Introduction
Gadoxetic acid (Gd-EOB-DTPA) is a
hepatocyte-specific magnetic resonance (MR) contrast agent
(1,2), which has being used increasingly in
recent years for imaging diagnosis of hepatocellular carcinoma
(HCC), liver metastasis, and other diseases (3–7). Its
advantage lies on the addition of offering hepatobiliary phase
(HBP) imaging (8). After intravenous
injection, Gd-EOB-DTPA is gradually taken up by functional
hepatocyte and eventually excreted via biliary and renal system
(9,10). Owing to this property, Gd-EOB-DTPA
has been thought to be superior to the conventional extracellular
Gd-based contrast agents, especially in the early detection of
focal liver lesions (FLLs) (11–13).
However, despite the advantages of HBP imaging in
Gd-EOB-DTPA-enhanced MR, arterial phase enhancement remains
critical for the detection and characterization of FLLs (14). In previous study, it has been
reported that transient severe motion (TSM) often occurred after
administration of Gd-EOB-DTPA (15).
Our previous study showed that TSM is more often observed in
patients receiving Gd-EOB-DTPA than those receiving Gd-DTPA
(16). TSM has been described by
patients as a temporary, self-limiting phenomenon that lasts about
10–20 sec. The mechanism of such phenomena still remains unclear,
and some authors have attributed its severity to
Gd-EOB-DTPA-related dose-toxicity (17,18). In
order to minimize the issue related TSM, a body-weight calibrated
dose and a slower injection rate of contrast has been adopted in
previous studies (14,17). Since TSM may have a serious effect on
image quality during the arterial phase, and affect diagnostic
accuracy of liver disease, it is crucial to provide optimal
arterial phase imaging.
Recently, a newly developed technique introduced by
Michaely et al (19), has
been shown to significantly increase the temporal resolution during
multiphasic imaging. This technique has demonstrated that within a
single breath-hold, in combination of volume interpolated
breath-hold examination (VIBE) with controlled aliasing in parallel
imaging results in higher acceleration (CAIPIRINHA), view-sharing
time-resolved imaging with interleaved stochastic trajectories
(TWIST), and Dixon fat suppression (CAIPIRINHA-Dixon-TWIST-VIBE,
CDT-VIBE), is capable of obtaining the high spatial and temporal
resolution of multiple hepatic arterial subphases (19,20). To
the best of our knowledge, there is no published research utilizing
such a technique in order to investigate TSM on arterial phase
imaging.
This study, therefore, aims to investigate the
comparison between patient receiving single phase of VIBE
(single-VIBE) technique and those receiving five subphases of
CDT-VIBE (5-CDT-VIBE) technique during arterial phase with
Gd-EOB-DTPA, and whether 5-CDT-VIBE technique can provide adequate
diagnostic information in patients with TSM.
Materials and methods
Patients
This retrospective study was approved by the ethics
committee of the Second Xiangya Hospital of Central South
University. All clinical investigations were conducted according to
the principles of the Declaration of Helsinki. Written informed
consent was obtained from all patients before inclusion.
From January 2013 to March 2016, a total of
consecutive 463 patients (310 males and 153 females) who were the
first time suggested undergoing Gd-EOB-DTPA-enhanced MR of the
liver with known or unknown liver lesions were included in the
study. Eighty-five patients who were referred for the evaluation of
the liver with Gd-EOB-DTPA-enhanced MRI of the liver did not
undergo the requested study because they met the exclusion
criteria: known pulmonary disease (which may influence the breath
holding) such as chronic obstructive pulmonary disease (COPD),
pleural effusion, and MR examination contraindication such as known
renal inadequacy (glomerular filtration rate, GFR <30 ml/min),
existence of claustrophobia, post-implantation of pacemaker, breath
holding time less than 20 sec, and related adverse reaction of
gadolinium chelates. Finally, the total study population comprised
378 patients (239 males, 139 females, ranged in age from 26–85
years with a mean age of 57.3±18.2 years), which was consisted of
HCC (n=170), liver metastasis (n=95), focal nodular hyperplasia
(FNH, n=45), cholangiocellular carcinoma (CCC, n=32), hemangioma
(n=17), hepatocellular adenoma, (HCA, n=13), regenerative nodules
in liver cirrhosis (RN, n=6). The final diagnosis of the liver
lesions was based on either histopathology (resection, n=112;
biopsy, n=46), the 2014 version of Liver Imaging Reporting and Data
System (LI-RADS) criteria (n=146), or existence of primary
extrahepatic tumor (n=74). The potential risk factors that might
have an influence on the respiratory motion artifact were recorded,
which included age, gender, body mass index (BMI), Child-Pugh
grading, and the relative volume of ascites. The relative volume of
ascites was scored according to the imaging qualitative scale of
1–3, 1 was classified as absent of ascites, 2 was classified as
small amount of ascites, and 3 was classified as moderate to large
amount of ascites. The detailed demographic of all patients is
listed in Table I.
 | Table I.Demographic data for all patients. |
Table I.
Demographic data for all patients.
| Factor | All | Single-VIBE |
5-CDT-VIBE |
|---|
| Age, years | 57.3±18.2 | 53.1±14.2 | 59.8±19.4 |
| Sex |
|
|
|
| Male | 239 | 135 | 104 |
|
Female | 139 | 81 | 58 |
| Etiology |
|
|
|
| HCC | 170 | 98 | 72 |
| Liver
metastasis | 95 | 53 | 42 |
| FNH | 45 | 25 | 20 |
| CCC | 32 | 17 | 15 |
|
Hemangioma | 17 | 11 | 6 |
| HCA | 13 | 8 | 5 |
| RN | 6 | 4 | 2 |
MR imaging technique
MR imaging was performed under a clinical 3.0 Tesla
superconducting MR system (Magnetom Skyra; Siemens Healthcare,
Germany) with the 18-channel body matrix coil and the inbuilt
24-channel spine matrix coil. The comprehensive MRI protocol
including T1-weighted fat saturation gradient recalled echo
sequence as well as T2-weighted half fourier acquisition
single-shot turbo spin-echo sequence were obtained prior to
Gd-EOB-DTPA administration. Among 378 patients, either 5-CDT-VIBE
technique (n=162) or single-VIBE technique (n=216) was performed on
the unenhanced phase, arterial phase, portovenous phase (90 sec),
the delayed phase (180 sec), and the HBP (20 min). In order to
catch the same contrast-enhanced phase in every patient, a MR
automated injector pump was used to administer Gd-EOB-DTPA through
an 18-gauge cubital intravenous access at a dose of 0.1 ml/kg body
weight and an injection rate of 1 ml/s. The 5-CDT-VIBE of initial
arterial phase started at a standard timing of 18 sec after the
start of contrast agent injection with a temporal resolution of 2.6
sec. The detailed MR parameters are listed in Table II.
 | Table II.MR sequence parameters. |
Table II.
MR sequence parameters.
| Parameters | Single-VIBE | 5-CDT-VIBE | T2 HASTE |
|---|
| TR/TE,
milliseconds | 2.9/1.4 | 3.8/1.2 | 2000/80 |
| Subphases | 1 | 5 | 1 |
| Sequence type | VIBE | TWIST-VIBE | HASTE |
| Voxel size,
mm3 | 1.3×1.3×3.0 | 1.3×1.3×3.0 | 1.3×1.3×3.0 |
| FOV, mm | 380 | 380 | 380 |
| Slice number | 72 | 72 | 72 |
| Flip angle,
degree | 9 | 9 | 180 |
| Respiratory
control | Breath hold | Breath hold | Triggered |
| Fat
suppression | Spectral
saturation | Dixon | Spectral
saturation |
| Temporal
resolution, s | – | 2.6 | – |
| TWIST size of
k-center, % | – | 20 | – |
| TWIST size of
k-periphery, % | – | 25 | – |
| Breath holding
time, seconds | 9–22 | 20 | 10–15 |
Imaging analysis
Two radiologists with 20-year and 12-year liver
imaging experience independently and randomly reviewed all images.
Both radiologists were blinded to the clinical data and each
other's results. Respiratory motion artifact was evaluated using a
scoring system, as previously described, with high reproducibility
(15). The degree of respiratory
motion artifact was scored from 1 to 5, 1 was defined as no motion
artifact, 2 was defined as minimal motion artifact without effect
on diagnostic quality, 3 was defined as moderate motion artifact,
with some, but not severe effect on diagnostic quality, 4 was
defined as severe motion artifact, but images still interpretable,
and 5 was defined as extensive motion artifact and images
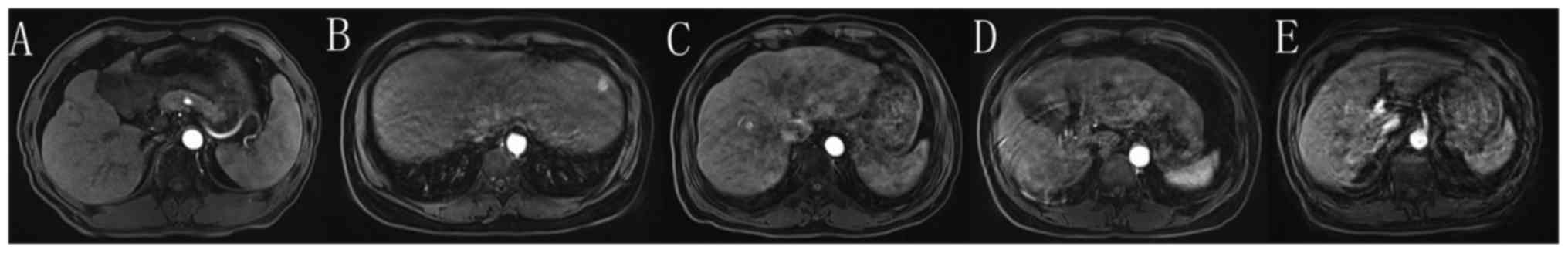
non-diagnostic quality. The scoring system and the typical imaging
of motion artifact are presented in Fig.
1. TSM was defined as motion score 2 or less on unenhanced
phase (or every one of motion score on 5 unenhanced subphases),
meanwhile motion score 4 or more on arterial phase (or any one of
motion score on 5 arterial subphases). The average motion score
between two radiologists was calculated in each subphase.
Statistical analysis
The intraclass correlation coefficients (ICCs) were
calculated to evaluate the inter-observer agreement between two
reviewers. Agreement was classified as poor (ICC, 0–0.40), fair to
good (ICC, 0.40–0.75), or excellent (ICC, >0.75). Differences in
age and BMI between single-VIBE group and 5-CDT-VIBE group were
compared using Mann-Whitney U Test. Results of Child-Pugh grading,
gender, and volume of ascites grading between single-VIBE group and
5-CDT-VIBE group were assessed using χ2 test or Fisher
exact test (if appropriate). Comparison of the above parameters was
also performed in the overall study population, patients with TSM,
and patients without TSM. Mean score between unenhanced phase and
arterial phase was compared using Wilcoxon Rank Sum Test. All
statistical analysis was performed under SPSS 19.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Study population
The patients' BMI and age between single-VIBE group
and 5-CDT-VIBE group were compared, but were not found to be
statistically significant (20.1±2.5 vs. 21.9±2.1, P=0.14; 53.1±14.2
vs. 59.8±19.4, P=0.073; respectively). However, in the overall
study population, single-VIBE group, as well as 5-CDT-VIBE group,
patients with TSM showed significantly higher BMI and volume of
ascites grading than those of patients without TSM, which might
indicate that TSM more frequently occurs in patients with a higher
BMI or ascites volume. The characteristics of patients were listed
in Table III.
 | Table III.Patient characteristics and risk
factors in patients with or without TSM. |
Table III.
Patient characteristics and risk
factors in patients with or without TSM.
|
| Single-VIBE | 5-CDT-VIBE | Overall |
|---|
|
|
|
|
|
|---|
| Characteristic | TSM | Non-TSM | P-value | TSM | Non-TSM | P-value | TSM | Non-TSM | P-value |
|---|
| BMI | 25.1±2.1 | 19.7±2.6 | 0.03 | 27.1±2.5 | 20.4±1.8 | 0.03 | 26.7±3.4 | 19.8±4.5 | 0.02 |
| Age, years | 52.7±13.7 | 56.3±16.4 | 0.47 | 60.1±17.7 | 57.6±19.6 | 0.39 | 58.5±17.1 | 56.4±19.5 | 0.45 |
| Child grading,
% | 25 | 191 | 0.87 | 22 | 140 | 0.35 | 47 | 331 | 0.84 |
| A | 13 (52) | 108 (56.5) | – | 8
(36.4) | 74 (52.9) | – | 21 (44.7) | 162
(48.9) | – |
| B | 9
(36) | 59
(30.9) | – | 10 (45.4) | 48 (34.3) | – | 19 (40.4) | 127
(38.4) | – |
| C | 3
(12) | 24
(12.6) | – | 4
(18.2) | 18 (12.8) | – | 7
(14.9) | 42
(12.7) | – |
| Ascites grading,
%a | 25 | 191 | <0.01 | 22 | 140 | <0.01 | 47 | 331 | <0.01 |
| 1 | 8
(32) | 143 (74.9) | – | 7
(31.8) | 97 (69.3) | – | 15 (31.9) | 240
(72.5) | – |
| 2 | 7
(28) | 29
(15.2) | – | 5
(22.7) | 29 (20.7) | – | 12 (25.5) | 58
(17.5) | – |
| 3 | 10 (40) | 19 (9.9) | – | 10 (45.5) | 14 (10) | – | 20 (42.6) | 33 (10) | – |
| Sex, % | 25 | 191 | 0.78 | 22 | 140 | 0.68 | 47 | 331 | 0.93 |
|
Male | 15 (60) | 120 (62.8) | – | 15 (68.2) | 89 (63.6) | – | 30 (63.8) | 209
(63.1) | – |
|
Female | 10 (40) | 71
(37.2) | – | 7
(31.8) | 51 (36.4) | – | 17 (36.2) | 122
(36.9) | – |
Consistency analysis
Reviewers' agreement of motion score calculation was
evaluated by ICC. There was fair to good to excellent
reproducibility of mean motion score in each phase between two
reviewers, with the ICCs ranging from 0.68 (the third arterial
phase) to 0.93 (the fifth unenhanced phase), indicating an
acceptable inter-observer agreement. The detailed ICCs between two
reviewers were listed in Table
IV.
 | Table IV.ICCs between two reviewers. |
Table IV.
ICCs between two reviewers.
|
| Unenhanced
subphases | Arterial
subphases |
|---|
|
|
|
|
|---|
| Technique | First | Second | Third | Fourth | Fifth | First | Second | Third | Fourth | Fifth |
|---|
| 5-CDT-VIBE | 0.86 | 0.91 | 0.74 | 0.88 | 0.93 | 0.89 | 0.71 | 0.68 | 0.78 | 0.87 |
| Single-VIBE |
|
| 0.91 |
|
|
|
| 0.85 |
|
|
Comparison between two groups
In the overall study population, there were 216
patients in the single-VIBE technique group, and 162 patients in
the 5-CDT-VIBE technique group. TSM occurred in 47 patients
(47/378, 12.4%) in total, with 25 patients (25/216, 11.6%) in the
single-VIBE group, and 22 patients (22/162, 13.6%) in the
5-CDT-VIBE group. The frequency rate of TSM between the two groups
was not statistical significant (11.6 vs. 13.6%, P>0.05).
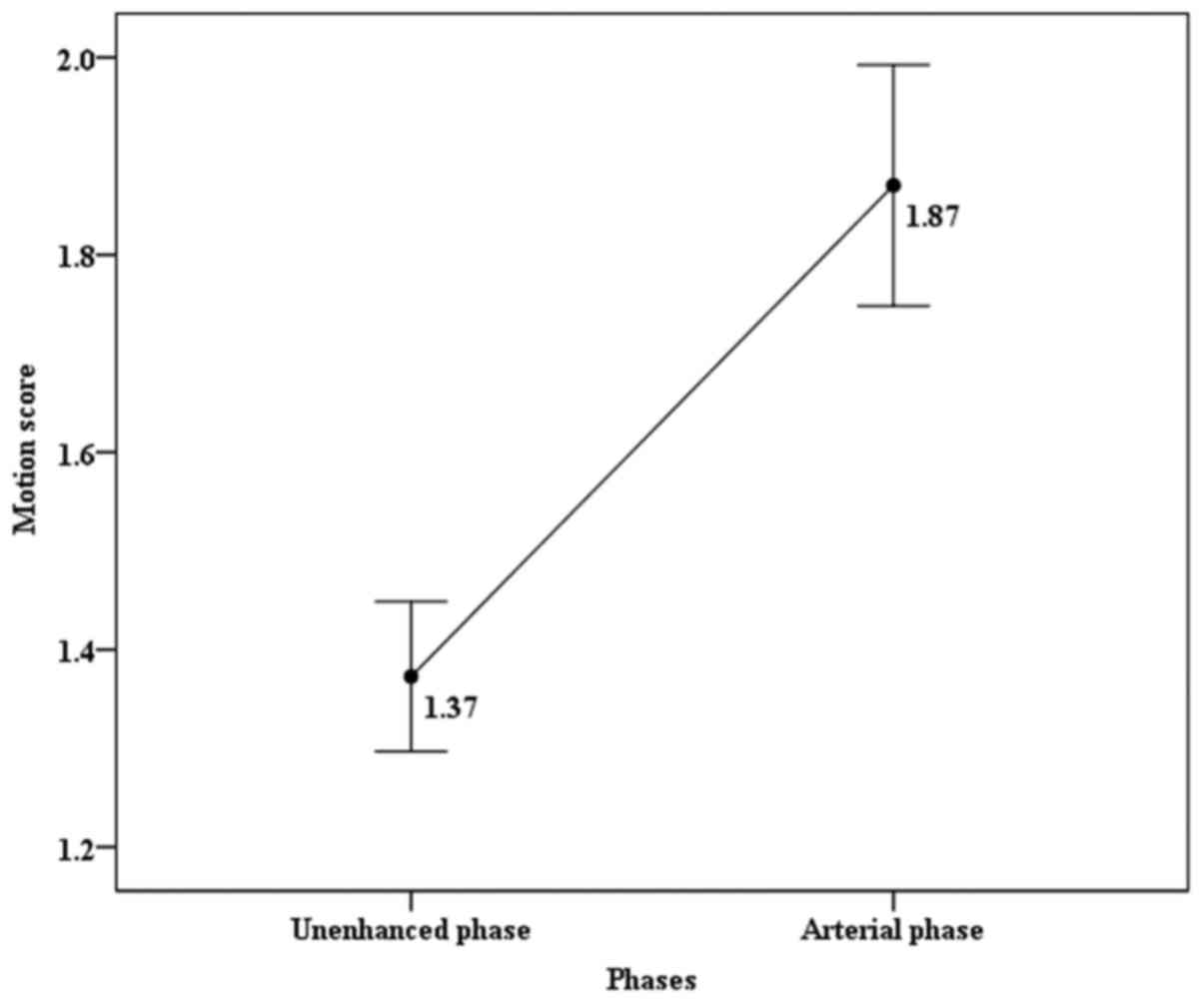
In the single-VIBE group, the motion score on
arterial phase was significantly higher than those on unenhanced
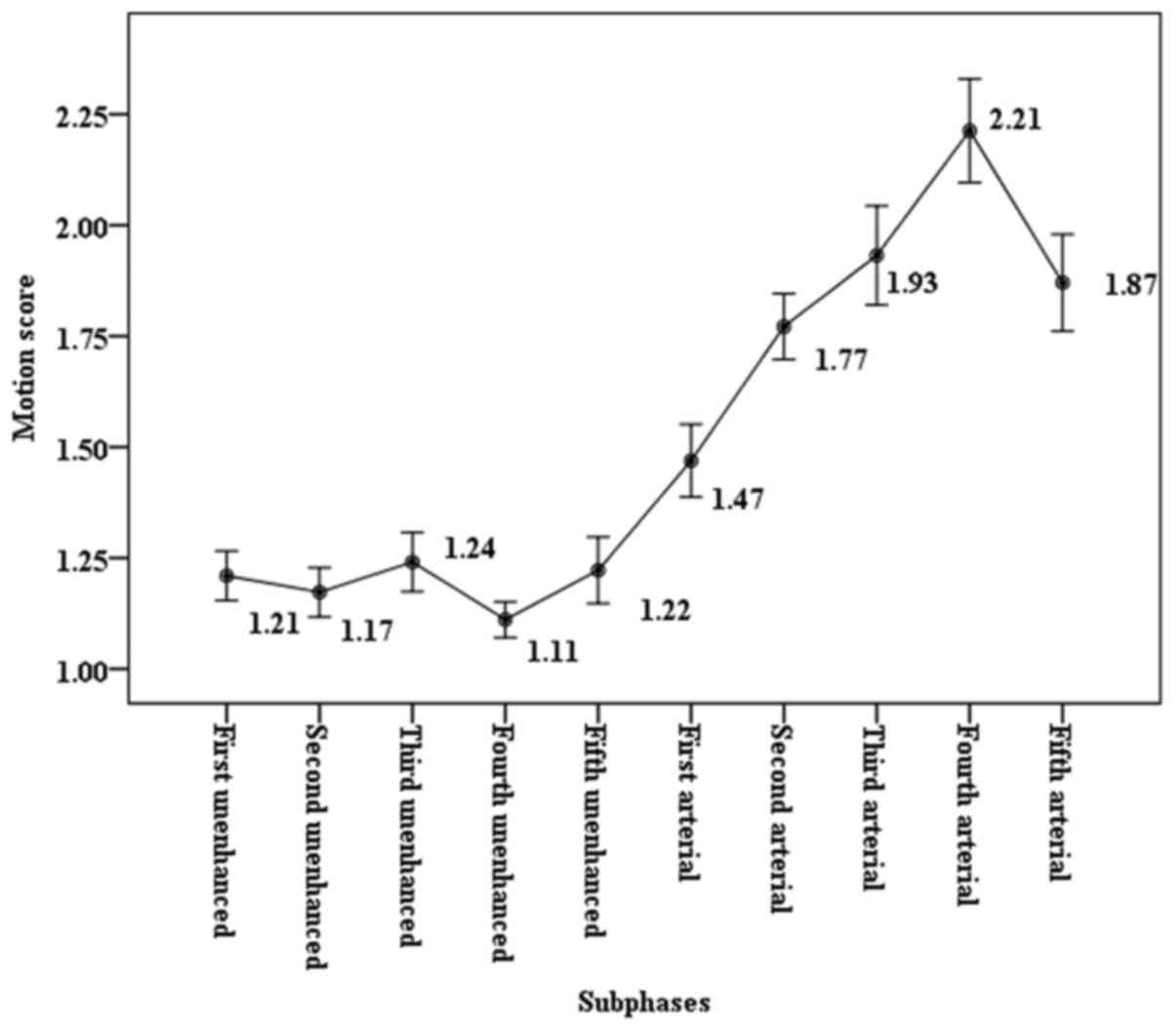
phase (1.87 vs. 1.37, P<0.05). In the 5-CDT-VIBE group, motion
scores on five arterial subphases (1.47, 1.77, 1.93, 2.21 and 1.87,
respectively corresponding to the first, second, third, fourth, and
fifth arterial phases) were significantly higher (P<0.05 for all
five comparisons) than those on five unenhanced subphases (1.21,
1.17, 1.24, 1.11 and 1.22, respectively corresponding to the first,
second, third, fourth, and fifth unenhanced phases). The motion
score among the five arterial subphases increased progressively up
to the peak at the fourth arterial phase, and then slowly decreased
until the fifth arterial phase. The change of motion score is shown
in Figs. 2 and 3. For patients with TSM in the 5-CDT-VIBE
group, the highest motion scores occurred during the fourth
arterial phase (12/22, 54.5%), the third arterial phase (7/22,
31.8%), and the fifth arterial phase (3/22, 13.7%), and 20 out of
22 (20/22, 90.9%) patients with TSM provided adequate arterial
phase diagnostic information attributed to the remaining arterial
subphases. Nevertheless, 0 of the 25 patients with TSM could
provide adequate diagnostic imaging in the single-VIBE group.
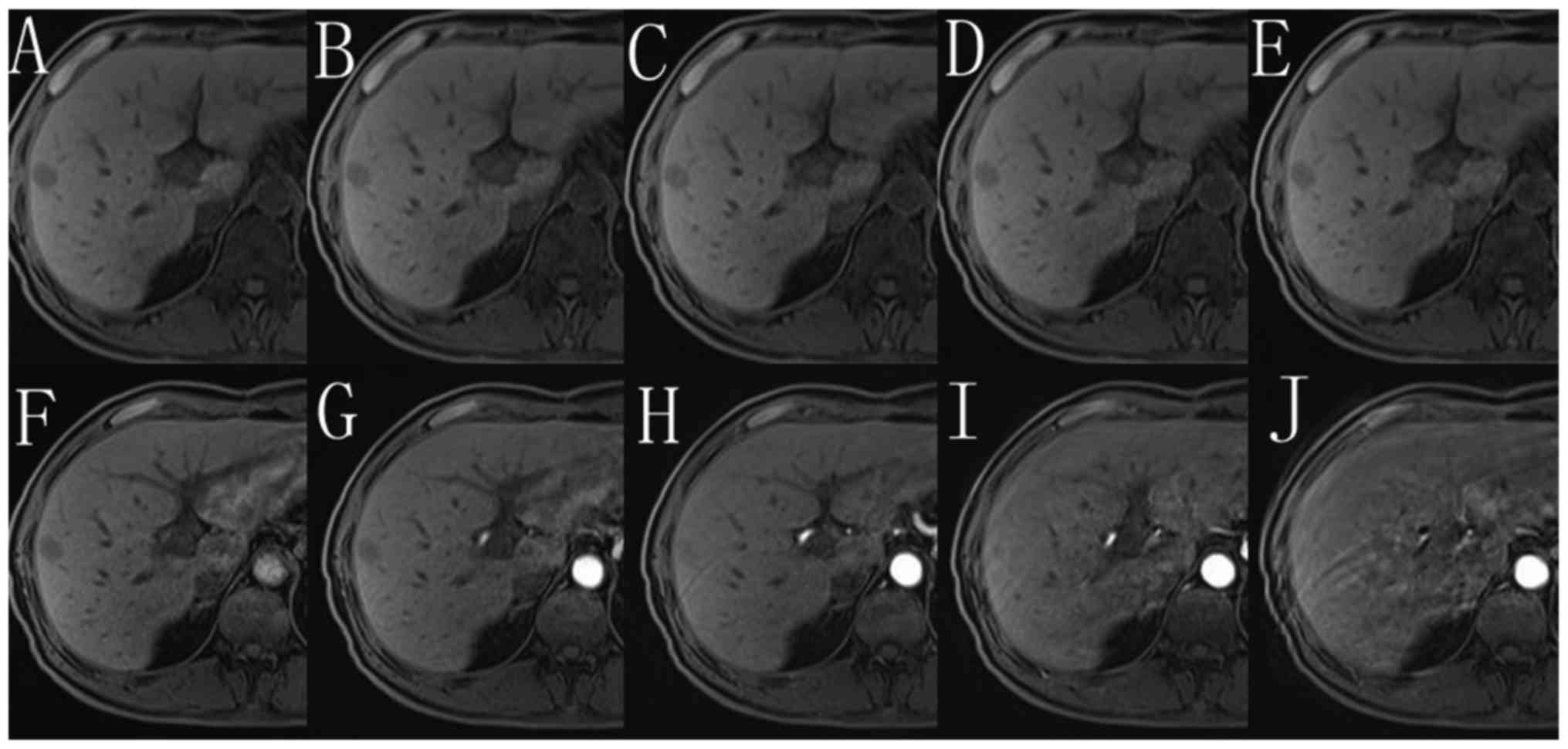
Figs. 4 and 5, respectively demonstrates typical imaging
of the single-VIBE group compared with the 5-CDT-VIBE group.
Fig. 4 shows a patient without
motion artifact on unenhanced phase, but on arterial phase, severe
motion artifact is observed, indicating the presence of TSM. In
this patient, only one arterial phase was obtained, leading to
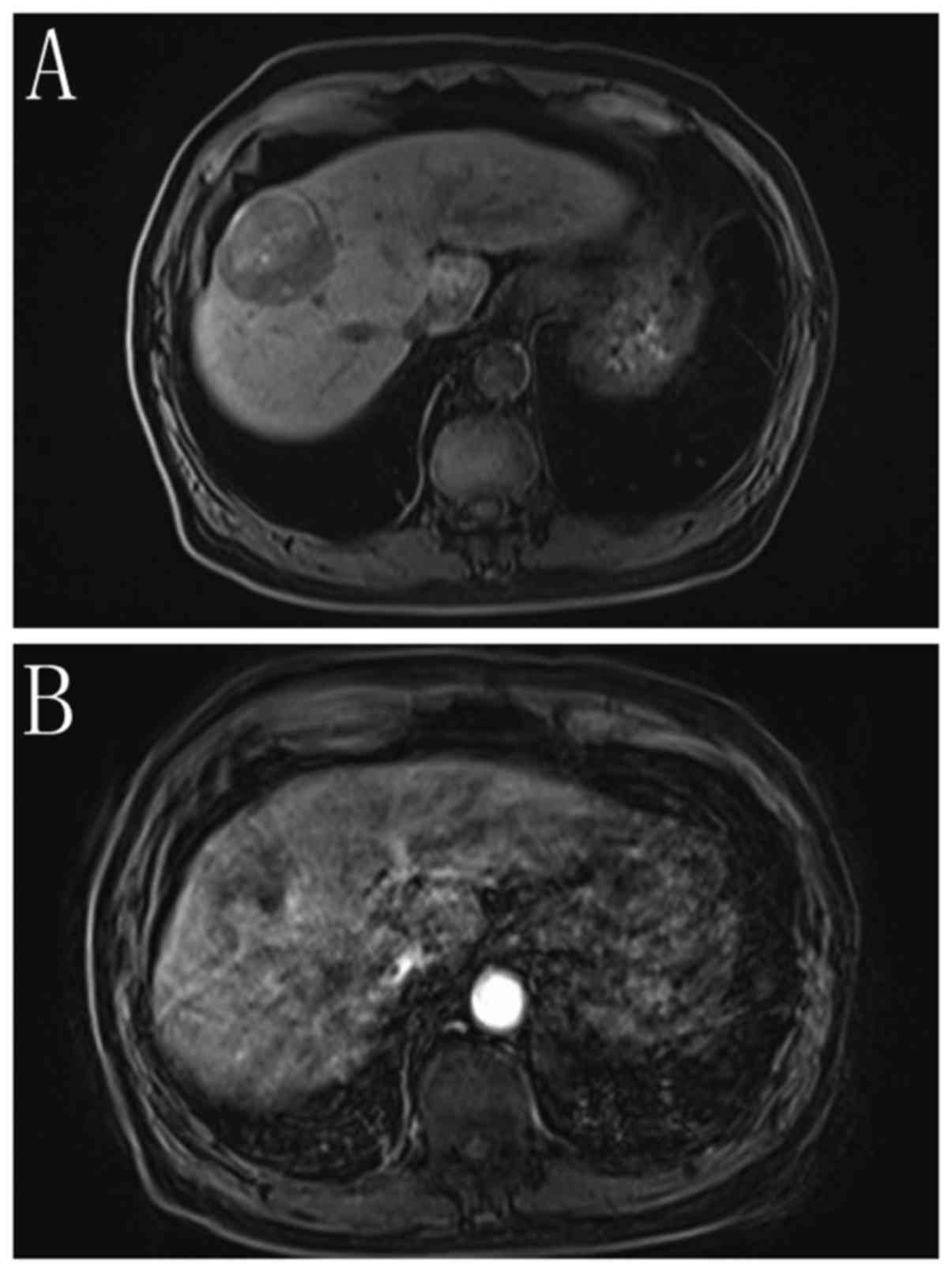
inadequate image quality for diagnosis. Fig. 5 shows a patient without motion
artifact on each of five unenhanced subphases, while among five
arterial subphases, motion artifact can be observed, most severe at
the fifth phase. Despite the severe motion artifact of the fifth
arterial subphase, the remaining subphases are sufficient to
provide adequate quality images.
Discussion
TSM on arterial phase imaging during Gd-EOB-DTPA
acquisition has been frequently described (21–23).
Since arterial phase enhancement is accepted as one of the
diagnostic criteria for HCC in LI-RADS, it is crucial to provide
optimal arterial phase imaging. In the present study, we confirm
that 5-CDT-VIBE technique can provide sufficient diagnostic
information on arterial phase for patients who display TSM during
Gd-EOB-DTPA-enhanced MR. In a single-phase technique group, 0 of 25
patients with TSM obtained adequate image quality on arterial
phase, in contrast however, in a 5-CDT-VIBE technique group, 20 out
of 22 patients with TSM could provide adequate diagnostic
information on arterial phase attributing to the other arterial
subphases, which were artifact free.
The prevalence of TSM related to Gd-EOB-DTPA
administration has been reported to range from 2 to 19% (15,17,20–25). In
the present study, prevalence of TSM is 12.4% (47/378) in the
overall study population, which shows a lower rate than the studies
demonstrated by Haradome et al (20 of 108 patients, 19%)
(24) and Davenport et al (17
of 99 patients, 17%) (15). Possible
reason for this discrepancy is the total volume of contrast agent
administered. In previous studies, 10 to 20 ml total volume of
contrast agent were utilized for examination, while in our study a
less volume was used (0.1 ml/kg body weight). Higher doses have
previously been reported to correlate with TSM (17). Likewise, several studies reveal that
the body weight of the patient is an independent risk factor for
TSM (22). We compare five patient
characteristics such as BMI, age, Child-Pugh grading, gender, and
volume of ascites gradingbetween patients with TSM and without TSM.
The results reveal that TSM more frequently occurs in patients with
elevated BMI or ascites volume grading. Because we use a body
weight-tailored dose administration strategy, in those of patients
with higher BMI or volume of ascites, they may have a higher degree
of adiposity or body weight, as a result more contrast dose
received.
Interestingly, the results of this study also
demonstrate that in 22 patients with TSM among the 5-CDT-VIBE
group, the highest motion score occurs during the fourth arterial
phase (mean motion score is 2.21, 12/22, 54.5%), the third arterial
phase (mean motion score of 1.93, 7/22, 31.8%), and the fifth
arterial phase (mean motion score of 1.87, 3/22, 13.7%). In our
study, using the 5-CDT-VIBE technique, initial arterial phase
starts at a standard timing of 18 sec following the administration
of contrast with a temporal resolution of 2.6 sec. The fourth
arterial phase starts at 25.8 to 28.4 sec. In a cohort study
conducted by Pietryga et al showed the highest motion score
occurred at the third arterial phase from the timing of 30 to 37.5
sec after initiation of contrast agent injection (25). This discrepancy is most likely due to
the difference in temporal resolution. In their study, the temporal
resolution in each arterial subphase acquisition is 7.5 sec, but in
our study, the temporal resolution is only 2.6 sec. As it has been
reported that higher temporal resolution affords more accurate
measurement of peak and dynamic changes in signal intensity
(26), our study shows an early
arrival of the highest motion score.
In Pietryga et al study, they used a triple
arterial subphase technique to reduce the effect of TSM on arterial
phase with Gd-EOB-DTPA administration (25). However, this technique had some
disadvantages, such as reduction of spatial resolution and a
relatively thicker slice thickness, which may reduce the signal to
noise ratio of each individual phase. In the present study, we use
a 5-CDT-VIBE technique to obtain 5 arterial subphases with a single
breath holding. 5-CDT-VIBE is a newly developed technique, which
can achieve robust contrast-enhanced imaging of the liver with high
temporospatial resolution (19,27).
Our study has some limitations. Firstly, this study
is retrospectively designed. We did not randomly make the decision
for which technique the patient would receive. Secondly, only the
patient with liver lesions is enrolled, other liver diseases such
as liver cirrhosis is not included in the study. Liver cirrhosis
may have an impact on patients' breath holding. Thus, selection
bias may have been presented. Thirdly, we do not compare liver
lesion detection between single-VIBE group and 5-CDT-VIBE group. A
study conducted by Kazmierczak et al reveals that 5-CDT-VIBE
technique can significantly improve the detection and differential
diagnosis of hypervascular FLLs (28). We have not assessed the utility of
5-CDT-VIBE technique in patients with or without TSM for FLLs
detection. Thirdly, as breath-holding is important in the reduction
of respiratory motion artifacts (29), we did not keep a unified
breath-holding time in two groups. The reason may be that a fixed
breath-holding time of 20 sec is used in 5-CDT-VIBE technique,
while for single-vibe technique, the breath-holding time is
unfixed. Finally, we have not evaluated the nonarterial
post-contrast image and the adequacy of arterial phase timing.
Pietryga et al (25) reviewed
the motion artifact by using an additional scoring system to
evaluate the adequacy of late hepatic arterial phase timing, which
may have higher accuracy.
In conclusion, Gd-EOB-DTPA related TSM mostly occurs
between the 25.8 to 28.4 sec (the fourth arterial phase) following
contrast administration. We conclude that 5-CDT-VIBE technique can
provide images with diminished artifact in patients with TSM.
Competing interests
The authors declare that they have no competing
interests
Glossary
Abbreviations
Abbreviations:
|
Gd-EOB-DTPA
|
gadoxetic acid
|
|
MR
|
magnetic resonance
|
|
HCC
|
hepatocellular carcinoma
|
|
HBP
|
hepatobiliary phase
|
|
FLL
|
focal liver lesion
|
|
TSM
|
transient severe motion
|
|
VIBE
|
volume interpolated breath-hold
examination
|
|
CAIPIRINHA
|
controlled aliasing in parallel
imaging results in higher acceleration
|
|
TWIST
|
time-resolved imaging with interleaved
stochastic trajectories
|
|
CDT-VIBE
|
CAIPIRINHA-Dixon-TWIST-VIBE
|
|
ICC
|
intraclass correlation coefficient
|
|
BMI
|
body mass index
|
References
|
1
|
Nishie A, Kakihara D, Asayama Y, Ushijima
Y, Takayama Y, Fujita N, Shimamoto D, Shirabe K, Hida T and Honda
H: Detectability of hepatocellular carcinoma on gadoxetic
acid-enhanced MRI at 3 T in patients with severe liver dysfunction:
Clinical impact of dual-source parallel radiofrequency excitation.
Clin Radiol. 70:254–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verloh N, Utpatel K, Haimerl M, Zeman F,
Fellner C, Fichtner-Feigl S, Teufel A, Stroszczynski C, Evert M and
Wiggermann P: Liver fibrosis and Gd-EOB-DTPA-enhanced MRI: A
histopathologic correlation. Sci Rep. 5:154082015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esterson YB, Flusberg M, Oh S, Mazzariol
F, Rozenblit AM and Chernyak V: Improved parenchymal liver
enhancement with extended delay on Gd-EOB-DTPA-enhanced MRI in
patients with parenchymal liver disease: Associated clinical and
imaging factors. Clin Radiol. 70:723–729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haimerl M, Verloh N, Fellner C, Zeman F,
Teufel A, Fichtner-Feigl S, Schreyer AG, Stroszczynski C and
Wiggermann P: MRI-based estimation of liver function:
Gd-EOB-DTPA-enhanced T1 relaxometry of 3T vs. the MELD score. Sci
Rep. 4:56212014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang J, Kim YK, Jeong WK, Choi D, Rhim H
and Lee WJ: Nonhypervascular hypointense nodules at gadoxetic
acid-enhanced MR imaging in chronic liver disease:
Diffusion-weighted imaging for characterization. Radiology.
276:137–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tse JR, Naini BV, Lu DS and Raman SS:
Qualitative and quantitative gadoxetic acid-enhanced MR imaging
helps subtype hepatocellular adenomas. Radiology. 279:118–127.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao YD, Paudel R, Liu H, Zhang B, Ma C
and Zhou SK: Gadolinium ethoxybenzyl diethylenetriamine pentaacetic
acid-enhanced magnetic resonance imaging: A potential utility for
the evaluation of regional liver function impairment following
transcatheter arterial chemoembolization. Oncol Lett. 9:1191–1196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubota K, Tamura T, Aoyama N, Nogami M,
Hamada N, Nishioka A and Ogawa Y: Correlation of liver parenchymal
gadolinium-ethoxybenzyl diethylenetriaminepentaacetic acid
enhancement and liver function in humans with hepatocellular
carcinoma. Oncol Lett. 3:990–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamada T, Ito K, Sone T, Kanki A, Sato T
and Higashi H: Gd-EOB-DTPA enhanced MR imaging: Evaluation of
biliary and renal excretion in normal and cirrhotic livers. Eur J
Radiol. 80:e207–e211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao YD, Paudel R, Liu J, Ma C, Zhang ZS
and Zhou SK: MRI contrast agents: Classification and application
(Review). Int J Mol Med. 38:1319–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haradome H, Grazioli L, Al Manea K, Tsunoo
M, Motosugi U, Kwee TC and Takaraha T: Gadoxetic acid
disodium-enhanced hepatocyte phase MRI: Can increasing the flip
angle improve focal liver lesion detection? J Magn Reson Imaging.
35:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE
and Choi JY: Added value of gadoxetic acid-enhanced hepatobiliary
phase MR imaging in the diagnosis of hepatocellular carcinoma.
Radiology. 255:459–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao YD, Ma C, Liu J, Li HB, Zhang ZS and
Zhou SK: Evaluation of hypointense liver lesions during
hepatobiliary phase MR imaging in normal and cirrhotic livers: Is
increasing flip angle reliable? Sci Rep. 6:189422016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aran S, Shaqdan KW and Abujudeh HH:
Adverse allergic reactions to linear ionic gadolinium-based
contrast agents: Experience with 194, 400 injections. Clin Radiol.
70:466–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davenport MS, Viglianti BL, Al-Hawary MM,
Caoili EM, Kaza RK, Liu PSC, Maturen KE, Chenevert TL and Hussain
HK: Comparison of acute transient dyspnea after intravenous
administration of gadoxetate disodium and gadobenate dimeglumine:
Effect on arterial phase image quality. Radiology. 266:452–461.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Xiao Y, Wang S, Li Y, Zhong X, Situ
W, Xiao E and Zhang Z: TWIST-VIBE five-arterial-phase technology
decreases transient severe motion after bolus injection of
Gd-EOB-DTPA. Clin Radiol. 72:800 e1–800.e6. 2017. View Article : Google Scholar
|
|
17
|
Davenport MS, Bashir MR, Pietryga JA,
Weber JT, Khalatbari S and Hussain HK: Dose-toxicity relationship
of gadoxetate disodium and transient severe respiratory motion
artifact. AJR Am J Roentgenol. 203:796–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujinaga Y, Ueda H, Kitou Y, Tsukahara Y,
Sugiyama Y and Kadoya M: Time-intensity curve in the abdominal
aorta on dynamic contrast-enhanced MRI with high temporal and
spatial resolution: Gd-EOB-DTPA versus Gd-DTPA in vivo. Jpn J
Radiol. 31:166–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Michaely HJ, Morelli JN, Budjan J, Riffel
P, Nickel D, Kroeker R, Schoenberg SO and Attenberger UI:
CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold
examination (VIBE): A new technique for fast time-resolved dynamic
3-dimensional imaging of the abdomen with high spatial resolution.
Invest Radiol. 48:590–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo JL, Lee CH, Park YS, Kim JW, Lee J,
Kim KA, Seol HY and Park CM: The short breath-hold technique,
controlled aliasing in parallel imaging results in higher
acceleration, can be the first step to overcoming a degraded
hepatic arterial phase in liver magnetic resonance imaging: A
prospective randomized control study. Invest Radiol. 51:440–446.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bashir MR, Castelli P, Davenport MS,
Larson D, Marin D, Hussain HK and Jaffe TA: Respiratory motion
artifact affecting hepatic arterial phase MR imaging with
gadoxetate disodium is more common in patients with a prior episode
of arterial phase motion associated with gadoxetate disodium.
Radiology. 274:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hayashi T, Saitoh S, Tsuji Y, Takahashi J,
Tagaya N, Hiramoto M, Fukuzawa K, Tano M, Miyati T and Kumada H:
Influence of gadoxetate disodium on oxygen saturation and heart
rate during dynamic contrast-enhanced MR imaging. Radiology.
276:756–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Motosugi U, Bannas P, Bookwalter CA, Sano
K and Reeder SB: An investigation of transient severe motion
related to gadoxetic acid-enhanced MR imaging. Radiology.
279:93–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haradome H, Grazioli L, Tsunoo M, Tinti R,
Frittoli B, Gambarini S, Morone M, Motosugi U and Colagrande S: Can
MR fluoroscopic triggering technique and slow rate injection
provide appropriate arterial phase images with reducing artifacts
on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J
Magn Reson Imaging. 32:334–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pietryga JA, Burke LMB, Marin D, Jaffe TA
and Bashir MR: Respiratory motion artifact affecting hepatic
arterial phase imaging with gadoxetate disodium: Examination
recovery with a multiple arterial phase acquisition. Radiology.
271:426–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujinaga Y, Ohya A, Tokoro H, Yamada A,
Ueda K, Ueda H, Kitou Y, Adachi Y, Shiobara A, Tamaru N, et al:
Radial volumetric imaging breath-hold examination (VIBE) with
k-space weighted image contrast (KWIC) for dynamic gadoxetic acid
(Gd-EOB-DTPA)-enhanced MRI of the liver: Advantages over cartesian
VIBE in the arterial phase. Eur Radiol. 24:1290–1299. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Budjan J, Ong M, Riffel P, Morelli JN,
Michaely HJ, Schoenberg SO and Haneder S: CAIPIRINHA-Dixon-TWIST
(CDT)-volume-interpolated breath-hold examination (VIBE) for
dynamic liver imaging: comparison of gadoterate meglumine,
gadobutrol and gadoxetic acid. Eur J Radiol. 83:2007–2012. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kazmierczak PM, Theisen D, Thierfelder KM,
Sommer WH, Reiser MF, Notohamiprodjo M and Nikolaou K: Improved
detection of hypervascular liver lesions with
CAIPIRINHA-Dixon-TWIST-volume-interpolated breath-hold examination.
Invest Radiol. 50:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gutzeit A, Matoori S, Froehlich JM, von
Weymarn C, Reischauer C, Kolokythas O, Goyen M, Hergan K,
Meissnitzer M, Forstner R, et al: Reduction in respiratory motion
artefacts on gadoxetate-enhanced MRI after training technicians to
apply a simple and more patient-adapted breathing command. Eur
Radiol. 26:2714–2722. 2016. View Article : Google Scholar : PubMed/NCBI
|