Introduction
Skin cancer can be divided into two groups,
non-melanoma skin cancer (NMSC) and malignant melanoma (MM). MM is
the most related death skin cancer; incidence of melanoma is
increasing worldwide (1–4). Melanoma developed from melanocyte,
which is pigment-producing cell. There are several methods to treat
melanoma depending on stage and severity of disease such as
surgical excision, chemotherapy, radiotherapy, immunotherapy and
biological therapy. All of them kill cancer cells but also kill
normal cells, so new therapy is developing everyday (5).
Apoptosis or program cell death is an essential
mechanism in multicellular organism. This process plays a critical
role including normal cell turnover, development of embryo,
functioning of the immune system, tissue homeostasis and
elimination of damaged cells. During the early process of
apoptosis, cells were initially described by its morphological
characteristics including cell shrinkage, membrane blebbing,
chromatin condensation and nuclear fragmentation. Abnormal
apoptosis induces the processes of oncogenesis including
initiation, progression and metastasis in abnormal cell. Recently,
there are studies about apoptosis with cancer cells, which studied
about signaling process in cell (6–10).
Goniothalamin is extracted from root and bark of
plant in family Annonaceae genus Goniothalamus. Studies show
effect of goniothalamin as apoptotic agent and anti-proliferative
agent in cancer cell, antibiotics, and antifungal (11,12).
Recent research has demonstrated that goniothalamin showed
cytotoxicity and apoptosis induction in various tumor cell lines.
However, the study of goniothalamin in MM has not yet been
reported.
In the present study, we found that goniothalamin
inhibited cell proliferation and induce apoptosis associated with
mitochondria dysfunction, caspase activation, and the upregulation
of c-Jun, p-p38 and p-ERK1/2, but downregulation of Akt signaling
pathway in melanoma A375 cells.
Materials and methods
Materials
RPMI-1640 medium were purchased from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Hoechst 33342,
3-(4,5-dimethylthaiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide) and phenylmethylsulphonylfluoride (PMSF) were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). DMSO was
purchased from Calbiochem (San Diego, CA, USA). Guava Cell
Cycle® reagent for cell cycle analysis and fetal bovine
serum (FBS) were purchased from Merck KGaA. MEK1/2 inhibitor
(U0126) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Goniothalamin was obtained from Assoc. Prof.
Wilawan Mahabusarakam, Faculty of Science, Prince of Songkla
University (Hat Yai, Thailand) in purified powder form (13).
Cell culture
MM cell line A375 was obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
maintained as a monolayer in RPMI-1640 supplemented with 10% FBS
(GE Healthcare, UK), 100 U/ml penicillin and 100 µg/ml streptomycin
(PAA Laboratories, Pasching, Austria). The cells were cultured in
5% CO2 at 37°C and subcultured 2–3 times/week.
Cell proliferation and cell viability
assays
MTT assay was used to determine the cytotoxicity of
goniothalamin. A375 cells were seeded in a 96-well plate at
5×103 cells/well and allowed to grow for 24 h. Then,
cells were treated with goniothalamin at 0.1, 0.3, 0.5, 1, 3, 5 and
10 µg/ml for 24 h, whereas the control group was treated with 0.5%
DMSO. After incubation, 100 µl of 0.5 mg/ml MTT solution was added
to each well and incubated for 2–4 h at 37°C, then supernatant was
removed and DMSO was added to solubilize the formazan crystals. The
absorbance was measured by using a microplate reader at 570 nm
(Multiskan EX; Thermo Electron Corp., Vantaa, Finland), and the
IC50 value was calculated by using the GraphPad Prism
3.03 (GraphPad Software, Inc., San Diego, CA, USA).
Nuclear morphological staining with
Hoechst 33342
A375 cells were seeded at 4×105
cells/well for 24 h. Then, the cells were treated with 3, 5 and 10
µg/ml goniothalamin for 24 h, while, the control group was treated
with 0.5% DMSO. After incubation, cells were stained with 5 µM
Hoechst 33342 for 30 min at 37°C and examined under a fluorescence
microscope (IX73; Olympus, Tokyo, Japan).
Cell cycle analysis
To examine apoptosis induction via upregulation of
sub-G1 population, flow cytometry was carried out. Cells were
treated with 3, 5 and 10 µg/ml goniothalamin for 24 h and 0.5% DMSO
was used as the control group. Upon treatment, cells were washed
with PBS and fixed with ice cold 70% ethanol at 4°C for more than 1
h. After fixation, cells were stained according to the
manufacturer's instructions (Guava Cell Cycle® reagent
from Merck KGaA). The DNA content was observed by Guava
easyCyte™ flow cytometer and GuavaSoft™
software (Merck KGaA).
Measurement of mitochondrial membrane
potential (ΔΨm)
JC-1 was used to determine the function of the ΔΨm,
which specific to mitochondria that is incorporated into the
mitochondrial membrane. A375 cells were seeded at
3×105/well for 24 h. Then, cells were treated with 3, 5
and 10 µg/ml goniothalamin for 24 h, whereas the control group was
treated with 0.5% DMSO. Cells were stained with 5 µg/ml of JC-1 in
the dark at 37°C for 10 min and washed with PBS for 3 times before
analysis by fluorescence microscopy.
Western blot analysis
A375 cells were seeded at 3×105/well for
24 h. Cells were treated with 3, 5 and 10 µg/ml goniothalamin for
24 h, whereas the control group was treated with 0.5% DMSO and
harvested at designated time points. The pellet cells were lysed
with RIPA lysis buffer (50 mM Tris-HCL, pH 7.5, 5 mM EDTA, 250 mM
NaCl, 0.5% Triton X-100) supplemented complete mini protease
inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany).
Protein expression by using western blot analysis was carried out
according to a previously reported protocol (13).
Statistical analysis
All data presented were obtained from at least three
independent experiments and were presented as mean ± standard
deviation (SD). Statistical significance was assessed by one-way
analysis of variance (ANOVA). Statistical analysis was performed by
using SPSS statistical software package (version 15; SPSS, Inc.,
Chicago, IL, USA) also carried out using the software GraphPad
Prism 3.03 (GraphPad Software, Inc., La Jolla, CA, USA). The
western blotting band intensity was quantified by Image J
densitometer.
Results
The effect of goniothalamin on cell
anti-proliferation and apoptosis induction in A375 cells
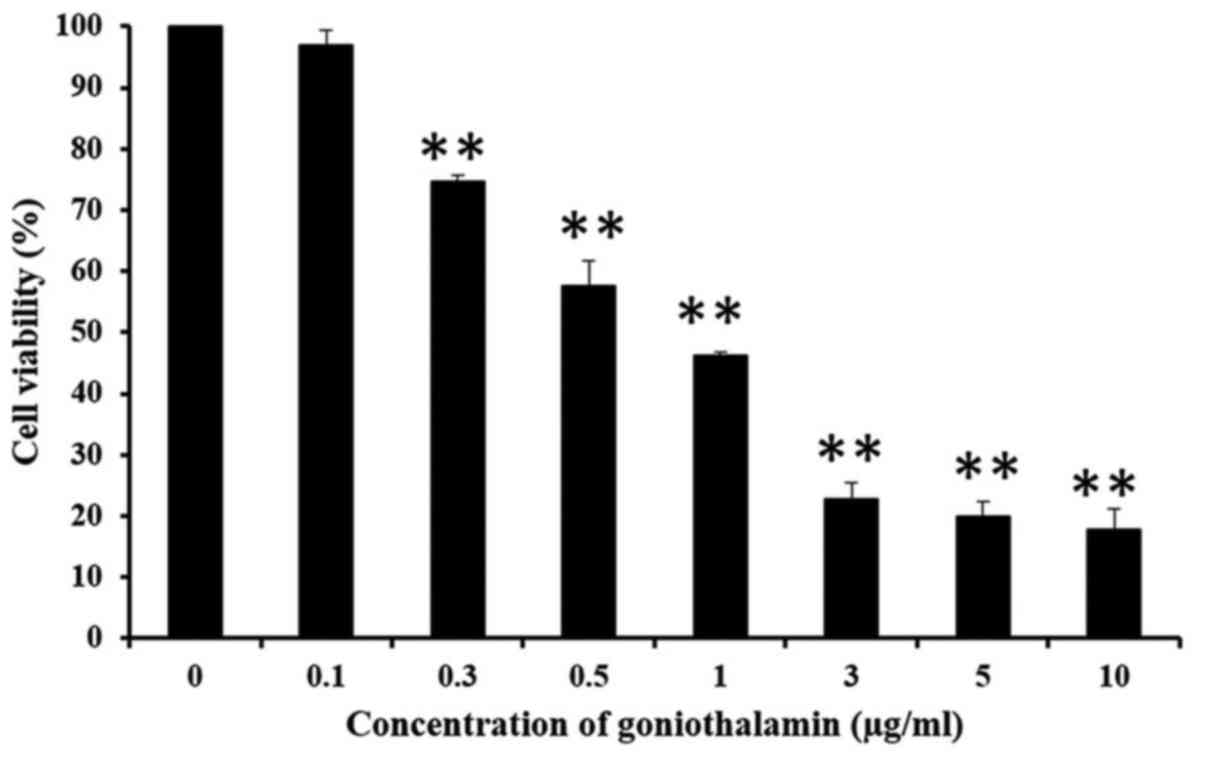
MTT assay was used to determine the
anti-proliferation activity of goniothalamin in A375 cells. The
results showed that goniothalamin inhibited cell proliferation in a
dose-dependent manner in A375 treated cells with the
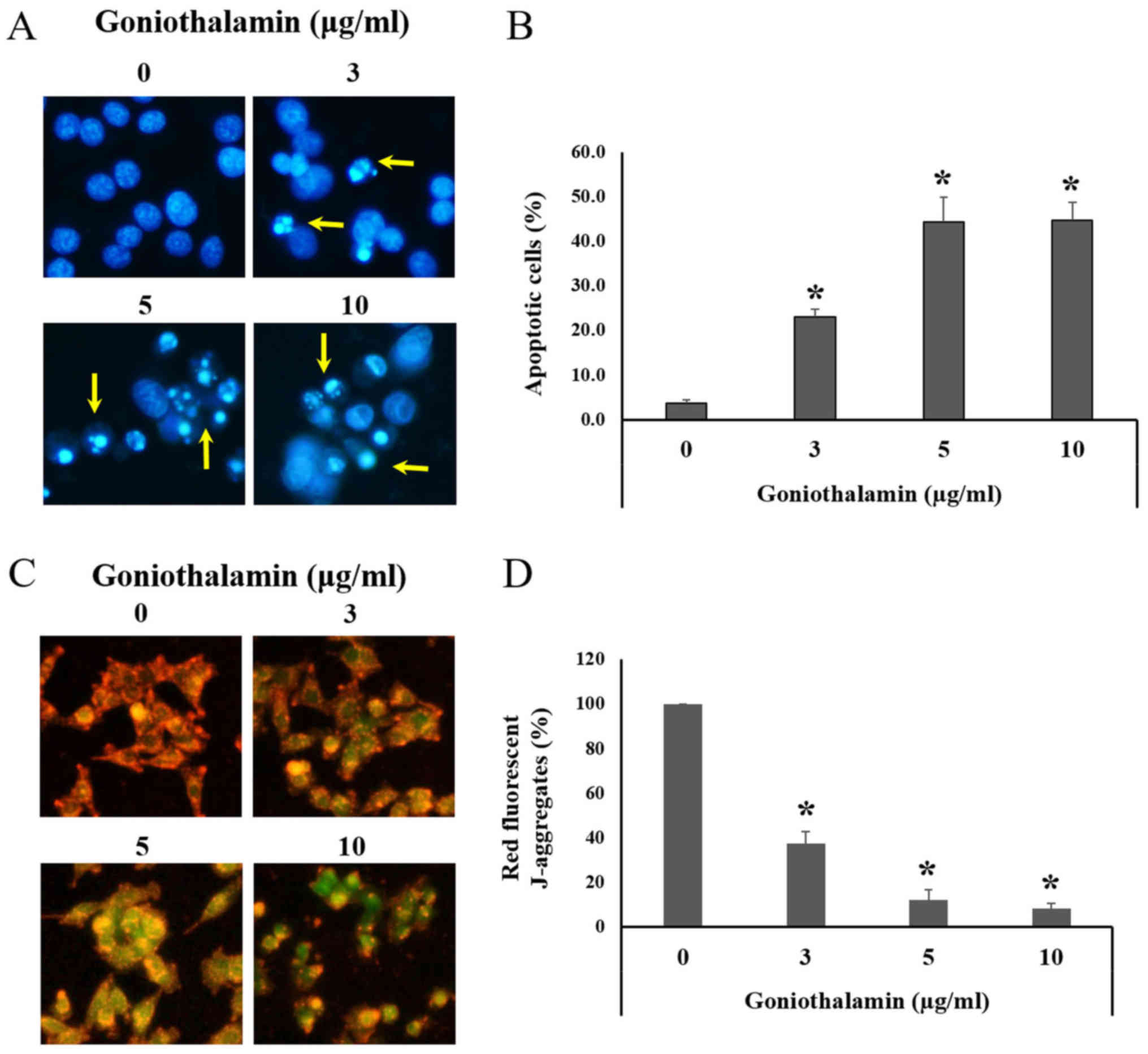
IC50 value was 1.7±0.627 µg/ml (Fig. 1). Hoechst 33342 staining was used to
determine the nuclear morphological changes mediated apoptosis.
Morphological changes in apoptotic cell include chromatin
condensation and apoptotic bodies. Hoechst 33342 is a fluorescence
dye and a cell-permeable DNA stain used for labeling DNA, although
the dyes can bind to all nucleic acid enhancing fluorescence
considerably (14). The results
revealed that goniothalamin induced chromatin condensation and
apoptotic bodies in A375 cells following treatment with
goniothalamin (Fig. 2A). Our results
showed that goniothalamin significantly induced apoptotic cells at
3, 5 and 10 µg/ml (P<0.05; Fig.
2B). This result suggested that goniothalamin could induce
apoptosis in A375 cells.
Mitochondrial membrane potential
(ΔΨm)
During apoptosis, mitochondria was disturbed by
pro-apoptotic proteins activation leading to the loss of ΔΨm. JC-1
staining was carried out to measure the ΔΨm. In normal cells with
high ΔΨm, JC-1 forms J-aggregates complexes as shown in red
fluorescence. Whereas, apoptotic cells with low ΔΨm, JC-1 remains
in the monomeric form as shown in green fluorescence (15). Our study showed that goniothalamin
significantly induced the loss of ΔΨm in A375 treated cells showing
decreased red fluorescence comparing with the control cells
(P<0.05; Fig. 2C and D). This
result indicated that goniothalamin induced apoptosis through
mitochondria dysfunction in A375 treated cells.
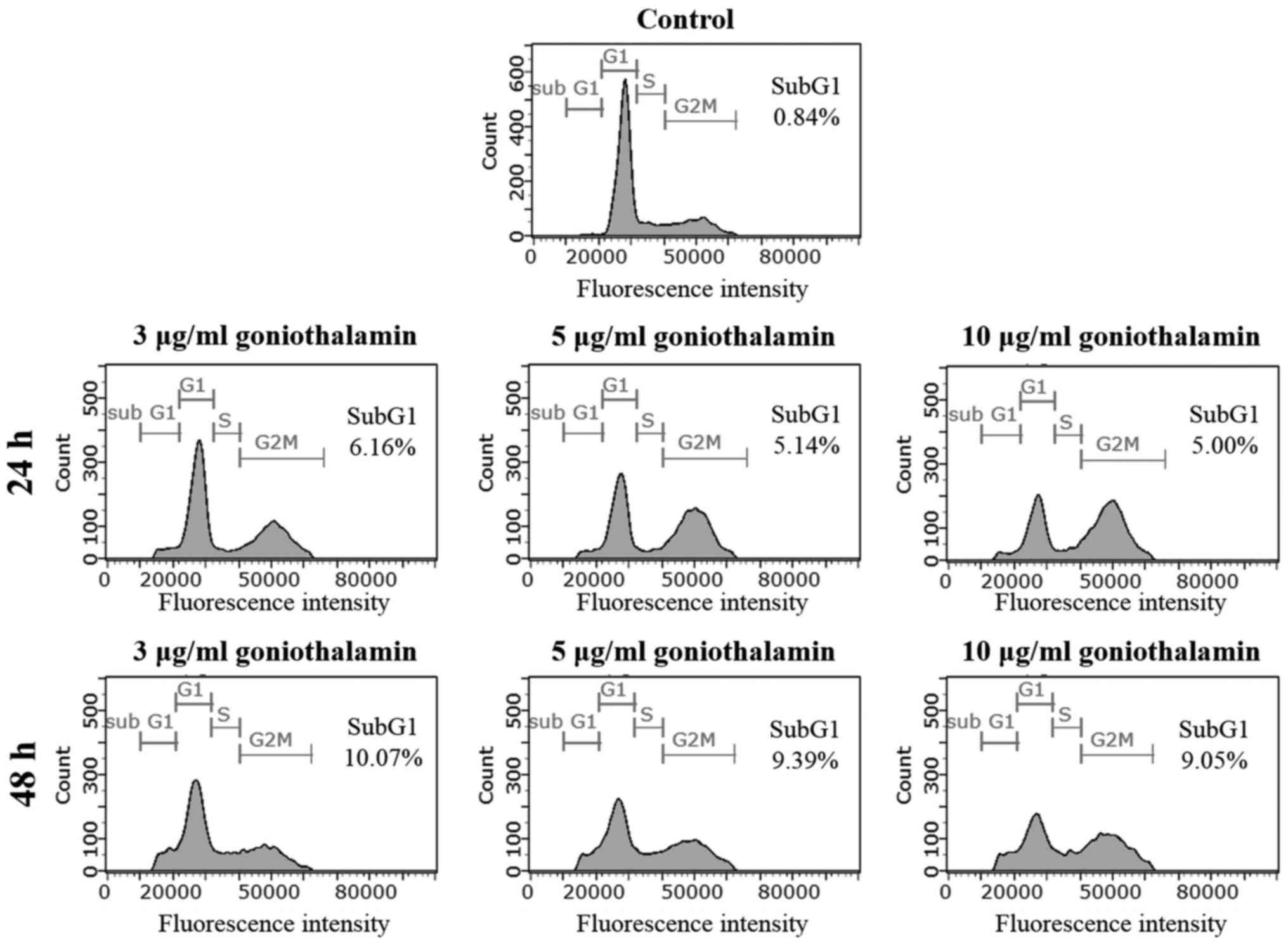
Cell cycle distribution
To further verify the inhibitory effect of
goniothalamin on apoptosis induction, cells were stained with PI
and histogram analysis-related DNA contents were measured by flow
cytometry. The treatment of A375 cells with goniothalamin at 3
µg/ml for 24 and 48 h showed increasing of sub-G1 population peak
to 6.16 and 10.07%, respectively, whereas the control cells showed
0.84% of sub-G1 population (Fig. 3).
This result confirmed that goniothalamin induced cell death by
apoptosis induction in A375 treated cells.
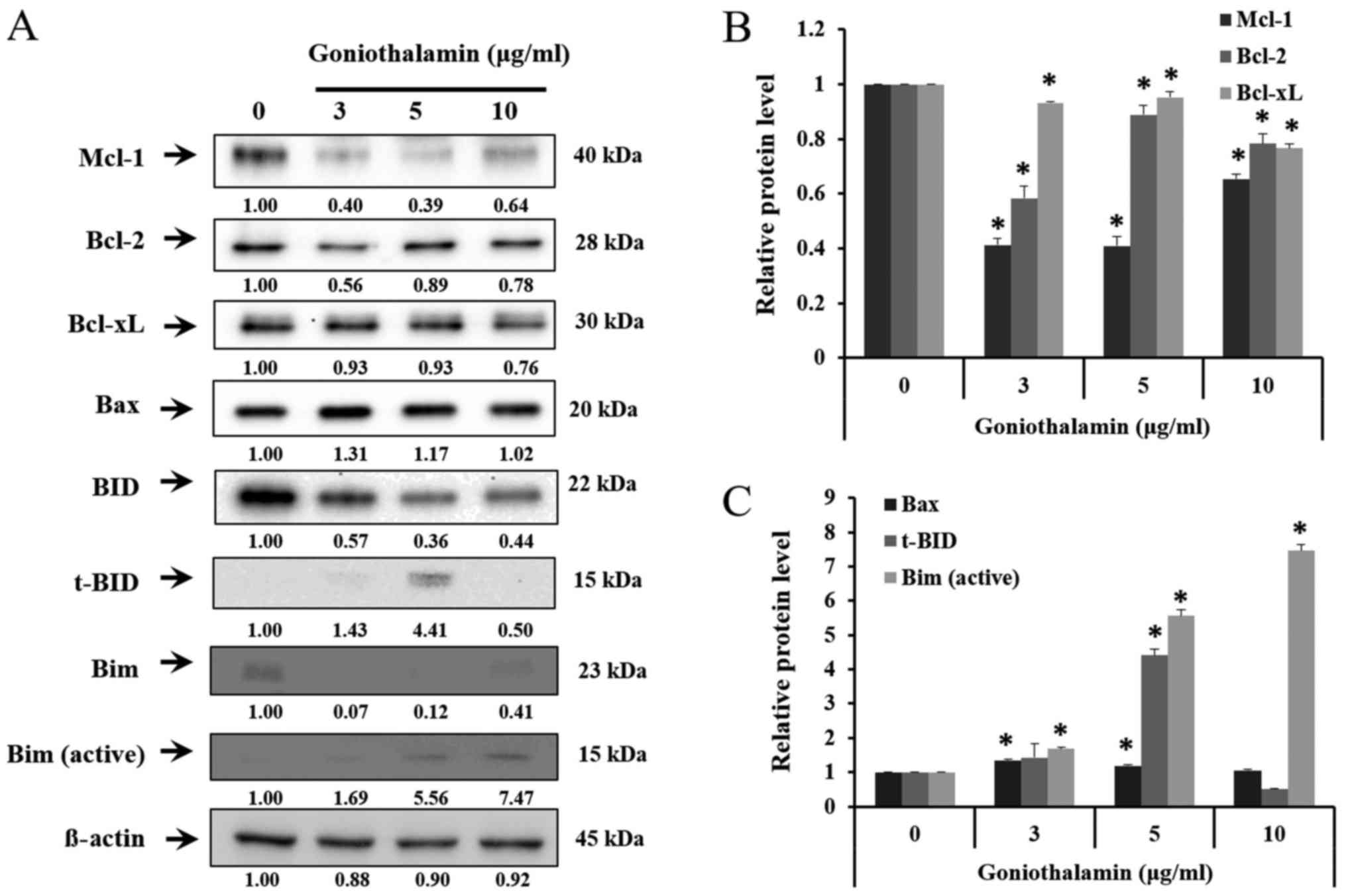
Bcl-2 family, caspase-7, caspase-9 and
cleaved-PARP expression
The results showed that goniothalamin decreased the
level of anti-apoptotic proteins Mcl-1, Bcl-2 and Bcl-xL in A375
treated cells (P<0.05; Fig. 4A and
B), whereas increased the level of pro-apoptotic proteins Bax,
t-BID and Bim in A375 treated cells (P<0.05; Fig. 4A and C). In addition, goniothalamin
induced caspase-9, caspase-7 and cleaved-PARP activation in A375
treated cells (P<0.05; Fig. 5A and
B). The results indicated that goniothalamin could induce
apoptosis via intrinsic pathway.
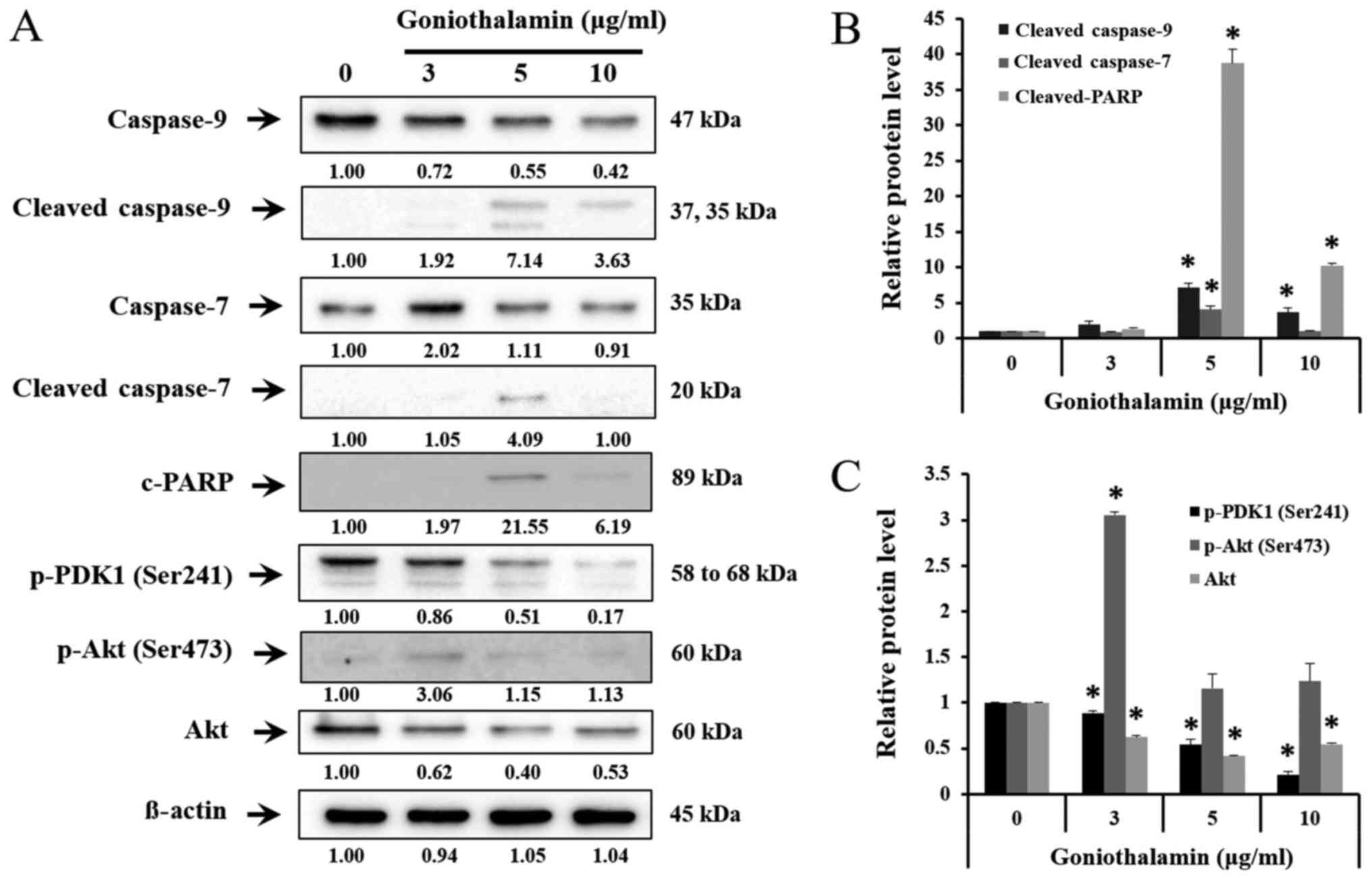 | Figure 5.Effect of goniothalamin on caspase,
cleaved-PARP induction and Akt signaling molecules. Cells were
treated with 3, 5, and 10 µg/ml goniothalamin for 24 h. (A) Effect
of goniothalamin on caspase-9, −7, cleaved-PARP, p-PDK1, p-Akt at
Ser473 and total Akt proteins in A375 cells were determined by
western blot analysis. (B) Relative protein level of
cleaved-caspase-9, cleaved-caspase-7 and cleaved-PARP proteins. (C)
Relative protein level of p-PDK1, p-Akt and Akt proteins. β-actin
was used as an internal control, *P<0.05 significantly compared
with the control. |
Effect of goniothalamin on AKT
signaling pathway
AKT signaling pathway is one of the most important
pathways, which promote cell proliferation, cell growth,
transcription and cell migration. The results showed that
goniothalamin downregulated p-PDK1 (Ser241) and total Akt
indicating that goniothalamin could inhibit A375 cell survival
(P<0.05; Fig. 5A and C).
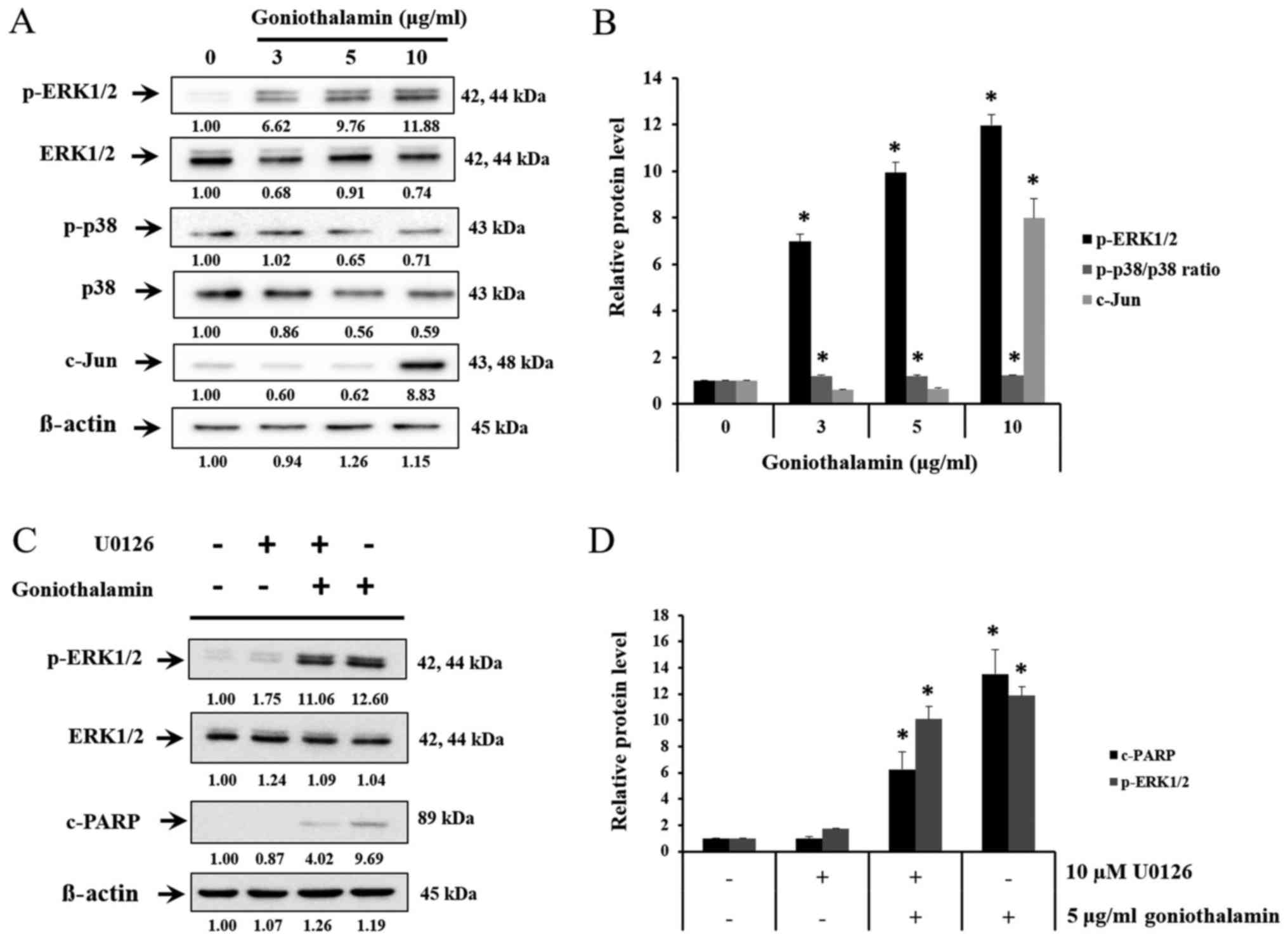
The effect of goniothalamin on MAPK
signaling pathway
MAPK signaling pathway is the pathway that involved
both apoptosis and cell survival. Upon treatment with
goniothalamin, the level of c-Jun, p-ERK1/2 and p-p38/p38 ratio
were increased (P<0.05; Fig. 6A and
B). These results indicated that goniothalamin induced
apoptosis via MAPK signaling pathway. In order to determine the
effects of p-ERK1/2 on apoptosis induction by goniothalamin in A375
cells, MEK1/2 inhibitor (U0126) was used. The result showed that
U0126 simultaneously blocked p-ERK1/2, in contrast, co-treatment
between U0126 and goniothalamin showed upregulation of p-ERK1/2 and
cleaved-PARP indicating that goniothalamin induced apoptosis
through ERK1/2 signaling (P<0.05; Fig. 6C and D). These results suggested that
goniothalamin induced apoptosis in A375 cells by promoting cell
death through p-ERK1/2 activation.
Discussion
In this study, A375 cell line was used as a model
for MM. MM is one of the most related death skin cancer which
originated from melanocyte (pigment cells). The treatments of MM
depending on stage and severity of disease, surgical excision is
the main therapy of MM. However adjuvant therapy is needed for
advanced stage disease but most of therapy have side effects and
not significantly increase survival rate in patients.
Recent research group has demonstrated that
goniothalamin showed cytotoxicity and apoptosis induction in
various tumor cell lines, H1299 (non-small cell lung cancer)
(16), HepG2 (human hepatoblastoma)
(17), SK-BR-3 (human breast cancer)
(13), A549 (lung carcinoma)
(18), HL60 (promyelocytic leukemia)
(19,20), SGC7901 (stomach cancer) (21), HT29 (colon cancer) (22,23),
HeLa (cervical cancer) (24), and
MDA-MB231 (invasive breast cancer) (25). However, the study of goniothalamin in
MM have not yet been reported.
The results showed goniothalamin inhibited A375 cell
growth in a dose-dependent manner. Innajak et al
demonstrated that goniothalamin inhibited SK-BR-3 cell growth in a
time- and dose-dependent manner with an IC50 value of
10±0.45 µg/ml (13). At 72 h,
goniothalamin completely inhibited cell viability in MDA-MB-231
with an IC50 value of about 1.46 µM (25).
Hoechst staining was used to confirm nuclear
morphological changes via apoptosis induction. Hoechst staining
showed condensed chromatin and apoptotic bodies in the A375 cells
after treatment with goniothalamin (Fig.
2A). In other cell line, Chen et al reported that after
treat MDA-MB-231 cells with 30 µM goniothalamin for 48 h, chromatin
condensation and nuclear fragmentation were detected (25).
Moreover the JC-1 staining assay showing
significantly decreased red fluorescence while increased green
indicating that the loss of ΔΨm and leading to apoptosis induction
(Fig. 2C and D).
To confirm signaling pathway of apoptosis induction,
Bcl-2 family proteins, caspase proteins, Akt and MAPK pathway were
analyzed by western blotting.
The anti-apoptotic proteins Bcl-2 family protein,
Bcl-2, Mcl-1 and Bcl-xL was deceased (Fig. 4A) whereas pro-apoptotic proteins Bax,
t-BID and Bim were increased upon treatment with goniothalamin
(Fig. 4A). In addition, there are
two types of caspase, initiator and effector caspase, caspase-9
(initiator caspase) can activate caspase-7 (effector caspase) and
deactivate PARP, which is DNA repairing protein. The results showed
that caspase-7 and caspase-9 were increased which then induced
cleaved-PARP activation (Fig. 5A).
These results correlated with previous study revealing that
goniothalamin induced apoptosis in different cancer cell types
including HeLa (26), SK-BR-3
(13), Colo 205, SW480 and LoVo
cells (27).
Akt is signaling pathway that promotes cell growth
and anti-apoptosis. From previous studies, goniothalamin down
regulated phosphorylated Akt at Ser473, Thr308 and total Akt in
SK-BR-3 cells leading to apoptosis induction (13). These studies showed the decrease of
p-PDK1 (Ser241) and total Akt (Fig.
5A) indicating that goniothalamin induced apoptosis and
inhibited cell proliferation.
Another group is protein in MAPK signaling pathway
playing important role both in cell survival and cell death.
Conventional MAPKs in mammalian include the ERK1/2, JNK1/2 and p38.
ERK1/2 activates Bax protein and caspase then deactivates Akt
pathway, which leads to apoptosis. JNK1/2 can activate the
transcriptional factors including c-Jun, which express Bim. p38 is
tumor suppressor, which induce apoptosis and inhibit cell
proliferation. p38 can activate p53, which is tumor suppressor
(Fig. 6A). This result showed that
goniothalamin induced p-ERK1/2, p-p38/p38 ratio and c-Jun
upregulation in A375 treated cells leading to apoptosis. In
general, ERK is important in cell proliferation, cell
differentiation, cell growth or cell survival, however, we found
that goniothalamin induced p-ERK1/2 upregulation in A375 treated
cells. These results correlated with previous report by Bee-Jen Tan
et al, that ERK1/2 could activate caspase and pro-apoptotic
protein in Bcl-2 family, moreover ERK1/2 could deactivated Akt
signaling pathway leading to apoptosis induction (28). Therefore, the results showed that
c-Jun and p-ERK1/2 were increased implying that goniothalamin
induced apoptosis in A375 cell.
Indeed, we also confirmed the effect of
goniothalamin on apoptosis induction through p-ERK1/2 activation
using U0126. The results showed that goniothalamin induced
apoptosis in A375 cells via ERK1/2 signaling (Fig. 6C). Our study correlated with previous
report that ERK is involved in apoptosis induction of Moringa
oleifera fruit (MOF) extract in human melanoma A2058 cells
(29).
In summary, goniothalamin has an effect as
anti-proliferation and apoptosis induction in A375 cells associated
with upregulated p-ERK1/2, c-Jun and downregulated p-PDK1 (Ser241),
total Akt in A375 cells. Studying the effect of Goniothalamin in
other MM cell lines. In the future, the effect of goniothalamin in
primary epidermal melanocytes (normal) will be studied to confirm
that this compound could affect melanoma but not the normal cell.
Furthermore the effect of goniothalamin should be studied in animal
model.
Acknowledgements
We would like to thank Research Division, Faculty of
Medicine, Research Unit in Biological Aactivities of Bioactive
Compounds, Strategic Wisdom and Research Institute Srinakharinwirot
University.
References
|
1
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Armstrong BK and Kricker A: The
epidemiology of UV induced skin cancer. J Photochem Photobiol B.
63:8–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diepgen TL and Mahler V: The epidemiology
of skin cancer. Br J Dermatol. 146 Suppl 61:S1–S6. 2002. View Article : Google Scholar
|
|
4
|
Strickland PT, Vitasa BC, West SK,
Rosenthal FS, Emmett EA and Taylor HR: Quantitative carcinogenesis
in man: Solar ultraviolet B dose dependence of skin cancer in
Maryland watermen. J Natl Cancer Inst. 81:1910–1913. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mukherjee AK, Basu S, Sarkar N and Ghosh
AC: Advances in cancer therapy with plant based natural products.
Curr Med Chem. 8:1467–1486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis RE, Yuan JY and Horvitz HR:
Mechanisms and functions of cell death. Annu Rev Cell Biol.
7:663–698. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wiederschain G: Essentials of apoptosis. A
guide for basic and clinical research. Springer; New York, NY:
2005
|
|
8
|
Klein G: Cancer, apoptosis, and nonimmune
surveillance. Cell Death Differ. 11:13–17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun T, Miao X, Zhang X, Tan W, Xiong P and
Lin D: Polymorphisms of death pathway genes FAS and FASL in
esophageal squamous-cell carcinoma. J Natl Cancer Inst.
96:1030–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mosaddik MA and Haque ME: Cytotoxicity and
antimicrobial activity of goniothalamin isolated from Bryonopsis
laciniosa. Phytother Res. 17:1155–1157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiu CC, Liu PL, Huang KJ, Wang HM, Chang
KF, Chou CK, Chang FR, Chong IW, Fang K, Chen JS, et al:
Goniothalamin inhibits growth of human lung cancer cells through
DNA damage, apoptosis, and reduced migration ability. J Agric Food
Chem. 59:4288–4293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Innajak S, Mahabusrakum W and
Watanapokasin R: Goniothalamin induces apoptosis associated with
autophagy activation through MAPK signaling in SK-BR-3 cells. Oncol
Rep. 35:2851–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Portugal J and Waring MJ: Assignment of
DNA binding sites for 4′,6-diamidine-2-phenylindole and
bisbenzimide (Hoechst 33258). A comparative footprinting study.
Biochim Biophys Acta. 949:158–168. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lecoeur H: Nuclear apoptosis detection by
flow cytometry: Influence of endogenous endonucleases. Exp Cell
Res. 277:1–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pihie A, Stanslas J and Din LB:
Non-steroid receptor-mediated antiproliferative activity of
styrylpyrone derivative in human breast cancer cell lines.
Anticancer Res. 18:1739–1743. 1998.PubMed/NCBI
|
|
17
|
Al-Qubaisi M, Rosli R, Subramani T, Omar
AR, Yeap SK, Ali AM and Alitheen NB: Goniothalamin selectively
induces apoptosis on human hepatoblastoma cells through caspase-3
activation. Nat Prod Res. 27:2216–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiart C: Goniothalamus species: A source
of drugs for the treatment of cancers and bacterial infections?
Evid Based Complement Alternat Med. 4:299–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petsophonsakul P, Pompimon W and
Banjerdpongchai R: Apoptosis induction in human leukemic
promyelocytic HL-60 and monocytic U937 cell lines by goniothalamin.
Asian Pac J Cancer Prev. 14:2885–2889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inayat-Hussain S, Annuar BO, Din LB, Ali
AM and Ross D: Loss of mitochondrial transmembrane potential and
caspase-9 activation during apoptosis induced by the novel
styryl-lactone goniothalamin in HL-60 leukemia cells. Toxicol In
Vitro. 17:433–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Fátima A, Modolo LV, Conegero LS, Pilli
RA, Ferreira CV, Kohn LK and De Carvalho JE: Styryl lactones and
their derivatives: Biological activities, mechanisms of action and
potential leads for drug design. Curr Med Chem. 13:3371–3384. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vendramini-Costa DB, Alcaide A,
Pelizzaro-Rocha KJ, Talero E, Ávila-Román J, Garcia-Mauriño S,
Pilli RA, de Carvalho JE and Motilva V: Goniothalamin prevents the
development of chemically induced and spontaneous colitis in
rodents and induces apoptosis in the HT-29 human colon tumor cell
line. Toxicol Appl Pharmacol. 300:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sophonnithiprasert T, Nilwarangkoon S,
Nakamura Y and Watanapokasin R: Goniothalamin enhances
TRAIL-induced apoptosis in colorectal cancer cells through DR5
upregulation and cFLIP downregulation. Int J Oncol. 47:2188–2196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alabsi AM, Ali R, Ali AM, Al-Dubai SA,
Harun H, Abu Kasim NH and Alsalahi A: Apoptosis induction, cell
cycle arrest and in vitro anticancer activity of gonothalamin in a
cancer cell lines. Asian Pac J Cancer Prev. 13:5131–5136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WY, Wu CC, Lan YH, Chang FR, Teng CM
and Wu YC: Goniothalamin induces cell cycle-specific apoptosis by
modulating the redox status in MDA-MB-231 cells. Eur J Pharmacol.
522:20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sophonnithiprasert T, Mahabusarakam W,
Nakamura Y and Watanapokasin R: Goniothalamin induces
mitochondria-mediated apoptosis associated with endoplasmic
reticulum stress-induced activation of JNK in HeLa cells. Oncol
Lett. 13:119–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sophonnithiprasert T, Mahabusarakam W,
Nakamura Y and Watanapokasin R: Antiproliferation and apoptosis
induction in colorectal cancer cells by goniothalamin. J Med Assoc
Thai. 98 Suppl 9:S146–S151. 2015.PubMed/NCBI
|
|
28
|
Tan BJ and Chiu GN: Role of oxidative
stress, endoplasmic reticulum stress and ERK activation in
triptolide-induced apoptosis. Int J Oncol. 42:1605–1612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guon TE and Chung HS: Moringa oleifera
fruit induce apoptosis via reactive oxygen species-dependent
activation of mitogen-activated protein kinases in human melanoma
A2058 cells. Oncol Lett. 14:1703–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|