Introduction
Liver cancer is one of the most common malignancies
worldwide, with ~600,000 new cases diagnosed annually (1). However, the 5-year survival rate of
liver cancer is <9% (2). This
tumor is the fourth cause of cancer-associated mortality, ranking
second in men and sixth in women, with >250,000 mortalities
annually (1,3–5). At
present, surgical resection is the major therapeutic strategy for
liver cancer, although a high rate of recurrence remains (6,7).
Stem cells are a type of cells harboring the ability
to self-renew and differentiate (8).
Cancer stem cells are a subset of cancer cells with stem cell
properties. Although radiotherapy and chemotherapy can eliminate
the majority of tumor cells, cancer stem cells have the ability to
self-renew and differentiate to generate tumor cell heterogeneity,
and thus resist these therapies (9).
Liver cancer stem cells (LCSCs) are considered to account for the
chemotherapy resistance and recurrence of liver cancer (10,11).
Multiple signals pathways, including Notch and Wnt/β-catenin, are
found to serve important roles in the stemness of LCSCs (12). The regulation of LCSCs via the
manipulation of internal signaling pathways may become a feasible
treatment for patients with liver cancer.
Curcumin is a yellow natural compound derived from
Rhizoma curcumae longae and is widely used as a spice in
Asia. Curcumin has been demonstrated to exert anti-inflammatory,
antioxidant and antiangiogenic effects (13–15),
while it also exerts a potential antitumor effect (15–17).
However, the effects of curcumin on LCSCs remain unclear.
Therefore, the aim of the present study was to examine the effects
of curcumin on the growth of LCSCs, as well as the underlying
mechanism of its action. The study demonstrated that treatment with
curcumin is able to inhibit the growth of LCSCs, and this compound
may be a promising treatment agent for liver cancer.
Materials and methods
Cell culture
The human liver cancer cell line HepG2 was obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were grown in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), and cultured in a
humidified atmosphere at 37°C with 5% CO2.
Isolation of LCSCs by magnetic
activated cell sorting (MACS)
CD133 is a marker of stem cells (18), and thus the present study used a
CD133 MicroBead kit (MiltenyiBiotec GmbH, Bergisch Gladbach,
Germany) for the isolation of LCSCs. Briefly, HepG2 cells were
digested with trypsin, washed with phosphate-buffered saline (PBS)
and made into a single-cell suspension. The cells were then
centrifuged at 300 × g for 10 min at 4°C, and 1×107
cells were resuspended in 60 µl buffer. Next, 20 µl FcR blocking
reagent and 20 µl CD133 MicroBeads supplied in the kit were added
into the cells and incubated at 4°C for 15 min. Subsequent to
washing with buffer, the cells were centrifuged at 300 × g for 10
min at 4°C, and then added into an appropriate MACS column that was
placed in the magnetic field of a MACS separator (both
MiltenyiBiotec GmbH). When the column reservoir was empty, the
column was washed with 500 µl buffer, removed from the separator
and eluted with an 1 ml buffer. The isolated cells were grown in
DMEM with 10% FBS and cultured in a humidified atmosphere at 37°C
with 5% CO2.
MTT assay for cell viability
determination
The LCSCs were seeded into 96-well plates with 3,000
cells in each well. At 24 h later, the cells were treated with 0,
10, 20, 40 or 80 µM curcumin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C for 24 h, and then the cell viability was assessed
by MTT assay. Briefly, MTT reagent (Sigma-Aldrich; Merck KGaA) at a
final concentration of 0.5 mg/ml was added into each well and
incubated for an additional 4 h. Subsequently, the media were
removed, and 200 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA)
was added into each well. The absorbance at 570 nm was then
measured by a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis detection
The LCSCs were seeded into 6-well plates at a
density of 1×105 cells in each well and treated with 0
or 20 µM curcumin at 37°C for 24 h. Next, the cell apoptosis was
detected by flow cytometry with a cell apoptosis detection kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). Briefly, the
cells were collected, washed twice with PBS and then resuspended in
500 µl binding buffer. Subsequently, 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl propidium iodide were added into the cells
and incubated for an additional 15 min. The cell apoptosis was then
analyzed by flow cytometry.
Western blot analysis
The LCSCs were seeded into 6-well plates
(1×105 cells/well) and treated with 0 or 20 µM curcumin
at 37°C for 24 h. Then the cells were collected by centrifugation
(speed, 300 × g) at 4°C for 10 min. Total proteins in the cells
from each group were extracted using radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
with 1% phenylmethanesulfonyl fluoride (Beyotime Institute of
Biotechnology). The mitochondrial and cytoplasmic proteins were
extracted using a cell mitochondria isolation kit (Beyotime
Institute of Biotechnology). Subsequent to measuring the
concentration of proteins with a BCA protein assay kit (Beyotime
Institute of Biotechnology), 40 µg proteins from each group were
separated by 12% SDS-PAGE. Next, the separated proteins were
transferred onto a polyvinylidene fluoride membranes (EMD
Millipore, Bedford, MA, USA). After blocking with 5% skim milk, the
membranes were incubated at 4°C overnight with primary antibodies
against caspase-3 (1:500; cat. no. ab44976; Abcam, Cambridge, UK),
caspase-9 (1:1,000; cat. no. ab25758; Abcam), B-cell lymphoma-2
(Bcl-2; 1:1,000; cat. no. 4223; Cell Signaling Technology, Inc.,
Danvers, MA, USA), Bcl-2 associated X protein (Bax; 1:1,000; cat.
no. 5023; Cell Signaling Technology), cytochrome c (1:200; cat. no.
sc-8385; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
phosphatidylinositol 3-kinase (PI3K; 1:200; cat. no. sc-293172;
Santa Cruz Biotechnology, Inc.), protein kinase B (AKT; 1:200; cat.
no. sc-55523; Santa Cruz Biotechnology, Inc.), phosphorylated AKT
(p-AKT; 1:1,000; cat. no. 4051; Cell Signaling Technology, Inc.),
mammalian target of rapamycin (mTOR; 1:200; cat. no. sc-8319; Santa
Cruz Biotechnology, Inc.), p-mTOR (1:200; cat. no. sc-101738; Santa
Cruz Biotechnology, Inc.), cytochrome c oxidase subunit IV
(1:1,000; cat. no. 11242-1-AP; ProteinTech Group, Inc., Chicago,
IL, USA), serving as internal reference for mitochondrial proteins)
and β-actin (1:1,000; cat. no. AF0003; Beyotime Institute of
Biotechnology), which served as the internal reference for total
proteins. Following washing with Tris-buffered saline with Tween
20, the membranes were incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies (cat. no. A0181, A0216,
A0208; all 1:1,000; Beyotime Institute of Biotechnology) at 37°C
for 2 h. Finally, the membranes were visualized with an enhanced
chemiluminescence detection system (Beyotime Institute of
Biotechnology). The protein levels were quantified using Quantity
One software (version 4.6; Bio-Rad Laboratories, Inc.).
Treatment with PI3K/AKT signal
activator, 740Y-P
LCSCs were seeded into 6-well plates
(1×105 cells/well) and treated with 20 µM curcumin for
24 h. Next, 20 µM 740Y-P (R&D Systems, Inc., Minneapolis, MN,
USA) was added into cells and incubated for a further 1 h. The
cells were then collected and subjected to MTT assay, apoptosis
detection and western blot analysis, as described earlier.
Statistical analysis
All experiments were repeated more than three times
and the results are presented as the mean ± standard deviation.
Differences between each group were analyzed using Student's t-test
in GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Curcumin inhibits the proliferation of
LCSCs
Following isolation, the LCSCs were treated with
different concentrations of curcumin, and then an MTT assay was
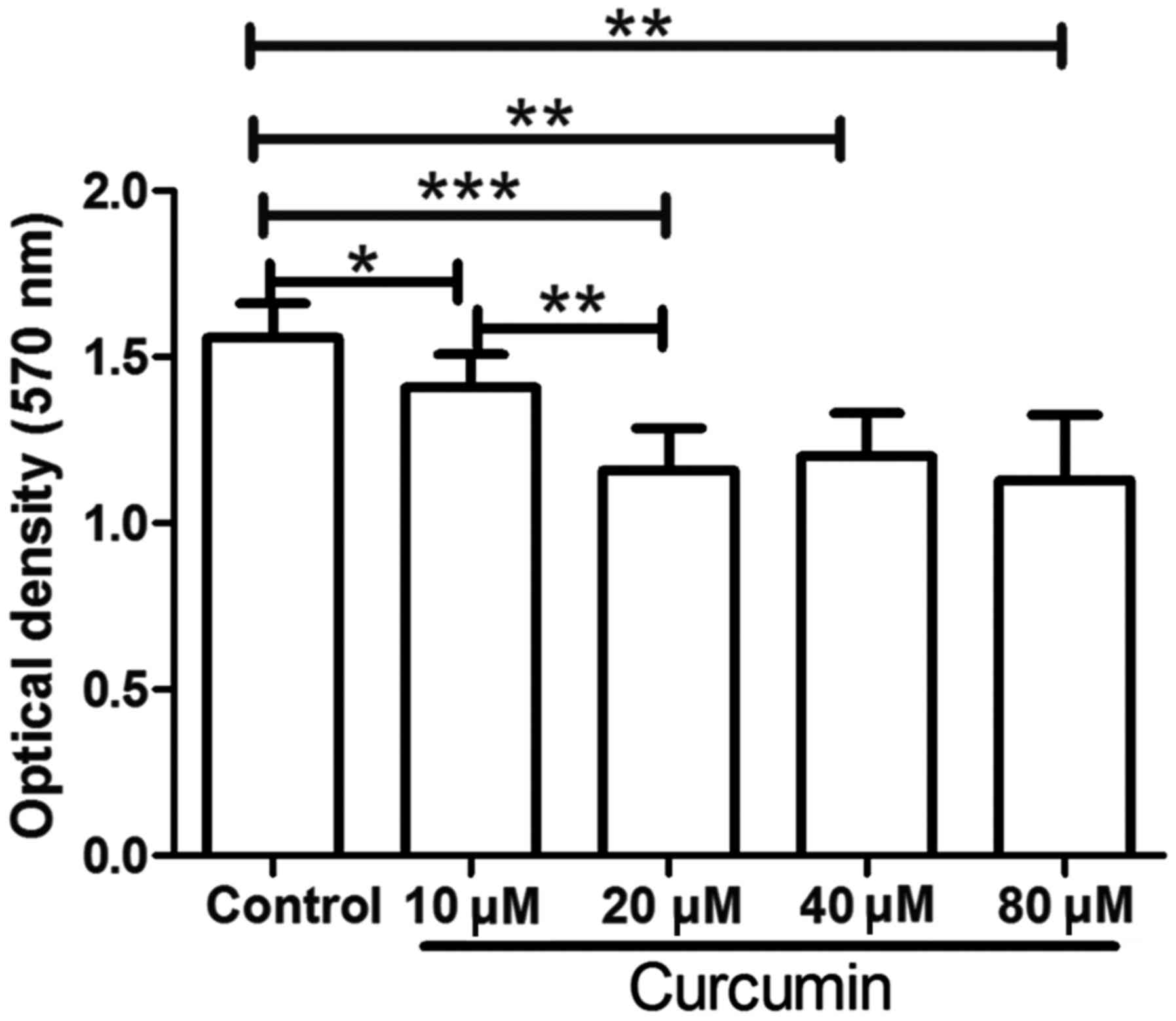
used to detect the proliferation of LCSCs. As shown in Fig. 1, following treatment with 10, 20, 40
or 80 µM curcumin, the proliferation of LCSCs was significantly
decreased compared with that in the untreated control group
(P<0.05). These results demonstrated that curcumin treatment
markedly inhibited the proliferation of LCSCs. In addition,
compared with the 10 µM curcumin group, treatment with 20 µM
curcumin further decreased the proliferation of LCSCs (P<0.01).
However, LCSCs treated with 40 or 80 µM curcumin demonstrated no
significant difference when compared with LCSCs treated with 20 µM
curcumin. Thus, the concentration of 20 µM curcumin was used in the
subsequent experiments.
Curcumin induces the apoptosis of
LCSCs
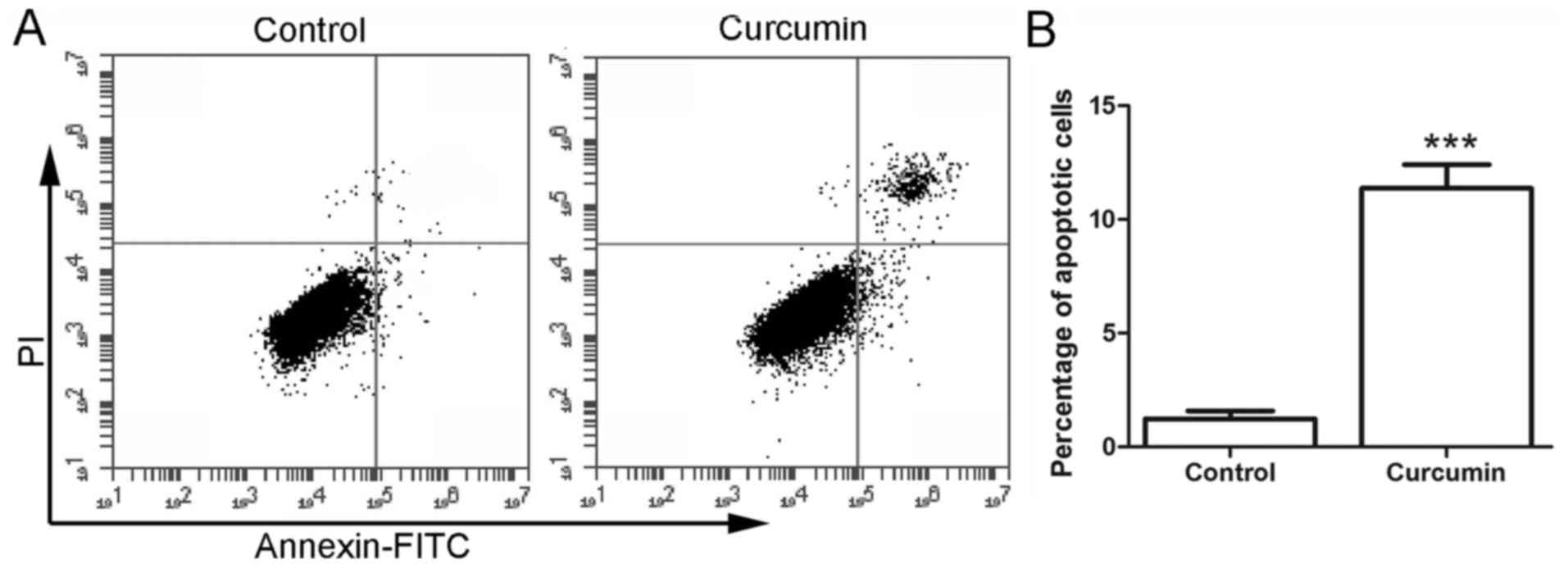
Subsequent to treatment with curcumin, the cell
apoptosis was detected by flow cytometry. As shown in Fig. 2, the percentage of apoptotic cells in
the control group was 1.23±0.35%. However, following treatment with
20 µM curcumin, the percentage of apoptotic cells was increased to
11.37±1.04%, which was significantly higher in comparison with that
in the control group (P<0.001). These results demonstrated that
treatment with curcumin induced the apoptosis of LCSCs.
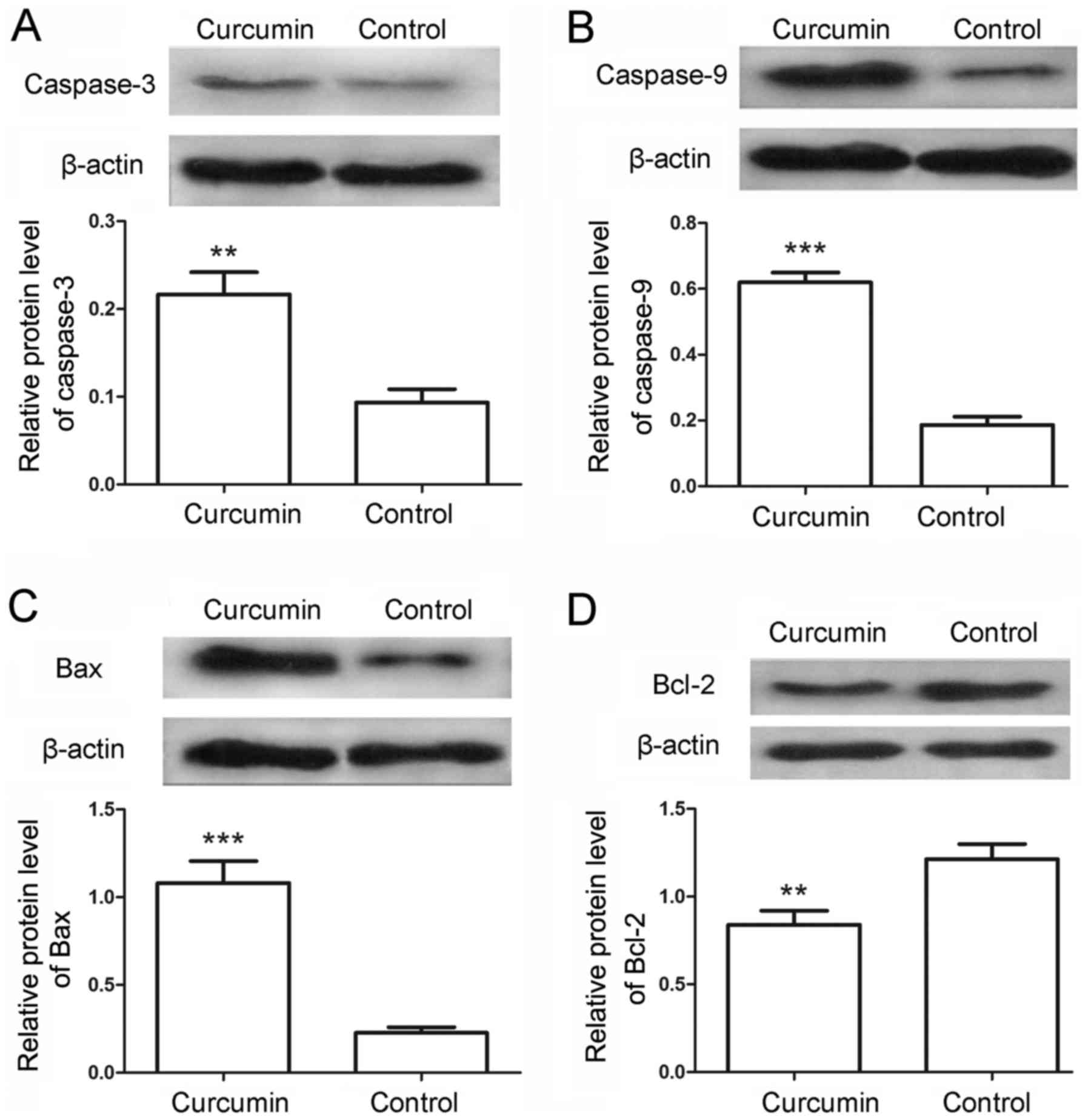
To further examine the cell apoptosis, the protein
levels of caspase-3 and caspase-9 in the LCSCs were also detected
by western blot analysis. Following treatment with curcumin, the
relative protein levels of caspase-3 were markedly increased from
0.09±0.02 in the control to 0.22±0.03 in the treated cells
(Fig. 3A; P<0.01). Similarly, the
protein levels of caspase-9 were significantly increased from
0.19±0.03 in the control cells to 0.62±0.03 in the curcumin-treated
cells (Fig. 3B; P<0.001). The
levels of apoptosis-associated proteins Bax and Bcl-2 were also
detected in the present study. The results indicated that the
protein levels of Bax were evidently increased from 0.23±0.03 to
1.08±0.13 (Fig. 3C; P<0.001),
whereas the protein levels of Bcl-2 were decreased from 1.21±0.09
to 0.84±0.08 (Fig. 3D; P<0.01) in
the control and curcumin-treated cells, respectively. Furthermore,
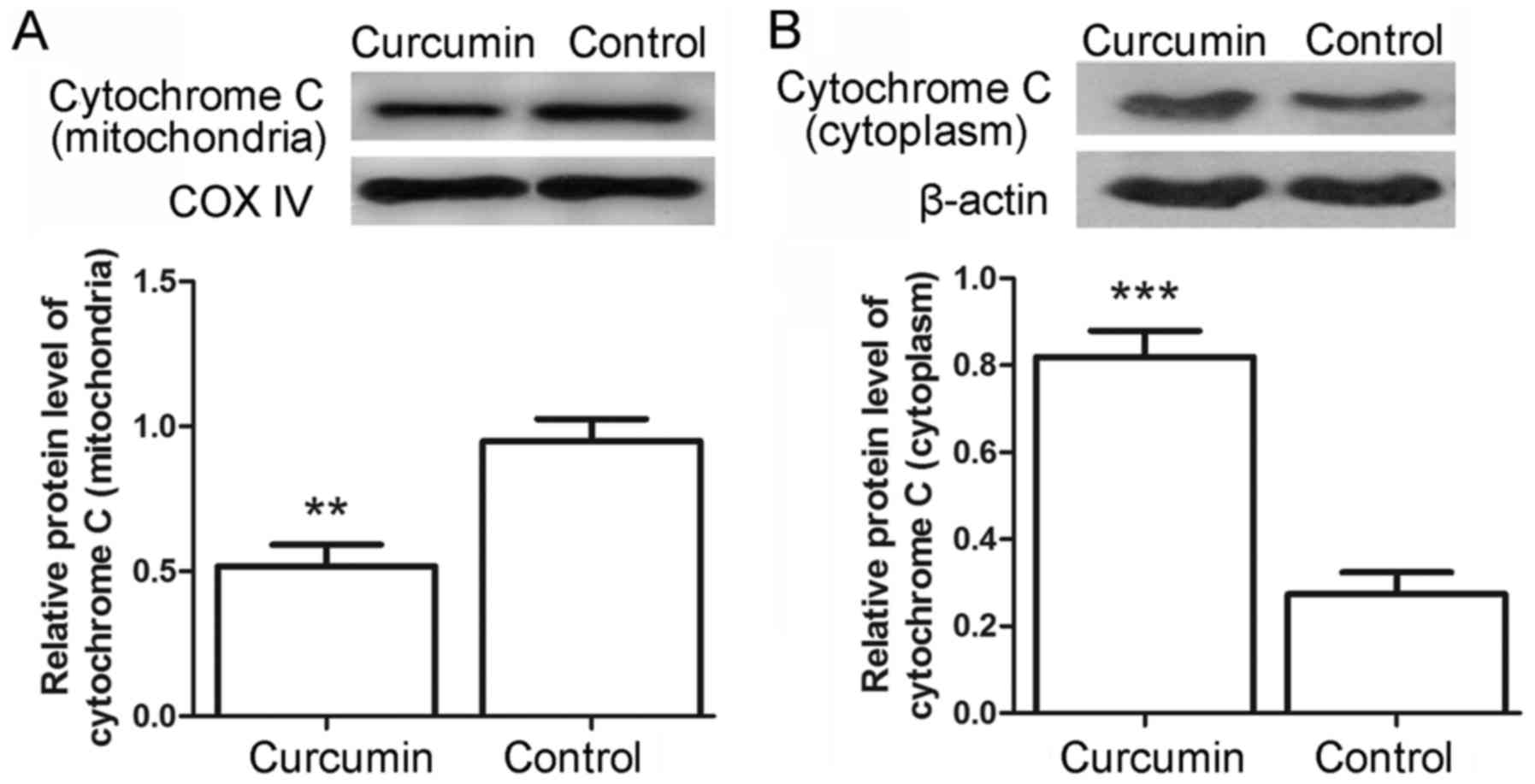
the release of cytochrome c from mitochondria was assessed
by western blot analysis. As shown in Fig. 4A and B, the levels of cytochrome
c in the mitochondria were decreased from 0.95±0.08 in the
control cells to 0.52±0.08 in the curcumin-treated cells
(P<0.01), whereas the levels of cytochrome c in the
cytoplasm were increased from 0.27±0.05 to 0.82±0.06 (P<0.001).
These results provided additional evidence for the
apoptosis-induced effects of curcumin.
Curcumin inhibits the activation of
the PI3K/AKT/mTOR signaling pathway
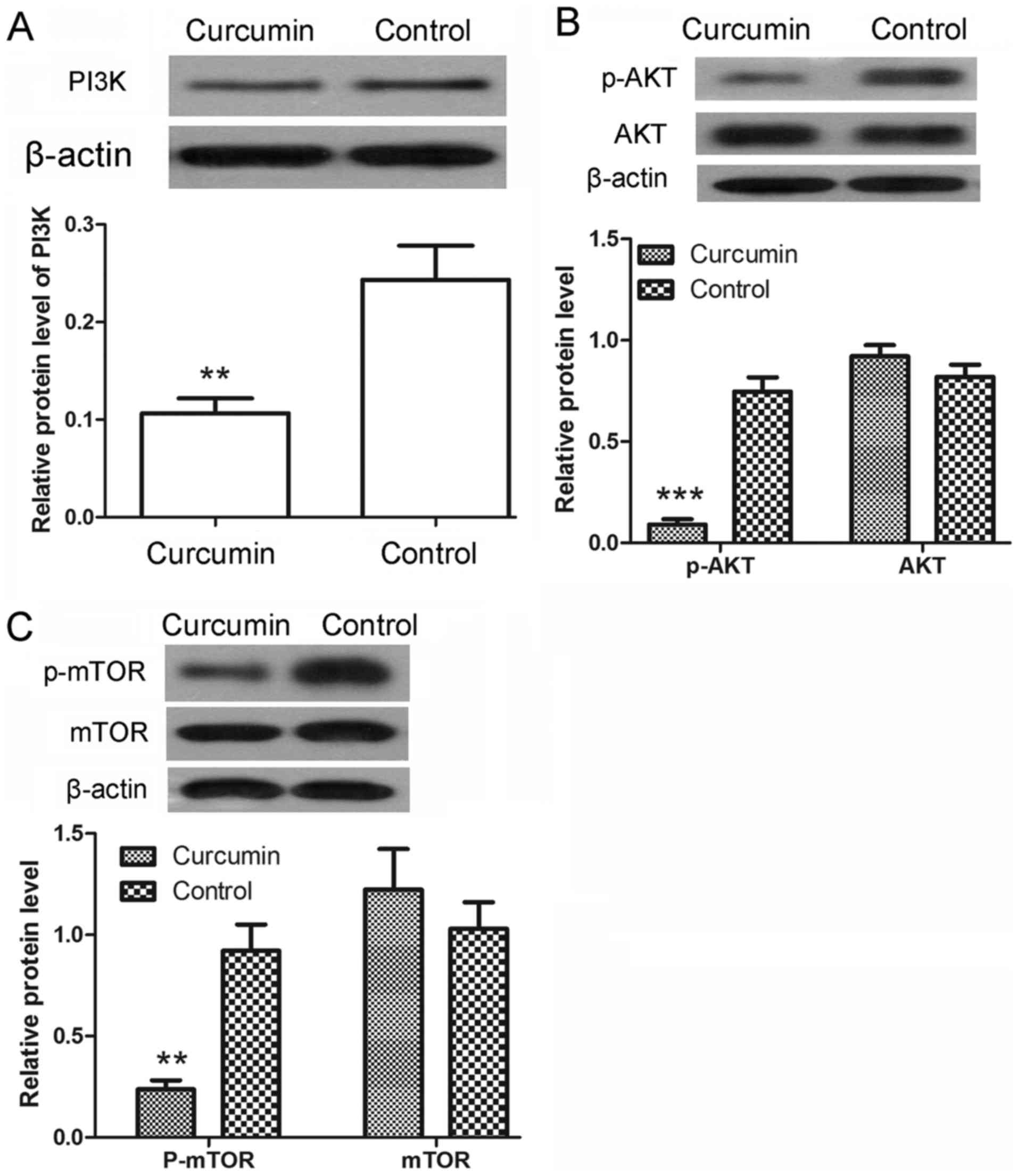
Following treatment with curcumin, the protein
expression levels of PI3K were detected by western blot analysis.
As shown in Fig. 5A, the protein
levels of PI3K were significantly decreased upon curcumin treatment
(P<0.01). The phosphorylation of AKT and mTOR was also detected
by western blot analysis. The results revealed that the protein
levels of p-AKT were significantly decreased after treatment with
curcumin (P<0.001), whereas the protein levels of AKT showed no
significant changes (Fig. 5B). There
was also a marked decrease in the protein levels of p-mTOR
(P<0.01), with no evident alteration observed in the protein
levels of mTOR (Fig. 5C). These
results demonstrated that the activation of PI3K/AKT/mTOR signaling
pathway was inhibited by curcumin.
PI3K/AKT activator 740Y-P reverses the
effects of curcumin
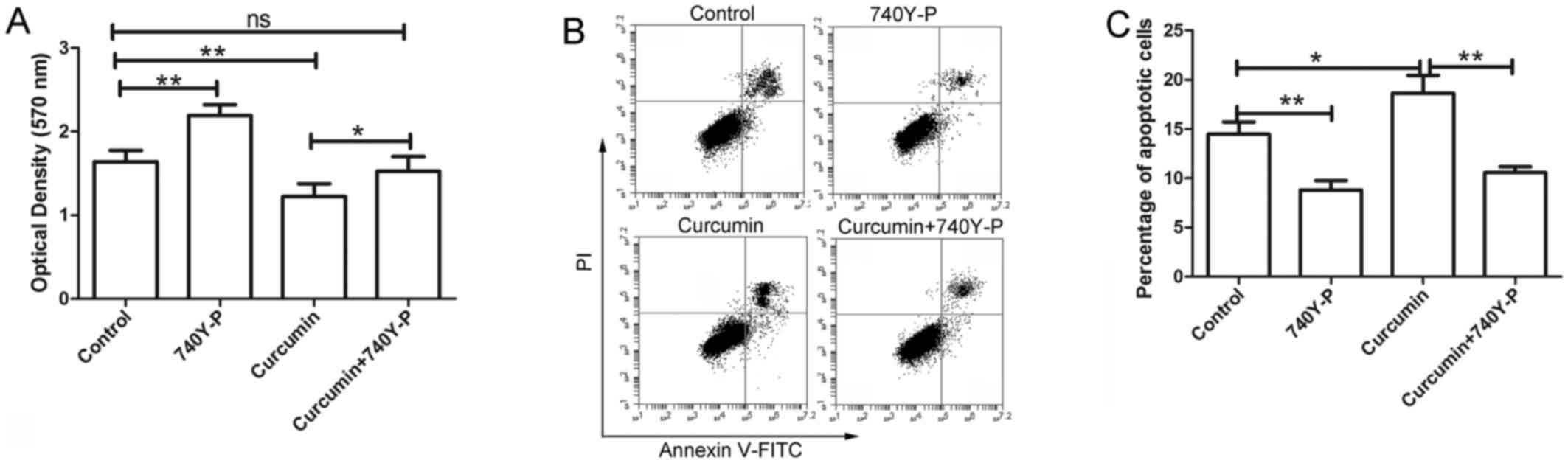
Following treatment with curcumin and/or 740Y-P, an
activator of PI3K/AKT signaling, the cell viability of LCSCs was
detected by MTT assay. The results of MTT assay revealed that
curcumin inhibited the proliferation of LCSCs, which was consistent
with the earlier observations of the present study. However, after
treatment with 740Y-P, the inhibitory effect of curcumin on the
proliferation of LCSCs was significantly reversed compared with the
cursumin group (Fig. 6A; P<0.05).
The cell apoptosis of LCSCs was also detected by flow cytometry
following treatment with 740Y-P. As shown in Fig. 6B and C, the apoptosis of LCSCs was
significantly increased after treatment with curcumin compared with
the control group (P<0.05), but was significantly reversed by
treatment with 740Y-P compared with the curcumin group
(P<0.01).
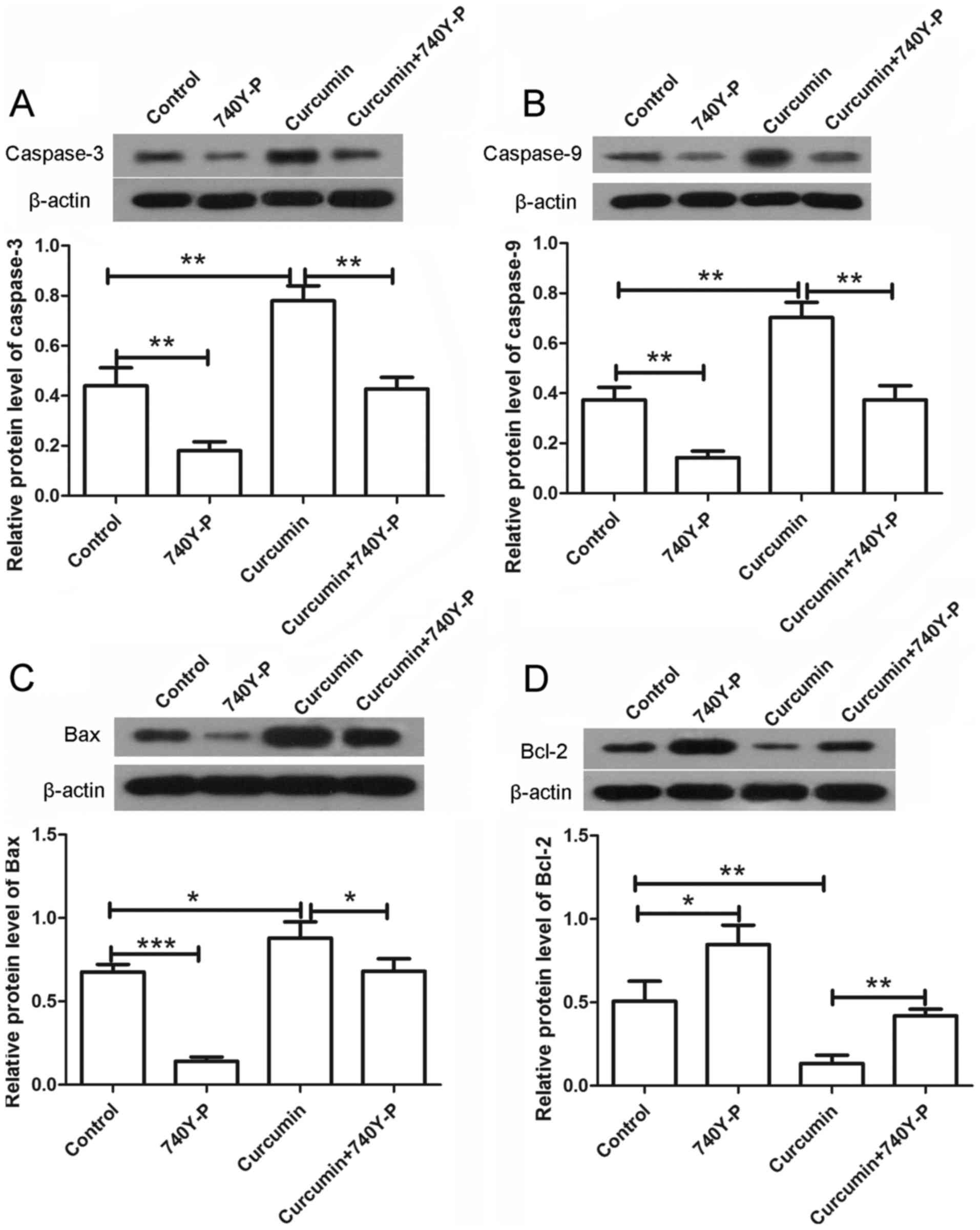
The expression levels of various
apoptosis-associated proteins were also detected by western blot
assay following treatment with curcumin and/or 740Y-P. As shown in
Fig. 7, the expression levels of
caspase-3 and caspase-9 were significantly increased upon treatment
with curcumin alone compared with the control group (P<0.01),
but were significantly decreased to nearly a normal level after
combined treatment with curcumin and 740Y-P compared with the
curcumin group (Fig. 7A and B;
P<0.01). In addition, the expression of Bax was increased and
the expression of Bcl-2 was decreased following treatment with
curcumin alone. However, upon treatment with curcumin and 740Y-P,
the expression of Bax was significantly decreased (P<0.05) and
the expression of Bcl-2 was significantly increased compared with
the curcumin group (Fig. 7C and D;
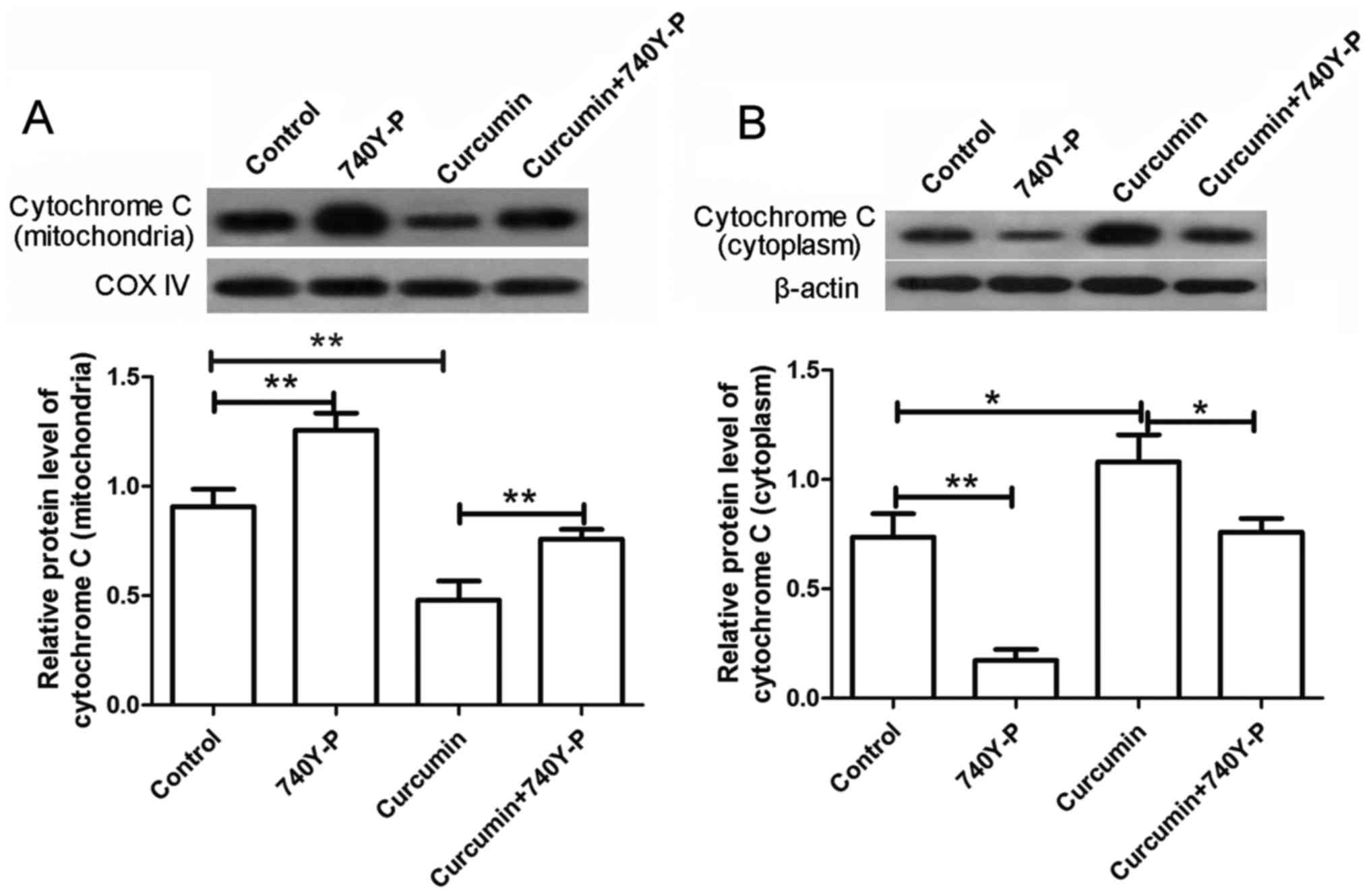
P<0.01). The levels of cytochrome c in the mitochondria
and cytoplasm were also detected by western blot analysis. The
results revealed that, after treatment with curcumin alone, the
levels of cytochrome c in the mitochondria were markedly
decreased, whereas the levels of cytochrome c in the
cytoplasm were markedly increased. By contrast, subsequent to
co-treatment with 740Y-P, the decreased levels of cytochrome
c in the mitochondria and increased levels in the cytoplasm
were significantly reversed compared with the curcumin group
(Fig. 8; P<0.01 and P<0.05,
respectively). These results revealed that, upon treatment with the
PI3K/AKT activator 740Y-P, the effects of curcumin on LCSCs were
reversed.
Discussion
Liver cancer is a type of malignancy with a strong
impact on human health, and LCSCs are important in the recurrence
of this tumor. In the present study, the effects of curcumin on the
growth of LCSCs were investigated. The results revealed that
curcumin inhibited the proliferation and induced apoptosis in
LCSCs. Further experiments demonstrated that the PI3K/AKT/mTOR
signaling pathway was involved in the growth-inhibitory effect of
curcumin.
Curcumin has been suggested to ameliorate liver
damage induced by various factors (19–22), and
to exert an anticancer effect in liver cancer (23–29). In
the current study, it was demonstrated that curcumin inhibited the
proliferation of LCSCs. As LCSCs are closely associated with the
recurrence of liver cancer, the present study suggests that
curcumin may have an excellent anticancer effect, reducing the
recurrence of liver cancer. Furthermore, curcumin has been observed
to exert a potential anticancer effect in multiple cancer types,
inhibiting the cancer-associated proliferation, migration and
angiogenesis (30–33).
Cancer stem cells are a population of cancer cells
with the ability to self-renew, differentiate, as well as initiate
and sustain tumor growth. It is considered that cancer stem cells
are responsible for cancer recurrence and chemoresistance (34,35).
Therefore, agents targeting cancer stem cells may have an improved
therapeutic effect in cancer and control tumor recurrence. In the
present study, it was observed that curcumin inhibited the growth
of LCSCs. Consistent with these findings, curcumin has previously
been reported to inhibit the growth of breast cancer and
glioblastoma stem cells (34,36).
Curcumin has chemopreventive and chemotherapeutic
effects in cancer, while Phase I and II clinical trials
demonstrated that this compound was well-tolerated in cancer
patients (37,38). Previous studies revealed that the
growth-inhibitory effect of curcumin on cancer cells was linked
with its apoptosis-inducing effect (39,40). In
the present study, curcumin was identified to induce apoptosis in
LCSCs. In addition, the protein levels of apoptosis-associated
caspase-3 and caspase-9 in LCSCs were also increased, which
provided additional evidence for the apoptosis-inducing effect of
curcumin. The current study also demonstrated an increased Bax
level and decreased Bcl-2 level in LCSCs following treatment with
curcumin, further suggesting the apoptosis-inducing effect of
curcumin. Furthermore, the ratio of Bcl-2/Bax is known to be
associated with the opening of the mitochondrial permeability
transition pores, and alterations in mitochondrial membrane
potential lead to the release of cytochrome c (41,42). In
the present study, release of cytochrome c was also detected
following treatment with curcumin. These results indicate that the
apoptosis-inducing effect of curcumin may be associated with the
mitochondrion-mediated apoptosis. The release of cytochrome
c leads to the activation of caspase-9, which is an
initiator of cell death (43). The
activation of caspase-9, in turn, leads to the cleave of caspase-3
and then the cleave of poly (ADP-ribose) polymerase 1, which is a
nuclear protein associated with programmed cell death (44). Finally, curcumin also affects the
cell cycle progression, autophagy, invasion, epithelial-mesenchymal
transition, angiogenesis and drug resistance of cancer cells
(45–51).
The PI3K/AKT signaling pathway is closely associated
with cell growth and is a critical target of chemotherapeutics. In
the present study, the results observed that treatment with
curcumin inhibited the activation of the PI3K/AKT/mTOR signaling
pathway; however, co-treatment with an activator of PI3K/AKT
reversed the effects of curcumin. These results demonstrate that
curcumin performs its growth-inhibitory effect in LCSCs through the
PI3K/AKT/mTOR signaling pathway. Curcumin can also impact the cell
cycle progression, autophagy, invasion, epithelial-mesenchymal
transition and angiogenesis of cancer cells through the PI3K/AKT
signaling pathway, thus inhibiting the growth and metastasis of
cancer (45–49). In addition, curcumin exerts an
anti-inflammatory effect and protects cardiomyocytes against high
glucose-induced apoptosis through this pathway (9,52).
Additionally, previous studies demonstrated that the Wnt/β-catenin,
Notch-1, nuclear factor-κB and mitogen-activated protein kinase
signaling pathways were also involved in the effects of curcumin in
cancer cells (51,53–57), and
these signaling pathways may also be involved in the
growth-inhibitory effect of curcumin in LCSCs; however, this
requires further investigation in future studies.
In conclusion, the present study demonstrated that
curcumin inhibited the growth of LCSCs through the PI3K/AKT/mTOR
signaling pathway. These results indicated that curcumin may be an
effective anticancer agent in the treatment of liver cancer and may
reduce the recurrence of liver cancer.
Acknowledgements
The authors would like to thank Dr Wei Xi (Jiangsu
Cancer Hospital, Nanjing, China) for his assistance in performing
the experiments, statistical analysis and manuscript drafting.
References
|
1
|
Hussain SA, Ferry DR, El-Gazzaz G, Mirza
DF, James ND, McMaster P and Kerr DJ: Hepatocellular carcinoma. Ann
Oncol. 12:161–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, risk factors, and screening. Semin Liver Dis.
25:143–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases, :
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu B, Sun D, Sun C, Sun YF, Sun HX, Zhu
QF, Yang XR, Gao YB, Tang WG, Fan J, et al: A polymeric
nanoparticle formulation of curcumin in combination with sorafenib
synergistically inhibits tumor growth and metastasis in an
orthotopic model of human hepatocellular carcinoma. Biochem Biophys
Res Commun. 468:525–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Visvader JE: Cells of origin in cancer.
Nature. 469:314–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tork OM, Khaleel EF and Abdelmaqsoud OM:
Altered cell to cell communication, autophagy and mitochondrial
dysfunction in a model of hepatocellular carcinoma: Potential
protective effects of curcumin and stem cell therapy. Asian Pac J
Cancer Prev. 16:8271–8279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu W, Zha W, Ke Z, Min Q, Li C, Sun H and
Liu C: Curcumin protects neonatal rat cardiomyocytes against high
glucose-induced apoptosis via PI3K/Akt signalling pathway. J
Diabetes Res. 2016:41585912016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seto K, Sakabe T, Itaba N, Azumi J, Oka H,
Morimoto M, Umekita Y and Shiota G: A novel small-molecule WNT
inhibitor, IC-2, has the potential to suppress liver cancer stem
cells. Anticancer Res. 37:3569–3579. 2017.PubMed/NCBI
|
|
11
|
Xiao Y, Lin M, Jiang X, Ye J, Guo T, Shi Y
and Bian X: The recent advances on liver cancer stem cells:
Biomarkers, separation, and therapy. Anal Cell Pathol (Amst).
2017:51086532017.PubMed/NCBI
|
|
12
|
Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo
J, Huang H, Du Q, Geller DA and Cheng B: Notch and Wnt/β-catenin
signaling pathway play important roles in activating liver cancer
stem cells. Oncotarget. 7:5754–5768. 2016.PubMed/NCBI
|
|
13
|
Sandur SK, Ichikawa H, Pandey MK,
Kunnumakkara AB, Sung B, Sethi G and Aggarwal BB: Role of
pro-oxidants and antioxidants in the anti-inflammatory and
apoptotic effects of curcumin (diferuloylmethane). Free Radic Biol
Med. 43:568–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suckow BK and Suckow MA: Lifespan
extension by the antioxidant curcumin in Drosophila melanogaster.
Int J Biomed Sci. 2:402–405. 2006.PubMed/NCBI
|
|
15
|
Yoysungnoen P, Wirachwong P, Changtam C,
Suksamrarn A and Patumraj S: Anti-cancer and anti-angiogenic
effects of curcumin and tetrahydrocurcumin on implanted
hepatocellular carcinoma in nude mice. World J Gastroenterol.
14:2003–2009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masuelli L, Benvenuto M, Fantini M,
Marzocchella L, Sacchetti P, Di Stefano E, Tresoldi I, Izzi V,
Bernardini R, Palumbo C, et al: Curcumin induces apoptosis in
breast cancer cell lines and delays the growth of mammary tumors in
neu transgenic mice. J Biol Regul Homeost Agents. 27:105–119.
2013.PubMed/NCBI
|
|
17
|
Zhang CY, Zhang L, Yu HX, Bao JD and Lu
RR: Curcumin inhibits the metastasis of K1 papillary thyroid cancer
cells via modulating E-cadherin and matrix metalloproteinase-9
expression. Biotechnol Lett. 35:995–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JH, Luo Q, Liu LL and Song GB: Liver
cancer stem cell markers: Progression and therapeutic implications.
World J Gastroenterol. 22:3547–3557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Afrin R, Arumugam S, Rahman A, Wahed MI,
Karuppagounder V, Harima M, Suzuki H, Miyashita S, Suzuki K,
Yoneyama H, et al: Curcumin ameliorates liver damage and
progression of NASH in NASH-HCC mouse model possibly by modulating
HMGB1-NF-κB translocation. Int Immunopharmacol. 44:174–182. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong W, Qian K, Xiong J, Ma K, Wang A and
Zou Y: Curcumin alleviates lipopolysaccharide induced sepsis and
liver failure by suppression of oxidative stress-related
inflammation via PI3K/AKT and NF-κB related signaling. Biomed
Pharmacother. 83:302–313. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, Guo Y, Deng Y, Li C, Zuo C and Peng
W: Involvement of protoporphyrin IX accumulation in the
pathogenesis of isoniazid/rifampicin-induced liver injury: The
prevention of curcumin. Xenobiotica. 47:154–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zabihi NA, Pirro M, Johnston TP and
Sahebkar A: Is there a role for curcumin supplementation in the
treatment of non-alcoholic fatty liver disease? The data suggest
yes. Curr Pharm Des. 23:969–982. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellerkamp V, Bortel N, Schmid E, Kirchner
B, Armeanu-Ebinger S and Fuchs J: Photodynamic therapy potentiates
the effects of curcumin on pediatric epithelial liver tumor cells.
Anticancer Res. 36:3363–3372. 2016.PubMed/NCBI
|
|
24
|
Bortel N, Armeanu-Ebinger S, Schmid E,
Kirchner B, Frank J, Kocher A, Schiborr C, Warmann S, Fuchs J and
Ellerkamp V: Effects of curcumin in pediatric epithelial liver
tumors: Inhibition of tumor growth and alpha-fetoprotein in vitro
and in vivo involving the NFkappaB- and the beta-catenin pathways.
Oncotarget. 6:40680–40691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duan W, Chang Y, Li R, Xu Q, Lei J, Yin C,
Li T, Wu Y, Ma Q and Li X: Curcumin inhibits hypoxia inducible
factor-1α-induced epithelial-mesenchymal transition in HepG2
hepatocellular carcinoma cells. Mol Med Rep. 10:2505–2510. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiablaem K, Lirdprapamongkol K,
Keeratichamroen S, Surarit R and Svasti J: Curcumin suppresses
vasculogenic mimicry capacity of hepatocellular carcinoma cells
through STAT3 and PI3K/AKT inhibition. Anticancer Res.
34:1857–1864. 2014.PubMed/NCBI
|
|
27
|
Xu MX, Zhao L, Deng C, Yang L, Wang Y, Guo
T, Li L, Lin J and Zhang L: Curcumin suppresses proliferation and
induces apoptosis of human hepatocellular carcinoma cells via the
wnt signaling pathway. Int J Oncol. 43:1951–1959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai XZ, Yin HT, Sun LF, Hu X, Zhou C, Zhou
Y, Zhang W, Huang XE and Li XC: Potential therapeutic efficacy of
curcumin in liver cancer. Asian Pac J Cancer Prev. 14:3855–3859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Park SY, Park OJ and Kim YM:
Curcumin suppresses migration and proliferation of Hep3B
hepatocarcinoma cells through inhibition of the Wnt signaling
pathway. Mol Med Rep. 8:282–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sa G and Das T: Anti cancer effects of
curcumin: Cycle of life and death. Cell Div. 3:142008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sinha D, Biswas J, Sung B, Aggarwal BB and
Bishayee A: Chemopreventive and chemotherapeutic potential of
curcumin in breast cancer. Curr Drug Targets. 13:1799–1819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhandarkar SS and Arbiser JL: Curcumin as
an inhibitor of angiogenesis. Adv Exp Med Biol. 595:185–195. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dandawate PR, Subramaniam D, Jensen RA and
Anant S: Targeting cancer stem cells and signaling pathways by
phytochemicals: Novel approach for breast cancer therapy. Semin
Cancer Biol 40–41. 1–208. 2016.
|
|
35
|
Subramaniam D, Kaushik G, Dandawate P and
Anant S: Targeting cancer stem cells for chemoprevention of
pancreatic cancer. Curr Med Chem. Jan 26–2017.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gersey ZC, Rodriguez GA, Barbarite E,
Sanchez A, Walters WM, Ohaeto KC, Komotar RJ and Graham RM:
Curcumin decreases malignant characteristics of glioblastoma stem
cells via induction of reactive oxygen species. BMC Cancer.
17:992017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF,
Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
38
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
40
|
Ravindran J, Prasad S and Aggarwal BB:
Curcumin and cancer cells: How many ways can curry kill tumor cells
selectively? AAPS J. 11:495–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bagci EZ, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: Roles of bax,
bcl-2, and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eissing T, Waldherr S, Allgöwer F,
Scheurich P and Bullinger E: Response to bistability in apoptosis:
Roles of bax, bcl-2, and mitochondrial permeability transition
pores. Biophys J. 92:3332–3334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wurstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Y, Kong Y, Liu S, Zeng L, Wan L and
Zhang Z: Curcumin induces apoptosis in human leukemic cell lines
through an IFIT2-dependent pathway. Cancer Biol Ther. 18:43–50.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang C, Zhang X, Teng Z, Zhang T and Li Y:
Downregulation of PI3K/Akt/mTOR signaling pathway in
curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J
Pharmacol. 740:312–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu X, Qin J and Liu W: Curcumin inhibits
the invasion of thyroid cancer cells via down-regulation of
PI3K/Akt signaling pathway. Gene. 546:226–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen WC, Lai YA, Lin YC, Ma JW, Huang LF,
Yang NS, Ho CT, Kuo SC and Way TD: Curcumin suppresses
doxorubicin-induced epithelial-mesenchymal transition via the
inhibition of TGF-β and PI3K/AKT signaling pathways in
triple-negative breast cancer cells. J Agric Food Chem.
61:11817–11824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lopes-Rodrigues V, Oliveira A,
Correia-da-Silva M, Pinto M, Lima RT, Sousa E and Vasconcelos MH: A
novel curcumin derivative which inhibits P-glycoprotein, arrests
cell cycle and induces apoptosis in multidrug resistance cells.
Bioorg Med Chem. 25:581–596. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kang Y, Hu W, Bai E, Zheng H, Liu Z, Wu J,
Jin R, Zhao C and Liang G: Curcumin sensitizes human gastric cancer
cells to 5-fluorouracil through inhibition of the NFκB
survival-signaling pathway. Onco Targets Ther. 9:7373–7384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cianciulli A, Calvello R, Porro C, Trotta
T, Salvatore R and Panaro MA: PI3k/Akt signalling pathway plays a
crucial role in the anti-inflammatory effects of curcumin in
LPS-activated microglia. Int Immunopharmacol. 36:282–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zheng R, Deng Q, Liu Y and Zhao P:
Curcumin inhibits gastric carcinoma cell growth and induces
apoptosis by suppressing the Wnt/β-catenin signaling pathway. Med
Sci Monit. 23:163–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Prasad CP, Rath G, Mathur S, Bhatnagar D
and Ralhan R: Potent growth suppressive activity of curcumin in
human breast cancer cells: Modulation of Wnt/β-catenin signaling.
Chem Biol Interact. 181:263–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang J, Wang C, Zhang Z, Chen X, Jia Y,
Wang B and Kong T: Curcumin inhibits the survival and metastasis of
prostate cancer cells via the Notch-1 signaling pathway. APMIS.
125:134–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tong W, Wang Q, Sun D and Suo J: Curcumin
suppresses colon cancer cell invasion via AMPK-induced inhibition
of NF-κB, uPA activator and MMP9. Oncol Lett. 12:4139–4146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dai C, Lei L, Li B, Lin Y, Xiao X and Tang
S: Involvement of the activation of Nrf2/HO-1, p38 MAPK signaling
pathways and endoplasmic reticulum stress in furazolidone induced
cytotoxicity and S phase arrest in human hepatocyte L02 cells:
Modulation of curcumin. Toxicol Mech Methods. 27:165–172. 2017.
View Article : Google Scholar : PubMed/NCBI
|