Introduction
Vulnerable plaques are plaques that are more likely
to rupture and cause cerebrovascular events. The likelihood that a
plaque will become vulnerable depends on a number of factors
including the degree of stenosis, plaque morphology and plaque
pathophysiology, such as intraplaque hemorrhage (1–4).
Angiogenesis is important in determining plaque development and
vulnerability. Plaque neovascularization is more extensive in
symptomatic and vulnerable carotid artery plaques (5,6) and
immunohistochemical studies in humans have confirmed that the
increased density of microvessels is associated with plaque rupture
(7,8). It has been demonstrated that vessel
density is two times higher in vulnerable plaques than in stable
plaques, which results in severe luminal narrowing. Furthermore,
vessel density is up to four times higher in ruptures than in
stable plaques (9).
It would be ideal to develop a noninvasive method
capable of analyzing intraplaque angiogenesis and assessing whether
these plaques are vulnerable to rupture. Sophisticated techniques,
including computerized tomography angiography with contrast agents
and positron emission tomography, have been developed to perform
carotid artery plaque imaging in vivo (10,11).
However, these techniques are expensive and require substantial
exposure to radiation, making them unsuitable for use during
routine follow-up. Furthermore, they are unable to identify
neovascularization. Although magnetic resonance imaging can
determine the neovascularity of plaques, it requires the use of
expensive apparatus and operation by trained practitioners. This is
not feasible in many parts of the world. Standard ultrasonography
can provide information on plaque morphology based on ultrasonic
echolucency (12) but is inadequate
for assessing plaque neovascularization (13). Due to these challenges, no imaging
technique has been established as a ‘gold standard’ for analyzing
vulnerable carotid plaques (14) and
little is known about how inflammation or morphological changes in
such plaques lead to cerebrovascular events.
Contrast-enhanced ultrasonography (CEUS) is a
promising noninvasive tool for the visualization of plaque
neovascularization. It combines the high spatial and temporal
resolution of standard vascular ultrasonography with the properties
of contrast agent microbubbles, which behave as intravascular
tracers (15). CEUS reveals plaque
biological activity and vascularization in vivo and a number
of studies have suggested that CEUS may be useful for plaque risk
stratification and assessing atherosclerosis progression and
regression (13,16,17).
However, to the best of our knowledge, no studies have directly
compared the effectiveness of CEUS with standard ultrasonography
for assessing plaque neovascularization in patients at risk of
atherosclerosis.
Statins are widely used to reduce cholesterol levels
in patients at risk of atherosclerosis and changes in carotid
plaques revealed by ultrasound are usually observed following large
doses of atorvastatin (80 or 40 mg/day) (18). Few studies have examined the effects
of small doses of statins administered over a long time on carotid
artery neovascularization. Thus the current study used CEUS to
determine whether carotid plaque echogenicity and intra-plaque
neovascularization were decreased following two-year atorvastatin
therapy (20 mg/day) in Chinese patients.
Patients and methods
Patients
Patients scheduled to undergo standard
ultrasonography of the carotid artery in the Department of
Ultrasound, Fuxing Hospital (Beijing, China) between March 2009 and
May 2012 were recruited. Among the 62 patients initially recruited,
10 patients (7 male patients and 3 female patients) were included
in the current study.
Patients were eligible as long as they had at least
one atherosclerotic plaque in the carotid artery that was thicker
than 2.0 mm (19) and which was
determined to be uniformly or predominantly echolucent by standard
ultrasonography. Atherosclerotic plaques were defined according to
the Mannheim consensus, which was the presence of focal structures
encroaching into the arterial lumen by >0.5 mm, by 50% of the
thickness of the surrounding intima-media complex or by the
thickness of the intima-media layer if this was >1.5 mm
(20). Patients were excluded from
the current study if they had known allergies to albumin or to
standard ultrasonography contrast medium.
Each patient was administered atorvastatin calcium
tablets (Pfizer Pharmaceutical Co., Ltd., Dalian, China; 20 mg/day
taken orally once a day) for 2 years and all patients were able to
continue their medications throughout. The dose of 20 mg/day was
selected as the majority of Chinese people do not have high
cholesterol levels; levels of <1.04 mmol/l are very common
(21). In addition, as atorvastatin
induces side effects in the liver, many patients are unable to
tolerate higher doses. The current prospective pilot study was
approved by the Research Ethics Committee of Fuxing Hospital and
written informed consent was obtained from each patient.
Analysis of carotid plaques
All subjects were analyzed using standard
ultrasonography and CEUS at baseline prior to initiation of
atorvastatin therapy and the same examination was performed
following 1 and 2 years of treatment. Standard ultrasonography and
CEUS were performed using an Acuson Sequoia 512 ultrasound machine
(Siemens Medical Solutions; Mountain View, CA, USA) equipped with
an 8-L probe and operated at a transmission frequency of 8–15 MHz.
These procedures were performed by a trained vascular technologist
who was blinded to the history of the participant. Prior to
re-examination, the technologist reviewed the previous examination
results of each patient to ensure that the same plaques were
assessed that had been assessed previously.
As each patient lay in the supine position, the left
and right carotid arteries were examined with the head supported at
a 45° angle and turned to the contralateral side. The common
carotid artery, extracranial segments of the internal carotid
artery and external carotid artery were examined in the
longitudinal and transverse planes using standard ultrasonography.
Maximal plaque thickness was measured as maximal intima-media
thickness. Uniformly or predominantly echolucent lesions with
plaques thicker than 2.0 mm were recorded during standard
ultrasonography and CEUS, and were analyzed later.
Following standard ultrasonography, the same plaques
were examined using real-time CEUS. Coded pulse inversion imaging
was activated, image contrast was maximized and the mechanical
index was reduced to 0.18–0.35. Using a region below the plaques of
interest, the technician adjusted the time gain compensation to
achieve homogeneous signal intensity for the carotid artery, while
minimizing noise from the carotid artery wall and the plaque. All
settings were kept constant throughout each examination.
CEUS imaging was performed following injection of
the intravascular tracer SonoVue (Bracco SpA, Milan, Italy),
consisting of sulfur hexafluoride phospholipid-stabilized
microbubbles with a mean diameter of 2.5 µm and a concentration of
1–5×108/ml (22). The
microbubbles could freely flow through the tissues of tiny
capillaries. However, they could not enter the tissue space through
the vascular endothelial cells, which is a perfusion area limited
in the vascular bed and is not involved in the outer region
(23). Prior to use, 5 ml saline
solution was added to the lyophilized powder under a sulfur
hexafluoride atmosphere and shaken thoroughly prior to use. The
contrast agent was injected via the antecubital vein as a 2.2 ml
bolus within 2–3 sec, followed by a 5 ml saline bolus. The
appearance of the contrast effect was observed inside the lumen of
the carotid artery 15–30 sec following the injection. A
contrast-enhanced carotid cineloop was acquired starting at least 3
sec prior to injection of the contrast material and this ended 5
min following the appearance of the contrast effect in the carotid
artery lumen. Videos were digitally stored for later analysis.
Participants were observed for 30 min before they were allowed to
leave, in case any complications developed.
Analysis of standard ultrasound
images
Maximal plaque thickness was measured from the
media-adventitia to the intima-lumen boundaries and determined as
the maximal intima-media thickness in a longitudinal image.
Echolucent and mixed plaques were analyzed. Homogeneous echo
plaques were defined as plaques with an echogenicity less than that
of the surrounding adventitia for >80% of the plaque area,
without acoustic shadowing. Mixed plaques were defined as plaques
containing <90% of the circumferential calcification or with
associated echo-dense and anechoic regions that occupied <80% of
the plaque area (24). Homogeneous
echo plaques were named as 1 and mixed as 2.
The dynamics of echogenic reflections from
microspheres in the lumen of the carotid artery and intraplaque
microvessels were observed. Subsequently, the region of interest
(ROI), a circle constructed within the interested region to
generate the time-intensity curve, was determined within the plaque
(ROI-P) as a whole and in the lumen of the carotid artery near the
plaque (ROI-L).
Plaque enhancement was quantified offline using the
time-signal intensity curve analysis software (Research-Arena;
Unterschleissheim, Germany). installed on the Acuson Sequoia 512.
This software is able to exhibit the signal intensity-time curve in
ROIs during enhancement. The following time-intensity curve
parameters were noted: Baseline intensity (BI), arrival time (AT),
time to peak (TTP) and peak intensity (PI). Due to the ultrasound
contrast agent, the intra-plaque signal intensity increased. Thus,
enhanced intensity (EI) was calculated as follows: EI = PI - BI. EI
is a parameter that measures the intensity differences between pre-
and post-injections of the intravascular tracer, SonoVue, within
the plaque ROI. Relative plaque enhancement (EI-R), measured at the
separate peak enhancement point in the blood and plaque, was
calculated as the ratio of enhanced intensity in the carotid artery
lumen (EI-L) to the enhanced intensity in the plaque (EI-P) using
the following formula: EI-R=EI-L/EI-P.
Quantitative data were retrospectively and
independently analyzed by two investigators, who were blinded to
the identity of the patient. Any disagreements were resolved by
discussion.
Statistical analysis
Values were reported as mean ± standard deviation,
where appropriate. Data analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Repeated-measurement analysis of
variance (ANOVA) was used to compare CEUS parameters at baseline
and after one and two years of atorvastatin treatment. Post-hoc
statistical tests (Bonferroni test) were performed after ANOVA to
account for the multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients
During the study period, 10 consecutive subjects
were enrolled: 7 males with a mean age of 68±9 years and 3 females
with a mean age of 67±10 years (Table
I). The number of patients with different risk factors
(diabetes mellitus, hypertension, smoking history, coronary artery
disease, stroke and peripheral arterial disease) were as follows: 8
patients had diabetes mellitus, 10 patients had hypertension, 5
patients had an active smoking history, 8 patients had coronary
artery disease, 10 patients had experienced stroke and 1 patient
had peripheral arterial disease. All patients presented with
nonspecific neurologic symptoms including vertigo and syncope. The
10 patients included in the current study presented with a total of
13 carotid plaques.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
|
Characteristics | Number |
|---|
| Age (years) | 68.9±9.2 |
| Sex |
|
|
Male | 7 |
|
Female | 3 |
| Diabetes | 8 |
|
Hypertension | 10 |
|
Smoking | 5 |
| Clinical
history |
|
|
Coronary artery disease | 8 |
|
Stroke | 10 |
|
Peripheral arterial
disease | 1 |
Blood lipid levels
A total of 4 ml blood was drawn using BD Vacationer
vacuum blood collection tubes (Suzhou BD Medical Devices Co., Ltd)
and blood lipid parameters (total cholesterol, triglycerides,
high-density lipoprotein cholesterol and low-density lipoprotein
cholesterol) were measured using an analyzer (Beijing Zhou Tian Hua
Feng Medical Instruments Co. Ltd.) to detect blood fat at baseline
and 1 and 2 years following atorvastatin treatment. All blood lipid
indexes are presented in Table II,
which was simultaneously checked during the ultrasound examination.
From these results, it could be observed that there were no
significant differences in blood lipid parameters among any of the
different time points.
 | Table II.Blood lipid parameters. |
Table II.
Blood lipid parameters.
| Variable | Baseline | 1 year | 2 years | P-value |
|---|
| TCHO (mmol/l) | 3.40±0.21 | 3.19±0.28 | 3.37±0.24 | 0.540 |
| TG (mmol/l) | 1.69±0.42 | 1.52±0.37 | 1.40±0.20 | 0.166 |
| HDL-C (mmol/l) | 1.16±0.10 | 1.05±0.08 | 1.11±0.09 | 0.673 |
| LDL-C (mmol/l) | 2.14±0.73 | 2.09±0.87 | 2.16±0.69 | 0.695 |
Ultrasound examination for carotid
artery plaques
For each of the plaques, standard ultrasonography
was used to evaluate lesion echogenicity, while CEUS was used to
perform the visual and quantitative analysis of neovascularization.
Each technique was applied at baseline (at the time of study
enrollment) and following 1 and 2 years of atorvastatin
treatment.
At baseline, standard ultrasonography revealed seven
uniformly echolucent lesions and six predominantly echolucent
lesions. Following atorvastatin therapy for 1 year, the same
technique revealed that all plaques were predominantly echogenic.
Following 2 years of therapy, all plaques appeared uniformly
echogenic or extensively calcified (Table III).
 | Table III.Carotid plaque echogenicity
features. |
Table III.
Carotid plaque echogenicity
features.
| Echogenicity
features | Baseline | 1 year | 2 years |
|---|
| Homogeneous Echo
Plaque | 7 | 4 | 0 |
| Mixed Plaque | 6 | 9 | 13 |
| Total | 13 | 13 | 13 |
The carotid plaque sizes at pre- and
post-atorvastatin therapy are presented in Table IV. ANOVA indicated a significant
difference in carotid plaque size between pre- and
post-atorvastatin therapy (P=0.016). These results demonstrate that
carotid plaque size shrunk each year following atorvastatin
therapy.
 | Table IV.Carotid plaque sizes and contrast
enhancement. |
Table IV.
Carotid plaque sizes and contrast
enhancement.
| Variable | Baseline | 1 year | 2 years | P-value |
|---|
| Plaque sizes
(mm2) | 40.98±15.94 | 29.58±12.75 | 24.57±13.33 | 0.016a |
| EI-P (dB) | 10.55±2.08 | 8.96±2.80 | 7.27±2.57 |
<0.001a |
| ΔTTP (sec) | 2.20±1.70 | 3.45±1.59 | 3.62±1.60 | 0.011a |
| ΔAT (sec) | 1.44±1.22 | 2.69±2.11 | 3.20±2.07 | 0.062 |
| EI-R | 3.11±1.08 | 3.61±1.33 | 4.96±2.99 | 0.022a |
CEUS analysis at baseline revealed an average EI-P
of 10.55±2.08 decibels (dB) and an average EI-R of 3.11±1.08 for
all 13 plaques. Following 1-year atorvastatin therapy, EI-P
decreased to 8.96±2.80 dB, while EI-R increased to 3.61±1.33.
Following 2 years of therapy, these values were 7.27±2.57 dB and
4.96±2.99, respectively. Values at both follow-ups differed
significantly from those at baseline (Table IV, Fig.
1).
In comparison with the baseline, average EI-P
decreased and EI-R increased after atorvastatin therapy 1 year and
2 years (Tables V and VI).
 | Table V.Multiple comparisons of enhanced
intensity in the plaque at different time points. |
Table V.
Multiple comparisons of enhanced
intensity in the plaque at different time points.
|
| P-value |
|---|
|
|
|
|---|
| Time point | Baseline | 1 year | 2 years |
|---|
| Baseline | – | 0.002 | 0.000 |
| 1 year | 0.002 | – | 0.012 |
| 2 year | 0.000 | 0.012 | – |
 | Table VI.Multiple comparisons of enhanced
intensity in the plaque ratio at different time points. |
Table VI.
Multiple comparisons of enhanced
intensity in the plaque ratio at different time points.
|
| P-value |
|---|
|
|
|
|---|
| Time point | Baseline | 1 year | 2 years |
|---|
| Baseline | – | 0.007 | 0.032 |
| 1
year | 0.007 | – | 0.079 |
| 2
year | 0.032 | 0.079 | – |
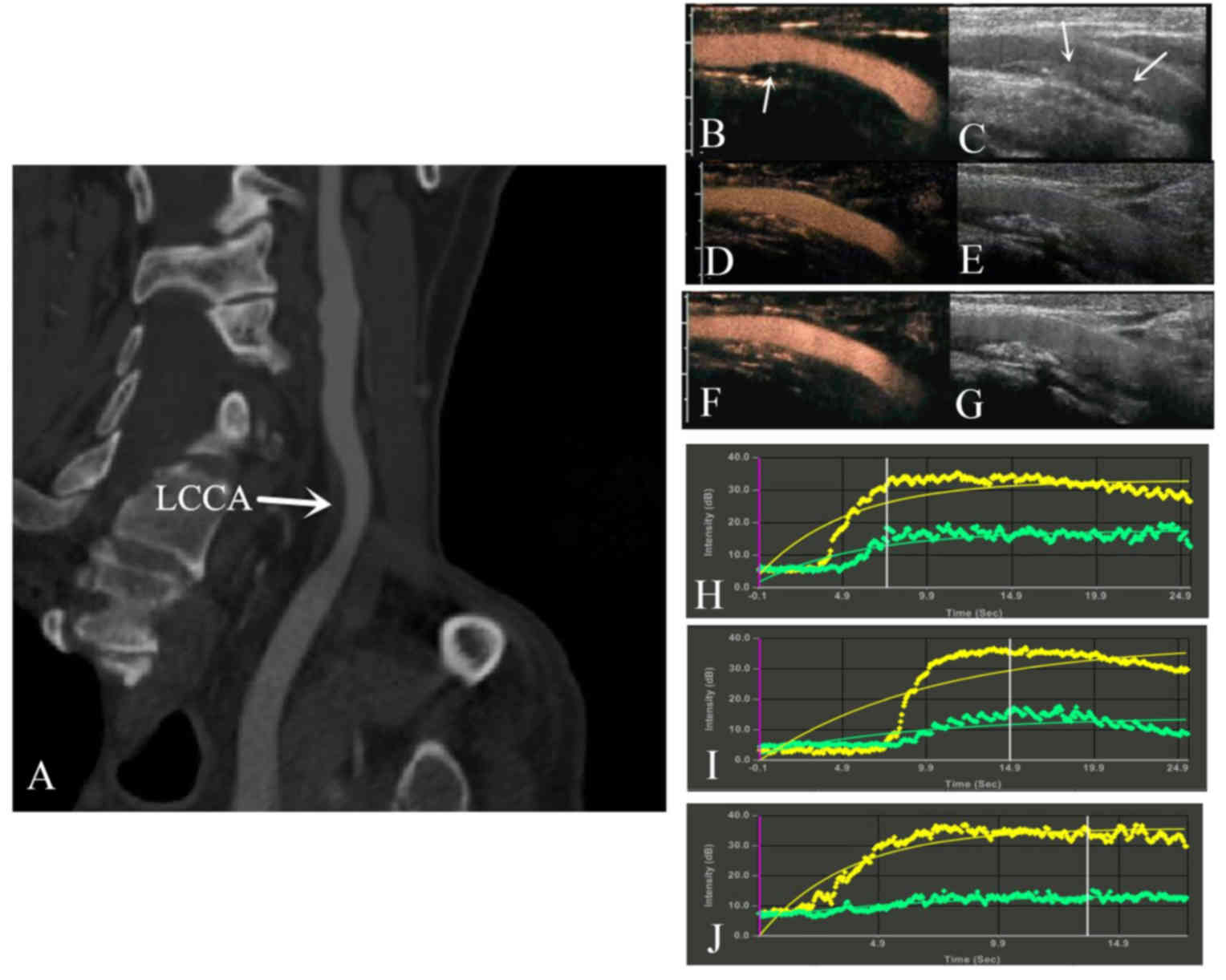
Fig. 2 presents a
patient who had a large plaque in the common carotid artery that
was identified by computed tomography angiography. CEUS and
time-signal intensity curves analysis revealed enhanced intensity
in the plaque and lumen. After 2 years of treatment, the echo of
the plaques was extensively enhanced by calcification, and the
plaque neovascularization in the carotid plaques decreased.
Discussion
In the current pilot study, it was identified that
CEUS is superior to standard ultrasonography at measuring plaque
neovascularization in patients at risk of atherosclerosis. Since
poor neovascularization is associated with plaque vulnerability,
CEUS may provide a noninvasive method for assessing the risk of
cerebrovascular events. CEUS also determined that 2-year
atorvastatin (20 mg/day) therapy is able to significantly reduce
neovascularization, suggesting that it induces a plaque-stabilizing
effect.
A previous study has detected change in carotid
plaques by ultrasound following administration of 40 or 80 mg/day
atorvastatin in Western populations, and the administration of 20
mg/day atorvastatin as a homeopathic dose appeared to induce no
strong response in the plaques in Western populations (25). However, some studies have indicated
that small doses of atorvastatin are safe and effective to
administer to ethnic Chinese patients (21,26).
Colhoun et al (27) reported
that 10 mg/day atorvastatin was safe and effective at reducing the
risk of first cardiovascular events including stroke in the UK and
Ireland with type II diabetes, without elevating low-density
lipoprotein (LDL) cholesterol levels. This may be due to ethnic
differences. Studies have confirmed that 20 mg/day atorvastatin
reduces LDL levels, as well as inflammation and thrombogenesis, in
Chinese patients with acute ischemic stroke caused by large artery
atherosclerosis (21,26,28–30). A
possible reason for the greater effect of moderate statin doses in
Asian compared with Western populations may be the difference in
statin pharmacokinetics (31). In
addition, a previous study indicated that a double dose or
increased statin administration did not bring significant clinical
effectiveness (32). In addition,
the results of the CHILLAS study may be considered a representative
of what can be achieved by lipid-lowering treatment (29). In Taiwan, the PAPAGO-T study
conducted among high-risk patients including those with type II
diabetes mellitus revealed that 10 mg/day atorvastatin was
well-tolerated, lowered LDL-C levels and improved the lipid profile
to a comparable degree in high-risk ethnic Chinese patients with
hypercholesterolemia (33).
Plaque inflammation and neovascularization are
histological markers of vulnerable plaques and predictors of
unstable atherosclerotic lesions in patients with cerebro- or
cardiovascular disease (17). This
has led to increasing interest in the inflammatory and histological
processes that occur in atherosclerotic lesions and give rise to
cerebrovascular events (34,35). A noninvasive method capable of
evaluating plaque neovascularization is required to evaluate these
processes. The microbubbles used with CEUS in the current study
could freely move through the tissues of tiny capillaries but could
not enter the tissue space through vascular endothelial cells.
Hence, CEUS was used to assess neovascularity in a noninvasive
manner.
Homogeneous echo plaques on standard ultrasonography
B-mode images reflect the histological features of plaque
instability. Such plaques are prone to rupture due to increased
lipid content, macrophage density and intraplaque hemorrhage.
Homogeneous echo plaques are also associated with an increased risk
of ischemic stroke (12,35). Standard ultrasonography of the
patients in the current study at baseline revealed either uniform
or predominant echolucent lesions. Following 2 years atorvastatin
therapy, plaques became uniformly echogenic or extensively
calcified, suggesting a lower risk of rupture. Furthermore, the
size of the carotid plaques shrunk each year following atorvastatin
therapy, suggesting that atorvastatin may inhibit the growth of
plaques. However, the carotid plaque size shrunk less during the
second year of therapy, compared with the decrease observed over
the first year. This may be explained by the fact that when blood
drug concentrations reach a certain degree following long-term
atorvastatin therapy, patients develop a degree of tolerance to the
drug. However, plaque stability does not appear to be associated
with blood lipid levels and a previous study confirmed that blood
lipid levels were not linearly associated with the stability of the
plaque (36). CEUS analysis through
the same treatment period has been proven to be effective at
assessing neovascularization, and determined that atorvastatin
significantly reduced EI-P and increased EI-R. EI-R is a
straightforward ratio of signal intensities within the plaque ROI
and the artery lumen at the time of peak intensity in the artery
lumen. This ratio was selected as it can cancel the interference
factor of different patients and represent the absolute value of
enhancement (37,38).
The results of the current study demonstrate the
usefulness of CEUS, supporting the results of previous studies
suggesting that this method is a promising noninvasive tool to
visualize plaque neovascularization (12,36).
Shah et al (35) identified a
strong correlation between CEUS analysis of plaque
neovascularization in carotid arteries and histological scores on
surgical specimens. Coli et al (39) demonstrated that CEUS measurements of
contrast enhancement correlated well with histologically determined
neovessel density in plaques.
The results of the current study provide some of the
first direct evidence that prolonged statin therapy reduces plaque
neoangiogenesis. Angiogenesis in plaques may be triggered by
hypoxia and inflammation (40,41) and
it has been demonstrated that statins reduce inflammation as well
as lowering lipid levels (42,43).
Therefore, the anti-inflammatory effects of statins may mediate
their therapeutic effect on plaque neovascularization. Larger
controlled trials are required to validate CEUS as a routine
screening and monitoring method for patients at risk of
atherosclerosis. Such studies should also examine the mechanism of
statin action in more detail.
In conclusion, the current pilot study suggests that
CEUS is a promising potential surrogate end point in clinical
trials that examine risk factors and treatments for
atherosclerosis. Using this technique, it was suggested that
long-term atorvastatin therapy may reduce plaque neovascularization
and thereby reduce the risk of cerebrovascular events
occurring.
Acknowledgements
The authors are grateful to Dr Lu Zhao Ling, for
providing technical assistance. The present study was funded by the
Chinese Medical Association Special Fund as part of the project
‘Neovascularization within carotid atherosclerotic plaques with
contrast-enhanced US angiography: Clinical Research’ (grant no.
09010410196) and by the Beijing Natural Science Foundation Program
and Scientific Research Key Program of Beijing Municipal Commission
of Education (Item No: KZ201510025031).
References
|
1
|
McCarthy MJ, Loftus IM, Thompson MM, Jones
L, London NJ, Bell PR, Naylor AR and Brindle NP: Angiogenesis and
the atherosclerotic carotid plaque: An association between
symptomatology and plaque morphology. J Vasc Surg. 30:261–268.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finn AV, Nakano M, Narula J, Kolodgie FD
and Virmani R: Concept of vulnerable/unstable plaque. Arterioscler
Thromb Vasc Biol. 30:1282–1292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michel JB, Delbosc S, Ho-Tin-Noé B,
Leseche G, Nicoletti A, Meilhac O and Martin-Ventura JL: From
intraplaque haemorrhages to plaque vulnerability: Biological
consequences of intraplaque haemorrhages. J Cardiovasc Med
(Hagerstown). 13:628–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Virmani R, Kolodgie FD, Burke AP, Finn AV,
Gold HK, Tulenko TN, Wrenn SP and Narula J: Atherosclerotic plaque
progression and vulnerability to rupture: Angiogenesis as a source
of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol.
25:2054–2061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Stefano R, Felice F and Balbarini A:
Angiogenesis as risk factor for plaque vulnerability. Curr Pharm
Des. 15:1095–1196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sluimer JC and Daemen MJ: Novel concepts
in atherogenesis: Angiogenesis and hypoxia in atherosclerosis. J
Pathol. 218:7–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dunmore BJ, McCarthy MJ, Naylor AR and
Brindle NP: Carotid plaque instability and ischemic symptoms are
linked to immaturity of microvessels within plaques. J Vasc Surg.
45:155–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kodama T, Narula N, Agozzino M and
Arbustini E: Pathology of plaque haemorrhage and neovascularization
of coronary artery. J Cardiovasc Med (Hagerstown). 13:620–627.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Guo L, Chen B, Sun L and Cui M:
Association of serum angiopoietin-1, angiopoietin-2 and
angiopoietin-2 to angiopoietin-1 ratio with heart failure in
patients with acute myocardial infarction. Exp Ther Med. 5:937–941.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warburton L and Gillard J: Functional
imaging of carotid atheromatous plaques. J Neuroimaging.
16:293–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Laufer EM, Winkens HM, Corsten MF,
Reutelingsperger CP, Narula J and Hofstra L: PET and SPECT imaging
of apoptosis in vulnerable atherosclerotic plaques with
radiolabeled Annexin A5. Q J Nucl Med Mol Imaging. 53:26–34.
2009.PubMed/NCBI
|
|
12
|
Grønholdt ML, Nordestgaard BG, Schroeder
TV, Vorstrup S and Sillesen H: Ultrasonic echolucent carotid
plaques predict future strokes. Circulation. 104:68–73. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannoni Fabrizia M, Vicenzini E, Monaco C
and Cao P: Contrast enhanced ultrasonography and carotid plaque
imaging: From the hemodynamic evaluation to the detection of
neoangiogenesis-The new approach to the identification of the
unstable plaque: From morphology to pathophysiologyUltrasound
Imaging. Tanabe M: InTech; pp. 171–188. 2011
|
|
14
|
Faggioli GL, Pini R, Mauro R, Pasquinelli
G, Fittipaldi S, Freyrie A, Serra C and Stella A: Identification of
carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography:
Correlation with plaque histology, symptoms and cerebral computed
tomography. Eur J Vasc Endovasc Surg. 41:238–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feinstein SB: The powerful microbubble:
From bench to bedside, from intravascular indicator to therapeutic
delivery system, and beyond. Am J Physiol Heart Circ Physiol.
287:H450–H457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feinstein SB: Contrast ultrasound imaging
of the carotid artery vasa vasorum and atherosclerotic plaque
neovascularization. J Am Coll Cardiol. 48:236–243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staub D, Patel MB, Tibrewala A, Ludden D,
Johnson M, Espinosa P, Coll B, Jaeger KA and Feinstein SB: Vasa
vasorum and plaque neovascularization on contrast-enhanced carotid
ultrasound imaging correlates with cardiovascular disease and past
cardiovascular events. Stroke. 41:41–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Della-Morte D, Moussa I, Elkind MS, Sacco
RL and Rundek T: The short-term effect of atorvastatin on carotid
plaque morphology assessed by computer-assisted gray-scale
densitometry: A pilot study. Neurol Res. 33:991–994. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Touboul PJ, Hennerici MG, Meairs S, Adams
H, Amarenco P, Desvarieux M, Ebrahim S, Fatar M, Hernandez
Hernandez R, Kownator S, et al: Mannheim intima-media thickness
consensus. Cerebrovasc Dis. 18:346–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Touboul PJ, Hennerici MG, Meairs S, Adams
H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S,
Hernandez Hernandez R, et al: Mannheim carotid intima-media
thickness and plaque consensus (2004-2006-2011). An update on
behalf of the advisory board of the 3rd, 4th and 5th watching the
risk symposia, at the 13th, 15th and 20th European Stroke
Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and
Hamburg, Germany, 2011. Cerebrovasc Dis. 34:290–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: Prevalence of diabetes among
men and women in China. N Engl J Med. 362:1090–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kern R, Szabo K, Hennerici M and Meairs S:
Characterization of carotid artery plaques using real-time compound
B-mode ultrasound. Stroke. 35:870–875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrara KW, Merritt CR, Burns PN, Foster
FS, Mattrey RF and Wickline SA: Evaluation of tumor angiogenesis
with US: Imaging, Doppler, and contrast agents. Acad Radiol.
7:824–839. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Staub D, Partovi S, Schinkel AF, Coll B,
Uthoff H, Aschwanden M, Jaeger KA and Feinstein SB: Correlation of
carotid artery atherosclerotic lesion echogenicity and severity at
standard US with intraplaque neovascularization detected at
contrast-enhanced US. Radiology. 258:618–626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kadoglou NP, Sailer N, Moumtzouoglou A,
Kapelouzou A, Gerasimidis T and Liapis CD: Aggressive
lipid-lowering is more effective than moderate lipid-lowering
treatment in carotid plaque stabilization. J Vasc Surg. 51:114–21.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gupta M, Martineau P, Tran T, Després JP,
Gaw A, de Teresa E, Farsang C, Gensini GF, Leiter LA, Blanco-Colio
LM, et al: Low-density lipoprotein cholesterol and high-sensitivity
C-reactive protein lowering with atorvastatin in patients of South
Asian compared with European origin: Insights from the achieve
cholesterol targets fast with atorvastatin stratified titration
(ACTFAST) study. J Clin Pharmacol. 52:850–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colhoun HM, Betteridge DJ, Durrington PN,
Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI,
Charlton-Menys V and Fuller JH: CARDS investigators: Primary
prevention of cardiovascular disease with atorvastatin in type 2
diabetes in the collaborative atorvastatin diabetes study (CARDS):
Multicentre randomised placebo-controlled trial. Lancet.
364:685–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min L, Shao S, Wu X, Cong L, Liu P, Zhao H
and Luo Y: Anti-inflammatory and anti-thrombogenic effects of
atorvastatin in acute ischemic stroke. Neural Regen Res.
8:2144–2154. 2013.PubMed/NCBI
|
|
29
|
Zhao SP, Yu BL, Peng DQ and Huo Y: The
effect of moderate-dose versus double-dose statins on patients with
acute coronary syndrome in China: Results of the CHILLAS trial.
Atherosclerosis. 233:707–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia W and Zhou L: Effect of 20 mg/day
atorvastatin: Recurrent stroke survey in Chinese ischemic stroke
patients with prior intracranial hemorrhage. J Clin Neurol.
9:139–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee E, Ryan S, Birmingham B, Zalikowski J,
March R, Ambrose H, Moore R, Lee C, Chen Y and Schneck D:
Rosuvastatin pharmacokinetics and pharmacogenetics in white and
Asian subjects residing in the same environment. Clin Pharmacol
Ther. 78:330–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao SP, Yu BL, Peng DQ and Huo Y: The
effect of moderate-dose versus double-dose statins on patients with
acute coronary syndrome in China: Results of the CHILLAS trial.
Atherosclerosis. 233:707–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu PY, Lin LY, Lin HJ, Hsia CH, Hung YR,
Yeh HI, Wu TC, Chen JY, Chien KL and Chen JW: Pitavastatin and
atorvastatin double-blind randomized comparative study among
high-risk patients, including those with type 2 diabetes mellitus,
in Taiwan (PAPAGO-T Study). PLoS One. 8:e762982013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Desai MY and Schoenhagen P: Emergence of
targeted molecular imaging in atherosclerotic cardiovascular
disease. Expert Rev Cardiovasc Ther. 7:197–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shah F, Balan P, Weinberg M, Reddy V,
Neems R, Feinstein M, Dainauskas J, Meyer P, Goldin M and Feinstein
SB: Contrast-enhanced ultrasound imaging of atherosclerotic carotid
plaque neovascularization: A new surrogate marker of
atherosclerosis? Vasc Med. 12:291–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong LF, Yan XN, Fan Y, Wu Q, Luo SH, Yang
B and Li JJ: Is the ratio of apoB/apoA-1 the best predictor for the
severity of coronary artery lesions in Chinese diabetics with
stable angina pectoris? An assessment based on Gensini scores. J
Geriatr Cardiol. 12:402–409. 2015.PubMed/NCBI
|
|
37
|
Zhang Q, Li C, Han H, Dai W, Shi J, Wang Y
and Wang W: Spatio-temporal quantification of carotid plaque
neovascularization on contrast enhanced ultrasound: Correlation
with visual grading and histopathology. Eur J Vasc Endovasc Surg.
50:289–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hoogi A, Adam D, Hoffman A, Kerner H,
Reisner S and Gaitini D: Carotid plaque vulnerability:
Quantification of neovascularization on contrast-enhanced
ultrasound with histopathologic correlation. AJR Am J Roentgenol.
196:431–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coli S, Magnoni M, Sangiorgi G,
Marrocco-Trischitta MM, Melisurgo G, Mauriello A, Spagnoli L,
Chiesa R, Cianflone D and Maseri A: Contrast enhanced ultrasound
imaging of intraplaque neovascularization in carotid arteries:
Correlation with histology and plaque echogenicity. J Am Coll
Cardiol. 52:223–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Coll B, Nambi V and Feinstein SB: New
advances in noninvasive imaging of the carotid artery: CIMT,
contrast-enhanced ultrasound, and vasa vasorum. Curr Cardiol.
12:497–502. 2010. View Article : Google Scholar
|
|
41
|
Kolodgie FD, Narula J, Yuan C, Burke AP,
Finn AV and Virmani R: Elimination of neoangiogenesis for plaque
stabilization: Is there a role for local drug therapy? J Am Coll
Cardiol. 49:2093–2101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jain RK, Finn AV, Kolodgie FD, Gold HK and
Virmani R: Antiangiogenic therapy for normalization of
atherosclerotic plaque vasculature: A potential strategy for plaque
stabilization. Nat Clin Pract Cardiovasc Med. 4:491–502. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Doyle B and Caplice N: Plaque
neovascularization and antiangiogenic therapy for atherosclerosis.
J Am Coll Cardiol. 49:2073–2080. 2007. View Article : Google Scholar : PubMed/NCBI
|