Introduction
Monoclonal antibodies (mAbs) have revolutionised the
treatment of oncological and autoimmune diseases over the past 10
years. Moreover, they are also successfully used in the management
of asthma, hypersensitivity reactions, osteoporosis,
skeletal-related events in patients with bone metastases from solid
tumours, neovascular (wet) age-related macular degeneration,
hyperlipidaemia, and many others. According to data from the U.S.
National Institutes of Health and other publications, various
ongoing clinical studies look promising for indications like
Alzheimer's disease, infections, and type-1 diabetes (1,2).
According to our local drug registration database
(EU-Slovenia), there are currently 64 medical products that contain
40 active mAbs (data retrieved in November 2017). Among these, 39
are intended for intravenous administration, 22 for subcutaneous,
and one each for intramuscular and intravitreal administration. For
their pharmaceutical forms, 50 are solutions or concentrates for
solution, 13 are in the form of powder for solutions, and one is a
kit for radiopharmaceutical preparations for infusion. The data
from the Agency for Medicines and Medical Devices of the Republic
of Slovenia show a wide spectrum of the designs, forms and routes
of administration of pharmaceutical drugs. In the earlier years of
clinical use, these preparations were compounded by healthcare
professionals in controlled environments, and administered in
healthcare facilities. However, with the introduction of TNF-α
inhibitors in pre-filled syringes in the last 10 years, medicinal
products have left such controlled environments, and can now be
stored and administered by the patients themselves (3). Therefore, in many cases the
responsibility for the appropriate storage conditions has shifted
from the healthcare professional to the patients.
The correct handling of biologicals at all times is
extremely important, from their production to their being released
to the market. As protein molecules retain their biological
activities and pharmacokinetics and pharmacodynamics profiles only
when the higher-order protein structure is maintained, various
factors that can lead to immune reactions should be avoided. Risks
to protein stability can arise from not only a low percentage of
‘foreignness’ of the protein, but also from minimal product
impurities and the formation of aggregates during handling. These
can result in unwanted immunogenicity and anti-drug Ab responses in
the patient, with subsequent effects on their treatment outcome.
The desired clinical outcome can be lost through immune reactions,
creating the potential need to switch to another drug with a
different mechanism of action; e.g., a switch from a TNF-α
inhibitor to an interleukin-6 inhibitor in patients with rheumatoid
arthritis. Such loss of efficacy of a treatment and the subsequent
lower interest in a medication can be attributed to human as well
as clinical outcomes. Also, considering the high value of the these
medicinal products, there are economic consequences that should not
be ignored regarding the waste from discarded medicines, potential
unwanted side-effects, and the costs of new medicines and hospital
staff and facilities.
Potential instabilities of medicinal
products and pharmaceutical preparations containing monoclonal
antibodies
Compared to a broad range of known proteins, the
molecular structure of Abs provides them with one of the most
stable and resistant forms against changes to their environment.
However, due to their therapeutic use, even small deviations from
the native structure of these proteins can severely affect one or
more of the pharmaceutical standards of quality, efficacy and
safety of the therapeutic product.
The protein structure of therapeutic mAbs is highly
similar to the structure of Abs produced daily by lymphocyte B
cells in response to any invasion of microorganisms in our body.
The majority of the biotechnological pipelines provide Abs of the
IgG1k type (~150 kDa), although other derivatives are also
produced, such as certolizumab pegol (48 kDa pegylated Fab'
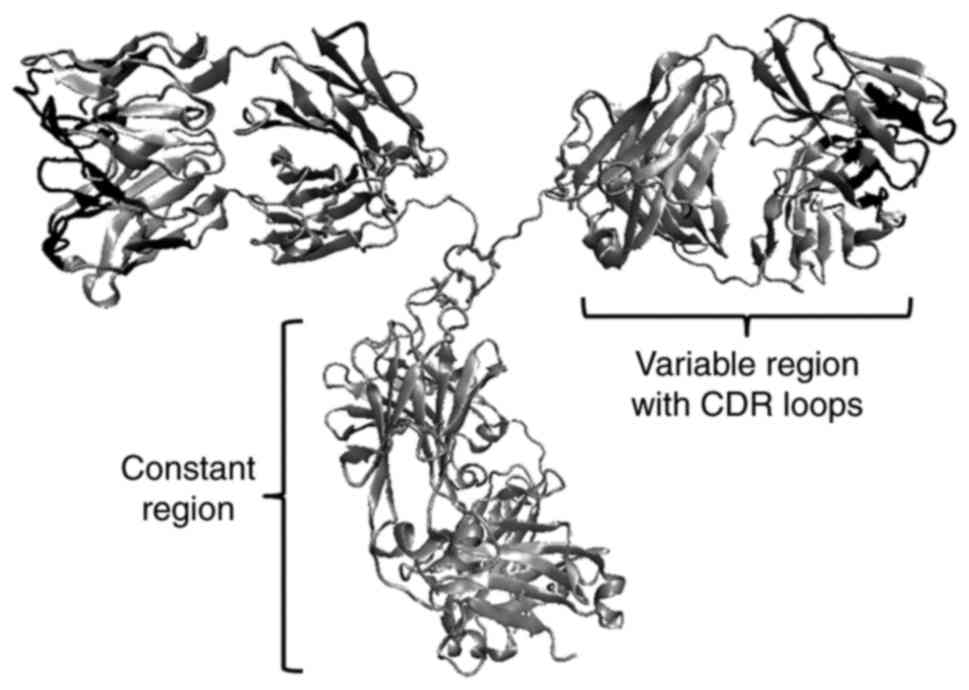
fragment) and radio-nuclide Ab drugs. The most important parts of
the IgG molecule that contribute to binding specificity with the
antigen are the CDR loops-the complementarity determining
regions-of the variable regions (Fig.
1). Small variations in the amino-acid side chains in CDR loops
can result in diversity of the molecular surface, and the
subsequent loss of recognition and specificity for binding to the
antigen.
Chemical instability
Oxidation
Although not all proteins are uniformly susceptible
to oxidative damage, oxidation remains one of the most important
mechanisms of covalent protein modification. This can lead to
altered or diminished biological functions because of
fragmentation, dimerisation, aggregation or denaturation of the
protein. The native conformation of proteins in a medicinal product
is usually protected by the addition of stabilisers. Oxidation
reactions are catalysed by free radicals, light, and trace amounts
of metal ions (4–8). Many studies have confirmed that
oxidation of cysteine or methionine in the Fc region can alter the
effector functions of mAbs, and decrease their binding to Fc
receptors on immune cells (9,10). The
amino-acids histidine, tyrosine and tryptophan are potential sites
of oxidation, and at the same time, they are also highly
concentrated in antigen-binding sites of Ab molecules (11). The effects of the oxidation of these
amino-acids in the binding regions have not been clearly defined to
date, although important alterations to Ab specificity and
proinflammatory activities have been reported in in-vitro studies
(12,13).
Deamidation
Deamidation is one of the most common modifications
to mAb structure, which introduces a high level of charge
heterogeneity into both their light and heavy chains. Removal of
the functional amide group from asparagine residues is generally
favoured, and to some extent also from glutamine. These reactions
are highly selective for individual Ab (14), and they have a detrimental effect on
potency if a negative charge is introduced into the antigen-binding
region (15).
Hydrolysis
Hydrolysis is a chemical process that affects the
primary structure of a protein, and it can result in loss of
protein conformation and mAb function. An example from the
literature shows that even when stored at 5°C, muromonab-CD3
(withdrawn in 2010) undergoes hydrolysis in the hinge region
between the cysteine in the variable region and the proline in the
Fc region (2). Today, such
hydrolysis of mAbs would not be expected under the conditions that
these medicinal products are exposed to during normal formulation
and storage (5), due to the use of
excipients.
Physical instability
During processing and handling of proteins, changes
in their molecule structure can result in many structural variants
of their conformation as they adapt to the changes in their
environment. Non-physiological conditions, shear stress, agitation
and stirring represent stress factors that are routinely
encountered during synthesis, purification, shipping and
preparation of such medicinal products. These structural changes
alter the physical properties of mAbs and can introduce physical
instability into the protein molecule. This can include adsorption
onto surfaces (e.g., containers, syringes, needles), unfolding and
formation of soluble aggregates, or formation of insoluble
precipitates. This instability will result in loss of efficacy of
the therapeutic protein, and also potential immunogenicity in
vivo.
In a complex, structure-function related protein, as
in the case of immunoglobulin, the different regions/domains
denature independently: The Fab region is more sensitive to heat
treatment, and the Fc region is more sensitive to lower pH
(16). Aggregates can form due to
changes to pH, ionic strength or surface tension, or to the
presence of organic solvents, although these are often reversible.
However, elevated temperature usually leads to irreversible
aggregation and loss of protein function. Aggregates are
organoleptically recognized as turbidity of a solution, although,
there can also be a period of transparency at the very beginning of
the nucleation process. Aggregation poses greater problems for the
use of pharmaceutical forms for subcutaneous and intramuscular
administration, where the protein concentrations in the solution
can reach 125 mg/ml, while the concentrates used for solutions for
infusion are usually lower, from 1 to 25 mg/ml. For
self-administration, a low solution volume is preferred due to
lower levels of site reactions and greater tolerability for the
patient (14).
Elevated temperatures
The stability of mAbs in pharmaceutical preparations
greatly depends on the temperatures they are exposed to. Longer
exposure to less-elevated temperatures mainly accelerates their
chemical instability. Studies have shown than a mAb exposed to 40°C
for 6 months mainly showed deamidation and hydrolysis (17). When mAbs are exposed to temperatures
near their unfolding temperature (defined as the temperature at
which 50% of the protein molecules are unfolded), the prevailing
instability mechanism is aggregation (4,5).
The stability of pharmaceutical preparations with
mAbs also depends on the pharmaceutical form (and consequently, the
use of excipients) and the properties of the protein itself. Ye
(18) exposed abciximab
(Reopro®) and trastuzumab (Herceptin®) to
temperatures up to 70°C for 15 min, and to room temperature for up
to 42 days. Under these conditions, 95% of abciximab was degraded
or aggregated with the 70°C treatment, while it remained stable at
room temperature; trastuzumab did not undergo any physical or
chemical changes under either condition. During the development of
equine venom Abs that are suitable for storage at room temperature
in climate zone IV, Segura et al (19) managed to achieve stability at 37°C
for 1 year using sorbitol and phenol as excipients.
Freeze-thaw cycles
The main mechanisms of protein instability during
their freezing and thawing result from their aggregation. This can
be due to their exposure to elevated concentrations of excipients
in the non-water phase [that do not freeze; (20)] and to pH changes (21), to their adsorption onto ice-liquid
interfaces or the walls of the vessels (5,22), as
well as gas-liquid interfaces caused by accelerated cooling
(23). The effects of freeze-thaw
cycles are also cumulative, where faster cooling can denaturate
proteins at rates up to 11-fold greater than seen for slower
cooling (24).
Protein stabilisation in pharmaceutical
forms
Diverse instability mechanisms are prevalent under
conditions that cause changes to pH or temperature. To achieve
greater stability and longer shelf-life of mAbs, these parameters
need to be adjusted accordingly during the development phase. The
U.S. Food and Drug administration criteria on stability of
pharmaceutical forms state that no more than 10% of the active
ingredient should deteriorate over 2 years (25).
Excipients
Various excipients are used in all mAb preparations
to assure their appropriate pharmacokinetics properties and
stability, to enable the formulation of the pharmaceutical product,
and to enhance the tolerability of the patient. Medicinal products
that contain mAbs are generally either powders for solutions or
concentrates for solutions for parenteral administration (Table I). Here, excipients are used to
maintain the pH (e.g., Tris, acetate, histidine, citrate buffers),
to enhance the protein stability and prevent oxidation (e.g.,
sugars, polyols), to achieve an appropriate viscosity, or to bind
metal ions and free radicals (e.g., chelators, antioxidants). Their
addition can therefore stabilise solutions or lyophilisates (see
section 3.2) over long periods of time (6).
 | Table I.Roles of excipients used in powder
for concentrate for the solution for infusion with belimumab
(Benlysta®). |
Table I.
Roles of excipients used in powder
for concentrate for the solution for infusion with belimumab
(Benlysta®).
| Excipient | Role of the
excipient |
|---|
| Citric acid
monohydrate | Buffer
component-weak acid (pH regulation) |
| Sodium citrate | Buffer
component-strong base (pH regulation) |
| Sucrose | Cryoprotectant,
bulking agent |
| Polysorbate 80 | Surfactant |
Lyophilisation
Hydrophilic solutions provide a favourable milieu
for physico-chemical changes to proteins. Aqueous media allow the
transfer of the electrons needed for oxidation and deamidation
reactions, and have an important role in protein aggregation. The
exclusion of water from pharmaceutical forms can therefore provide
important enhancement to the stability of proteins (14). The most commonly used method for this
water exclusion is the three-phase process of freeze-drying,
lyophilisation, and addition of cryoprotectants.
Unwanted immunogenicity of biologicals that
contain monoclonal antibodies
In general, the immunogenicity of drugs refers to
the formation of Abs against a certain drug (i.e., anti-drug Abs;
ADAs). All such biologicals can potentially induce unwanted immune
responses that can activate the mechanisms of innate and acquired
immunity. It has been recognized that mAb drugs or their novel mAb
derivatives (e.g., Fab fragments, scFv, nanobodies, fusion
proteins) can induce both humoral and cellular immune responses.
ADAs can alter the pharmacokinetics, pharmacodynamics and
bioavailability of mAbs, thus affecting the safety and efficacy of
these drugs.
There are two main mechanisms of immunogenicity: i)
activation of classical immune reactions that are triggered by
foreign proteins, which results in synthesis of ADAs and induction
of memory cells, and leads to enhanced reactions upon rechallenge
(26); and ii) breach of B-cell and
T-cell immune tolerance. The clinical consequences of these can
vary widely among individuals, and they are mostly unpredictable.
The major safety concerns related to immunogenicity are induction
of anaphylaxis, cytokine storms (the rapid release of
proinflammatory cytokines), serum sickness disease, and delayed
hypersensitivity. These can be accompanied by various clinical
symptoms, including fever, rash, myalgia, haematuria, proteinuria
and haemolytic anaemia, and can even induce autoimmune
reactions.
Two types of ADA responses have been defined: i)
Neutralising or blocking Abs (NAbs) which block the effects of the
exogenous drug or destroy the drug itself. These can neutralise the
biological activity of the drug by either blocking the cell-surface
molecule needed for its activity, or interfering with the binding
of the drug to its receptor on the target cells; and ii) binding
Abs, which (BAbs) bind to the drug but do not sterically hinder its
biological actions (27).
However, many studies have confirmed the altered
pharmacokinetics of a drug due to the formation of the immune
complex between the drug and the binding Ab, and the resulting
enhanced clearance (27–29). The levels of neutralising/blocking or
binding Abs that are produced depend on the dose and the frequency
and mode of injection or application of a drug (i.e., skin >
intramuscular > intravenous > per os). There was a well-known
case in 1998 where an increase in the incidence of pure red-cell
aplasia (PRCA) was associated with the formation of
anti-erythropoietin Abs after subcutaneous use of epoetin alpha
(Eprex®) in patients under chronic dialysis (30). Also, in 2006, a phase I clinical
study was being conducted for a humanised CD28 superagonist Ab,
TGN1412, as a potential drug candidate for the treatment of B-cell
lymphoma and rheumatoid arthritis. Within 8 h of the first infusion
at a dose 500-fold more dilute than that shown to be safe in animal
studies, all six of the human volunteers faced multiorgan failure.
This was later recognized to have been the result of cytokine storm
(31).
The earliest mAbs used as drugs originated from
mice, and their degree of ‘foreignness’ was a pivotal force for
development of immunogenicity. Indeed, overall some 90% of these
treated patients produced human anti-mouse Abs (32), which greatly diminished the clinical
objectives. The second generation of mAbs were chimeric mAbs, which
were fusions between the murine epitope-specific variable region
and the human constant region, and were produced by genetic
engineering methods. These Abs were much more successful for
therapeutic purposes. This technological advance from murine origin
to humanised Abs greatly improved their in vivo
tolerability, although 50% of treated patients still produced human
anti-chimeric Abs (HACA) (32).
The more recent production of fully human Abs
further decreased the unwanted immune response, although
anti-idiotypic response remain, which might influence the outcome
of immune responses. Anti-idiotypic Abs are raised against the
antigen-binding site because the individual T-cell receptors and
immunoglobulins are also immunogenic by virtue of the unique
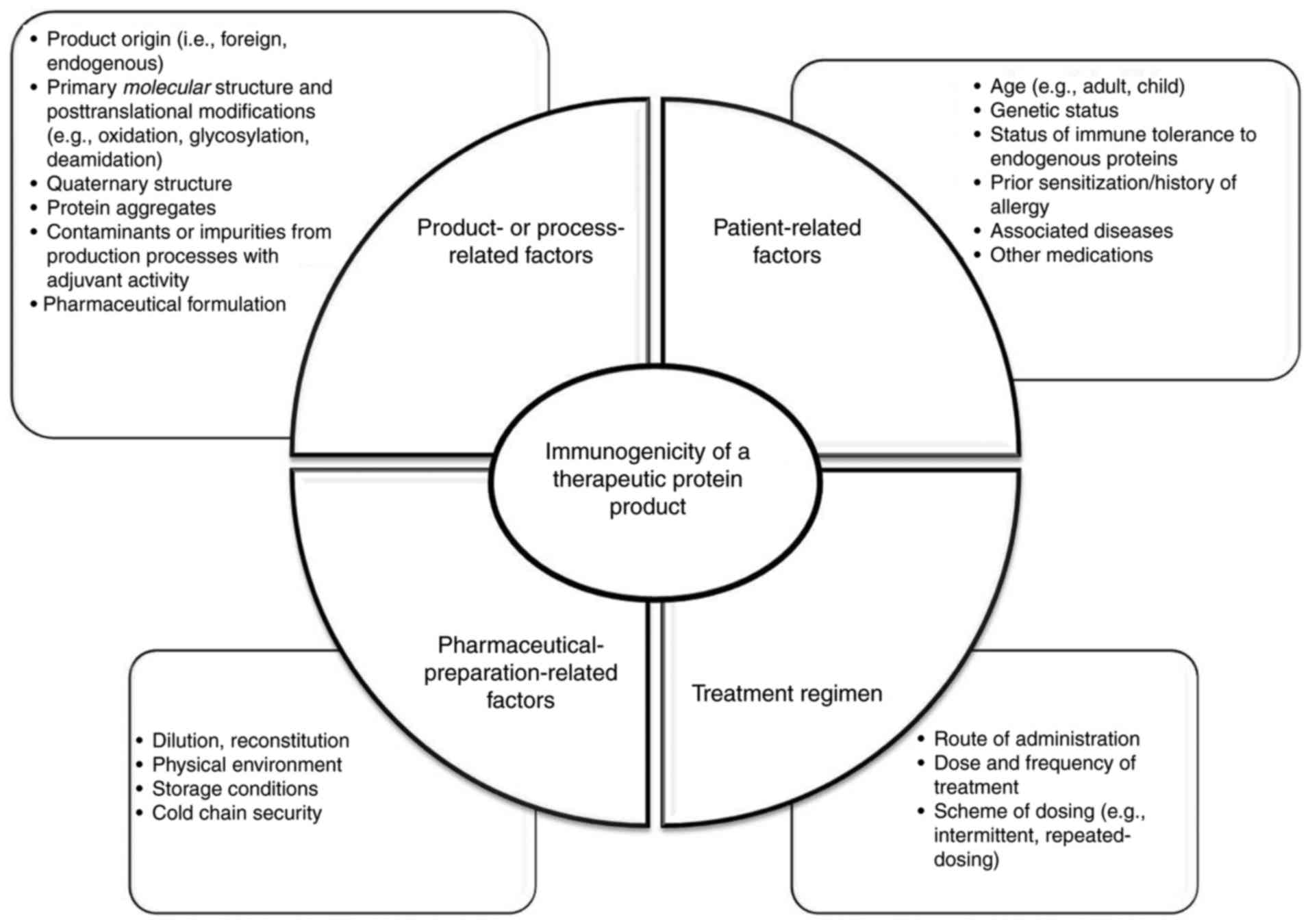
sequence within their variable regions. Therefore, even with human
mAbs it is not reasonable to expect immunogenicity rates of zero,
as this can be affected by numerous patient-related and
product-related factors (Fig. 2).
Aggregation, polymerization, denaturation or partial unfolding of
native proteins, expose neoepitopes, cryptic epitopes or repeated
epitopes. Subsequent immune response to the aggregates involves
previously mentioned mechanisms of the immunogenicity.
Protein aggregates in the size range of 0.1–10 µm
are believed to be the most immunogenic (33). Recently, new animal testing methods
have been developed that together with in-silico predication
models, can provide additional help to explain potential
immunogenicity (34,35). For mAbs intended for in-vivo
clinical use, the European Medicines Agency has issued regulatory
guidance about screening and confirmatory assays, as well as risk
assessment (EMA/CHMP/BMWP/86289/2010). Precise control of the
manufacturing processes with concomitant systematic evaluation of
immunogenicity would help to bridge this gap between product
quality and clinical immunogenicity and understanding of the
complex immune responses to mAbs. Due to the biological variability
and broad spectrum of biological agents that contain mAbs, their
different origins and different manufacturing processes, the ‘one
size-fits all’ strategy is no longer acceptable. Therapies should
be personalised and adapted to the individual patient, and
supported by therapeutic drug monitoring. Together with the assured
quality of mAb drugs, this will assure optimal treatment efficacy
with minimal toxicity.
Handling of biologicals
The main critical phases that can affect the
stability of mAb biologicals include the transportation and storing
of these medicinal products, and the reconstitution of
pharmaceutical preparations. Handling by the patient or a caregiver
can also prove critical, as the medicinal products are no longer
kept in a controlled environment.
Handling by pharmacy staff and
healthcare professionals
The transport to the healthcare provider is well
established, with the maintenance of the cold-chain conditions, and
this does not generally represent a risk to a medication. We
therefore describe here critical preparation steps and procedures
after the delivery of a biological to a healthcare facility. In
1976, the Breckenridge report stated that if possible, all
parenteral pharmaceutical preparations administered to a patient
should be compounded centrally in a hospital pharmacy, to ensure
patient safety and maintain the sterility of the final product
(36). The U.K. National Health
Service guidelines HC(76)9 from 2008, the American Society of
Clinical Oncology guidelines from 2012, and the South Australian
Health Service guidelines from 2015 all added pharmaceutical
preparations with mAbs used for oncological indications to their
list of medications that should be compounded in the hospital
pharmacy setting. In rare cases, where centralised preparation is
neither possible nor feasible, such preparations can be carried out
by a specially trained nurse (37).
Even though the establishing of a clean room is
associated with high costs, this investment returns through
rationalisation of the use of high-cost medicinal products that
contain mAbs. This is achieved by using large-volume vials and
being able to store the unused drug through the use of aseptic
conditions. The dosing of mAbs is usually based on patient weight
or body surface area. Due to limitations in the availability of
such powders or solutions of mAbs, during on-ward preparation, the
unused drug has to be discarded, according to the manufacturer
instructions.
The preparation of intravenous pharmaceutics should
be carried out by a pharmacy technician under the supervision of a
registered pharmacist, and more recently this can be guided by
specific compounding computer software. Two methods are commonly in
use. For volumetric preparation, every addition of a drug to the
base solution has to be confirmed by a pharmacist. As this requires
additional staff, more recently the gravimetric method is in
greater use as the workflow is guided by a predefined protocol
provided by the computer software (BD Cato™ medication
workflow solutions compounding), and the validity of every step
involving additions of the drug is confirmed by weighing. The use
of bar codes, defined drug solution densities, and predefined
tolerance levels, can exclude the human factor, and this ensures
the traceability and safety of every pharmaceutical preparation, as
well as providing better management of the unused drug.
Nevertheless, both methods require skilled staff who have undergone
training for working under aseptic conditions, as well as for
handling of medicinal products containing mAbs.
Especially in cases where prescriptions are not
verified in the pharmacy, it is crucial that medical doctors
consider incompatibility issues regarding medicinal products with
mAbs, such as the use of the correct dilution media and the
concentration ranges needed. In some cases, it is recommended that
the patients receive premedication prior to administration. Also,
administration of medicinal products with mAbs can be accompanied
by various adverse reactions. Again, it is crucial for physicians
to recognize and manage these. Based on local guidelines, medical
doctors are usually obligated to report these adverse reactions.
The European Medicines Agency pharmacovigilance legislation has
also placed mAbs under so-called ‘additional monitoring’, and these
medicines are labelled with an inverted black triangle to encourage
healthcare professionals, and especially medical doctors, to report
any suspected adverse effects (EMA/169546/2012).
Practical points for pharmacists and
healthcare professionals
i) Elevated temperature. All currently commercially
available pharmaceutical forms that contain mAbs have to be kept
between 2 and 8°C until the compounding of the pharmaceutical
preparation, or the administration of the drug itself (Agency of
the Republic of Slovenia for Medicines and Medical Devices). It is
highly unlikely that a product with mAbs would be exposed to
temperatures that would threaten their instability during storage
in a pharmacy, except in the case of refrigerator failure.
In rare cases, when such a product is accidentally
exposed to elevated temperatures, the manufacturer should be
consulted to obtain the necessary stability studies data, to
determine whether the drug is safe or should be discarded.
ii) Freeze-thaw cycles. Although medical products
with mAbs are not expected to be subjected to freeze-thaw cycles
after the formulation of the final drug product, the possibility of
accidental freezing cannot be fully ignored.
iii) Reconstitution of lyophilisates. The required
solvent should be added slowly to a lyophilisate that contains
mAbs, to minimise foaming and promote the formation of the native
conformation of the protein. During lyophilisation, the protein
molecules can form certain non-native conformations, due to
interactions with the molecules that substitute the water during
this process (14). Solvation can be
accelerated with rotation of the vial around its axis. Newer
medicines with lyophilised powders also provide the end-user with
parameters for use with mechanical reconstitution devices (European
Medicines Agency, Summary of product characteristics Benlysta). The
reconstitution of pharmaceutical preparations from lyophilisates is
technically challenging and time consuming.
iv) The effects of interfaces and adsorption.
Manufacturers often state that the shaking of vials with
concentrates for infusions and with the pharmaceutical preparations
themselves should be avoided. Solutions of mAbs can be exposed to
an air-liquid interface while shaking, and also during removal from
the vial and addition into the dilution medium. The transfer from a
syringe should be as slow as feasible, with the needle always
submerged in the dilution medium to avoid unnecessary contact of
the solution with the air. It has become common practice to use
polymer spikes with separate channels for fluid transfers and
air-pressure equalisation. These are usually equipped with 0.22 or
0.45-µm hydrophobic air filters (38). Before removal of an Ab solution from
the vial or after its reconstitution, the solution has to be
visually checked for turbidity or the presence of floating
non-transparent particles that indicate protein aggregation. Newer
products that are prepared for longer periods of use (e.g., up to
96 h) can include a separate stabilising solution that is added to
the infusion bag immediately before preparation of the
pharmaceutical (European Medicines Agency, Summary of product
characteristics Blincyto).
v) Protection from light. Until the compounding of
the pharmaceutical preparation, the medicinal products containing
mAbs are kept in secondary packaging to protect the solution from
light. Should the duration of administration to the patient last
several hours, the pharmaceutical preparation should be further
protected from light. The same applies to any remaining solutions
kept in preparation areas.
vi) Long-term stability of pharmaceutical
preparations with mAbs. There are several situations when a
solution that contains a mAbs cannot be administered immediately
after its preparation. The manufacturers define the time limits in
which prepared infusions or injections should be used if they were
not prepared under aseptic conditions. Pharmaceutical preparations
with mAbs do not contain preservatives. If preparations have been
compounded under validated aseptic conditions, these periods can be
longer. It appears that even for aseptic preparations, storage
times for diluted solutions are based on microbiological integrity,
rather than physico-chemical stability. For example, for diluted
rituximab solutions, the manufacturer states that these are
physically and chemically stable for up to 24 h at temperatures
between 2 and 8°C, and then for an additional 12 h at room
temperature (European Medicines Agency, Summary of product
characteristics MabThera). On the other hand, Paul et al
(39) reported that 1% rituximab
solutions in 0.9% NaCl stored at 4°C for 6 months did not show any
physical or chemical instability. The direct cytotoxic effects of
rituximab were also fully retained. The availability of
ready-to-use solutions with mAbs that are prepared in a controlled
environment by the manufacturers themselves would cut the
healthcare staff preparation costs and lessen their burden.
Handling by patients
The use of medicinal products containing mAbs for
self-administration has steadily been rising worldwide. As more
prescriptions for medications with mAbs are filled, the number of
patients or caregivers that handle them also rises. All of these
people should be properly informed about the concept of the cold
chain, and they should know to follow the recommendations of the
manufacturers and healthcare professionals.
The number of prescriptions dispensed and the total
cost of medicines in Slovenia are given in Table II. The rise of prescriptions filled
in 2012 and 2013 without a corresponding rise in costs can be
attributed to the use of denosumab in the prevention of
osteoporosis. The unusual increase in the number of prescriptions
filled in 2015 can be attributed to changes in dispensing
regulations, where most medicinal products that contain mAbs can
now only be dispensed for 1 month at a time, instead of the
previous 3 months limit (Health Insurance Institute of
Slovenia).
 | Table II.Details of prescriptions filled for
medicinal products containing mAbs for self-administration in
Slovenia EU (estimated population, ~2 million) from 2004 to
2016.a |
Table II.
Details of prescriptions filled for
medicinal products containing mAbs for self-administration in
Slovenia EU (estimated population, ~2 million) from 2004 to
2016.a
| Year | Number of
prescriptions | Total value
(€) |
|---|
| 2004 |
278 |
150,697 |
| 2005 |
827 |
1,154,331 |
| 2006 |
1,308 |
2,370,870 |
| 2007 |
1,966 |
4,491,672 |
| 2008 |
2,495 |
7,432,900 |
| 2009 |
3,367 | 10,854,609 |
| 2010 |
4,185 | 13,284,280 |
| 2011 |
7,490 | 17,141,873 |
| 2012 | 14,133 | 21,542,757 |
| 2013 | 20,115 | 24,701,471 |
| 2014 | 27,330 | 26,892,197 |
| 2015 | 39,507 | 30,276,936 |
| 2016 | 50,233 | 31,996,181 |
The first time a patient receives a medicinal
product that contains mAbs, they are usually given a package that
is prepared by the manufacturer. This contains information about
the safety of the drug and several practical aspects of its
handling. The roles of all of the healthcare professionals involved
in the care of a patient include the education of patients about
the correct transport and storage of their drug. The risks include
both the low and high temperatures that medicines can be exposed
to.
The conditions when a medicinal product containing
mAbs might be exposed to higher temperatures, and especially those
above the protein unfolding temperature, are frequent in the warmer
months of the year. The temperatures inside vehicles exposed to
direct sunlight can reach 90°C in summer and 60°C in spring and
autumn (40). Although these maximum
temperatures were measured on sunny days, on cloudy summer days the
temperatures are only 10°C lower (40). The colour of a vehicle is not a major
contributing factor. The patient has to be informed that the
transport time from the pharmacy should be as short as possible,
and that the medicine should not be left in a vehicle for long
periods under any conditions. Even greater risk is posed by
refrigerators used for storing medicines at the home of the
patient. As it would be unrealistic to expect that patients have a
medical-grade refrigerator for the sole purpose of storing their
medicinal products, these are mostly kept in domestic
refrigerators. Domestic refrigerators use heat exchange via the
walls, where the chilling liquid runs through. The temperature near
the walls where the heat exchangers are placed can reach −5°C
(41), while for the doors or in the
corners of the refrigerator, this can rise to 15°C (42). Domestic refrigerators also work in
cycles that can pose additional risks to medicines, and especially
those kept near the wall. As temperature can vary from below to
above freezing point, a medicine might be subjected to freeze-thaw
cycles. Patients are advised to keep medicines in the central part
of the refrigerator, where the temperature is closest to the
pre-set temperature (42), and to
frequently monitor the temperature conditions using a
thermometer.
Conclusions
Various factors have to be taken into consideration
to assure the maximum safety of medicinal products containing mAbs.
Current guidelines recommend compounding of pharmaceutical
preparations with mAbs under the controlled aseptic conditions of
the hospital pharmacy, with this performed by trained and
experienced pharmacy staff. As well as chemical and physical
reactions, a panel of patient characteristics and degrees of
‘foreignness’ of a protein are important for individual treatments.
The main goal is to determine the bioavailability and to use data
on unwanted immunogenicity to optimise the therapeutic dose for
each individual while reducing possible immune reactions and
preventing incorrect therapy choice. Due to the high economic cost
of these biologicals and to safety concerns because of potential
protein instability, healthcare professionals (i.e., medical
doctors, nurses, pharmacists) have to be properly and continuously
educated in terms of the prescribing and preparation of biological
medicinal products. Furthermore, their knowledge should also be
transferred to the patient, to ensure the correct handling of these
medicinal products at home. Where adverse reactions do occur, it is
highly recommended that they are reported, according to local
legislation. Only with strict and regular supervision all the way
from production to final use will a medicinal products that
contains mAbs provide the desired clinical, humanistic and economic
effects designed for the therapy.
Acknowledgements
The authors acknowledge Dr. Chris Berrie for editing
of the manuscript.
References
|
1
|
Das G and Rees A: Use of monoclonal
antibodies for proprotein convertase subtilisin kexin type 9
inhibition: Issues with efficacy, tolerability and safety. Curr
Opin Lipidol. 25:96–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elvin JG, Couston RG and van der Walle CF:
Therapeutic antibodies: Market considerations, disease targets and
bioprocessing. Int J Pharm. 440:83–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shukla D, Schneider CP and Trout BL:
Molecular level insight into intra-solvent interaction effects on
protein stability and aggregation. Adv Drug Deliv Rev.
63:1074–1085. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W: Instability, stabilization, and
formulation of liquid protein pharmaceuticals. Int J Pharm.
185:129–188. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berrill A, Biddlecombe J and Bracewell D:
Product quality during manufacture and supplyPeptide and protein
delivery. Chris Van Der W: Academic Press; Boston, MA: pp. 313–339.
2011, View Article : Google Scholar
|
|
6
|
Kamerzell TJ, Esfandiary R, Joshi SB,
Middaugh CR and Volkin DB: Protein-excipient interactions:
Mechanisms and biophysical characterization applied to protein
formulation development. Adv Drug Deliv Rev. 63:1118–1159. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bee JS, Nelson SA, Freund E, Carpenter JF
and Randolph TW: Precipitation of a monoclonal antibody by soluble
tungsten. J Pharm Sci. 98:3290–3301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davies MJ: The oxidative environment and
protein damage. Biochim Biophys Acta. 1703:93–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Margiloff L, Chaplia L, Chow A, Singhal PC
and Mattana J: Metal-catalyzed oxidation of immunoglobulin G
impairs Fc receptor-mediated binding to macrophages. Free Radic
Biol Med. 25:780–785. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu D, Ren D, Huang H, Dankberg J,
Rosenfeld R, Cocco MJ, Li L, Brems DN and Remmele RL Jr: Structure
and stability changes of human IgG1 Fc as a consequence of
methionine oxidation. Biochemistry. 47:5088–5100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mian IS, Bradwell AR and Olson AJ:
Structure, function and properties of antibody binding sites. J Mol
Biol. 217:133–151. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dimitrov JD, Ivanovska ND,
Lacroix-Desmazes S, Doltchinkova VR, Kaveri SV and Vassilev TL:
Ferrous ions and reactive oxygen species increase antigen-binding
and anti-inflammatory activities of immunoglobulin G. J Biol Chem.
281:439–446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omersel J, Jurgec I, Cucnik S, Kveder T,
Rozman B, Sodin-Semrl S and Bozic B: Autoimmune and proinflammatory
activity of oxidized immunoglobulins. Autoimmun Rev. 7:523–529.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daugherty AL and Mrsny RJ: Formulation and
delivery issues for monoclonal antibody therapeutics. Adv Drug
Deliv Rev. 58:686–706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang L, Lu J, Wroblewski VJ, Beals JM and
Riggin RM: In vivo deamidation characterization of monoclonal
antibody by LC/MS/MS. Anal Chem. 77:1432–1439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vermeer AW and Norde W: The thermal
stability of immunoglobulin: Unfolding and aggregation of a
multi-domain protein. Biophys J. 78:394–404. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Gaza-Bulseco G and Sun J:
Characterization of the stability of a fully human monoclonal IgG
after prolonged incubation at elevated temperature. J Chromatogr B
Analyt Technol Biomed Life Sci. 837:35–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye H: Simultaneous determination of
protein aggregation, degradation, and absolute molecular weight by
size exclusion chromatography-multiangle laser light scattering.
Anal Biochem. 356:76–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Segura A, Herrera M, González E, Vargas M,
Solano G, Gutiérrez JM and León G: Stability of equine IgG
antivenoms obtained by caprylic acid precipitation: Towards a
liquid formulation stable at tropical room temperature. Toxicon.
53:609–615. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arakawa T, Prestrelski SJ, Kenney WC and
Carpenter JF: Factors affecting short-term and long-term
stabilities of proteins. Adv Drug Deliv Rev. 46:307–326. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Nema S and Teagarden D: Protein
aggregation-pathways and influencing factors. Int J Pharm.
390:89–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hawe A, Kasper JC, Friess W and Jiskoot W:
Structural properties of monoclonal antibody aggregates induced by
freeze-thawing and thermal stress. Eur J Pharm Sci. 38:79–87. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W: Lyophilization and development of
solid protein pharmaceuticals. Int J Pharm. 203:1–60. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang BS, Kendrick BS and Carpenter JF:
Surface-induced denaturation of proteins during freezing and its
inhibition by surfactants. J Pharm Sci. 85:1325–1330. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cleland JL and Langer R: Formulation and
Delivery of Proteins and Peptides: Design and Development
Strategies. (ACS Symposium Series 567). American Chemical Society;
Washington, DC: 1994, View Article : Google Scholar
|
|
26
|
Hermeling S, Crommelin DJ, Schellekens H
and Jiskoot W: Structure-immunogenicity relationships of
therapeutic proteins. Pharm Res. 21:897–903. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosenberg AS: Effects of protein
aggregates: An immunologic perspective. AAPS J. 8:E501–E507. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Groot AS and Scott DW: Immunogenicity
of protein therapeutics. Trends Immunol. 28:482–490. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keiserman M, Codreanu C, Handa R,
Xibillé-Friedmann D, Mysler E, Briceño F and Akar S: The effect of
antidrug antibodies on the sustainable efficacy of biologic
therapies in rheumatoid arthritis: Practical consequences. Expert
Rev Clin Immunol. 10:1049–1057. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schellekens H and Jiskoot W:
Eprex-associated pure red cell aplasia and leachates. Nat
Biotechnol. 24:613–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suntharalingam G, Perry MR, Ward S, Brett
SJ, Castello-Cortes A, Brunner MD and Panoskaltsis N: Cytokine
storm in a phase 1 trial of the anti-CD28 monoclonal antibody
TGN1412. N Engl J Med. 355:1018–1028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang WY and Foote J: Immunogenicity of
engineered antibodies. Methods. 36:3–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joubert MK, Hokom M, Eakin C, Zhou L,
Deshpande M, Baker MP, Goletz TJ, Kerwin BA, Chirmule N, Narhi LO
and Jawa V: Highly aggregated antibody therapeutics can enhance the
in vitro innate and late-stage T-cell immune responses. J Biol
Chem. 287:25266–25279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brinks V, Jiskoot W and Schellekens H:
Immunogenicity of therapeutic proteins: The use of animal models.
Pharm Res. 28:2379–2385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swanson SJ and Bussiere J: Immunogenicity
assessment in non-clinical studies. Curr Opin Microbiol.
15:337–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Breckenridge A: Report of the working
party on the addition of drugs to intravenous infusion fluids
(HC(76)9). Department of Health and Social Security; London:
1976
|
|
37
|
Langford S, Fradgley S, Evans M and Blanks
C: Assessing the risk of handling monoclonal antibodies. Hospital
Pharm. 15:60–64. 2008.
|
|
38
|
Odou P: Medical devices for safe handling
of cytotoxic drugs. Eur J Oncol Pharm. 4:17–19. 2010.
|
|
39
|
Paul M, Vieillard V, Jaccoulet E and
Astier A: Long-term stability of diluted solutions of the
monoclonal antibody rituximab. Int J Pharm. 436:282–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grundstein A, Meentemeyer V and Dowd J:
Maximum vehicle cabin temperatures under different meteorological
conditions. Int J Biometeorol. 53:255–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laguerre O and Flick D: Temperature
prediction in domestic refrigerators: Deterministic and stochastic
approaches. Int J Refrigeration. 33:41–51. 2010. View Article : Google Scholar
|
|
42
|
James SJ, Evans J and James C: A review of
the performance of domestic refrigerators. J Food Eng. 87:2–10.
2008. View Article : Google Scholar
|