Introduction
Oxybuprocaine, as an ophthalmic topical anesthetic,
is widely applied in a variety of eye surgery procedures. After
drop administration with anesthetic, corneal sensation decreases or
disappears and the epithelium loses its regularity and easily
becomes dry, so it has certain toxic effects (1). One study (2)reported that allergic conjunctivitis and
severe corneal damage have occurred after drop administration of
oxybuprocaine after operation in clinical practice. Another study
(3) revealed that 0.5–4 g/l
oxybuprocaine has an obvious inductive effect on apoptosis of human
corneal endothelial cells (HCECs), displaying a significant
concentration- and time-dependent manner, but the concentration of
oxybuprocaine used in clinical practice is 4 g/l, so oxybuprocaine
with clinical concentration has a strong inductive effect on
apoptosis of HCECs. Thus, there is a need to develop efficient new
drugs for HCECs to inhibit oxybuprocaine toxicity.
Licorice, belonging to Leguminosae Glycyrrhiza, is
derived from the dry roots and rhizomes of Glycyrrhiza
uralensis, Glycyrrhiza glabra and Glycyrrhiza inflata
Batal., which is commonly added into a variety of traditional
Chinese medicine compounds as an adjuvant or messenger drug; it
tastes sweet and is neutral in nature, with effects such as
invigorating spleen and replenishing qi, clearing away heat
and toxic materials, expelling phlegm and arresting coughing,
relieving spasm and stopping pain and moderating the property of
herbs (1–3). Liquiritin is one of the main flavonoids
in Glycyrrhiza uralensis, which has antidepressant,
neuroprotective and therapeutic effects on heart system diseases
(3–7). In Chinese traditional medicine,
licorice has been used to treat eye disease, for example, viral
keratitis, ulcerative keratitis, and irritability of keratitis.
Liquiritin can significantly reduce apoptosis of human umbilical
vein endothelial cells (HUVECs) induced by AGEs (8,9) and play
a strong protective effect on vascular endothelial cells in
myocardial ischemia-reperfusion injury model (6,10). It
can also protect smoking-induced lung epithelial cell injury
(11). Our past studies
(unpublished)showed liquiritin was worthy of further study by
HPLC-MS analysis and biological experiments. However, whether it
can resist corneal epithelial cell damage by oxybuprocaine has not
been reported yet.
This study investigated the protective effect of
liquiritin on oxybuprocaine-induced apoptosis of HCECs, so as to
provide some experimental foundation and theoretical basis for its
application in clinical protection of corneal epithelial cells from
injury.
Materials and methods
Cell culture
The HCEC-12 cells were purchased from
Creative-Bioarray Co. (cat. no. CSC-C3457; New York, NY, USA) and
placed in the RPMI-1640 medium containing 10% fetal bovine serum
(FBS) (both from HyClone, Logan, UT, USA), followed by placement in
a cell culture incubator (37°C, 5% CO2). Penicillin and
streptomycin with each concentration of 1.0×105 µl were
added into nutrient solution to resist bacterial contamination.
Microscopic observation showed that cells were in the adherent
growth in culture fluid, with multiplication every 26–48 h. The
cell concentration was controlled at 106 cells/ml. The
fluid was changed every two days, and cells were subcultured once
every four days. Oxybuprocaine: 0.4 g oxybuprocaine powder was
dissolved in 100 ml Dulbecco's modified Eagle's medium (DMEM)/F12
for preparation of 4 g/l solution, adding medium to the desired
concentration. Cells were cultured and oxybuprocaine was added into
the cells on the second day for the required time.
Cell counting kit-8 (CCK-8)
The HCEC-12 in logarithmic growth phase was
inoculated to the wells of a 96-well plate, followed by adjustment
of density to 2×103 in each well. Subsequently, 200 µl
RPMI-1640 medium containing 10% FBS was added. Six duplicated wells
were set in each group. After culture for 24 h, 10 µl CCK-8
solution was added into each well, and then the sample was
incubated in an incubator containing CO2 for 4 h. The
well with phosphate-buffered solution (PBS) was regarded as the
control, and the absorbance A value at 450 nm was detected by the
enzyme analyzer. The growth curve was drawn.
Detection of apoptosis by flow
cytometry
The adherent cells were digested with trypsin
without ethylene diamine tetraacetic acid (EDTA) and collected (the
digestion time was shortened as much as possible to avoid false
positive); cells were rinsed by PBS twice (centrifuged at 600 × g
for 5 min), and then 1–5×105 cells were collected. Cell
suspension (500 µl) with binding buffer was added. After 5 µl of
Annexin V-family of intracellular (FITC) protein was added,
followed by mixing well, then 5 µl propidium iodide (PI) was added.
The fluid was mixed well, and reacted at room temperature avoiding
light for 5–15 min. The sample was observed and determined by flow
cytometer within 1 h with excitation wavelength Ex=488 nm and
emission wavelength Em=530 nm. The green fluorescence of Annexin V
was detected by FITC channel (FL1). The red fluorescence of PI was
determined by PI channel using FL3. Statistical analysis was
performed by GraphPad Software, Inc. (La Jolla, CA, USA).
Detection of reactive oxygen species
(ROS)
The treated cells were digested by pancreatin,
followed by collection. The cells were re-suspended using
pre-cooling PBS. Subsequently, serum-free medium was used to
prepare 10 µM probe dyeing working fluid. The pre-cooling and
re-suspended cells were centrifuged and re-suspended in the probe
dyeing working fluid, followed by mixing well to make the probe
fully contact with cells. After incubation, cells were rinsed by
serum-free medium three times, so as to fully remove
2,7-dichlorodi-hydrofluorescein diacetate (DCFH-DA) that did not
enter the cells. The sample was detected by flow cytometry,
followed by excitation with 480 nm wavelength and determination of
emission light at 525 nm. ROS-positive cells showed strong green
fluorescence correspondening to FL1 detection channel of BD
Biosciences (Franklin Lakes, NJ, USA) flow cytometer.
Western blotting
Polyacrylamide gel electrophoresis (PAGE) was
conducted. The loading amount of protein in each well was 150 µg.
Eighty volts was changed to 100 V for electrophoresis when Marker
began to separate. When Marker was completely separated and the
target band could be obtained, the electrophoresis was stopped. The
protein was electrically transferred onto polyvinylidene fluoride
(PVDF) membrane with electric current of 350 mA for ~2 h. The
membrane was sealed with 5% bovine serum albumin (BSA)/milk at room
temperature for 1 h, followed by incubation with the diluted rabbit
anti-human primary monoclonal antibodies [NF-κB p65 (cat. no.
4764), caspase-3 (cat. no. 9665), Bax (cat. no. 2774), B-cell
lymphoma-2 (Bcl-2; cat. no. 2872) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. 2118); (all 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA)] according to the instructions
at 4°C overnight. On the second day, goat anti-rabbit secondary
polyclpnal antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) was added. Then, the sample was incubated at 37°C
for 1 h and added with exposure liquid, followed by photographing
using chemiluminescence apparatus.
Statistical analysis
Statistical results were analyzed by GraphPad Prism
5 software. The data are expressed as mean ± standard deviation.
The independent samples t-test was used for comparison of
difference between two groups, and analysis of variance was adopted
for comparison of multivariate means. P<0.05 indicates that the
difference was statistically significant.
Results
Oxybuprocaine inhibits the
proliferation of HCEC-12, HCEC-H9C1, and HCEC-B4G12
Oxybuprocaine is a common medicine for eye
anesthesia. In order to study its influence on different human
corneal endothelial cells, oxybuprocaine media with different
concentrations were adopted to induce HCEC-12, HCEC-H9C1 and
HCEC-B4G12 for 0, 3, 6, 12 and 24 h, and the effect of
oxybuprocaine on activity of HCEC-12, HCEC-H9C1 and HCEC-B4G12 was
detected by CCK-8. The results displayed that HCEC-12, HCEC-H9C1
and HCEC-B4G12 activity was inhibited and it was dependent on the
concentration of oxybuprocaine; moreover, the cell activity of
HCEC-12 was significantly inhibited after the reaction with
concentration of oxybuprocaine over 100 mg/l for 12 h compared with
those of HCEC-H9C1 and HCEC-B4G12 (P<0.05) (Fig. 1A-C). So we chose HCEC12 for the
study.
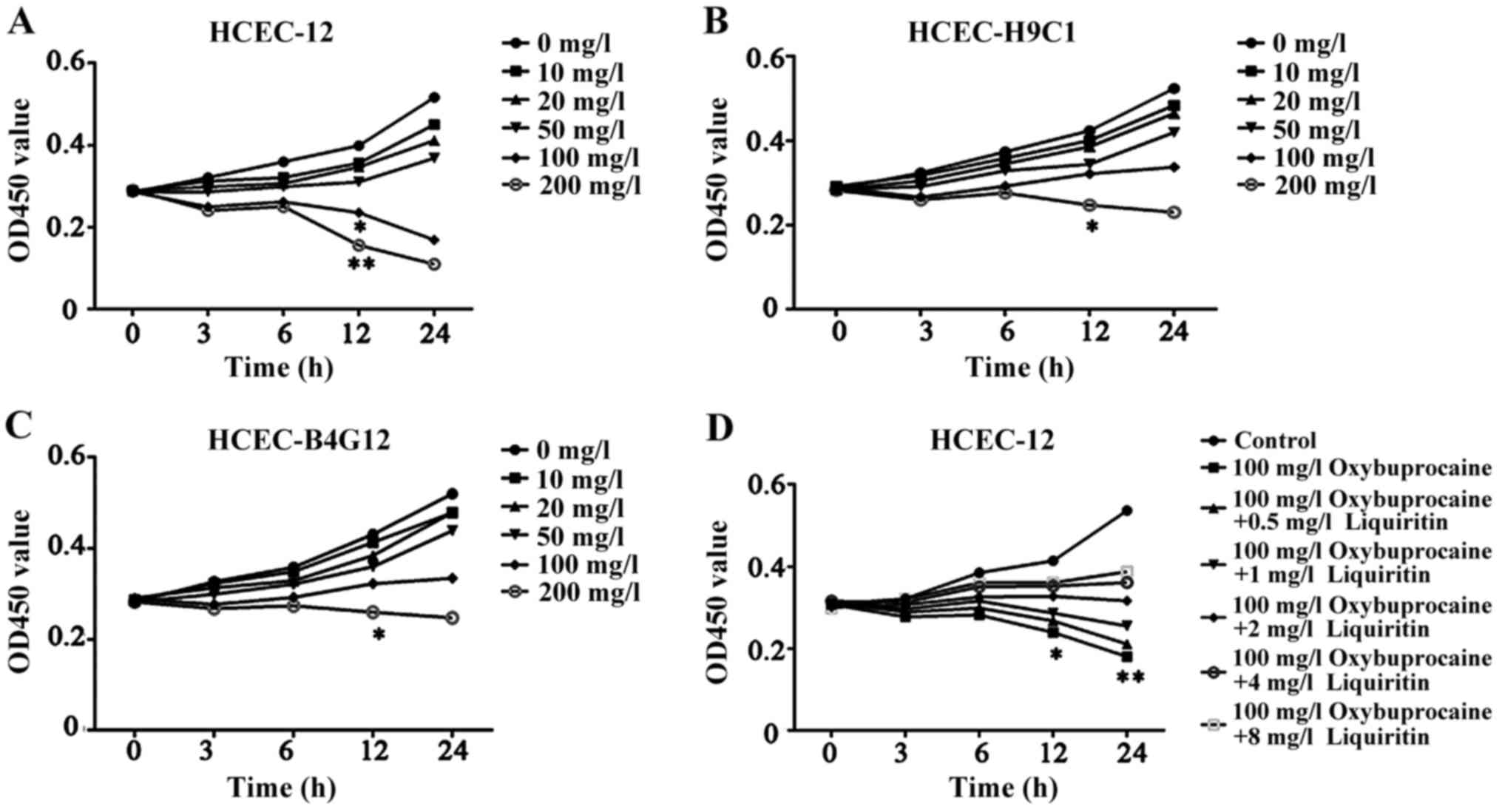 | Figure 1.Detection of proliferation of HCEC-12,
HCEC-H9C1, HCEC-B4G12 using CCK-8. (A-C) The effect of
oxybuprocaine (0, 10, 20, 50, 100 and 200 mg/l) on the
proliferation of HCEC-12, HCEC-H9C1 and HCEC-B4G12 is detected
using CCK-8. (D) Liquiritin (0.5, 1, 2, 4 and 8 mg/ml) resisting
the inhibitory effect of oxybuprocaine on the proliferation of
HCEC-12 was detected using CCK-8. *P<0.05 and **P<0.01. |
Liquiritin resists the proliferation
of HCEC-12 inhibited by oxybuprocaine
The high concentration of oxybuprocaine can induce
HCEC-12 thus significantly reducing the activity of HCEC-12. In
order to investigate the effect of liquiritin on proliferation of
HCEC-12 induced by oxybuprocaine, HCEC-12 was pretreated by
liquiritin in different concentrations, followed by being induced
by 100 mg/l oxybuprocaine for 0, 3, 6, 12 and 24 h. The activity of
cells in each group was detected by CCK-8. The results showed that
oxybuprocaine could significantly decrease cell activity compared
with that in control group (P<0.05); the cell activity in
pretreatment with liquiritin group was distinctly increased
compared with that in oxybuprocaine group, and it showed the most
significant increase in pretreatment with liquiritin group in the
concentration of 8 mg/ml (Fig. 1D),
indicating that liquiritin could resist the inhibitory effect of
oxybuprocaine on the proliferation of HCEC-12.
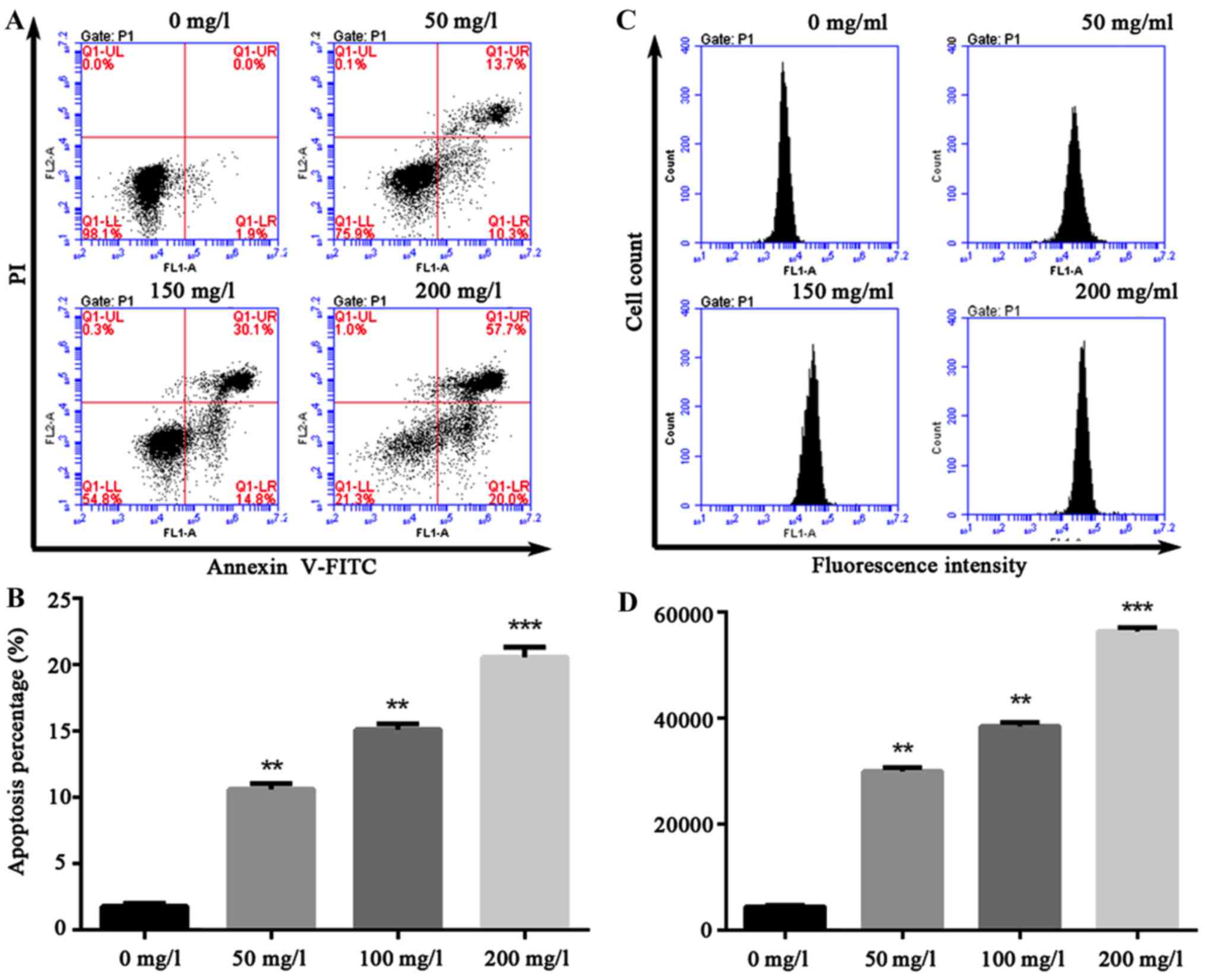
Oxybuprocaine induces HCEC-12
apoptosis and ROS production
A variety of pro-apoptotic signals (such as
unfavorable environmental factors, injury, radiation,
chemotherapeutic agents, excitatory amino acid and death ligand)
can cause increased cell endogenous or exogenous ROS or altered
redox equilibrium. The production of ROS can serve as a signal
triggering apoptosis in transduction pathway. Thus, HCEC-12 was
intervened by oxybuprocaine media in different concentrations for
12 h in this study, and apoptosis was assessed by flow cytometry,
revealing that compared with that in control group (1.9%), 200 mg/l
oxybuprocaine can significantly induce HCEC-12 apoptosis (20%)
(Fig. 2A and B). Additionally,
DCFH-DA staining and fluorescent-activated cell sorting (FACS) were
used to analyze the production of ROS after HCEC-12 was stimulated
by oxybuprocaine, suggesting that different concentrations of
oxybuprocaine could significantly induce the production of ROS in
HCEC-12, which was concentration-dependent (Fig. 2C and D).
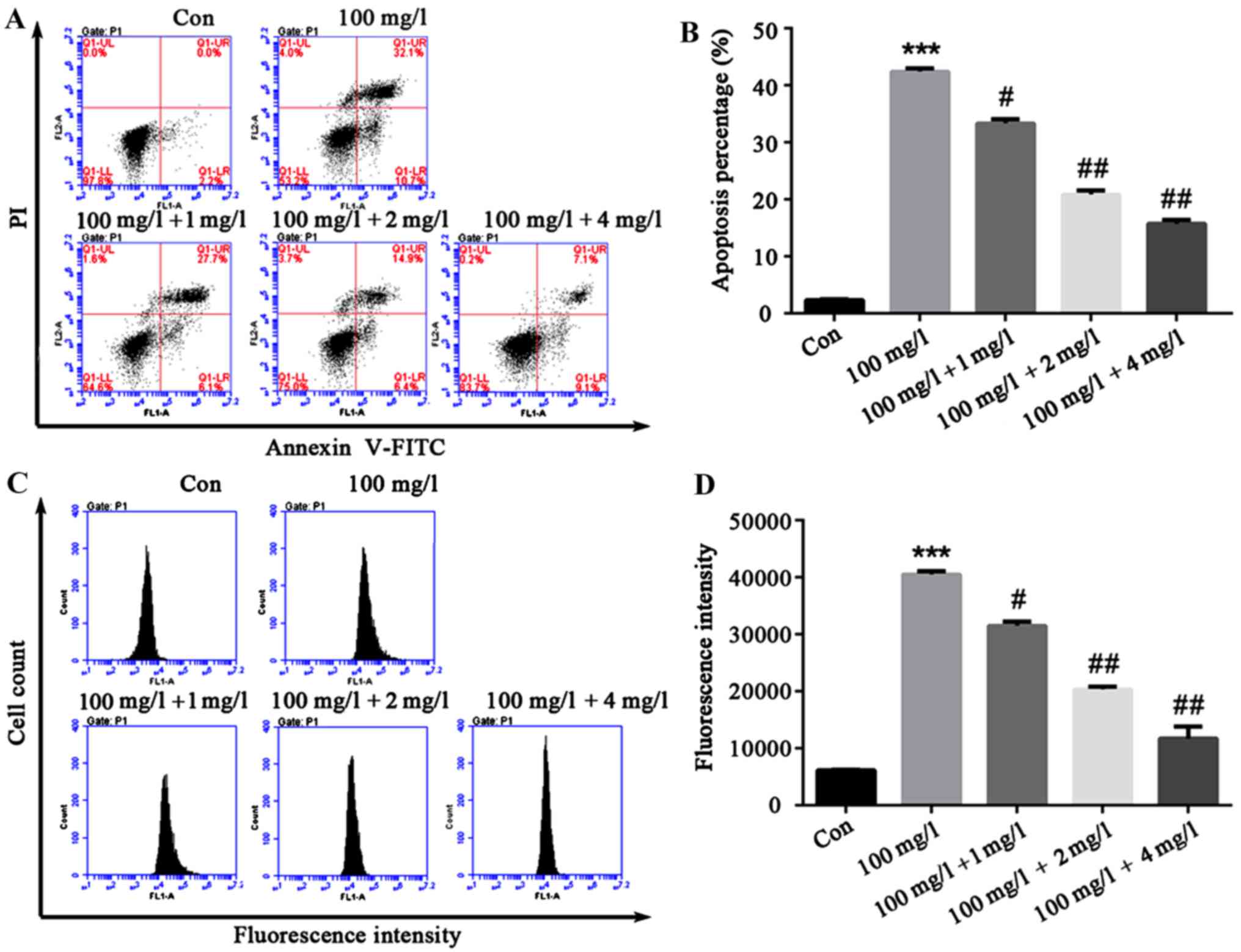
Liquiritin resists proliferation of
HCEC-12 apoptosis and ROS production is induced by
oxybuprocaine
In order to explore the effect of liquiritin on
HCEC-12 apoptosis and ROS production induced by oxybuprocaine,
HCEC-12 was pretreated by liquiritin in different concentrations
for 1h, and then induced by 100 mg/l oxybuprocaine for 12 h in this
study, and apoptosis and ROS production were detected by flow
cytometry. The results revealed that compared with that in control
group, apoptosis in oxybuprocaine group was distinctly increased,
and it was remarkably reduced in pretreatment with liquiritin group
compared with that in oxybuprocaine group (Fig. 3A and B). The production of ROS
analyzed by DCFH-DA staining and FACS obtained results that were
consistent with that of apoptosis, suggesting that liquiritin could
resist the production of ROS in HCEC-12 induced by oxybuprocaine
(Fig. 3C and D).
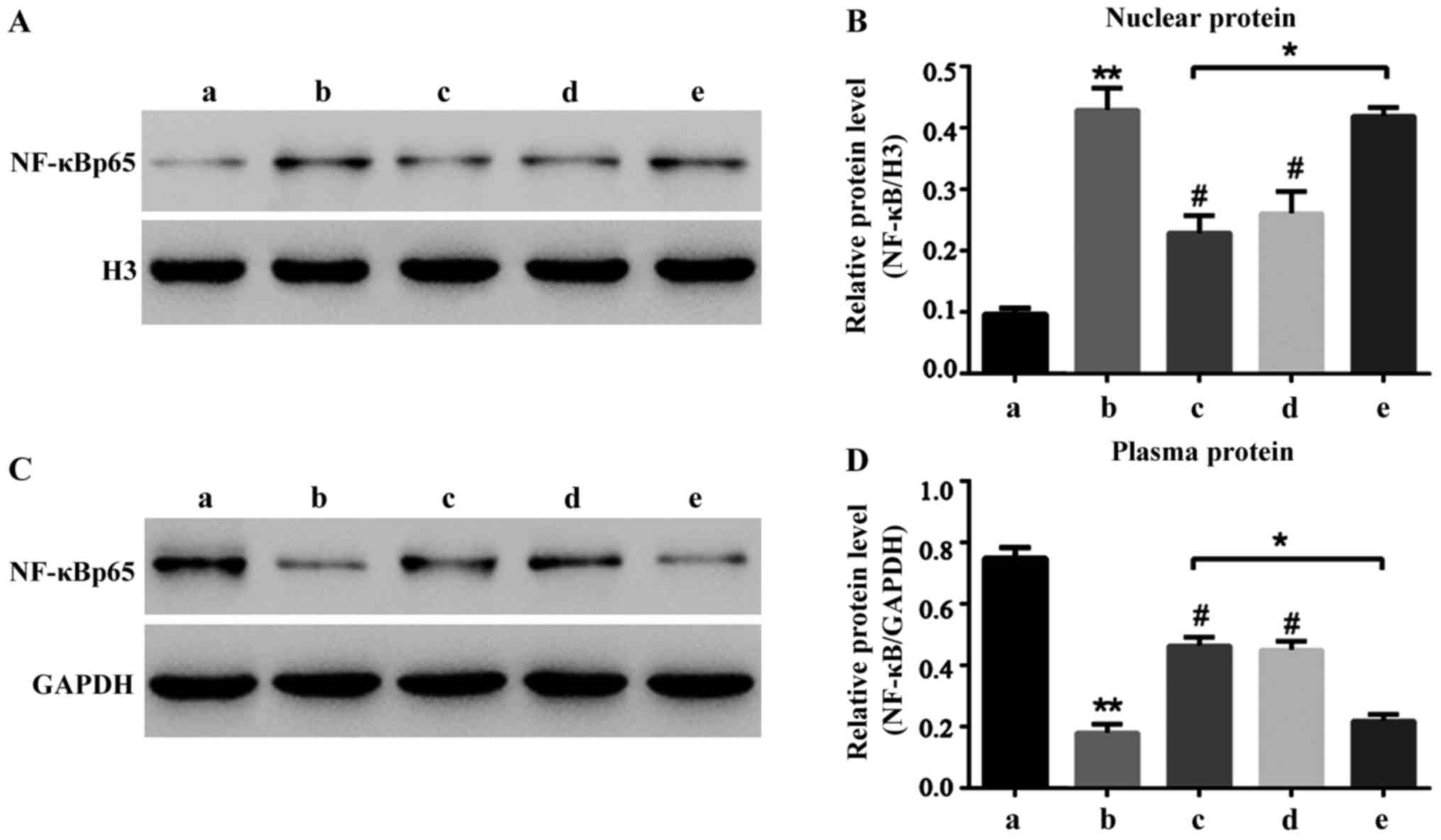
Liquiritin resists the NF-κB signal
pathway activated by oxybuprocaine
Liquiritin can significantly reduce HCEC-12
apoptosis induced by oxybuprocaine thus protecting HCEC-12. It is
well known that NF-κB signal pathway is widely involved in the
process of apoptosis in many cells (12–15).
Hence, this study aimed to investigate whether HCEC-12 apoptosis
induced by oxybuprocaine is dependent on the NF-κB signal pathway,
and whether liquiritin resists the induction of HCEC-12 apoptosis
by oxybuprocaine through inhibiting the NF-κB signal pathway. The
results revealed that 50 mg/l oxybuprocaine could significantly
increase the expression of NF-κB p65 in nuclear protein (Fig. 4A) and decrease the expression of
NF-κB p65 in plasmosin (Fig. 4B),
and the pretreatment with 2 mg/ml liquiritin and 50 µmol/l
pyrrolidinedithiocarbamic acid (PDTC) obviously blocked the
expression of NF-κB p65 in nuclear protein increased by
oxybuprocaine (Fig. 4A).
Additionally, 10 ng/ml tumor necrosis factor-α (TNF-α) blocked the
inhibitory effect of liquiritin on the expression of NF-κB p65 in
nuclear protein (Fig. 4A), thus
obstructing the protective effect of liquiritin, indicating that
liquiritin resists HCEC-12 apoptosis induced by oxybuprocaine
through inhibiting the NF-κB signal pathway.
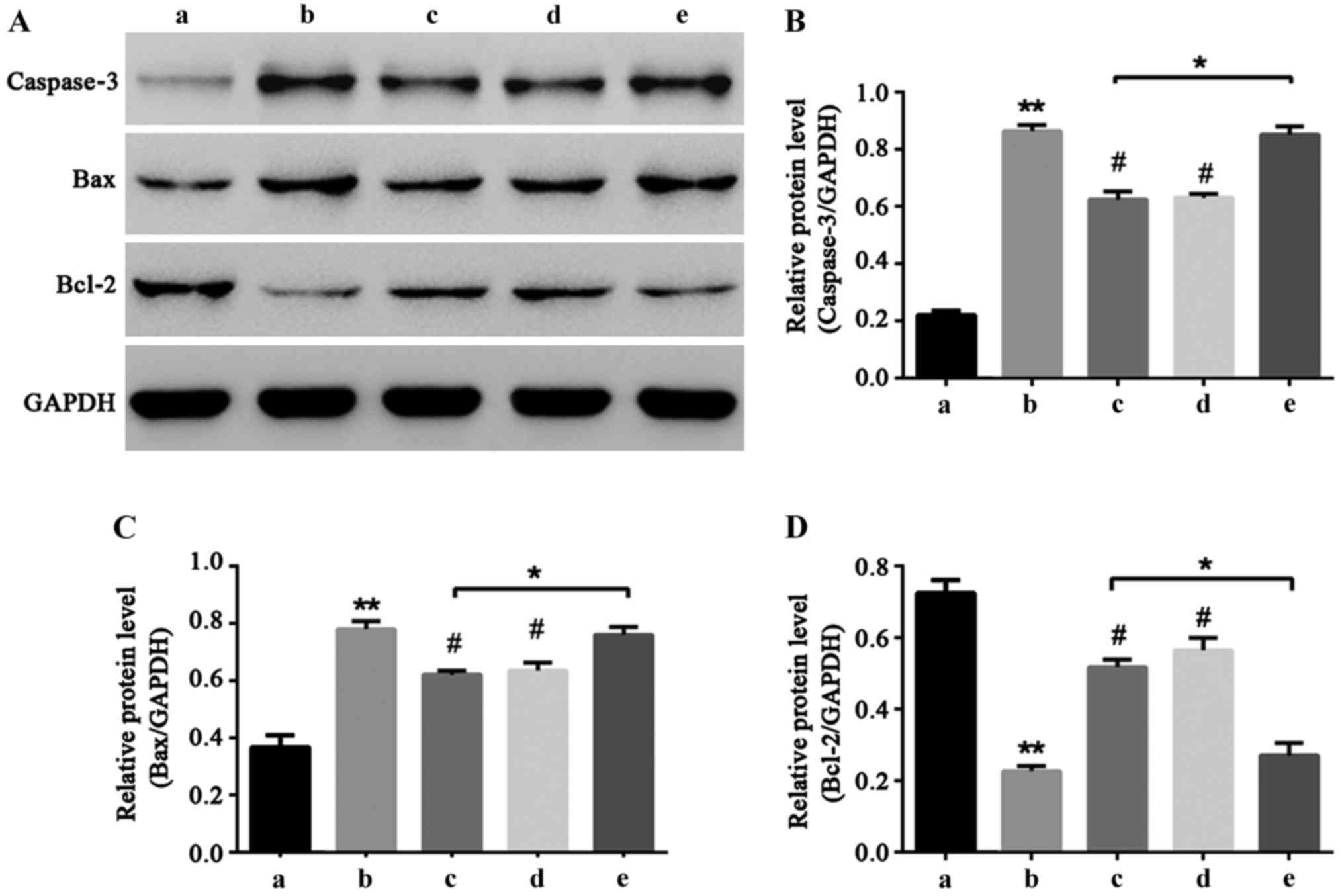
NF-κB signal pathway participates in
liquiritin resistance of HCEC-12 apoptosis induced by
oxybuprocaine
Caspase-3, Bax and Bcl-2 are important proteins that
can directly reflect the extent of apoptosis (16–18). In
order to explore the molecular mechanism of liquiritin resistance
of HCEC-12 induced by oxybuprocaine, western blot was utilized to
determine the expressions of caspase-3, Bax and Bcl-2 proteins. The
results showed that oxybuprocaine increased the expression levels
of caspase-3 and Bax and reduced the expression levels of
anti-apoptotic protein Bcl-2, and pretreatment with liquiritin and
PDTC inhibited the increasing effect of oxybuprocaine on expression
levels of caspase-3 and Bax proteins and reduced the inhibitory
effect of oxybuprocaine on Bcl-2 expression level; pretreatment
with TNF-α blocked the inhibitory effect of liquiritin on the
expression levels of caspase-3 and Bax proteins and reduced the
increasing effect of liquiritin on Bcl-2 expression (Fig. 5), revealing that liquiritin reduced
the expression of apoptosis proteins and increased the expression
of anti-apoptosis proteins through inhibiting the NF-κB signal
pathway, thus resisting HCEC-12 apoptosis induced by
oxybuprocaine.
Discussion
With the development of ophthalmic surgery
techniques and equipment, ophthalmic topical anesthetics have been
widely applied, and its side effects on cornea are getting
increasing attention. The most commonly used topical anesthetic is
oxybuprocaine (8–10). Cornea is mainly composed of
endothelium, stroma, epithelium and its derivatives, and corneal
endothelial cells in mammals (except for rabbits) lose their
regenerative ability in adulthood. Therefore, the damage of human
corneal endothelial cells cannot be repaired, and the study on HCEC
is of uppermost priority. Previous studies show that oxybuprocaine
can induce apoptosis of corneal epithelial cells, which causes
certain damage on corneal epithelial cells, so it is urgent to
develop drugs that can resist toxicity of oxybuprocaine (15–18).
Liquiritin is derived from Glycyrrhiza
uralensis which belongs to leguminous plants. It has good
anti-inflammatory and antioxidant activity (18). However, its protective effect on
corneal epithelial cell injury has not been reported. The
experimental results showed that oxybuprocaine inhibited the
proliferation of human corneal epithelial cells and induced its
apoptosis, which was concentration-dependent. The results of
pretreatment with liquiritin revealed that oxybuprocaine
significantly decreased cell activity compared with that in control
group (P<0.05); the cell activity in pretreatment with
liquiritin group was distinctly increased compared with that in
oxybuprocaine group, and it showed the most significant increase in
pretreatment with liquiritin group in the concentration of 8 mg/ml,
indicating that liquiritin could resist the inhibitory effect of
oxybuprocaine on the proliferation of HCEC-12. The results of
apoptosis experiment showed that compared with that in the control
group, apoptosis in oxybuprocaine group was distinctly increased,
and it was remarkably reduced in pretreatment with liquiritin group
compared with that in oxybuprocaine group. The production of ROS
analyzed by DCFH-DA staining and FACS obtained results which were
consistent with that of apoptosis, suggesting that liquiritin can
resist the production of ROS in HCEC-12 induced by
oxybuprocaine.
The results of molecular mechanism investigation
revealed that oxybuprocaine significantly increased the expression
of NF-κB p65 in nuclear protein and decreased NF-κB p65 in
plasmosin, and the pretreatment with liquiritin and PDTC obviously
blocked the expression of NF-κB p65 in nuclear protein increased by
oxybuprocaine. Additionally, TNF-α blocked the inhibitory effect of
liquiritin on the expression of NF-κB p65 in nuclear protein, thus
obstructing the protective effect of liquiritin. The results of
western blotting showed that oxybuprocaine increased the expression
levels of caspase-3 and Bax and reduced the expression levels of
anti-apoptotic protein Bcl-2, and pretreatment with liquiritin and
PDTC inhibited the increasing effect of oxybuprocaine on expression
levels of caspase-3 and Bax proteins and reduced the inhibitory
effect of oxybuprocaine on Bcl-2 expression level; pretreatment
with TNF-α blocked the inhibitory effect of liquiritin on the
expression levels of caspase-3 and Bax proteins. NF-κB has been
reported to inhibit apoptosis or promote apoptosis depending on the
contexts (19). The downregulation
of Bcl-2, upregulation of Bax, and activation of caspase-3 are
widely known in the occurrence of apoptosis. We found that
oxybuprocaine induced changes of protein levels of NF-κB, Bcl-2,
Bax and caspase-3, but did not further analyze the relationship
between NF-κB and Bcl-2, Bax, or caspase-3. Regarding the
relationship between NF-κB and Bcl-2, Bax, or caspase-3, Wier et
al (20) showed that despite the
cleavage of NF-κB p65 by caspase-3, the cleavage-generated p65
N-terminal fragment interferes with the RPS3/NF-κB-confering gene
transcription. Cao et al (21) also found that inhibition of NF-κB
lead to increase of Bcl-2 expression and attenuates caspase-3
activation. Therefore, there were interactions between NF-κB and
Bcl-2, Bax or caspase-3, but the detailed relationships in the
context of liquiritin against oxybuprocaine-induced apoptosis need
to be analyzed in further studies.
In conclusion, the results of this study indicated
that liquiritin reduces the expression of apoptosis proteins and
increases the expression of anti-apoptosis proteins through
inhibiting NF-κB signal pathway, thus resisting HCEC-12 apoptosis
induced by oxybuprocaine. The protective effect of liquiritin on
corneal epithelial cells is expected to be used in the clinical
practice to inhibit the toxicity of oxybuprocaine on corneal
epithelial cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
DL collected, analyzed and interpreted the patient
data, and drafted the manuscript. PZ conceived and designed the
study, and revised the manuscript for important intellectual
content. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hatano T, Yasuhara T, Miyamoto K and Okuda
T: Anti-human immunodeficiency virus phenolics from licorice. Chem
Pharm Bull (Tokyo). 36:2286–2288. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kelly-Pieper K, Patil SP, Busse P, Yang N,
Sampson H, Li XM, Wisnivesky JP and Kattan M: Safety and
tolerability of an antiasthma herbal Formula (ASHMI) in adult
subjects with asthma: A randomized, double-blinded,
placebo-controlled, dose-escalation phase I study. J Altern
Complement Med. 15:735–743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whorwood CB, Sheppard MC and Stewart PM:
Licorice inhibits 11 beta-hydroxysteroid dehydrogenase messenger
ribonucleic acid levels and potentiates glucocorticoid hormone
action. Endocrinology. 132:2287–2292. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamir S, Eizenberg M, Somjen D, Stern N,
Shelach R, Kaye A and Vaya J: Estrogenic and antiproliferative
properties of glabridin from licorice in human breast cancer cells.
Cancer Res. 60:5704–5709. 2000.PubMed/NCBI
|
|
5
|
Hatano T, Shintani Y, Aga Y, Shiota S,
Tsuchiya T and Yoshida T: Phenolic constituents of licorice. VIII.
Structures of glicophenone and glicoisoflavanone, and effects of
licorice phenolics on methicillin-resistant Staphylococcus aureus.
Chem Pharm Bull (Tokyo). 48:1286–1292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun YX, Tang Y, Wu AL, Liu T, Dai XL,
Zheng QS and Wang ZB: Neuroprotective effect of liquiritin against
focal cerebral ischemia/reperfusion in mice via its antioxidant and
antiapoptosis properties. J Asian Nat Prod Res. 12:1051–1060. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi T, Takasuka N, Iigo M, Baba M,
Nishino H, Tsuda H and Okuyama T: Isoliquiritigenin, a flavonoid
from licorice, reduces prostaglandin E2 and nitric oxide, causes
apoptosis, and suppresses aberrant crypt foci development. Cancer
Sci. 95:448–453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Song Y, Han X, Feng L, Wang R,
Zhang M, Zhu M, Jia X and Hu S: Liquiritin attenuates advanced
glycation end products-induced endothelial dysfunction via
RAGE/NF-κB pathway in human umbilical vein endothelial cells. Mol
Cell Biochem. 374:191–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng L, Zhu MM, Zhang MH, Wang RS, Tan XB,
Song J, Ding SM, Jia XB and Hu SY: Protection of glycyrrhizic acid
against AGEs-induced endothelial dysfunction through inhibiting
RAGE/NF-κB pathway activation in human umbilical vein endothelial
cells. J Ethnopharmacol. 148:27–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guoqiang Q and Guoping Z: Optimal
proportion of four effective components of Danggui Decoction on
vascular endothelial cell protection in rats with myocardial
ischemia reperfusion injury. Tradit Chin Med Mater. 34:580–584.
2011.(In Chinese).
|
|
11
|
Guan Y, Li FF, Hong L, Yan XF, Tan GL, He
JS, Dong XW, Bao MJ and Xie QM: Protective effects of liquiritin
apioside on cigarette smoke-induced lung epithelial cell injury.
Fundam Clin Pharmacol. 26:473–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koshimizu JY, Beltrame FL, de Pizzol JP
Jr, Cerri PS, Caneguim BH and Sasso-Cerri E: NF-κB overexpression
and decreased immunoexpression of AR in the muscular layer is
related to structural damages and apoptosis in cimetidine-treated
rat vas deferens. Reprod Biol Endocrinol. 11:292013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arora R, Yates C, Gary BD, McClellan S,
Tan M, Xi Y, Reed E, Piazza GA, Owen LB and Dean-Colomb W:
Panepoxydone targets NF-κB and FOXM1 to inhibit proliferation,
induce apoptosis and reverse epithelial to mesenchymal transition
in breast cancer. PLoS One. 9:e983702014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Decean H, Fischer-Fodor E, Tatomir C,
Perde-Schrepler M, Somfelean L, Burz C, Hodor T, Orasan R and Virag
P: Vitis vinifera seeds extract for the modulation of cytosolic
factors BAX-α and NF-κB involved in UVB-induced oxidative stress
and apoptosis of human skin cells. Clujul Med. 89:72–81. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuo N, Zheng X, Liu H and Ma X:
Fenofibrate, a PPARα agonist, protect proximal tubular cells from
albumin-bound fatty acids induced apoptosis via the activation of
NF-κB. Int J Clin Exp Pathol. 8:10653–10661. 2015.PubMed/NCBI
|
|
16
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi L, Teng H, Zhu M, Li C, Huang K, Chen
BI, Dai Y and Wang J: Paeoniflorin inhibits nucleus pulposus cell
apoptosis by regulating the expression of Bcl-2 family proteins and
caspase-9 in a rabbit model of intervertebral disc degeneration.
Exp Ther Med. 10:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Ding Y, Ye N, Wild C, Chen H and
Zhou J: Direct activation of bax protein for cancer therapy. Med
Res Rev. 36:313–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jing H and Lee S: NF-κB in cellular
senescence and cancer treatment. Mol Cells. 37:189–195. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wier EM, Fu K, Hodgson A, Sun X and Wan F:
Caspase-3 cleaved p65 fragment dampens NF-κB-mediated
anti-apoptotic transcription by interfering with the p65/RPS3
interaction. FEBS Lett. 589:3581–3587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao ZH, Yin WD, Zheng QY, Feng SL, Xu GL
and Zhang KQ: Caspase-3 is involved in IFN-γ- and TNF-α-mediated
MIN6 cells apoptosis via NF-κB/Bcl-2 pathway. Cell Biochem Biophys.
67:1239–1248. 2013. View Article : Google Scholar : PubMed/NCBI
|