AW: Fatal
infectious mononucleosis with liver failure in two sisters. Arch
Dis Child. 43:480–485. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McMahon JM, Elliott CW and Green RC:
Infectious mononucleosis complicated by hepatic coma. Am J
Gastroenterol. 51:200–207. 1969.PubMed/NCBI
|
|
19
|
Herbinger KH, Hanus I, Felbinger TW, Weber
C, Beissner M, von Sonnenburg F, Löscher T, Bretzel G, Nothdurft
HD, Hoelscher M and Alberer M: Elevated values of clinically
relevant transferases induced by imported infectious diseases: A
controlled cross-sectional study of 14,559 diseased german
travelers returning from the tropics and subtropics. Am J Trop Med
Hyg. 95:481–487. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wadsworth RC and Keil PG: Biopsy of the
liver in infectious mononucleosis. Am J Pathol. 28:1003–1025.
1952.PubMed/NCBI
|
|
21
|
Sullivan BH Jr, Irey NS, Pileggi VJ, Crone
RI and Gibson JR: The liver in infectious mononucleosis. Am J Dig
Dis. 2:210–223. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maines MD: The heme oxygenase system: A
regulator of second messenger gases. Annu Rev Pharmacol Toxicol.
37:517–554. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jangi S, Otterbein L and Robson S: The
molecular basis for the immunomodulatory activities of unconjugated
bilirubin. Int J Biochem Cell Biol. 45:2843–2851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kapitulnik J: Bilirubin: An endogenous
product of heme degradation with both cytotoxic and cytoprotective
properties. Mol Pharmacol. 66:773–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu TW, Fung KP and Yang CC: Unconjugated
bilirubin inhibits the oxidation of human low density lipoprotein
better than Trolox. Life Sci. 54:P477–P481. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schwertner HA, Jackson WG and Tolan G:
Association of low serum concentration of bilirubin with increased
risk of coronary artery disease. Clin Chem. 40:18–23.
1994.PubMed/NCBI
|
|
28
|
Akboga MK, Canpolat U, Sahinarslan A,
Alsancak Y, Nurkoc S, Aras D, Aydogdu S and Abaci A: Association of
serum total bilirubin level with severity of coronary
atherosclerosis is linked to systemic inflammation.
Atherosclerosis. 240:110–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghem C, Sarmento-Leite RE, de Quadros AS,
Rossetto S and Gottschall CA: Serum bilirubin concentration in
patients with an established coronary artery disease. Int Heart J.
51:86–91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu BJ, Chen K, Barter PJ and Rye KA:
Niacin inhibits vascular inflammation via the induction of heme
oxygenase-1. Circulation. 125:150–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basiglio CL, Arriaga SM, Pelusa HF, Almará
AM, Roma MG and Mottino AD: Protective role of unconjugated
bilirubin on complement-mediated hepatocytolysis. Biochim Biophys
Acta. 1770:1003–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Ma J, Fan ST, Schlitt HJ and Tsui
TY: Bilirubin derived from heme degradation suppresses MHC class II
expression in endothelial cells. Biochem Biophys Res Commun.
338:890–896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hansen TW, Mathiesen SB and Walaas SI:
Bilirubin has widespread inhibitory effects on protein
phosphorylation. Pediatr Res. 39:1072–1077. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng F, Deng X, Yu Y, Chen X, Shen L,
Zhong X, Qiu W, Jiang Y, Zhang J and Hu X: Serum bilirubin
concentrations and multiple sclerosis. J Clin Neurosci.
18:1355–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liechti FD, Grandgirard D and Leib SL:
Bacterial meningitis: Insights into pathogenesis and evaluation of
new treatment options: A perspective from experimental studies.
Future Microbiol. 10:1195–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vitek L, Muchová L, Jančová E, Pešičková
S, Tegzová D, Peterová V, Pavelka K, Tesař V and Schwertner H:
Association of systemic lupus erythematosus with low serum
bilirubin levels. Scand J Rheumatol. 39:480–484. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zuwała-Jagiełło J, Warwas M and
Pazgan-Simon M: Ischemia-modified albumin (IMA) is increased in
patients with chronic hepatitis C infection and related to markers
of oxidative stress and inflammation. Acta Biochim Pol. 59:661–667.
2012.PubMed/NCBI
|
|
38
|
Komaravelli N and Casola A: Respiratory
viral infections and subversion of cellular antioxidant defenses. J
Pharmacogenomics Pharmacoproteomics. 5:pii: 10001412014.
|
|
39
|
Ngondi JL, Oben J, Forkah DM, Etame LH and
Mbanya D: The effect of different combination therapies on
oxidative stress markers in HIV infected patients in Cameroon. AIDS
Res Ther. 3:192006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Checconi P, Salzano S, Bowler L, Mullen L,
Mengozzi M, Hanschmann EM, Lillig CH, Sgarbanti R, Panella S,
Nencioni L, et al: Redox proteomics of the inflammatory secretome
identifies a common set of redoxins and other glutathionylated
proteins released in inflammation, influenza virus infection and
oxidative stress. PLoS One. 10:e01270862015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chakraborty SP, Das S, Chattopadhyay S,
Tripathy S, Dash SK, Pramanik P and Roy S: Staphylococcus aureus
infection induced redox signaling and DNA fragmentation in
T-lymphocytes: Possible ameliorative role of nanoconjugated
vancomycin. Toxicol Mech Methods. 22:193–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aslan M, Nazligul Y, Horoz M, Bolukbas C,
Bolukbas FF, Aksoy N, Celik H and Erel O: Serum prolidase activity
and oxidative status in Helicobacter pylori infection. Clin
Biochem. 40:37–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kariya C, Chu HW, Huang J, Leitner H,
Martin RJ and Day BJ: Mycoplasma pneumoniae infection and
environmental tobacco smoke inhibit lung glutathione adaptive
responses and increase oxidative stress. Infect Immun.
76:4455–4462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rosenthal P, Pincus M and Fink D: Sex- and
age-related differences in bilirubin concentrations in serum. Clin
Chem. 30:1380–1382. 1984.PubMed/NCBI
|
|
45
|
Pottel H, Vrydags N, Mahieu B,
Vandewynckele E, Croes K and Martens F: Establishing age/sex
related serum creatinine reference intervals from hospital
laboratory data based on different statistical methods. Clin Chim
Acta. 396:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Uemura O, Honda M, Matsuyama T, Ishikura
K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, et al:
Age, gender, and body length effects on reference serum creatinine
levels determined by an enzymatic method in Japanese children: A
multicenter study. Clin Exp Nephrol. 15:694–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
O'Leary JG, Wong F, Reddy KR, Garcia-Tsao
G, Kamath PS, Biggins SW, Fallon MB, Subramanian RM, Maliakkal B,
Thacker L and Bajaj JS: Gender-specific differences in baseline,
peak, and delta serum creatinine: The NACSELD experience. Dig Dis
Sci. 62:768–776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meng Z, Liu M, Zhang Q, Liu L, Song K, Tan
J, Jia Q, Zhang G, Wang R, He Y, et al: Gender and age impacts on
the association between thyroid function and metabolic syndrome in
Chinese. Medicine (Baltimore). 94:e21932015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Meng Z, Liu M, Zhang Q, Liu L, Song K, Tan
J, Jia Q, Zhang G, Wang R, He Y, et al: Gender and age impact on
the association between thyroid-stimulating hormone and serum
lipids. Medicine (Baltimore). 94:e21862015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ren X, Meng Z, Liu M, Zhu M, He Q, Zhang
Q, Liu L, Song K, Jia Q, Jia Q, et al: No associations exist
between mean platelet volume or platelet distribution width and
thyroid function in Chinese. Medicine (Baltimore). 95:e45732016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou P, Meng Z, Liu M, Ren X, Zhu M, He Q,
Zhang Q, Liu L, Song K, Jia Q, et al: The associations between
leukocyte, erythrocyte or platelet, and metabolic syndrome in
different genders of Chinese. Medicine (Baltimore). 95:e51892016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang S, Zhang J, Zhu L, Song L, Meng Z,
Jia Q, Li X, Liu N, Hu T, Zhou P, et al: Association between liver
function and metabolic syndrome in Chinese men and women. Sci Rep.
7:448442017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Meng Z, Zhang Q, Liu L, Song K,
Tan J, Li X, Jia Q, Zhang G and He Y: Gender impact on the
correlations between subclinical thyroid dysfunction and
hyperuricemia in Chinese. Clin Rheumatol. 35:143–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nowak J, Borkowska B and Pawlowski B:
Leukocyte changes across menstruation, ovulation, and mid-luteal
phase and association with sex hormone variation. Am J Hum Biol.
28:721–728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pérez-de-Heredia F, Gómez-Martínez S, Díaz
LE, Veses AM, Nova E, Wärnberg J, Huybrechts I, Vyncke K,
Androutsos O, Ferrari M, et al: Influence of sex, age, pubertal
maturation and body mass index on circulating white blood cell
counts in healthy European adolescents-the HELENA study. Eur J
Pediatr. 174:999–1014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mandala WL, Gondwe EN, MacLennan JM,
Molyneux ME and MacLennan CA: Age- and sex-related changes in
hematological parameters in healthy Malawians. J Blood Med.
8:123–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pratley RE, Wilson C and Bogardus C:
Relation of the white blood cell count to obesity and insulin
resistance: Effect of race and gender. Obes Res. 3:563–571. 1995.
View Article : Google Scholar : PubMed/NCBI
|