Introduction
Bronchial asthma is a chronic inflammatory disease
of the airways affecting millions of people worldwide; there are
currently ~300,000,000 patients with asthma (1,2). It has
been demonstrated in a rat model of asthma that the interaction
between inflammation and hypercoagulable state aggravates the
severity of asthma and contributes to airway remodeling (3). In humans, lung inflammation in asthma
is accompanied by a pulmonary procoagulant and antifibrinolytic
environment (4). A number of cells,
including eosinophilia, mastocytes, T lymphocytes, neutrophils and
airway epithelium cells contribute to the development of chronic
airway inflammation in asthma (2).
Levels of coagulation parameters, platelets, fibrinogen, tissue
factor, thromboxane A2, thrombin and activated protein C may
reflect thrombosis formation during the pathophysiological process
of asthma (5,6). Platelets activated by the up-regulation
of cluster of differentiation 154 may release the platelet δ, α and
λ granules, induce pulmonary inflammation and enhance the T helper
cell 2 immune response, thus aggravating asthma severity (7). The extracellular signal-regulated
kinase 1/2 signaling pathway serves an important role in the
process of thrombus-promoting airway remodeling in ovalbumin
allergic rats (8). This suggests
that coagulation is closely associated with inflammation in
asthma.
Tissue factors (TF) are a key connector of the
coagulation and inflammation network and participate in thrombosis
formation through the extrinsic pathway of blood coagulation. They
may also contribute to the inflammation and remolding of the asthma
airway (9,10). Mononuclear cells are the key cell
type to express TF and are the primary source of blood-borne TF
in vitro and in vivo (11).
TF released by endothelial cells serves a major role in the initial
stage, whereas blood-borne TF serves a key role in the
amplification stage of thrombosis (11).
Thromobospondin-1 (TSP-1) is a mucoprotein involved
in the formation of thrombosis (12). It is secreted by platelets,
macrophages, mononuclear cells, vascular muscle cells, fibroblasts
and endothelial cells following the onset of inflammation (13). Platelet activation is an important
determinant of the severity of allergic asthma and TSP-1 is a
marker of platelet activation that represents a higher level in
severe asthma compared with non-severe asthma (14). In addition, TSP-1 may induce
chemotaxis of the macrophagocytes and induce a pro-inflammatory
response (15). However, it remains
unknown whether TIPE2 levels are correlated with TF or TSP-1 levels
in asthma.
Tumor necrosis factor-α-induced protein-8 like-2
(TIPE2) is a gene that was initially identified in a mouse model of
autoimmune encephalomyelitis (16).
As a type of tumor necrosis factor-α-induced protein-8, TIPE2 is
primarily expressed by the myeloid and lymphoid immune cells,
particularly by T lymphocytes, mononuclear cells and macrophages
(17,18). TIPE2 is a negative regulator of
inflammation in certain diseases including chronic hepatitis B
(19), chronic hepatitis C (20), systemic lupus erythematosus (SLE)
(21), diabetic nephropathy
(22) and abdominal aortic aneurysm
(23), suggesting that it serves an
important role in the pathogenesis of inflammatory diseases.
It has been demonstrated that TIPE2 is
down-regulated in the peripheral blood mononuclear cells (PBMCs) of
children with bronchial asthma and that TIPE2 expression is
negatively correlated with immunoglobulin (Ig)E, interleukin (IL)-4
and eosinophil counts (24).
However, the association between the inflammatory regulator TIPE2
and the coagulation substances TF and TSP-1 in asthma remains
unclear. In the current study, the relative expression of TIPE2 and
TF in PBMCs, as well as the levels of TF and TSP-1 in the sera,
were measured in patients with bronchial asthma and healthy
controls. The association between TIPE2, and TF and TSP-1 in
patients with asthma was subsequently analyzed.
Patients and methods
Patients
A total of 65 patients (male:female, 35:30) aged
38–88 years, diagnosed with acute asthma were recruited from the
First Affiliated Hospital of Zhengzhou University (Henan, China)
between November 2015 and May 2016. Asthma diagnoses were based on
the criteria established by Global Initiative for asthma (25). None of the patients had other
diseases, including pulmonary embolism, chronic bronchitis,
pulmonary tuberculosis, chronic obstructive pulmonary disease and
hematological tumors. Furthermore, patients had not received oral
corticosteroids and did not experience any upper respiratory
infections for 2 months prior to enrollment. A total of 40 healthy
individuals (male:female, 20:20) aged 34–85 years old were also
recruited from the First Affiliated Hospital of Zhengzhou
University (Henan, China). There were no statistical differences in
sex and age between the two groups. All participants gave their
written informed consent for inclusion in the current study and
ethical approval was granted by the local Ethics Committee of the
First Affiliated Hospital of Zhengzhou University.
Western blotting
Levels of TIPE2 and TF in the PBMCs were assessed as
previously described (24). Briefly,
PBMCs were respectively separated from 1 ml EDTA-K2 anticoagulation
peripheral blood from 65 asthmatic patients and 40 healthy controls
using density gradient centrifugation at 600 × g for 30 min at 25°C
with Human lymphocyte separation fluid (Dakewe Biotech Co., Ltd,
Shenzhen, China) according to the manufacturer's instructions.
Proteins of PBMCs were extracted using a western and IP lysate
solution (Beyotime Institute of Biotechnology, Shanghai, China).
Protein concentration was determined using a Bradford kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (10 µg) were
separated on 12% SDS-polyacrylamide gel and transferred onto 0.22
µm PVDF membranes (Merck KGaA, Darmstadt, Germany) by
electrotransfer. Following blocking with 5% skimmed powder milk in
TBST for 2 h at room temperature, the PVDF membrane was incubated
overnight at 4°C with a 1:500 dilution of rabbit anti-human TF
monoclonal antibodies (cat no. AB151748; Abcam, Cambridge, UK) or
1:1,000 dilution of mouse anti-human TIPE2 polyclonal antibodies
(cat no. 15940–1-AP; ProteinTech Group, Inc., Chicago, IL, USA). A
1:1,000 dilution of mouse anti-human β-actin monoclonal antibodies
(cat no. 66,009-1-Ig; ProteinTech Group, Inc.) was used as internal
reference. The membrane was then washed four times with TBST and
incubated with a 1:1,000 dilution of goat anti-rabbit IgG and goat
anti-mouse IgG secondary antibodies, respectively, for 1 h at room
temperature. Following four washes with TBST, the membrane was
visualized using an ECL western blot detection kit (Beyotime
Institute of Biotechnology). Images were analyzed using a Gel
analyzing Program analyzer version 4.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Enzyme-linked immunosorbent assay
(ELISA)
Sera were separated from the peripheral blood of
asthmatic patients and healthy controls at 1,000 × g for 5 min at
25°C. To assess levels of TF and TSP-1 in the sera of 65 patients
with asthma and 40 healthy controls, sandwich ELISA kits (cat nos.
E-EL-H0040c and E-EL-H1589c; Elabscience Biotechnology, Houston,
TX, USA) were used according to the manufacturers' protocols. Each
sera sample was diluted with sample dilution buffer from the kit
and then added to plates pre-coated with the TF or TSP-1
antibodies, which were also part of the kit. Plates were incubated
for 90 min at 37°C. Following three washes, 1:100 diluted
biotinylated TF or TSP-1 detection antibodies were added to the
plates and incubated at 37°C for 60 min. Plates were washed a
further three times, then horseradish peroxidase-conjugated
streptavidin was added and incubated at 37°C for 30 min.
Subsequently, etramethylbenzidine substrate solution was added to
the plates. Following 10 min incubation in the dark at room
temperature, the reaction was halted by the addition of the stop
solution and measured at 450 nm. The concentration of samples was
calculated according to the standard curve, which was produced by
different dilutions of reference standard.
Statistical analysis
When the data were normally distributed, an unpaired
student's t-test was used to compare the difference between
patients with bronchial asthma and healthy controls. If the data
were not normally distributed, the Mann-Whitney U-test was used.
Spearman's rank correlation coefficient was used to determine
whether there was a correlation between TIPE2 and either TF or
TSP-1. All statistical analyses were performed using Graphpad 5.0
software (Graphpad Software, Inc., La Jolla, CA, USA) and P<0.05
was considered to indicate a statistically significant
difference.
Results
The expression of TIPE2 and TF
proteins in PBMCs from patients with bronchial asthma compared with
healthy controls
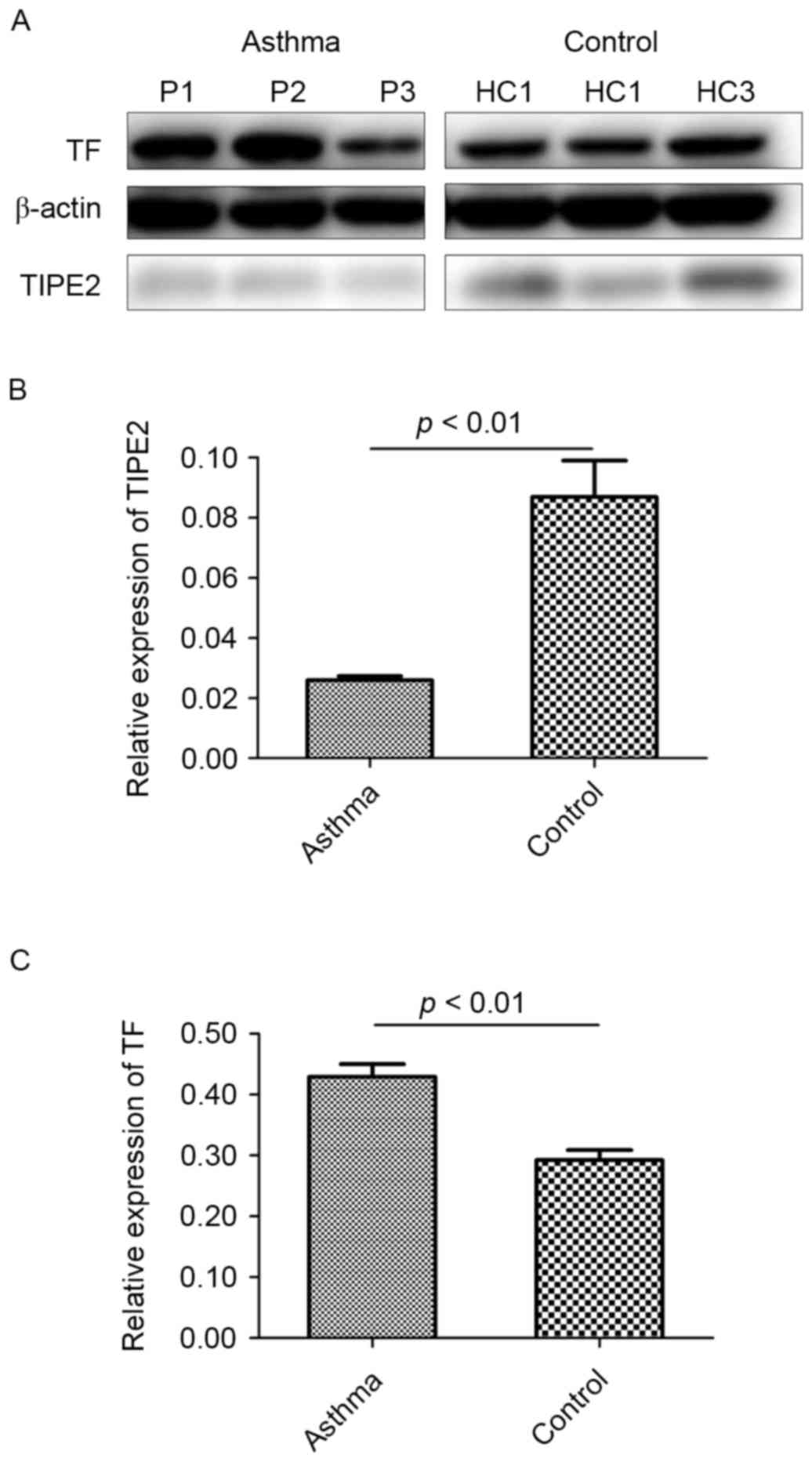
Relative protein levels of TIPE2 and TF in PBMCs
from patients with bronchial asthma and healthy controls were
detected and β-actin was used as an internal reference (Fig. 1A). The results demonstrated that
TIPE2 expression in PBMCs is significantly lower in patients with
asthma compared with healthy controls (P<0.01; Fig. 1B). However, TF expression in PBMCs
was significantly higher in patients with asthma compared with
healthy controls (P<0.01; Fig.
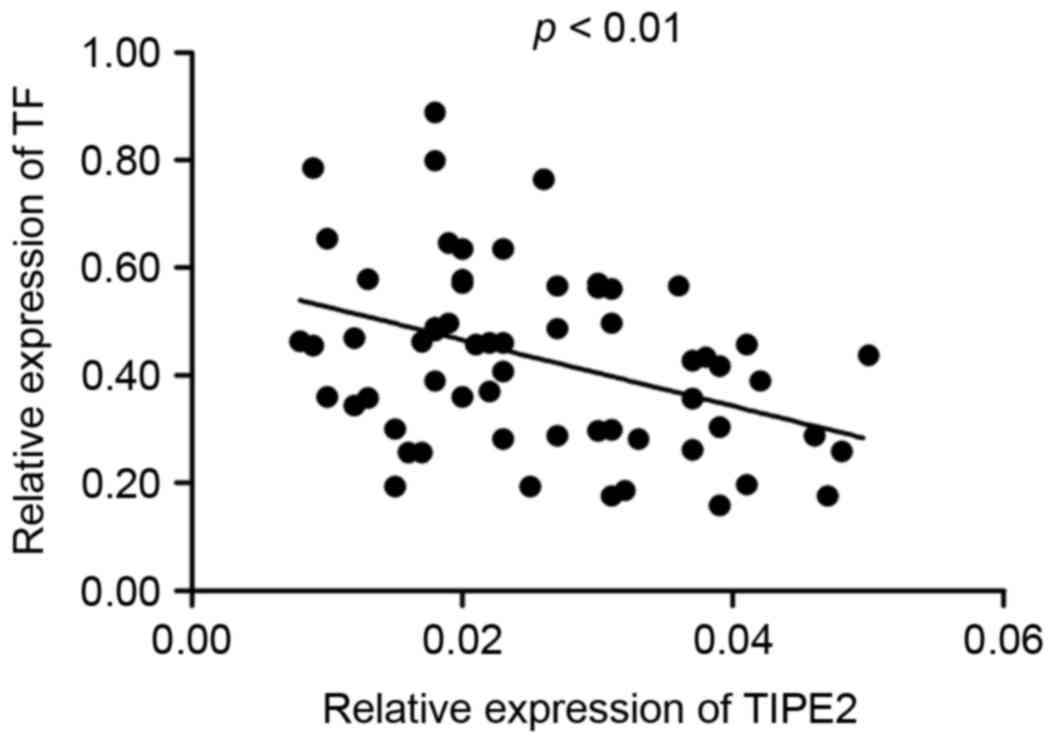
1C). Furthermore, it was determined that there was a negative
correlation between the expression of TIPE2 and TF in the PBMCs of
patients with asthma (r=−0.3828, P<0.01; Fig. 2).
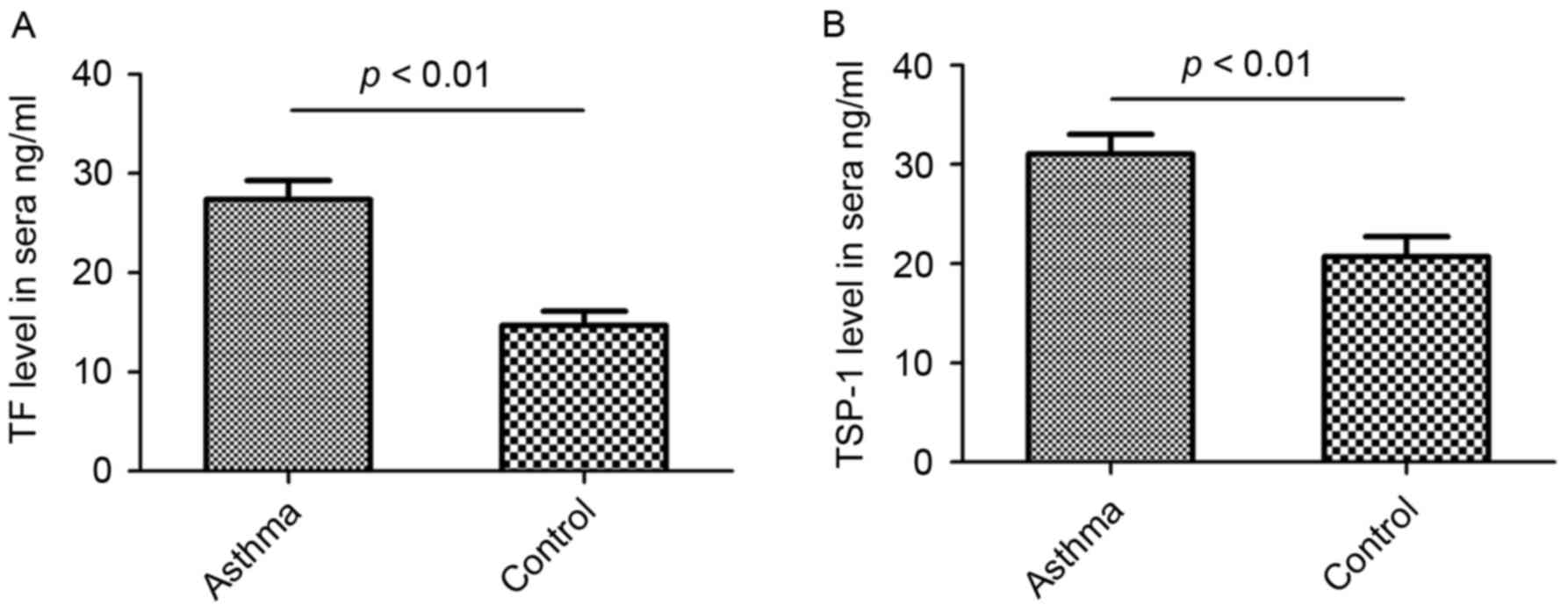
TF and TSP-1 levels in the sera of
patients with bronchial asthma compared with healthy controls
Bronchial asthma is a chronic inflammatory disease
of the airways and previous studies have determined that TF and
TSP-1 serve important roles in the inflammatory process (10,15).
Therefore, in the current study, the levels of TF and TSP-1 in the
sera of patients with bronchial asthma and healthy controls were
measured using sandwich ELISA. The results demonstrated that TF
levels in the sera of patients with bronchial asthma were
significantly higher than those of healthy individuals (P<0.01;
Fig. 3A). Furthermore, TSP-1 levels
in the sera of patients with bronchial asthma were significantly
higher than those of healthy controls (P<0.01; Fig. 3B).
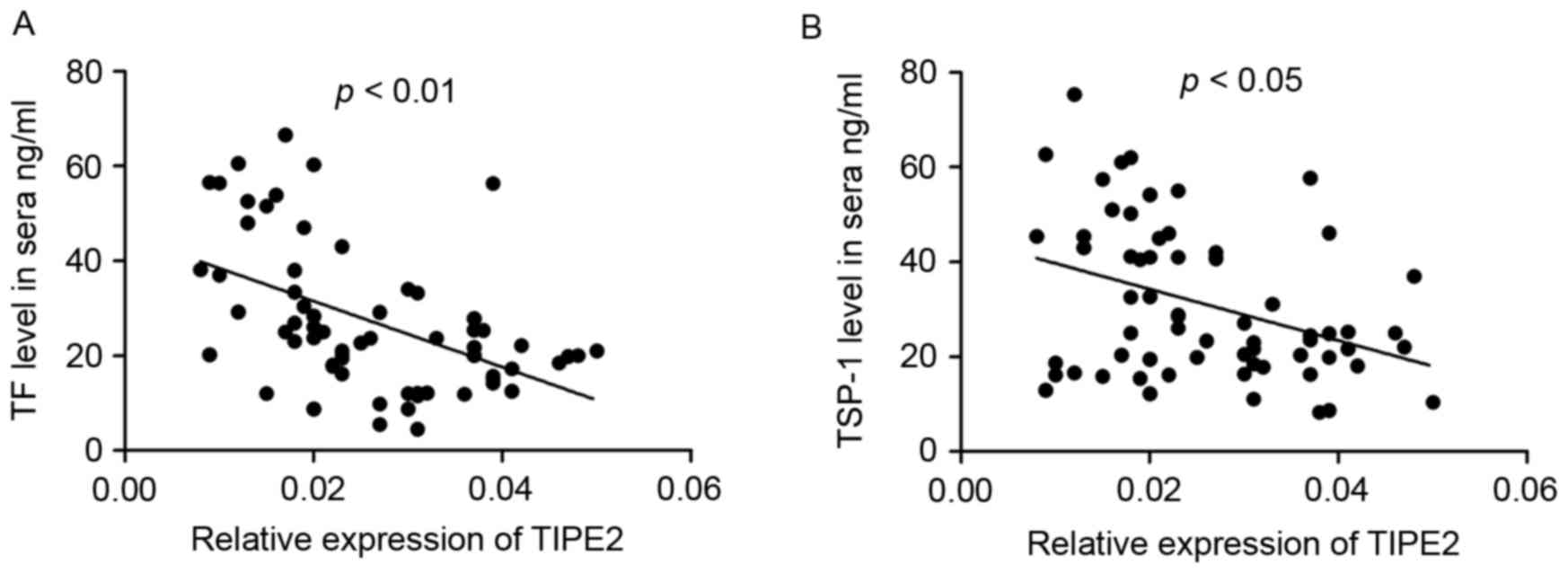
Correlation of TIPE2 expression with
TF and TSP-1
To further investigate the mechanism of TIPE2 in
patients with bronchial asthma, the correlation between TIPE2
expression and sera TF and TSP-1 levels was determined. The
relative expression of TIPE2 was negatively correlated with TF
levels in the sera of patients with asthma (r=−0.5422, P<0.01;
Fig. 4A). Furthermore, the relative
expression of TIPE2 in the PBMCs was negatively correlated with
TSP-1 levels in the sera of patients with asthma (r=−0.3013,
P<0.05; Fig. 4B).
Discussion
Asthma is chronic inflammatory disease of the
airways (2). It has been
demonstrated that patients with bronchial asthma also develop
hyper-coagulation (4). However, the
mechanism between inflammation and coagulation in asthma is not
fully understood. Therefore, the current study investigated the
association between the negative inflammatory regulator TIPE2 and
the coagulation substances TF and TSP-1 to provide innovative
insights into the pathological mechanism and clinical diagnosis of
asthma, as well as identify a potential novel method of treating
asthma.
In the present study, the relative expression of
TIPE2 and TF in the PBMCs of patients with bronchial asthma were
measured and compared with those of healthy controls. In addition,
levels of TF and TSP-1 in sera were assessed. It was determined
that the expression of TIPE2 in the PBMCs of patients with
bronchial asthma was significantly down regulated (Fig. 1B), which was consistent with the
results of a study by Ma et al (24). By contrast, levels of TF were
significantly up regulated in the PBMCs of patients with bronchial
asthma compared with healthy controls (Fig. 1C). Furthermore, significantly higher
levels of circulating TF and TSP-1 were detected in the sera
samples of patients with asthma compared with healthy controls
(Fig. 3). A negative correlation
between TIPE2 and TF in the PBMCs and sera was identified in
patients with bronchial asthma (Figs.
2 and 4A) and there was a
negative correlation between TIPE2 and levels of TSP-1 in the sera
of patients with bronchial asthma (Fig.
4B). These results suggest that there is a link between the
negative inflammatory regulator TIPE2 and activation of the
coagulation cascade in the peripheral blood circulation.
Previous studies have indicated that TIPE2 serves an
important role in the pathogenesis of inflammatory diseases
including chronic hepatitis B (19),
chronic hepatitis C (20) and SLE
(21). TIPE2 may alleviate SLE by
inducing macrophage polarization to an M2 phenotype and it has been
demonstrated that TIPE2 overexpression significantly decreases the
severity of SLE (21). In addition,
TIPE2 may inhibit the synthesis of inducible nitric oxide, which
inhibits inflammation (26).
However, silencing of TIPE2 expression may counteract the reduced
inflammation and myocardial injury in NOD2-/-ischemic mice
(27). Furthermore, down-regulation
of TIPE2 expression may increase the expression of pro-inflammatory
factors including IL-1, IL-10, IL-12 and tumor necrosis factor-α,
which in turn induce liver inflammation and the progression of
liver disease in TIPE2−/− mice (16). In the present study, it was
demonstrated that the expression of TIPE2 was reduced in the PBMCs
of patients with asthma. This was in accordance with the results of
a previous study, which identified the down-regulation of TIPE2 in
PBMCs of patients with asthma (24).
The results of the present demonstrated that there was a
significant correlation between TIPE2 expression in PBMCs and TF
expression in the PBMCs and sera, as well as with TSP-1 in sera
samples from patients with asthma.
As a key connector of the coagulation and
inflammation network, TF may contribute to the inflammation and
remolding of asthmatic airways, as well as participate in the
formulation of thrombosis via the extrinsic blood coagulation
pathway (3,9,10). The
serum pro-inflammatory cytokine IL-33, which is increased in
asthmatic patients, amplifies the coagulation function of human
endothelial cells by increasing the production and activity of TF,
which increases disease severity (28). The data collected in the current
study indicated that the expression of TF was unregulated in the
PBMCs and sera of patients with asthma. Furthermore, the results of
the current study indicated that the expression of the negative
inflammatory regulator TIPE2 was negatively correlated with TF.
These results suggest that TIPE2 may participate in the
pathogenesis of asthma by regulating the expression of TF.
TSP-1, which is known for its antiangiogenic
function (29), has been extensively
studied in cancer (30) and wound
healing (31). However, TSP-1 is a
multifunctional protein and serves a significant role in
inflammation. It has been demonstrated that TSP-1 contributes to
the development of vascular inflammation by regulating the cell
motility of monocytes in a mouse model of abdominal aortic aneurysm
(23). Furthermore, TSP-1
efficiently down-regulates TF-induced coagulation by binding to
TFPI and locating to surfaces within the extravascular space
following vascular injury (32).
Platelet activation is an important determinant of the severity of
allergic asthma. Levels of TSP-1, a marker of platelet activation,
are generally higher in patients with severe asthma than those with
less severe asthma (14). The
results of the current study identified high levels of TSP-1 in the
sera samples of patients with asthma compared with healthy
controls. Furthermore, a negative correlation between TSP-1 and
TIPE2 was identified in patients with asthma. Thus, TIPE2 may act
via TSP-1 to regulate inflammation and coagulation in asthma.
However, further studies are required to determine the specific
mechanism by which TIPE2 regulates the pathogenesis of asthma
through the coagulation substances TSP-1 and TF.
In conclusion, the results of the current study
indicated that patients with asthma exhibit reduced TIPE2
expression in the PBMCs but higher levels of TF and TSP-1 in the
sera compared with healthy controls. Furthermore, TIPE2 expression
is negatively correlated with TF expression in PBMCs and sera, and
negatively correlated with TSP-1 levels in the sera of patients
with bronchial asthma. TIPE2 may participate in the pathological
mechanism of asthma by interacting with the coagulating substances
TF and TSP-1. However, the specific mechanism by which TIPE2
regulates the coagulation substances TF and TSP-1 remains unknown
and requires further investigation. This may facilitate the
development of novel methods to diagnose and treat bronchial
asthma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81501715) and the
Key Project on Science and Technology Research (Henan, China; grant
no. 152102410067).
Glossary
Abbreviations
Abbreviations:
|
TIPE2
|
tumor necrosis factor α induced
protein-8 like-2
|
|
TF
|
tissue factor
|
|
TSP-1
|
thromobospondin-1
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
IgE
|
immunoglobulin E
|
|
IL
|
interleukin
|
|
TBST
|
Tris-buffered saline tween
|
|
IgG
|
immunoglobulin G
|
|
ELISA
|
Enzyme-linked immunosorbent assay
|
|
SLE
|
systemic lupus erythematosus
|
References
|
1
|
Lee YS, Baek S, Ko Y, Kim MY, Lee HK, Kim
TB, Cho YS, Moon HB, Lee SD and Oh YM: New scoring system for the
differentiation of chronic obstructive pulmonary disease and
asthma. Respirology. 20:626–632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner AM, Tamasi L, Schleich F, Hoxha M,
Horvath I, Louis R and Barnes N: Clinically relevant subgroups in
COPD and asthma. Eur Respir Rev. 24:283–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dong F, Wang C, Duan J, Zhang W, Xiang D
and Li M: Puerarin attenuates ovalbumin-induced lung inflammation
and hemostatic unbalance in rat asthma model. Evid Based Complement
Alternat Med. 2014:7267402014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sneeboer MM, Fens N, van de Pol MA, Majoor
CJ, Meijers JC, Kamphuisen PW, Lutter R, Sterk PJ and Bel EH: Loss
of asthma control and activation of coagulation and fibrinolysis.
Clin Exp Allergy. 46:422–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Boer JD, Majoor CJ, van 't Veer C, Bel
EH and van der Poll T: Asthma and coagulation. Blood.
119:3236–3244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lambrecht BN and Hammad H: Asthma and
coagulation. N Engl J Med. 369:1964–1966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian J, Zhu T, Liu J, Guo Z and Cao X:
Platelets promote allergic asthma through the expression of CD154.
Cell Mol Immunol. 12:700–707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bi M, Guo A, Zhao H, Sun X, Chen Q, Yu L,
Shi W, Wang Y, Shen G, Wang X, et al: Role of the extracellular
signal-regulated kinase 1/2 signaling pathway in the process of
thrombin-promoting airway remodeling in ovalbumin-allergic rats.
Immunopharmacol Immunotoxicol. 37:26–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JA, Sharif AS, Tschumperlin DJ, Lau
L, Limbrey R, Howarth P and Drazen JM: Tissue factor-bearing
exosome secretion from human mechanically stimulated bronchial
epithelial cells in vitro and in vivo. J Allergy Clin Immunol.
130:1375–1383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Witkowski M, Landmesser U and Rauch U:
Tissue factor as a link between inflammation and coagulation.
Trends Cardiovasc Med. 26:297–303. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackman N, Tilley RE and Key NS: Role of
the extrinsic pathway of blood coagulation in hemostasis and
thrombosis. Arterioscler Thromb Vasc Biol. 27:1687–1693. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riedl J, Hell L, Kaider A, Koder S, Marosi
C, Zielinski C, Panzer S, Pabinger I and Ay C: Association of
platelet activation markers with cancer-associated venous
thromboembolism. Platelets. 27:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lessey-Morillon EC and Roberts DD:
Thrombospondin-1: An extracellular message delivered by macrophages
that promotes aortic aneurysms. Circ Res. 117:113–115. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johansson MW, Han ST, Gunderson KA, Busse
WW, Jarjour NN and Mosher DF: Platelet activation, P-selectin, and
eosinophil β1-integrin activation in asthma. Am J Respir Crit Care
Med. 185:498–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Urao N, Mirza RE, Heydemann A, Garcia J
and Koh TJ: Thrombospondin-1 levels correlate with macrophage
activity and disease progression in dysferlin deficient mice.
Neuromuscul Disord. 26:240–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al: TIPE2, a negative
regulator of innate and adaptive immunity that maintains immune
homeostasis. Cell. 133:415–426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang
Y, Qu Z, Guo C, Chen Y, Zhang Y and Liu S: Tissue-specific
expression of TIPE2 provides insights into its function. Mol
Immunol. 47:2435–2442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang LY, Fan YC, Zhao J, Gao S, Sun FK,
Han J, Yang Y and Wang K: Elevated expression of tumour necrosis
factor-α-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral
blood mononuclear cells is associated with disease progression of
acute-on-chronic hepatitis B liver failure. J Viral Hepat.
21:64–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong L, Liu K, Zhang YZ, Jin M, Wu BR,
Wang WZ, Li W, Nan YM and Chen YH: Downregulation of TIPE2 mRNA
expression in peripheral blood mononuclear cells from patients with
chronic hepatitis C. Hepatol Int. 7:844–849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, Zhu X, Yang Y, Huang L and Xu J:
TIPE2 Alleviates systemic lupus erythematosus through regulating
macrophage polarization. Cell Physiol Biochem. 38:330–339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia L, Gui B, Tian P, Yao G, Fu R, Wang L,
Ge H and Ou Y: TIPE2, a novel biomarker for clinical chronic kidney
allograft rejection. Artif Organs. 37:221–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Morgan S, Ren J, Wang Q, Annis DS,
Mosher DF, Zhang J, Sorenson CM, Sheibani N and Liu B:
Thrombospondin-1 (TSP1) contributes to the development of vascular
inflammation by regulating monocytic cell motility in mouse models
of abdominal aortic aneurysm. Circ Res. 117:129–141. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Liu X, Wei Z and Wang X, Wang Z,
Zhong W, Li Y, Zhu F, Guo C, Zhang L and Wang X: The expression and
significance of TIPE2 in peripheral blood mononuclear cells from
asthmatic children. Scand J Immunol. 78:523–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Global initiative for asthma (GINA), .
Global strategy for asthma management and prevention: NHLBI/WHO
workshop report. Bethesda. National Institutes of Health, National
Heart, Lung and Blood Institute. 2002; Revised 2016.
|
|
26
|
Lou Y, Zhang G, Geng M, Zhang W, Cui J and
Liu S: TIPE2 negatively regulates inflammation by switching
arginine metabolism from nitric oxide synthase to arginase. PLoS
One. 9:e965082014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Zhu T, Liu W, Qu X, Chen Y, Ren
P, Wang Z, Wei X, Zhang Y and Yi F: TIPE2 acts as a negative
regulator linking NOD2 and inflammatory responses in myocardial
ischemia/reperfusion injury. J Mol Med (Berl). 93:1033–1043. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bahrami Mahneh S, Movahedi M, Aryan Z,
Bahar MA, Rezaei A, Sadr M and Rezaei N; Universal Scientific
Education and Research Network (USERN), : Serum IL-33 is elevated
in children with asthma and is associated with disease severity.
Int Arch Allergy Immunol. 168:193–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sims JN and Lawler J:
Thrombospondin-1-based antiangiogenic therapy. J Ocul Pharmacol
Ther. 31:366–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeanne A, Schneider C, Martiny L and
Dedieu S: Original insights on thrombospondin-1-related
antireceptor strategies in cancer. Front Pharmacol. 6:2522015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tie L, Chen LY, Chen DD, Xie HH, Channon
KM and Chen AF: GTP cyclohydrolase I prevents diabetic-impaired
endothelial progenitor cells and wound healing by suppressing
oxidative stress/thrombospondin-1. Am J Physiol Endocrinol Metab.
306:E1120–E1131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mast AE, Stadanlick JE, Lockett JM,
Dietzen DJ, Hasty KA and Hall CL: Tissue factor pathway inhibitor
binds to platelet thrombospondin-1. J BiolChem. 275:31715–31721.
2000.
|