Introduction
Esophageal cancer is the eighth most common cancer
worldwide, with ~480,000 new cases and 400,000 associated
mortalities per year (1).
Histologically, there are two main forms of esophageal cancer:
Esophageal adenocarcinoma and esophageal squamous cell carcinoma
(ESCC), which represents 90% of all esophageal cancer cases
(2). Currently, surgery is the only
way to treat patients with esophageal cancer and the overall 5-year
survival rate is 14–18%. This is due to the fact that surgery can
only be performed in a limited number of patients, as in many cases
tumors are inoperable (3). Although
genetic and epigenetic alterations underpin the development of
ESCC, the molecular mechanisms underlying neoplastic progression
remain unclear (4,5). Therefore, improving understanding of
the molecular biology of ESCC is critical to facilitate the
development of more effective diagnostic and therapeutic strategies
for ESCC.
MicroRNAs (miRs) are small non-coding RNAs ~22
nucleotides long that bind to the 3′-untranslated region (3′-UTR)
of target mRNA (6). Previous studies
have demonstrated that different types of miRs regulate the
proliferation, apoptosis, invasion, metastasis and the
epithelial-mesenchymal transition of tumor cells (7,8). The
aberrant expression of miRs has also been identified in ESCC
(9,10). The upregulated expression of miR-21
may promote cell proliferation, migration and resistance to
apoptosis via the phosphatase and tension homolog/phosphoinositide
3 kinase/protein kinase b signaling pathway in ESCC (11). miR-let-7 is downregulated in ESCC and
is considered to be a tumor suppressor, as it regulates the
interleukin-6/signal transducer and activator of transcription 3
signaling pathway during neoplastic progression (12). Additionally, it has been demonstrated
that miR-142-3p expression is associated with histological
differentiation and may be a potential independent prognostic
factor in patients with ESCC following surgery (13). It has been demonstrated that miR-133b
decreases the invasiveness of esophageal cancer by inhibiting the
expression of fascin actin-bundling protein 1 (FSCN1) (14). However, the molecular mechanisms
underlying the function of miR-133b in proliferation and apoptosis
of esophageal cancer cells remains unknown.
The results of the current study demonstrated that
miR-133b expression was downregulated in ESCC tissues and cell
lines and its low expression was associated with the
clinicopathological features of patients with ESCC. Overexpression
of miR-133b in ESCC cell lines decreased cell proliferation and
promoted cell apoptosis. Cullin 4B (CUL4B) has been identified as a
direct target of miR-133b and it was demonstrated that miR-133b
functions as a tumor suppressor by negatively regulating CUL4B
expression. Furthermore, it was demonstrated that CUL4B promotes
ESCC cell proliferation and inhibits apoptosis by activating the
protein kinase B/glycogen synthase kinase 3β/β-catenin
(AKT/GSK3β/β-catenin) pathway. Thus, the results of the present
study suggest that miR-133b/CUL4B may be a promising therapeutic
target for ESCC.
Materials and methods
Tissue collection
Primary ESCC tissues and adjacent non-tumor tissues
samples (>5 cm from the edge of tumor) were obtained from 47
untreated patients (27 males and 20 females; mean age of 57 years)
undergoing primary surgical resection at the Department of
Cardiothoracic Surgery, Jinling Hospital (Nanjing, China) between
January 2014 and August 2015. The clinical staging of tumors was
performed using the seventh edition of the American Joint Committee
on Cancer Staging Manual (15).
Samples were snap-frozen in liquid nitrogen prior to RNA isolation
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The current study was approved by the Human Ethics
Committee of Jinling Hospital and written informed consent was
obtained from each study participant.
Cell lines and cell culture
Six human ESCC cell lines (TE-1, TE-8, KYSE150,
KYSE450, Eca-109 and EC9706) and the normal human esophageal
epithelial cell line HEECs (cat. no. BNCC337729) were purchased
from the Shanghai Institute of the Chinese Academy of Sciences
(Shanghai, China). Another normal human esophageal epithelial cell
line Het-1A was purchased from American Type Culture Collection
(Manassas, VA, USA). TE-1, TE-8, Eca-109, EC9706 and Het-1A cells
were cultured in Roswell Park Memorial Institute medium-1640
(Thermo Fisher Scientific Inc., Waltham, MA, USA), and KYSE150,
KYSE450 and HEECs cells were cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc.). These media were
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
in a humidified atmosphere at 37°C with 5% CO2.
RT-qPCR
Tissues samples and culture cells were treated with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) for total RNA extraction, following the manufacturer's
protocols. For miR-133b quantification, 100 ng total RNA was
reverse transcribed using the specific stem-loop RT primer and the
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used. The cDNA were quantified
using a TaqMan MicroRNA assay (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and normalized to U6 RNA levels. The qPCR
reaction conditions were as follows: Initial denaturation at 95°C
for 10 min and subsequently 40 cycles of 95°C for 15 sec and 60°C
for 1 min. For CUL4B mRNA analysis, cDNA was synthesized from 1 µg
total RNA using PrimeScript™ RT reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China). qPCR was performed using a TaqMan
RT-qPCR assay (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and normalized to GAPDH mRNA levels. The qPCR reaction conditions
were as follows: Initial denaturation at 95°C for 5 min and
subsequently 40 cycles of 95°C for 15 sec, 60°C for 15 sec and 70°C
for 20 sec. Each sample was analyzed in triplicate using an ABI
7500 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Relative expression levels were evaluated using
the 2−ΔΔCq method (16).
The following primers were used for qPCR: miR-133b forward,
5′-TTTGGTCCCCTTCAACCAGCTA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
and CUL4B forward, 5′-GGGAAAGGAATGGTGAA-3′ and reverse,
5′-TGCATAGAGCCGGTTAG-3′. The primers used for the internal controls
were as follows: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′.
miR-133b transfection
The mature miR-133b sequence
(5′-UUUGGUCCCCUUCAACCAGCUA-3′) was obtained from the miRBase
database (http://www.mirbase.org/). Subsequently,
0.5 µg miR-133b cDNA sequence was synthesized and inserted into the
pcDNA3.1 vector to generate a human pcDNA-miR-133b plasmid. For
transfection, KYSE150 or Eca-109 cells (~5×105/well)
were seeded in 6-well plates. After 24 h, the cells were
transfected with 3 µg pcDNA-miR-NC control vector or pcDNA-miR-133b
plasmids using Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Cultures were incubated for 72 h prior to
collecting samples for subsequent western blotting and RT-qPCR
analysis.
Cell proliferation assay
Cell proliferation was determined by culturing
~5×103 cells/well on 96-well plates and cells were
transiently transfected with 2 µg pcDNA-miR-133b/miR-NC or
pcDNA-CUL4B/empty plasmids (FulenGen, Guangzhou, China) using
Lipofectamine™ 2000. After 72 h, 10 µl Cell Counting
kit-8 (CCK-8) solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added to each well and cells were incubated
for 1 h at 37°C. Optical density was then detected at a wavelength
of 450 nm using an Epoch Microplate Spectrophotometer (BioTek
Instruments, Inc., Winooski, VT, USA). Each sample was analyzed in
triplicate and data analysis was performed using the mean of the
results.
Cell apoptosis assay
Cell apoptosis was analyzed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Beyotime Institute of Biotechnology, Haimen, China). Trypsinized
cells were washed three times with PBS and stained with
FITC-labeled anti-Annexin V antibody and propidium iodide in the
dark for 10 min at room temperature. Cells were then analyzed using
the BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA) and FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR,
USA).
Western blot analysis
Transfected cells were washed once in PBS and lysed
in radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) and protein concentration was
determined using a BCA protein assay kit. A total of 50 µg/lane
protein was separated by 10% SDS-polyacrylamide gel electrophoresis
and transferred onto 0.22 µm polyvinylidene difluoride membranes,
which was incubated with 5% fat-free skimmed milk in Tris-buffered
saline containing 0.05% Tween-20 for 1 h at room temperature.
Membranes were incubated overnight at 4°C with rabbit anti-CUL4B
(cat. no. ab157103; 1:1,000; Abcam, Cambridge, UK), β-catenin (cat.
no. ab32572; 1:1,000; Abcam), GSK-3β (cat. no. 9315; 1:1,000; Cell
Signaling Technology, Danvars, MA, USA), phosphorylated
(p)-GSK-3βTyr216 (cat. no. ab75745; 1:1,000; Abcam) and
β-actin (cat. no. sc-130656; 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) primary antibodies. Following extensive
washing with PBS supplemented with 0.1% Triton X-100, membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (cat. no. ab205718; 1:1,500; Abcam)
for 1 h at room temperature. Images were visualized using an
enhanced chemiluminescent system (EMD Millipore, Billerica, MA,
USA). Relative band intensities were determined by densitometry
using Quantity One 4.6.2 software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Luciferase reporter assays
The 3′-UTR of CUL4B with wild-type or mutant binding
sites for miR-133b was amplified and subcloned into the pGL3 vector
(Promega Corp., Madison, WI, USA), respectively. Eca-109 and
KYSE150 cells were co-transfected with 150 ng miR-133b or
miR-negative control plasmids and 50 ng pGL3-CUL4B wild-type or
mutant reporter plasmids using Lipofectamine 2000. A total of 48 h
following transfection, the Dual-luciferase assay kit (Promega,
Madison, WI, USA) was used to determine the luciferase activity.
Renilla luciferase activity was normalized to the Firefly
luciferase activity. Each experiment was performed in
triplicate.
Bioinformatic analysis
The target gene of miR-133b was identified and
compared using the online target prediction algorithms miRanda
(http://www.microrna.org/), miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/predictedmirnagene.html)
and PicTar (http://www.pictar.org/).
Statistical analysis
All statistical analyses were performed using SPSS
software 15.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard error of the mean from ≥3 independent
experiments. Statistical significance was evaluated using a
Student's t-test (two-tailed), one-way analysis of variance
followed by a Tukey's post hoc test and the Mann-Whitney test.
Pearson's correlation analysis was used to measure the correlation
between CUL4B and miR-133b expression. P<0.05 was determined to
indicate a statistically significant difference.
Results
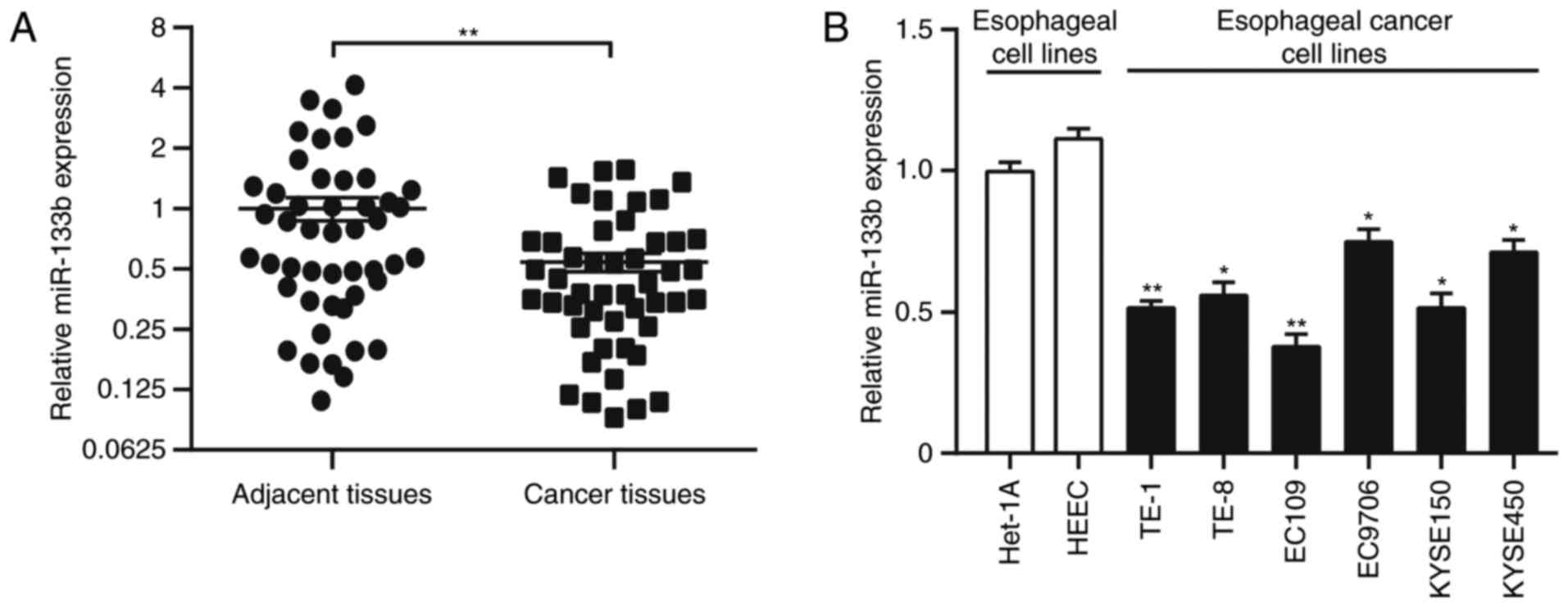
miR-133b expression is downregulated
in human ESCC tissues and cell lines
The biological function of miR-133b in the
pathogenesis of ESCC was investigated by examining miR-133b
expression in 47 paired ESCC and adjacent non-tumor tissues by
RT-qPCR. miR-133b expression was significantly downregulated in
ESCC samples compared with adjacent normal tissues (P<0.01;
Fig. 1A). Similarly, miR-133b
expression in the six ESCC cell lines TE-1 (P<0.01), TE-8
(P<0.05), KYSE150 (P<0.05), KYSE450 (P<0.05), Eca-109
(P<0.01), and EC9706 (P<0.05), was significantly lower than
that in the normal esophageal epithelial cell lines Het-1A and HEEC
(Fig. 1B). miR-133b expression was
also associated with tumor stage (P<0.05), tumor size
(P<0.05) and differentiation status (P<0.05); however, it was
not associated with other clinicopathological factors of patients
with ESCC (Table I).
 | Table I.Association between miR-133b
expression and clinicopathological features of patients with
ESCC. |
Table I.
Association between miR-133b
expression and clinicopathological features of patients with
ESCC.
|
|
| miR-133b
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Patients (n=47) | High (n=24) | Low (n=23) | P-value |
|---|
| Age (years) |
|
|
|
|
|
>60 | 29 | 15 | 14 | 0.417 |
|
<60 | 18 | 8 | 10 |
|
| Sex |
|
|
|
|
|
Male | 27 | 14 | 13 | 0.213 |
|
Female | 20 | 9 | 11 |
|
| Tumor
localization |
|
|
|
|
| Upper
third | 12 | 5 | 7 | 0.625 |
| Middle
third | 19 | 11 | 8 |
|
| Lower
third | 16 | 7 | 9 |
|
| Tumor stage |
|
|
|
|
|
I+II | 25 | 9 | 16 | 0.041a |
|
III | 19 | 14 | 8 |
|
| Tumor size
(cm) |
|
|
|
|
|
<5 | 28 | 8 | 20 | 0.034a |
| ≥5 | 19 | 12 | 7 |
|
| Lymph node
metastasis |
|
|
|
|
|
Negative | 32 | 15 | 17 | 0.102 |
|
Positive | 15 | 9 | 6 |
|
| Differentiation
status |
|
|
|
|
|
High | 28 | 13 | 15 | 0.048a |
|
Low | 19 | 10 | 9 |
|
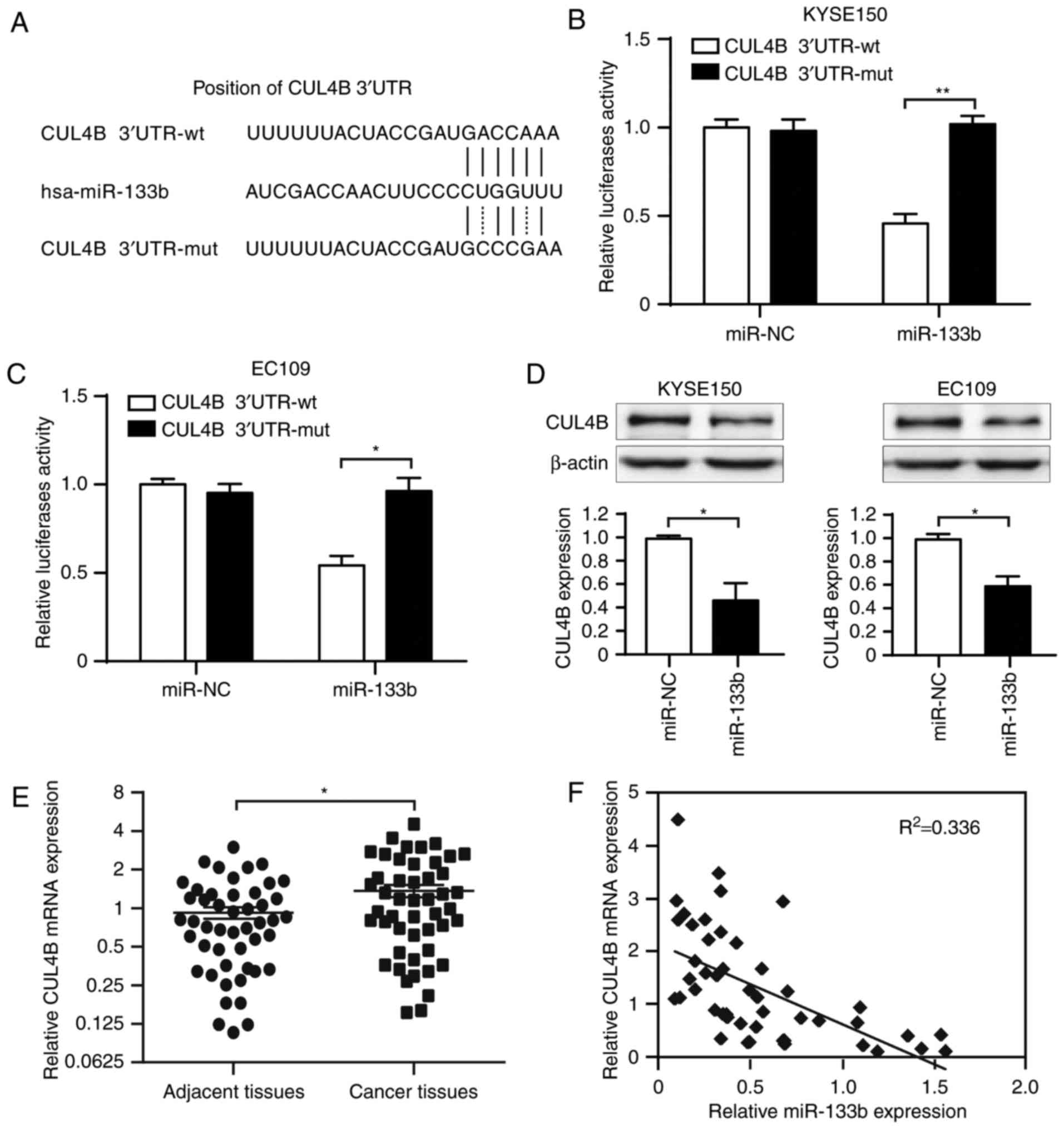
CUL4B is a correlated target gene of
miR-133b in ESCC cells
The target gene of miR-133b in ESCC was identified
using the online target prediction algorithms. Based on the Gene
Expression Omnibus database analysis (17), it was revealed that human CUL4B, an
important ubiquitilation molecule associated with apoptosis,
contained the conserved putative miR-133b target site (Fig. 2A). Dual-luciferase reporter analysis
indicated that co-expression of miR-133b significantly inhibited
luciferase activity in KYSE150 (P<0.01) and Eca-109 (P<0.05)
cells containing the CUL4B-3′-UTR reporter plasmid, compared with
those containing the mutant plasmid (Fig. 2B and C). Endogenous expression of
CUL4B was significantly inhibited following transfection of
miR-133b in KYSE150 and Eca-109 cells compared with the control
(P<0.05; Fig. 2D). Furthermore,
CUL4B expression was significantly upregulated in ESCC tissues
compared with adjacent non-tumor tissues (P<0.05; Fig. 2E) and there was a negative
correlation between miR-133b and CUL4B mRNA levels in ESCC tissues
(Fig. 2F). These results indicate
that CUL4B is a direct target of miR-133b and suggest that miR-133b
may exert its effect by inhibiting CUL4B expression.
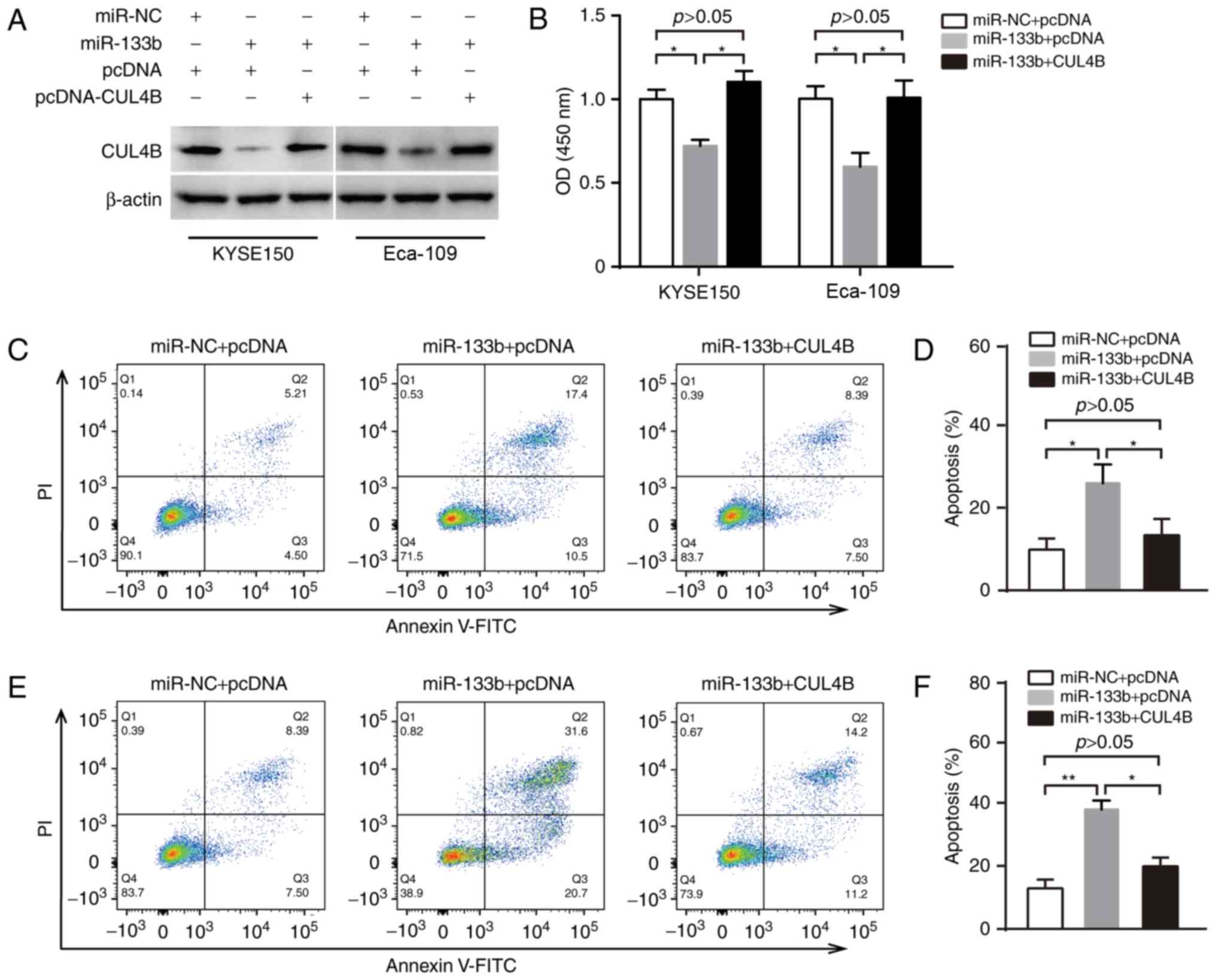
miR-133b inhibits cell proliferation
and promotes apoptosis by targeting CUL4B in ESCC
The biological function of miR-133b/CUL4B in ESCC
was analyzed by transfecting miR-133b and/or CUL4B into KYSE150 and
Eca-109 cells (Fig. 3A). The role of
miR-133b/CUL4B on the proliferation of ESCC cells was investigated
using a CCK-8 assay and the results indicated that overexpression
of miR-133b significantly decreased the proliferation of KYSE150
and Eca-109 cells compared with cells transfected with the negative
control (P<0.05; Fig. 3B).
However, reintroduction of CUL4B into miR-133b-transfected KYSE150
or Eca-109 cells significantly reversed the effects of miR-133b on
ESCCC cell proliferation (P<0.05; Fig. 3B). Additionally, overexpression of
miR-133b significantly increased apoptosis in KYSE150 cells, which
was significantly reversed when CUL4B was reintroduced into
miR-133b-transfected KYSE150 cells (all P<0.05; Fig. 3C and D). In Eca-109 cells, miR-133b
overexpression also significantly increased levels of apoptosis and
the reintroduction of CUL4B inhibited the effects of apoptosis
(P<0.05; Fig. 3E and F).
miR-133b inhibits the
AKT/GSK3β/β-catenin pathway by downregulating CUL4B in ESCC
It has been demonstrated that CUL4B activates
AKT/GSK3β/β-catenin signaling and may promote proliferation and
invasion in malignant neoplasms (18). Therefore, it was hypothesized that
miR-133b/CUL4B inhibits ESCC cell proliferation and promotes
apoptosis by inhibiting AKT/GSK3β/β-catenin signaling pathway.
After transfection of miR-133b or miR-NC plasmid, CUL4B, GSK-3β,
p-GSK3βTyr216 and β-catenin levels were measured using
western blot analysis. The results indicated that the expression of
GSK3β (P<0.01) and p-GSK3β (P<0.05) were significantly
increased in KYSE150 cells transfected with miR-133b compared with
those transfected with the negative control (Fig. 4A and B). By contrast, the expression
of the CUL4B (P<0.01) and downstream
proliferation/apoptosis-associated target protein β-catenin
(P<0.05) were significantly decreased. Similarly, levels of
GSK3β and p-GSK3β were significantly increased (P<0.05), but the
levels of CUL4B and β-catenin were significantly decreased
(P<0.05) in Eca-109 cells transfected with miR-133b compared
with cells transfected with negative control (Fig. 4C and D). Taken together, these
results indicate that miR-133b/CUL4B may affect ESCC cell
proliferation and apoptosis by regulating the AKT/GSK3β/β-catenin
pathway.
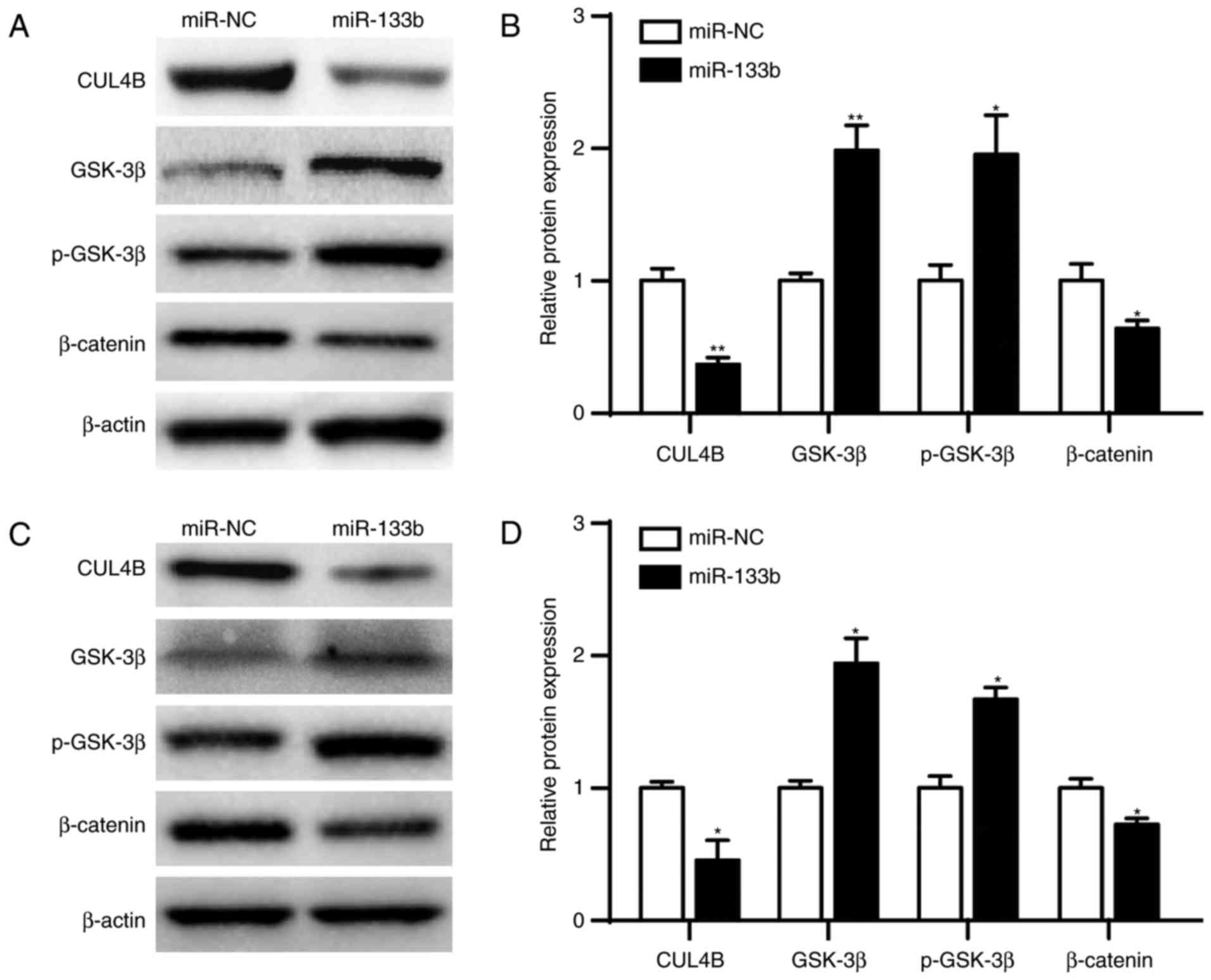 | Figure 4.CUL4B activates the
AKT/GSK3β/β-catenin pathway in ESCC. (A) Levels of CUL4B, GSK-3β,
p-GSK-3β and β-catenin expression were analyzed by western blot
analysis in KYSE150 cells transfected with miR-NC or miR-133b. (B)
Quantification of western blot analysis. (C) Levels of CUL4B,
GSK-3β, p-GSK-3β and β-catenin expression were analyzed by western
blot analysis in Eca-109 cells transfected with mi-NC or miR-133b.
(D) Quantification of western blot analysis. Data are expressed as
the mean ± standard error of the mean. Each sample was analyzed in
triplicate. *P<0.05, **P<0.01 vs. miR-NC. CULB4B, cullin 4B;
miR-133b, microRNA-133b; miR-NC, microRNA negative control; ESCC,
esophageal squamous cell carcinoma; Akt, protein kinase b; p-,
phosphorylated; GSK-3β, glycogen synthase kinase 3β; NC, negative
control. |
Discussion
miR-133b is a muscle-specific molecular marker,
which serves a role in skeletal muscle development, myoblast
differentiation and myogenic-associated disease (19,20). It
has been demonstrated that miR-133b serves a suppressive role
during tumor growth, invasion, metastasis and apoptosis. miR-133b
inhibits gastric cancer cell metastasis in vitro and in
vivo by directly suppressing the expression of zinc finger
protein Gli1 (21). miR-133b
promotes the apoptosis and inhibits the proliferation of
osteosarcoma cells by directly targeting B cell lymphoma-2 like
protein (22). Downregulation of
miR-133b in colorectal cancer tissues compared with adjacent
non-tumorous tissues is associated with the poor survival of
patients (23). Furthermore, it has
been demonstrated that miR-133b inhibits the invasiveness of
esophageal types of cancer by inhibiting FSCN1 expression (14). However, the molecular mechanisms of
miR-133b in ESCC cell apoptosis and proliferation remain
unknown.
The results of the present study demonstrated that
miR-133b significantly decreased tumor cell proliferation and
promoted apoptosis in vitro. These results indicate that
miR-133b may be used as a novel method of treating patients with
ESCC. miR-133b levels were also highly associated with tumor stage
and differentiation status; differentiation-associated miR-133b
levels may be used to predict tumor progression in patients with
ESCC that have undergone surgery; however, further studies are
required to validate this.
It was also demonstrated that miR-133b/CUL4B served
a role in ESCC cell growth and apoptosis. CUL4B is a member of the
cullin family and forms a complex that functions as an E3 ubiquitin
ligase and catalyzes the polyubiquitination of specific protein
substrates in the cell (24,25). Previous studies have demonstrated
that CUL4B expression is significantly upregulated in various types
of human cancer, promoting cell proliferation, invasion and
tumorigenesis (26–28). For example, CUL4B promotes the
proliferation and inhibits the apoptosis of osteosarcoma and
glioblastoma cells (26,27). In addition, CUL4B is a novel
prognostic marker correlating with colon cancer pathogenesis and
progression (28). The mechanism
underlying CUL4B function in cancer progression remains unclear;
however, CUL4B may serve a role in epigenetic changes, including
heterochromatin formation, histone modification, parental
imprinting or X-chromosome inactivation (29,30).
CUL4B also promotes cell cycle progression and tumorigenesis via
degradation of numerous cyclin-dependent kinase inhibitors or p53
protein (31,32). In addition, CUL4B serves an important
role in stabilizing β-catenin against proteasomal degradation in
multiple signaling pathways. The results of the current study
indicate that CUL4B activates the AKT/GSK3β/β-catenin pathway and
may affect ESCC cell proliferation and apoptosis by regulating this
pathway. These results provide important insights into the CUL4B
pathway and shed light on the functional importance of
proliferation and apoptosis in ESCC.
In conclusion, the current study demonstrated that
miR-133b is downregulated in ESCC, and its expression is associated
with advanced tumor stage and the differentiation status of
patients with ESCC. Additionally, it was determined that miR-133b
serves a crucial role in inducing proliferation and apoptosis by
directly targeting CUL4B and may therefore be a novel therapeutic
target to treat patients with ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contribution
HH designed methods and experiments, performed the
laboratory experiments and analyzed the data. YX and ZG co-designed
the cell proliferation and apoptosis experiments and collaborated
on associated data collection and their interpretation. XC and SJ
collaborated on bioinformatics analysis and statistical analysis.
ZX co-designed experiments, discussed analyses, interpretation,
presentation and wrote the paper. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The current study was performed in accordance with
the Declaration of Helsinki and approved by the Human Ethics
Committee of Jinling Hospital. Written informed consent was
obtained from each study participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou K, Zhang SS, Yan Y and Zhao S:
Overexpression of transient receptor potential vanilloid 2 is
associated with poor prognosis in patients with esophageal squamous
cell carcinoma. Med Oncol. 31:172014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toh Y, Egashira A and Yamamoto M:
Epigenetic alterations and their clinical implications in
esophageal squamous cell carcinoma. Gen Thorac Cardiovasc Surg.
61:262–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu J, Wang Y and Wu X: MicroRNA in the
pathogenesis and prognosis of esophageal cancer. Curr Pharm Des.
19:1292–1300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feber A, Xi L, Luketich JD, Pennathur A,
Landreneau RJ, Wu M, Swanson SJ, Godfrey TE and Litle VR: MicroRNA
expression profiles of esophageal cancer. J Thorac Cardiovasc Surg.
135:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He B, Yin B, Wang B, Xia Z, Chen C and
Tang J: MicroRNAs in esophageal cancer (Review). Mol Med Rep.
6:459–465. 2012.PubMed/NCBI
|
|
11
|
Li P, Mao WM, Zheng ZG, Dong ZM and Ling
ZQ: Down-regulation of PTEN expression modulated by dysregulated
miR-21 contributes to the progression of esophageal cancer. Dig Dis
Sci. 58:3483–3493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugimura K, Miyata H, Tanaka K, Hamano R,
Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori
M and Doki Y: Let-7 expression is a significant determinant of
response to chemotherapy through the regulation of IL-6/STAT3
pathway in esophageal squamous cell carcinoma. Clin Cancer Res.
18:5144–5153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin RJ, Xiao DW, Liao LD, Chen T, Xie ZF,
Huang WZ, Wang WS, Jiang TF, Wu BL, Li EM and Xu LY: MiR-142-3p as
a potential prognostic biomarker for esophageal squamous cell
carcinoma. J Surg Oncol. 105:175–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kano M, Seki N, Kikkawa N, Fujimura L,
Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M and
Matsubara H: miR-145, miR-133a and miR-133b: Tumor-suppressive
miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J
Cancer. 127:2804–2814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clough E and Barrett T: The gene
expression omnibus database. Methods Mol Biol. 1418:93–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian Y, Yuan J, Hu H, Yang Q, Li J, Zhang
S, Jiang B, Shao C and Gong Y: The CUL4B/AKT/β-catenin axis
restricts the accumulation of myeloid-derived suppressor cells to
prohibit the establishment of a tumor-permissive microenvironment.
Cancer Res. 75:5070–5083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams AH, Liu N, van Rooij E and Olson
EN: MicroRNA control of muscle development and disease. Curr Opin
Cell Biol. 21:461–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kirby TJ and McCarthy JJ: MicroRNAs in
skeletal muscle biology and exercise adaptation. Free Radic Biol
Med. 64:95–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao Y, Huang J, Zhang L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B and Zhu Z: MiR-133b is frequently decreased
in gastric cancer and its overexpression reduces the metastatic
potential of gastric cancer cells. BMC Cancer. 14:342014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion,
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu G, Chen D, Li X, Yang K, Wang H and Wu
W: miR-133b regulates the MET proto-oncogene and inhibits the
growth of colorectal cancer cells in vitro and in vivo. Cancer Biol
Ther. 10:190–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higa LA, Wu M, Ye T, Kobayashi R, Sun H
and Zhang H: CUL4-DDB1 ubiquitin ligase interacts with multiple
WD40-repeat proteins and regulates histone methylation. Nat Cell
Biol. 8:1277–1283. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kerzendorfer C, Hart L, Colnaghi R,
Carpenter G, Alcantara D, Outwin E, Carr AM and O'Driscoll M:
CUL4B-deficiency in humans: Understanding the clinical consequences
of impaired Cullin 4-RING E3 ubiquitin ligase function. Mech Ageing
Dev. 132:366–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong J, Wang XQ, Yao JJ, Li G and Li XG:
Decreased CUL4B expression inhibits malignant proliferation of
glioma in vitro and in vivo. Eur Rev Med Pharmacol Sci.
19:1013–1021. 2015.PubMed/NCBI
|
|
27
|
Chen Z, Shen BL, Fu QG, Wang F, Tang YX,
Hou CL and Chen L: CUL4B promotes proliferation and inhibits
apoptosis of human osteosarcoma cells. Oncol Rep. 32:2047–2053.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang T, Tang HM, Wu ZH, Chen J, Lu S,
Zhou CZ, Yan DW and Peng ZH: Cullin 4B is a novel prognostic marker
that correlates with colon cancer progression and pathogenesis. Med
Oncol. 30:5342013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jia S, Kobayashi R and Grewal SI:
Ubiquitin ligase component Cul4 associates with Clr4 histone
methyltransferase to assemble heterochromatin. Nat Cell Biol.
7:1007–1013. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dumbliauskas E, Lechner E, Jaciubek M,
Berr A, Pazhouhandeh M, Alioua M, Cognat V, Brukhin V, Koncz C,
Grossniklaus U, et al: The Arabidopsis CUL4-DDB1 complex interacts
with MSI1 and is required to maintain MEDEA parental imprinting.
EMBO J. 30:731–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Higa LA, Yang X, Zheng J, Banks D, Wu M,
Ghosh P, Sun H and Zhang H: Involvement of CUL4 ubiquitin E3
ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E
degradation. Cell Cycle. 5:71–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishitani H, Shiomi Y, Iida H, Michishita
M, Takami T and Tsurimoto T: CDK inhibitor p21 is degraded by a
proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway
during S phase and after UV irradiation. J Biol Chem.
283:29045–29052. 2008. View Article : Google Scholar : PubMed/NCBI
|