Introduction
The treatment process of cancer varies according to
the type, and it comprises different steps, including surgery,
radiotherapy, and chemotherapy. The agents used in chemotherapy
have different mechanisms of actions. Nevertheless, the agents with
the most efficient selective cytotoxicity features spread rapidly
in cells with active cell division and cause a variety of
toxicities revealed as neutropenia, anemia, thrombocytopenia, hair
follicle cell damage, and apoptosis of hemopoietic stem cells
(1,2). Some agents, such as cisplatin, taxon,
and vinca alkaloids damage the peripheral nervous system, resulting
in sensory-motor neuropathy. Also, cisplatin can damage the
testicles and cause infertility (1,2).
Despite the fact that cisplatin is one of the most
efficient antineoplastic drugs used in the treatment of many cancer
types in clinical oncology, the most important side effect
restricting its use is dose-dependent toxicity. Infertility is
proportional to testicular damage resulting from cisplatin
(1–4). In general, infertility problems occur
after chemotherapy and radiotherapy. Thus, the protection of
reproductive health is an important issue that needs to be resolved
in cancer patients with reproduction concerns. In this context,
many researchers have classified the chemotherapeutic agents with
wide clinical use as having high, intermediate, and low risk
(4–6).
Because amifostine reduced cumulative tissue
toxicity generated by cisplatin in patients with advanced stage
cancer, except for small cell lung carcinoma, it was approved by
the food and drug administration (FDA) in the United States in 1996
(7–10). Amifostine has been shown to be
effective in preventing the side effects such as nephrotoxicity,
ototoxicity, and neurotoxicity in both preclinical and clinical
studies.
Curcumin is a diarylheptanoid and exhibits
relatively powerful antioxidant, anti-inflammatory,
antiproliferative and anti-tumor effects. The phenolic-OH group of
curcumin, as well as its β diketone derivatives, react with some
reactive oxidative species. Thus, curcumin prevents lipid
peroxidation and oxidative DNA damage and reduces the release of
arachidonic acid as a result of inhibition of oxidative
lipoxygenase and cyclooxygenase enzymes. Also, it inhibits the
activation of NF-κB and induces an anti-inflammatory effect
(11–13).
CAPE is an active component of propolis extracts and
is known to have antioxidant properties (14–16) CAPE
exhibits different properties, such as balancing of intracellular
calcium and removal of free radicals (17,18).
Moreover, it also exhibits antioxidant properties by enhancing the
effects of superoxide dismutase, glutathione peroxidase,
glutathione reductase, and glucose-6-phosphate dehydrogenase
stimulation. Due to the aforementioned biological effects of CAPE,
it may demonstrate a protective effect in cancer treatment
concerning organ and tissue damage associated with chemotherapy
(19,20).
The aim of the present study was to investigate in
rats the protective efficacies of the antioxidant substances
amifostine, curcumin, and CAPE in cisplatin-induced testicular
damage and associated infertility that require sperm
cryopreservation prior to therapy.
Materials and methods
Animal study
Male Sprague Dawley rats, (3–4 months old weighing
200–300 g) were procured from the Ataturk University Animal Care
and Research Unit (Erzurum, Turkey). All animals received humane
care according to the criteria outlined in the ‘Guide for the Care
and Use of Laboratory Animals’ prepared by the National Academy of
Sciences and published by the National Institutes of Health. The
study was approved by the Ataturk University Institutional Animal
Ethical Committee (Erzurum, Turkey) (2013; 36643897-126).
Animal study
The animals were kept at 21±3°C and exposed to 12 h
light/dark cycles. Pellets containing 21% crude protein (Purina)
and water were provided ad libitum. Rats were allocated to five
groups of six, which were untreated (group I control) or treated
with cisplatin (group II), cisplatin+amifostine (group III),
cisplatin+curcumin (group IV) and cisplatin+CAPE (group V).
Drug preparation and experimental
protocols
All interventions to each experimental group, except
for the control group, were performed in the facilities of the
Experiment Animals Research Unit of the University. Group 1 helped
to determine baseline values of histopathological parameters.
Previous experimental studies demonstrated that at low doses
(1.1–2,5 mg/kg), cisplatin was selectively toxic for spermatogonia.
At higher doses (10–20 mg/kg), cisplatin had toxic effects on the
spermatocytes and spermatids in the adluminal compartment. We used
cisplatin at 5 mg/kg to bypass some of the higher dose side effects
(21–23). A single dose of 5 mg/kg cisplatin was
given to the indicated groups by intraperitoneal (IP) injection.
400 mg/kg/day of amifostine (in ethanol) was applied IP to group
III (10), starting 24 h prior to
the application of cisplatin injection and continued for seven
days. 100 mg/kg/day of curcumin (Sigma, St. Louis, MO, USA)
dissolved in dimethyl sulfoxide (DMSO) was given orally to group IV
(24,25), starting 24 h before the application
of cisplatin injection and continued for seven days. 10 µmol/kg/day
of CAPE (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) dissolved
in DMSO was applied IP to group V (17,18),
starting 24 h before the application of cisplatin injection and
continued for seven days.
Seven days after the application of cisplatin, the
rats were sacrificed by cervical dislocation under general
anaesthesia induced by 10 mg/kg xylazine (Rompun, Bayern, Istanbul,
Turkey) and 90 mg/kg ketamine (Ketalar, Eczacıbaşı Co., Istanbul,
Turkey). The testes were removed, histologic sections were taken
and processed for light and electron microscopic examinations.
The weights of all rats were recorded on day 1
(baseline), before cisplatin administration, and on day 7, after
the completion of the study and before sacrifice.
Histological preparation
Testicular tissue sections were fixed in bouin
solution and subsequently embedded in paraffin. 4–5 µm thick
sections were prepared for hematoxylin-eosin (H&E), Periodic
Acid Schiff (PAS), and Masson trichrome staining (Applichem GmbH,
Darmstadt, Germany). Preparations were analyzed under a light
microscope (DM6200; Leica Microsystems GmbH, Wetzlar, Germany).
Photos were taken using the Olympus DP20 camera (Olympus
Corporation, Tokyo, Japan).
Immunohistochemistrigical
preparation
Testicular tissue blocks were fixed in 2.5%
formaldehyde and were subsequently embedded in paraffin. 1–3 µm
sections were prepared and immunologically stained for NF-κB/p65
(1:100, Anti-NF-κB/p65 antibody, ab16502; Abcam, Cambridge, UK),
caspase-3 (1:100, rabbit anti-caspase 3 polyclonal antibody,
ab4051; Abcam) and 8-OHdG (1:50, KOG-HS10E, Dako Envision kit;
Japan Institute for the Control of Aging (JaICA), Nikken SEIL Co.,
Ltd., Shizuoka, Japan), using the Ventana Benchmark GX (USA)
device. Then the secondary antibody was applied (UltraView
Universal DAB Detection kit, 760–500; Ventana Medical Systems,
Inc., Tucson, AZ, USA). Sections were examined under a light
microscope and photos were taken as described above.
Stereological analysis
For stereological examination, software
(Stereoinvestigator 8; MBF Bioscience, Williston, VT, USA) and
camera attachment were used. Counting grid size and frame size were
run biased estimated. The measurement area was sampled
systematically and randomly over the fractionator probe. NF-κB/p65,
caspase-3, and 8-OHdG positive cells were counted at ×40
magnification. Slides were examined without any randomising in
their orientation. Positive cells were determined as described
previously (26–28). Eventually, the mean numerical density
of NF-κB/p65, caspase-3, and 8-OHdG positive cell were estimated by
the following formula:
Nv=QSxA
Nv: numerical density, Q: total markers counted, S:
no. of sampling sides, A: counting frame area.
Transmission electron microscopy
The tissues were fixed in 100 mM phosphate buffer
containing 2.5% glutaraldehyde for 1.5 h at 4°C. The samples were
rinsed in phosphate buffer and stored at 4°C for later processing.
These were post-fixed in 1% osmium tetroxide, dehydrated in ethanol
series and then embedded in Epoxy resin (Araldite CY212) kit (Agar
Scientific, Ltd., Stansted, UK). 750 nano meter thick sagittal
sections were cut and stained with toluidine blue for light
microscopic analysis. Stained sections were visualized and imaged
using a Leica DM 6400 (Leica Microsystems GmbH). For electron
microscopic analysis, 40–70 nm thick sections were cut using
ultramicrotome (LKB Nova, Sweden) set on 200-mesh copper or nickel
grids. The sections were stained with uranyl acetate and Reynold's
lead citrate for transmission electron microscopy. Imaging was
achieved with the JEOL 100SX transmission electron microscope,
(JEOL, Ltd., Tokyo, Japan) and photographed (Kodak 4489; Kodak,
Rochester, NY, USA).
Statistical analysis
The numerical density of NF-κB/p65, 8-OHdG, and
caspase-3 positive cells was calculated. Data were expressed as
mean ± SEM (standard error of the mean). Statistical analysis was
performed using one-way analysis of variance followed by Duncan's
test for each paired experiment value of P<0.05, which is
considered to be significant. Body weights were expressed as mean ±
SEM. Statistical analysis was performed using paired samples t test
for each paired experiment value of P<0.05, which was considered
to indicate a statistically significant difference. The package
used for statistical analyses was the IBM SPSS Statistics v20 (IBM
Corp., Armonk, NY, USA).
Results
Light microscopy results
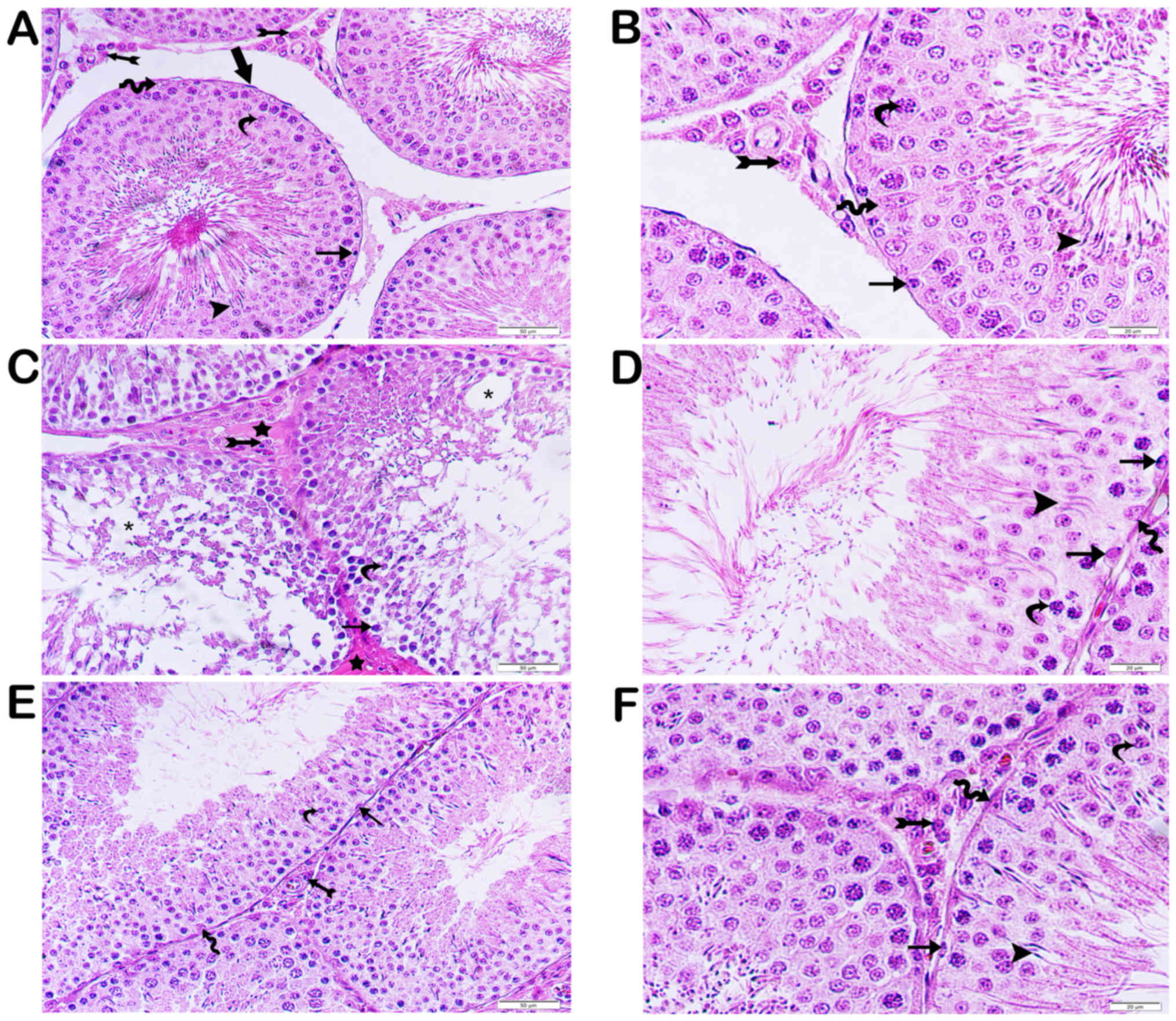
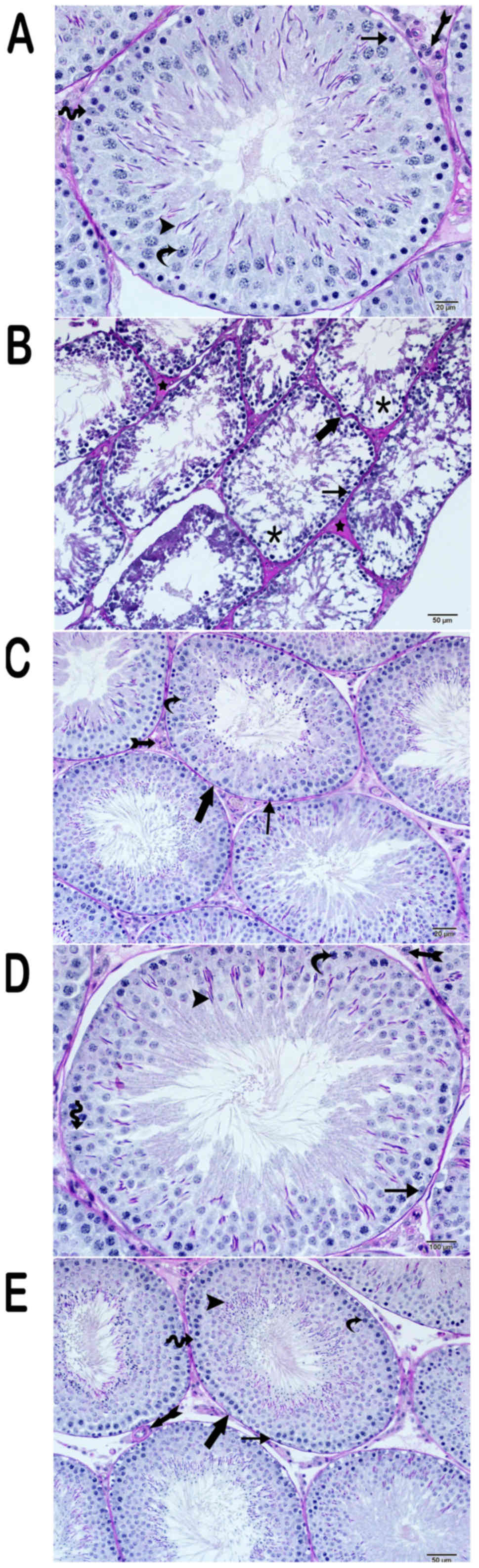
The testicular structure of the control group was
normal (Figs. 1 and 2). The connective tissue, vascular
structures, and Leydig cells formed a typical structure. The basal
lamina of the testicular seminiferous tubules was of usual
regularity (Figs. 1A and B, 2A).
The testicular tissue of the cisplatin group was
extensive oedematous as a result of the loss of the germinal
epithelial cells of the seminiferous tubules. In the remaining
germinative epithelium, the connection between spermatocytes and
spermatogonia with dense chromatin containing atypical nuclei was
lost. Also, the numbers of spermatozoa and spermatids were lower
than those of the control group. The basal lamina of the
seminiferous tubules was degenerate (Fig. 2B). There was intense hyalinization of
the interstitial spaces. In addition, there was vascular congestion
in the interstitial spaces and tunica albuginea. The Leydig cells
had fragmented nuclei, and their number was low in the interstitial
spaces. (Figs. 1C, 2B).
The testicular tissue structures of the amifostine
group (group III) had normal germinal epithelial cells and Leydig
cells. The basal lamina of the testicular seminiferous tubules was
regular (Figs. 1C, 2C).
In the curcumin-treated group, there was little
inter-epithelial cell vacuolization of the seminiferous tubules.
The spermatogonia and spermatids were normal. Spermatozoa in the
seminiferous tubules were far more numerous than the cisplatin
group. Hyalinization of the interstitial space was less intense
than that of the cisplatin group. (Figs.
1E, 2D). Similarly, the
regularity of the basal layer of the seminiferous tubules was less
than the corresponding cisplatin group (Fig. 2D).
Microscopically, in the testicular tissue sections
obtained from group V, the seminiferous tubules showed
irregularity. Hyalinization of the connective tissue decreased,
which was similar to the cisplatin treatment group (group IV)
(Figs. 1E, 2E). The germinal epithelium had a thickness
close to those of the control group, and the edema was reduced
between the serial cells. However, the spermatids were located in
both the adluminal and luminal compartments. They were similar to
the control and amifostine groups. The basal lamina of the
seminiferous tubules was regular.
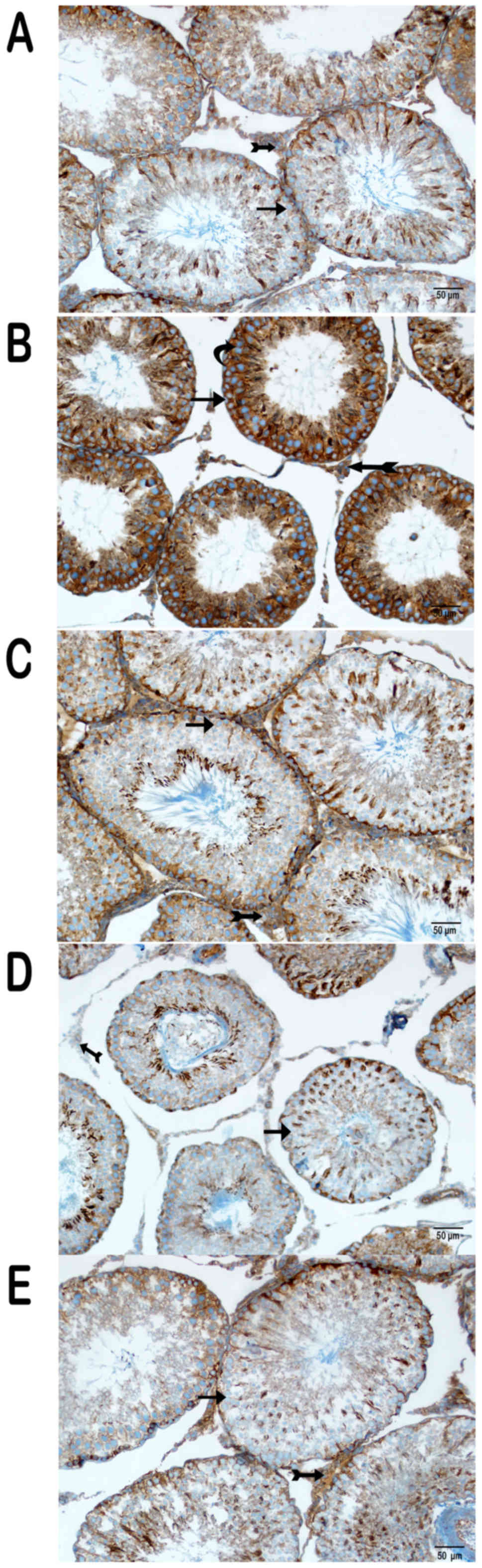
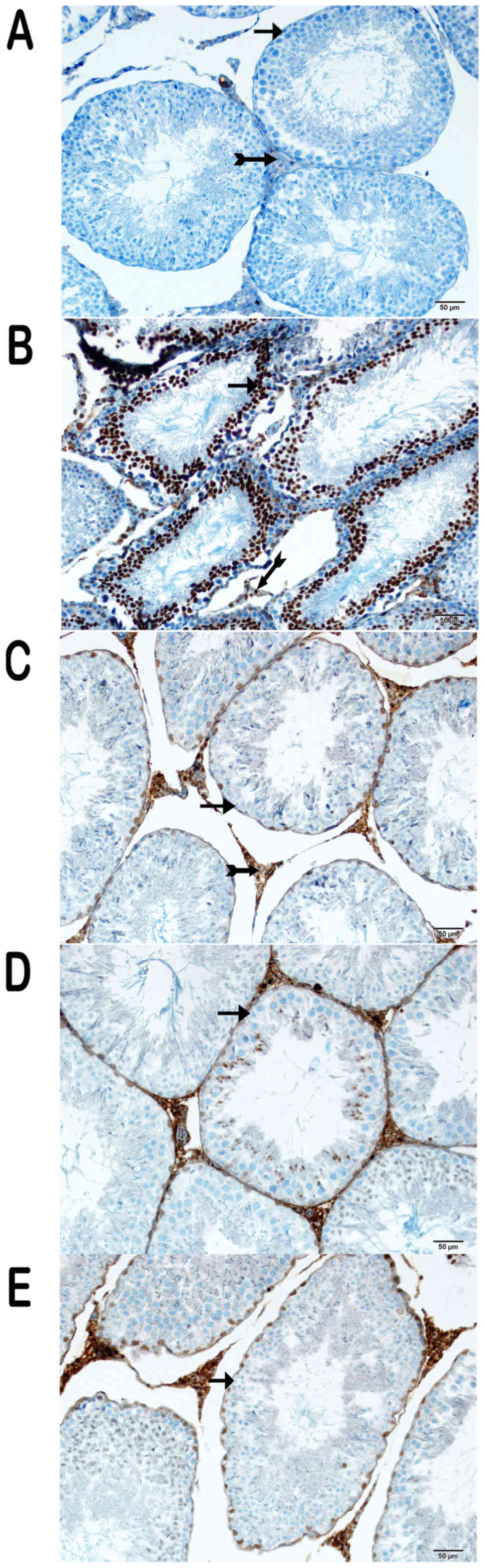
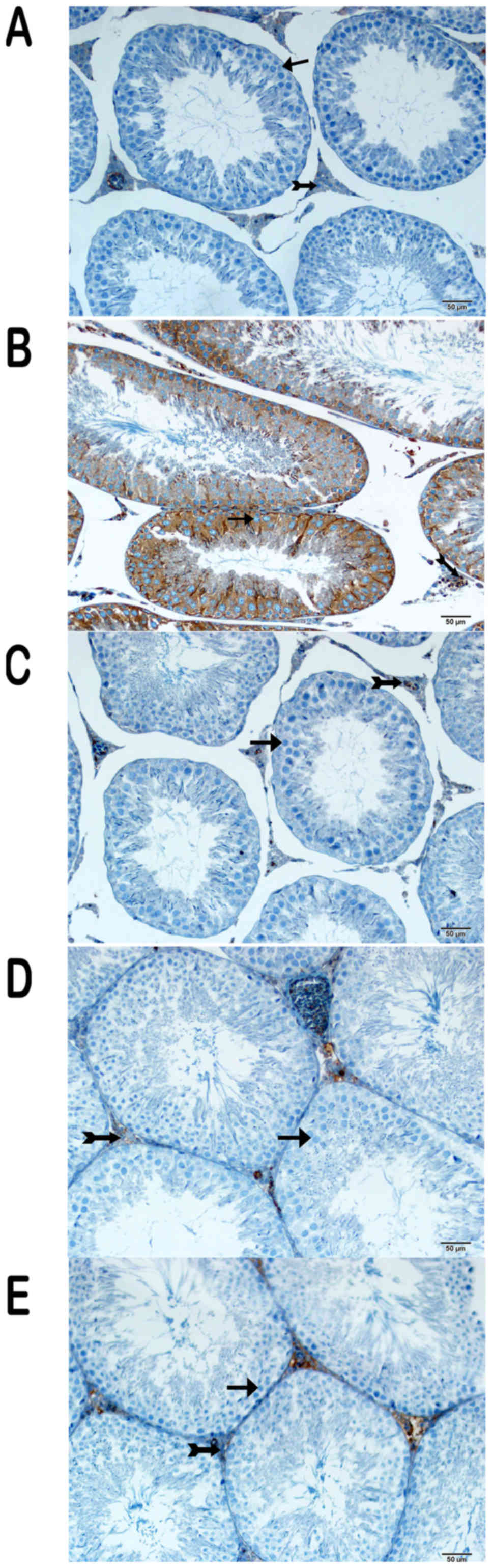
Immunohistochemical (IHC) results
IHC was performed to determine the expression of
various proteins (Figs. 3, 4 and 5).
There was intense cytoplasmic staining for NF-κB/p65, caspase-3 and
8-OHdG in the germinal epithelium of the seminiferous tubules,
particularly in type A and type B spermatogonia, primary
spermatocytes and spermatids in the cisplatin group (Figs. 3B, 4B
and 5B) (Table I). On the other hand, sections from
group III were negative for these markers (Figs. 3C, 4C
and 5C) (Table I), as were those of group IV
(Figs. 3D, 4D, and 5D)
(Table I), except for the weak
cytoplasmic staining in Sertoli cells and spermatids in the latter
group. Partial cytoplasmic staining was observed in the germinal
and Sertoli cells in group V (Figs.
3E, 4E, and 5E) (Table
I).
 | Table I.Immunohistochemistry positivity
numerical density. |
Table I.
Immunohistochemistry positivity
numerical density.
| Group | NF-κB numerical
density (mm2) | Caspase-3 numerical
density (mm2) | 8-OHdG numerical
density (mm2) |
|---|
| Control |
5±1.15 |
5.5±1.8 |
7.16±2.35 |
| cisplatin |
142.33±14.01a |
77.33±8.14a |
79±5.63a |
|
cisplatin+amifostine |
7.5±1.33b |
17.5±2.83b |
10.5±1.78b |
|
cisplatin+curcumin |
15.17±2.19b |
23.5±3.89b |
23.83±4.57b |
| cisplatin+CAPE |
23.33±4.93b |
29.5±3.93b |
26.33±5.05b |
Electron microscopy results
The basal lamina of the seminiferous tubules of the
sections had a regular fine structure under transmission electron
microscopy (Fig. 6A) in the control
group. On the other hand, the corresponding structures in the
cisplatin group showed degeneration. Also, apoptotic spermatogonia
and spermatocytes were present. Fragmented spermatogonia and
spermatid nuclei were also observed (Figs. 6B-D). The nucleolus of the
spermatogonia was condensed and lost its stellar appearance
(Fig. 6B-D). Vacuoles occurred as a
result of cytoplasmic loss were seen in spermatocytes and
spermatogonia (Fig. 6B-D). Apoptotic
spermatids were also noted. Mitochondrial vacuolizations were also
detected in spermatogonia and spermatocytes (Fig. 6B-D).
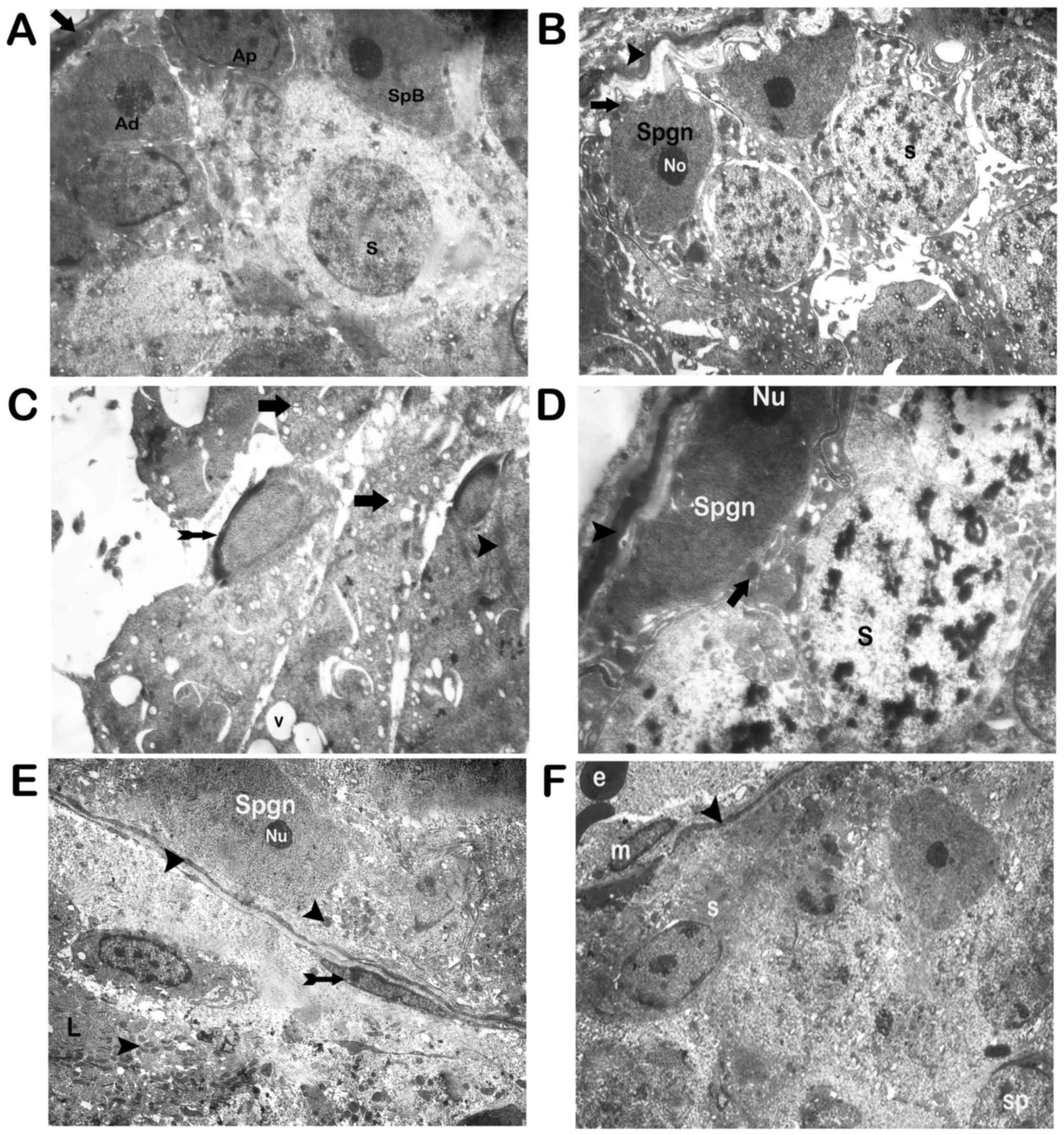 | Figure 6.Transmission electron microscope
micrographs of testicular tissue with Uranyl acetate and Reynold's
lead citrate staining. (A) Control group: A normal basal lamina of
the seminiferous tubule with the myoepithelial cell (indicated by
an arrow), containing dark type A spermatogonia (indicated by Ad);
and Pale type A spermatogonia (indicated by Ap) and type B
spermatogonia (indicated by SpB). Spermatocytes are indicated by s
(magnification, 3,000x). (B) Cis applied group: Deterioration of
integrity between spermatogonia and degenerative basal lamina were
monitored (indicated by arrowheads); Fragmented nucleus (indicated
by arrows) and Nucleolus (indicated by No; magnification, 3,000x).
(C) C is applied group: Degenerated mitochondria (indicated by
arrows); spermatid nuclear caps (indicated by tailed arrow); ring
formation (indicated by arrowhead); vacuoles (indicated by v;
magnification, 3,000x). (D) Cis+amifostine applied group:
Spermatocytes (indicated by s); typical basal lamina was observed
(indicated by arrowheads); spermatogonia (indicated by Spgn);
typical mitochondria with mitochondrial membranes were monitored
(indicated by arrows); Nucleolus (indicated by Nu; magnification,
3,000x). (E) Cis+curcumin applied group: Typical mitochondria with
dense mitochondrial were observed (indicated by arrowheads);
nucleus (indicated by Nu); myoepithelial cell (indicated by tailed
arrow); the regular Leydig cell was seen (indicated by L;
magnification, 3,000x). (F) Cis+CAPE applied group; Mitochondria
(indicated by m); basal lamina (indicated by arrowheads);
spermatocytes (indicated by s); spermatids (indicated by sp);
erythrocyte (indicated by e; magnification, 2,500x). Cis,
cisplatin; CAPE, caffeic acid phenethyl ester. |
Electron microscopic examination of testicular
sections of group III revealed normal basal lamina (Fig. 6E). Spermatogonium and spermatocytes
with typical fine structure were observed. Mitochondria with an
intense matrix and typical fine structure were seen in
spermatogonia and spermatocytes (Fig.
6F). The Leydig cells had normal fine structure and
mitochondria with typical crystals and intense matrix content.
Group IV sections displayed normal myoepithelial
cells with a normal fine structure of the basal lamina.
Spermatogonia and spermatocytes with a typical fine structure were
also observed. The mitochondria had an intense matrix with typical
fine structure. The mitochondria of the Leydig cells had a normal
fine structure (Fig. 6E).
Sections of group V revealed typical myoepithelial
cells with a fine structure of the basal lamina. Although the
appearance of the spermatogonia and spermatocytes on the basal
lamina were regular, vacuoles were present in spermatogonia and
spermatocytes in small numbers (Fig.
6F).
Statistical analysis
With the exception of the controls, there was a
statistically significant decrease in the body weights of the
animals after the treatment (P<0.05) (Table II).
 | Table II.Body weights of rats before and after
treatment. |
Table II.
Body weights of rats before and after
treatment.
| Group | Pre-treatment body
weights (gram) (mean ± standard deviation) | Post-treatment body
weights (gram) (mean ± standard deviation) |
|---|
| Control |
250.00±16.58 |
262.67±13.53 |
| cisplatin |
259.33±33.33 |
219.67±32.49a |
|
cisplatin+amifostin |
260.33±29.38 |
216.00±27.33 |
|
cisplatin+curcumin |
238.67±17,77 |
206.83±22.27 |
| cisplatin+CAPE |
260.50±21.77 |
218.67±22.37a |
The results also revealed larger NF-κB/p65,
caspase-3 and 8-OHdG staining of the germinal epithelium of the
cisplatin group compared to the control group (P<0.05). The
cisplatin+amifostine group showed less staining for NF-κB/p65
(P<0.05), caspase-3 and 8-OHdG expression than the cisplatin
group (P<0.05). The cisplatin+curcumin and cisplatin+CAPE groups
displayed less NF-κB/p65 (P<0.05), caspase-3 and 8-OHdG
expression than the cisplatin group (P<0.05). Intensity and
distribution of staining for the same markers in
cisplatin+amifostine sections were similar to those of the
cisplatin+curcumin and CAPE group (P>0.05) (Table I).
Discussion
Survival of young male cancer patients has increased
by 75% over the last 20 years as a result of early diagnosis and
intervention (6,29,30).
Typically, infertility occurs after chemotherapy and radiotherapy.
Currently, semen cryopreservation has accompanied the treatment of
cancer patients to partially compensate infertility. However, sperm
cryopreservation has shortages including low fertilization, genetic
anomalies due to DNA breakages, ethical issues, and high cost.
Therefore, more efficient alternative approaches are needed
(6,29,30).
Cisplatin is an essential antineoplastic agent that
is widely used in the treatment of solid tumors. cisplatin
interferes with DNA base pair cross-linking, resulting in
cytotoxicity of proliferating cells (5,12,31–33).
Agents causing DNA damage show more cytotoxic
effects on proliferating cells (29,34–37).
Spermatogenesis is a natural process, which is characterized by
cell division in the testis. Recent studies have reported that the
toxic effect of cisplatin on the testis results in a variety of
anomalies, such as atrophic seminiferous tubules, germinal
epithelium damage due to apoptosis and necrosis, loss of
connections between the spermatogonium and basal lamina,
inflammation and damage in the peritubular area (31,32,38–46). Our
histopathological findings appear to support the cytotoxic effects
of cisplatin except for peritubular inflammation. However, we
determined that cisplatin causes the cytoplasmic expression of the
transcription factors NF-κB/p65 and 8-OHdG in spermatogenic cells
and Sertoli cells in the germinal epithelium of the seminiferous
tubules, and an increase in nuclear caspase-3 expression in other
spermatogonial cells. On the contrary, the expression of NF-κβ,
caspase-3, and 8-OHdG decreased in all cisplatin groups treated
with amifostine. In the cisplatin group alone, we observed
degenerated basal lamina, apoptotic spermatogonia, spermatocytes,
fragmented spermatogonia, and spermatid nuclei under electron
microscopy. The spermatocytes were swollen and lost their stellar
appearance, demonstrating a condensed nucleolus, cytoplasm and
perinuclear area.
In the literature, cisplatin-induced cellular and
tissue damage have been attributed to its oxidative stress.
However, no other mechanisms of action have been investigated by
histopathological studies (33,41,43,47,48).
In an vitro study by Yang et al, cisplatin induced
apoptosis in concentrations of 10–50 µM in tubular cells (45). Apoptosis is reported to be induced
via the MAPK signaling pathways. The MAPK signaling pathway
regulates proliferation, differentiation, and survival of cells.
The MAPK signaling pathway incorporates serine/threonine kinases of
parallel cascades. These parallel cascades are activated by
different extracellular physical and chemical stresses. There are
three main parallel MAPK signaling cascades: the ERK pathway,
protein kinase (JNK GENE/FATH) pathway (which is activated by p38)
and Jun N-terminal Kinase/stress pathway. The ERK pathway is
typically activated by extracellular growth factors and is
associated with cell viability and cell death. P38 and JNK
GENE/FATH paths are activated by oxidation, UV irradiation,
hyperosmolality and inflammatory cytokines that are associated with
cell death. cisplatin induces its toxicity in vitro and
in vivo by activating these three MAPK signaling pathways.
The ERK and P38 signaling pathways activate the production of
TNF-α. ERK controls cell apoptosis and activates caspase-3
(38,43–47).
Several studies have reported that cisplatin
activates caspase-3 via the ERK pathway. The caspase cascade plays
a key role in inducing apoptosis. In particular, activation of
caspase-3 is an irreversible step that induces apoptosis. For this
reason, studies have used caspase-3 expression to evaluate
apoptotic cells (3,5,31–33,36,38,41–45,48,49).
In our study, we confirmed that the number of caspase-3 positive
cells in the cisplatin group significantly increased compared to
cells in the control group (Table I,
P<0,05). There was no significant difference between group III
and the control group (Table I,
P>0.05). There was also no significant difference between group
III (Table I, P>0.05) and both
groups IV and V (Table I,
P>0.05).
The Ras oncogenic protein plays a key role in the
ERK signaling pathway. Mutations of its gene in cancers induce
distortion of GTP. Thus, although an impact on the ERK pathway was
expected from an efficient chemotherapeutic agent such as
cisplatin, prevention of the distortion of GTP development depends
on the Ras gene mutation in healthy cells in the testicular tissue
with active cell division such as spermatogenesis, which is
essential for fertility. Amifostine, curcumin, and CAPE have
previously been reported to significantly decrease
caspase-3/caspase-8 activity (50–57). In
the present study, we observed that amifostine and curcumin
prevented caspase-3 activation and protected both testicular tissue
and spermatogenesis and that CAPE provided partial protection.
CAPE has been reported to reduce apoptosis (14,17,20,47).
Conversely, Severi-Aguiar et al (58) noticed that 10 mg of propolis/kg/day
induced Cx43 upregulation and caused morphological damage to germ
cells, Sertoli cells, and Leydig cells. They observed that
apoptotic cells were present in the germinal epithelium of the
testes in a dose-dependent manner in their studies. In our study,
as in other studies, we found that apoptosis was reduced by
cisplatin significantly despite the presence of low level of
caspase-3 positivity.
The toxicity induced by cisplatin on the testis via
the JNK/SAPK pathways has not yet been investigated thus far.
cisplatin triggers p38 activation via free oxygen radicals
(hydroxyl radicals). It was reported in the literature that p38
activation causes oxidative stress, which results in damage to the
epithelial cells of the seminiferous tubules by the formation of
free oxygen radicals.
Cisplatin also induces the formation of reactive
oxygen species (ROS) in mitochondria, by activating NADPH oxidase
and the xanthine oxidase. ROS-induced nuclear and mitochondrial DNA
damage could be detected by 8-OHdG. Therefore, 8-OHdG was used to
detect damage caused by cellular oxidative stress. It has been
established that ROS induced damage is counteracted by
antioxidants, such as CAPE, vitamin C and vitamin E (19,33,38,41,59).
Bhimani et al reported that CAPE decreased the tumor
promoter-mediated oxidative processes which decreased 8-OHdG
(60). Some studies demonstrated
that curcumin treatment significantly decreased 8-OHdG (61). There are no studies addressing the
effects of amifostine on 8-OHDG. However, previous studies have
reported that amifostine acts as a potent free radical scavenger
giving free radicals hydrogen ions (9,10,55,62).
In our study, the number of 8-OHdG positive cells in the cisplatin
group was significantly higher than those in the control group
(Table I, P<0.05). There was no
significant difference between group V and control group (Table I, P>0.05), nor was there a
significant difference between groups III and IV (Table I, P>0.05) and V (Table I, P>0.05). In accordance with the
previous reports, we observed that the antioxidants CAPE,
amifostine, and curcumin protect cells from ROS.
Free radicals generated by the MAPK pathways
activate the transcription factor, NF-κB/p65, resulting in
apoptosis. In this study, consistent with other studies, the number
of NF-κB/p65-positive cells in the cisplatin group was
significantly higher than the control group (Table II, P<0.05). There are no studies
investigating the effects of amifostine, curcumin, and CAPE on
NF-κB induced by cisplatin toxicity. However, Schwertheim et
al (63) reported curcumin
downregulation of NF-κB activity. In our findings, there was no
significant difference between group V and the control group
(Table I, P>0.05), nor was there
a significant difference between groups III and IV (Table I, P>0.05) and V (Table I, P>0.05). Recent studies revealed
that NF-κB/p65 could play a regulatory role in the cases of
oxidative stress. In addition to cellular damage of the
seminiferous tubules caused by cisplatin, we also observed
thickening of the basal lamina and distortion of its integrity.
In conclusion, we showed different mechanisms of
damage in the testis at play resulting from cisplatin, and their
reversal by the antioxidants amifostine, curcumin and, to a lesser
extent, CAPE. Further studies are needed to determine if amifostine
and cisplatin have an antagonistic effect on cancerous cells.
References
|
1
|
Sabanegh ES Jr and Ragheb AM: Male
fertility after cancer. Urology. 73:225–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schrier RW, Wang W, Poole B and Mitra A:
Acute renal failure: Definitions, diagnosis, pathogenesis and
therapy. J Clin Invest. 114:5–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin A and Hamza AA: Effects of roselle
and ginger on cisplatin-induced reproductive toxicity in rats.
Asian J Androl. 8:607–612. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colpi GM, Contalbi GF, Nerva F, Sagone P
and Piediferro G: Testicular function following chemo-radiotherapy.
Eur J Obstet Gynecol Reprod Biol. 113 Suppl 1:S2–S6. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cherry SM, Hunt PA and Hassold TJ:
cisplatin disrupts mammalian spermatogenesis, but does not affect
recombination or chromosome segregation. Mutat Res. 564:115–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dohle GR: Male infertility in cancer
patients: Review of the literature. Int J Urol. 17:327–331. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agarwal A and Prabakaran SA: Mechanism,
measurement and prevention of oxidative stress in male
reproductive. physiology. 43:1–974. 2005.
|
|
8
|
Tokatli F, Uzal C, Doganay L, Kocak Z,
Kaya M, Ture M, Kurum T, Alkaya F, Karadag H and Kutlu K: The
potential cardioprotective effects of amifostine in irradiated
rats. Int J Radiat Oncol Biol Phys. 58:1228–1234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uzal C, Durmus-Altun G, Caloglu M, Ergülen
A, Altaner S and Yigitbasi NO: The protective effect of amifostine
on radiation-induced acute pulmonary toxicity: Detection by
99mTc-DTPA transalveolar clearances. Int J Radiat Oncol Biol Phys.
60:564–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wasserman TH and Brizel DM: The role of
amifostine as a radioprotector. Oncology (Williston Park).
15:1349–1354. 2001.PubMed/NCBI
|
|
11
|
Choudhary D, Chandra D and Kale RK:
Modulation of radioresponse of glyoxalase system by curcumin. J
Ethnopharmacol. 64:1–7. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devasena T, Rajasekaran KN and Menon VP:
Bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione (a curcumin
analog) ameliorates DMH-induced hepatic oxidative stress during
colon carcinogenesis. Pharmacol Res. 46:39–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan MH, Huang TM and Lin JK:
Biotransformation of curcumin through reduction and glucuronidation
in mice. Drug Metab Dispos. 27:486–494. 1999.PubMed/NCBI
|
|
14
|
Uz E, Söğüt S, Sahin S, Var A, Ozyurt H,
Güleç M and Akyol O: The protective role of caffeic acid phenethyl
ester (CAPE) on testicular tissue after testicular torsion and
detorsion. World J Urol. 20:264–270. 2002.PubMed/NCBI
|
|
15
|
Grunberger D, Banerjee R, Eisinger K, Oltz
EM, Efros L, Caldwell M, Estevez V and Nakanishi K: Preferential
cytotoxicity on tumor cells by caffeic acid phenethyl ester
isolated from propolis. Experientia. 44:230–232. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akyol S, Akbas A, Butun I, Toktas M,
Ozyurt H, Sahin S and Akyol O: Caffeic acid phenethyl ester as a
remedial agent for reproductive functions and oxidative
stress-based pathologies of gonads. J Intercult Ethnopharmacol.
4:187–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dilber Y, Inan S, Ercan GA and Sencan A:
The role of CAPE in PI3K/AKT/mTOR activation and oxidative stress
on testis torsion. Acta Histochem. 118:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogeturk M, Kus I, Colakoglu N, Zararsiz I,
Ilhan N and Sarsilmaz M: Caffeic acid phenethyl ester protects
kidneys against carbon tetrachloride toxicity in rats. J
Ethnopharmacol. 97:273–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kus I, Colakoglu N, Pekmez H, Seckin D,
Ogeturk M and Sarsilmaz M: Protective effects of caffeic acid
phenethyl ester (CAPE) on carbon tetrachloride-induced
hepatotoxicity in rats. Acta Histochem. 106:289–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tolba MF, Omar HA, Azab SS, Khalifa AE,
Abdel-Naim AB and Abdel-Rahman SZ: Caffeic acid phenethyl ester: A
review of its antioxidant activity, protective effects against
ischemia-reperfusion injury and drug adverse reactions. Crit Rev
Food Sci Nutr. 56:2183–2190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ciftci O, Beytur A, Cakir O, Gurbuz N and
Vardi N: Comparison of reproductive toxicity caused by cisplatin
and novel platinum-N-heterocyclic carbene complex in male rats.
Basic Clin Pharmacol Toxicol. 109:328–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boekelheide K: Mechanisms of toxic damage
to spermatogenesis. J Natl Cancer Inst Monogr. 1–8. 2005.
|
|
23
|
Meistrich ML, Finch M, Cunha MF, Hacker U
and Au WW: Damaging effects of fourteen chemotherapeutic drugs on
mouse testis cells damaging effects of fourteen chemotherapeutic
drugs on mouse testis. 42:1–131. 1982.
|
|
24
|
Jain KK: The handbook of neuroprotection.
2011. View Article : Google Scholar
|
|
25
|
Ozgen SÇ, Dökmeci D, Akpolat M, Karadağ
CH, Gündüz O, Erbaş H, Benian O, Uzal C and Turan FN: The
protective effect of curcumin on ionizing radiation-induced
cataractogenesis in rats. Balkan Med J. 29:358–363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalkan Y, Kapakin KA, Kara A, Atabay T,
Karadeniz A, Simsek N, Karakus E, Can I, Yildirim S, Ozkanlar S and
Sengul E: Protective effect of panax ginseng against serum
biochemical changes and apoptosis in kidney of rats treated with
gentamicin sulphate. J Mol Histol. 43:603–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mercantepe T, Unal D, Selli J, Mercantepe
F and Unal B: Protective effects of estrogen and bortezomib in
kidney tissue of post-menopausal rats: An ultrastructural study.
Ren Fail. 38:1129–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Selli J, Unal D, Mercantepe F, Akaras N,
Kabayel R, Unal B and Atilay H: Protective effects of beta glucan
in brain tissues of post-menopausal rats: A histochemical and
ultra-structural study. Gynecol Endocrinol. 32:234–239. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bray F, Richiardi L, Ekbom A, Pukkala E,
Cuninkova M and Møller H: Trends in testicular cancer incidence and
mortality in 22 European countries: Continuing increases in
incidence and declines in mortality. Int J Cancer. 118:3099–3111.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
International Agency for Research on
Cancer: World cancer report 2008. 2008.
|
|
31
|
Duale N, Lindeman B, Komada M, Olsen AK,
Andreassen A, Soderlund EJ and Brunborg G: Molecular portrait of
cisplatin induced response in human testis cancer cell lines based
on gene expression profiles. Mol Cancer. 6:532007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mohammadnejad D, Abedelahi A,
Soleimani-rad J, Mohammadi-roshandeh A, Rashtbar M and Azami A:
Degenerative effect of cisplatin on testicular germinal epithelium.
Adv Pharm Bull. 2:173–177. 2012.PubMed/NCBI
|
|
33
|
Zhang X, Yamamoto N, Soramoto S and
Takenaka I: cisplatin-induced germ cell apoptosis in mouse testes.
Arch Androl. 46:43–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aitken RJ: The amoroso lecture. The human
spermatozoon-a cell in crisis? J Reprod Fertil. 115:1–7. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aitken RJ: Founders' lecture. Human
spermatozoa: Fruits of creation, seeds of doubt. Reprod Fertil Dev.
16:655–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Peltola V, Huhtaniemi I and Ahotupa M:
Antioxidant enzyme activity in the maturing rat testis. J Androl.
13:450–455. 1992.PubMed/NCBI
|
|
37
|
Zini A and Schlegel PN: Catalase mRNA
Expression in the male rat reproductive tract. J Androl.
17:473–480. 1996.PubMed/NCBI
|
|
38
|
Al-Bader M and Kilarkaje N: Effects of
bleomycin, etoposide and cisplatin treatment on Leydig cell
structure and transcription of steroidogenic enzymes in rat testis.
Eur J Pharmacol. 747:150–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Berger CC, Bokemeyer C, Schuppert F and
Schmoll HJ: Endocrinological late effects after chemotherapy for
testicular cancer. Br J Cancer. 73:1108–1114. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bieber AM, Marcon L, Hales BF and Robaire
B: Effects of chemotherapeutic agents for testicular cancer on the
male rat reproductive system, spermatozoa, and fertility. J Androl.
27:189–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Delbès G, Chan D, Pakarinen P, Trasler JM,
Hales BF and Robaire B: Impact of the chemotherapy cocktail used to
treat testicular cancer on the gene expression profile of germ
cells from male Brown-Norway rats. Biol Reprod. 80:320–327. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Howell SJ and Shalet SM: Spermatogenesis
after cancer treatment: Damage and recovery. J Natl Cancer Inst
Monogr 12–17. 2005. View Article : Google Scholar
|
|
43
|
Zirak MR, Rahimian R, Ghazi-Khansari M,
Abbasi A, Razmi A, Mehr SE, Mousavizadeh K and Dehpour AR:
Tropisetron attenuates cisplatin-induced nephrotoxicity in mice.
Eur J Pharmacol. 738:222–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pavkovic M, Riefke B and
Ellinger-Ziegelbauer H: Urinary microRNA profiling for
identification of biomarkers after cisplatin-induced kidney injury.
Toxicology. 324:147–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Liu H, Liu F and Dong Z:
Mitochondrial dysregulation and protection in cisplatin
nephrotoxicity. Arch Toxicol. 88:1249–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nudell DM, Monoski MM and Lipshultz LI:
Common medications and drugs: How they affect male fertility. Urol
Clin North Am. 29:965–973. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ilbey YO, Ozbek E, Cekmen M, Simsek A,
Otunctemur A and Somay A: Protective effect of curcumin in
cisplatin-induced oxidative injury in rat testis: Mitogen-activated
protein kinase and nuclear factor-kappa B signaling pathways. Hum
Reprod. 24:1717–1725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nalesnik JG, Sabanegh ES Jr, Eng TY and
Buchholz TA: Fertility in men after treatment for stage 1 and 2A
seminoma. Am J Clin Oncol. 27:584–588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ozyurt B, Parlaktas BS, Ozyurt H, Aslan H,
Ekici F and Atis O: A preliminary study of the levels of testis
oxidative stress parameters after MK-801-induced experimental
psychosis model: Protective effects of CAPE. Toxicology. 230:83–89.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Calaf GM, Echiburú-Chau C, Roy D, Chai Y,
Wen G and Balajee AS: Protective role of curcumin in oxidative
stress of breast cells. Oncol Rep. 26:1029–1035. 2011.PubMed/NCBI
|
|
51
|
Zhou M, Fan C and Tian N: Effects of
curcumin on the gene expression profile of L-02 cells. Biomed Rep.
3:519–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
He B, Wei W, Liu J, Xu Y and Zhao G:
Synergistic anticancer effect of curcumin and chemotherapy regimen
FP in human gastric cancer MGC-803 cells. Oncol Lett. 14:3387–3394.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jiang S, Yan H, Fan D, Song J and Fan C:
Multi-layer electrospun membrane mimicking tendon sheath for
prevention of tendon adhesions. Int J Mol Sci. 16:6932–6944. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu S, Tao L, Wang JN, Xu ZQ, Wang J, Xue
YJ, Huang KY, Lin JF, Li L and Ji KT: Amifostine pretreatment
attenuates myocardial ischemia/reperfusion injury by inhibiting
apoptosis and oxidative stress. Oxid Med Cell Longev.
2017:41308242017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chok MK, Conti M, Almolki A, Ferlicot S,
Loric S, Dürrbach A, Benoît G, Droupy S and Eschwège P:
Renoprotective potency of amifostine in rat renal
ischaemiareperfusion. Nephrol Dial Transplant. 25:3845–3851. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
İkinci A, Mercantepe T, Unal D, Erol HS,
Şahin A, Aslan A, Baş O, Erdem H, Sönmez OF, Kaya H and Odacı E:
Morphological and antioxidant impairments in the spinal cord of
male offspring rats following exposure to a continuous 900MHz
electromagnetic field during early and mid-adolescence. J Chem
Neuroanat. 75:99–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Du Q, Hao C, Gou J, Li X, Zou K, He X and
Li Z: Protective effects of p-nitro caffeic acid phenethyl ester on
acute myocardial ischemia-reperfusion injury in rats. Exp Ther Med.
11:1433–1440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Severi-Aguiar GD, Pinto SJ, Capucho C,
Oliveira CA, Diamante MA, Barbieri R, Predes FS and Dolder H:
Chronic intake of green propolis negatively affecting the rat
testis. Pharmacogn Res. 9:27–33. 2017. View Article : Google Scholar
|
|
59
|
Chan D, Delbès G, Landry M, Robaire B and
Trasler JM: Epigenetic alterations in sperm DNA associated with
testicular cancer treatment. Toxicol Sci. 125:532–543. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bhimani RS, Troll W, Grunberger D and
Frenkel K: Inhibition of oxidative stress in HeLa cells by
chemopreventive agents. Cancer Res. 53:4528–4533. 1993.PubMed/NCBI
|
|
61
|
Ho C, Hsu YC, Lei CC, Mau SC, Shih YH and
Lin CL: Curcumin Rescues Diabetic Renal Fibrosis by Targeting
Superoxide-Mediated Wnt Signaling Pathways. Am J Med Sci.
351:286–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jia J, Zhang L, Shi X, Wu M, Zhou X, Liu X
and Huo T: SOD2 mediates amifostine-induced protection against
glutamate in PC12 cells. Oxid Med Cell Longev. 2016:42024372016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schwertheim S, Wein F, Lennartz K, Worm K,
Schmid KW and Sheu-Grabellus SY: Curcumin induces G2/M arrest,
apoptosis, NF-κB inhibition and expression of differentiation genes
in thyroid carcinoma cells. J Cancer Res Clin Oncol. 143:1143–1154.
2017. View Article : Google Scholar : PubMed/NCBI
|