Introduction
Polycystic ovary syndrome (PCOS) is a common
difficult and complicatedgynecologic disease, which often occurs in
puberty and childbearing period, affecting 6–21% women (1). It is mainly characterized by
anovulation or rare ovulation, clinical or biochemical androgen
excess and polycystic ovary, and insulin resistance (IR) is an
important pathophysiological change (2). Due to long-term metabolic disorders,
the middle-aged and elderly people suffer from diabetes mellitus,
cardiovascular diseases and even tumors, which will affect their
whole lives if treated improperly. Studies have found that
non-alcoholic fatty liver disease (NAFLD) is closely related to
obesity, abnormal glucose tolerance, IR, dyslipidemia and other
factors (3). Both PCOS and NAFLD are
diseases with IR as the core pathogenesis, and previous studies
have suggested there is a correlation between them (4,5). Some
scholars believe that NAFLD may be one of the complications of
PCOS. The correlation between PCOS and NAFLD is a research hotspot
at present. In this study, the risk factors for PCOS complicated
with NAFLD were analyzed, so as to provide clinical references for
the early diagnosis and treatment of PCOS patients complicated with
NAFLD.
Patients and methods
Clinical data
According to the unified standard developed in
Rotterdam International Conference in 2003 (6), 188 PCOS patients with complete clinical
data, admitted into the Department of Obstetrics and Gynecology,
Shengli Oilfield Central Hospital (Dongying, China) from February
2014 to February 2015, were collected as PCOS group. A total of 65
healthy outpatients receiving physical examination during the same
period were collected as normal control group. Inclusion criteria
of normal group: i) patients with regular menstruation and without
biochemical and clinical androgen excess; ii) patients without
history of endocrine diseases; iii) patients without polycystic
manifestations in bilateral ovaries via B ultrasound. Exclusion
criteria: i) patients complicated with other endocrine diseases
(Cushing syndrome, thyroid dysfunction and premature ovarian
failure); ii) patients with smoking history, alcohol abuse history,
hypertension or coronary heart disease history; iii) patients with
history of diseases in important organs, such as heart, liver or
kidney; iv) patients who received drug therapy for PCOS in the past
3 months or took drugs affecting the glucose and lipid metabolism
(such as glucocorticoids); v) patients with history of chronic
liver disease (viral hepatitis, alcoholic hepatitis or other liver
diseases). Diagnostic criteria revised in the Guideline (2010) were
used for NAFLD. This study obtained ethics approval from the Ethics
Committee of Shengli Oilfield Central Hospital, and patients were
informed and signed the consent.
Research methods
General clinical data
General clinical data of the patients, including the
name, age, history of menstruation, history of marriage and
childbearing, smoking history, drinking history, previous history
(hypertension, diabetes mellitus, coronary heart disease, pituitary
disease, fatty liver, viral hepatitis), family history and
medication, were recorded.
Anthropometric parameters
The weight of the participants in fasting state and
single-layer garment was measured, the waist circumference and hip
circumference were measured under stable breathing, and the height
was measured. Each index was measured twice and the average was
taken. Body mass index (BMI) = height (kg)/ weight (m2),
and BMI ≥25 indicated obesity; waist-hip ratio (WHR) = waist
circumference/hip circumference.
Detection of serological indexes
Specimens were collected from all objects on the
3rd-5th days in menstruation. At 8 h after fasting, antecubital
venous blood was drawn from the patients in quiet and fasting
states in the morning, and detected as soon as possible. The levels
of follicle-stimulating hormone (FSH), luteinizing hormone (LH),
prolactin (PRL), estradiol (E2) and testosterone (T) were detected
via immunochemiluminescence method. Serum dehydroepiandrosterone
sulfate (DHEAs), sex hormone binding globulin (SHBG) and alanine
aminotransferase (ALT) were detected via double antibody sandwich
enzyme-linked immunosorbent assay (ELISA). Serum triglyceride (TG),
total cholesterol (TC), high-density lipoprotein (HDL), low-density
lipoprotein (LDL) and fasting blood glucose (FBG) were detected
using the enzymatic method. Moreover, fasting insulin (FINS) was
detected via radioimmunoassay, and homeostasis model assessment
(HOMA) was used for the evaluation of insulin resistance (IR);
HOMA-IR index = [FBG (mmol/l) × FINS (mU/l)]/22.5. Free androgen
index (FAI) = T (nmol/l) × 100/SHBG (nmol/l). All operations were
performed by experts in strict accordance with the
instructions.
Vaginal ultrasound examination
All the patients received vaginal ultrasound
examination to detect the antral follicle count (AFC) and bilateral
ovarian volume, and they all underwent abdominal liver B ultrasound
examination to exclude measurement errors. Each index was measured
twice.
Statistical analysis
Data were recorded using SPSS 20.0 software (SPSS
Inc., Chicago, IL, USA). Measurement data are presented as (mean ±
SD) and t-test was used for comparison. Measurement data in
abnormal distribution are presented as mean value [95% confidence
interval (CI)], and rank sum test was used for comparison. Logistic
regression analysis was used for the multivariate result analysis.
P<0.05 indicated that the difference was statistically
significant.
Results
Comparison of clinical data of
patients between control group and PCOS group
There were no statistically significant differences
in comparisons of age, BMI, WHR, FSH, DHEA and FBG between the two
groups (P>0.05). The levels of LH, LH/FSH, T, FAI, FINS and
HOMA-IR index in PCOS group were higher than those in normal
control group (P<0.05), but the SHBG level was lower than that
in normal control group (P<0.05), as shown in Table I.
 | Table I.Comparisons of clinical data of
patients between control group and PCOS group. |
Table I.
Comparisons of clinical data of
patients between control group and PCOS group.
| Index | Control group
(n=65) | PCOS group
(n=188) | P-value |
|---|
| Age (years) |
26.96±4.78 |
27.14±5.23 | 0.056 |
| BMI
(kg/m2) |
24.23±2.97 |
25.18±3.21 | 0.124 |
| WHR |
0.87±0.75 |
0.89±0.82 | 0.573 |
| LH (IU/l) |
4.52±2.25 |
8.10±2.76 | >0.05 |
| FSH (IU/l) |
6.73±2.85 |
6.16±3.12 | 0.212 |
| LH/FSH |
0.67±0.22 |
1.31±0.28 | >0.05 |
| T (nmol/l) |
1.69±0.58 |
2.19±0.88 | >0.05 |
| DHEA (µg/dl) |
215.86±84.37 |
225.37±101.69 | 0.975 |
| SHBG
(nmol/l)a | 45.12
(37.54–50.87) | 34.45
(28.68–40.13) | 0.001 |
| FAIa | 4.67 (3.85–5.59) | 10.82
(8.89–12.51) | >0.05 |
| FBG (mmol/l) |
4.83±0.41 |
4.85±0.46 | 0.659 |
| FINS
(mIU/l)a | 9.92
(8.50–11.42) | 15.12
(12.56–17.39) | 0.024 |
| HOMA-IR |
1.56±0.28 |
3.53±0.64 | >0.05 |
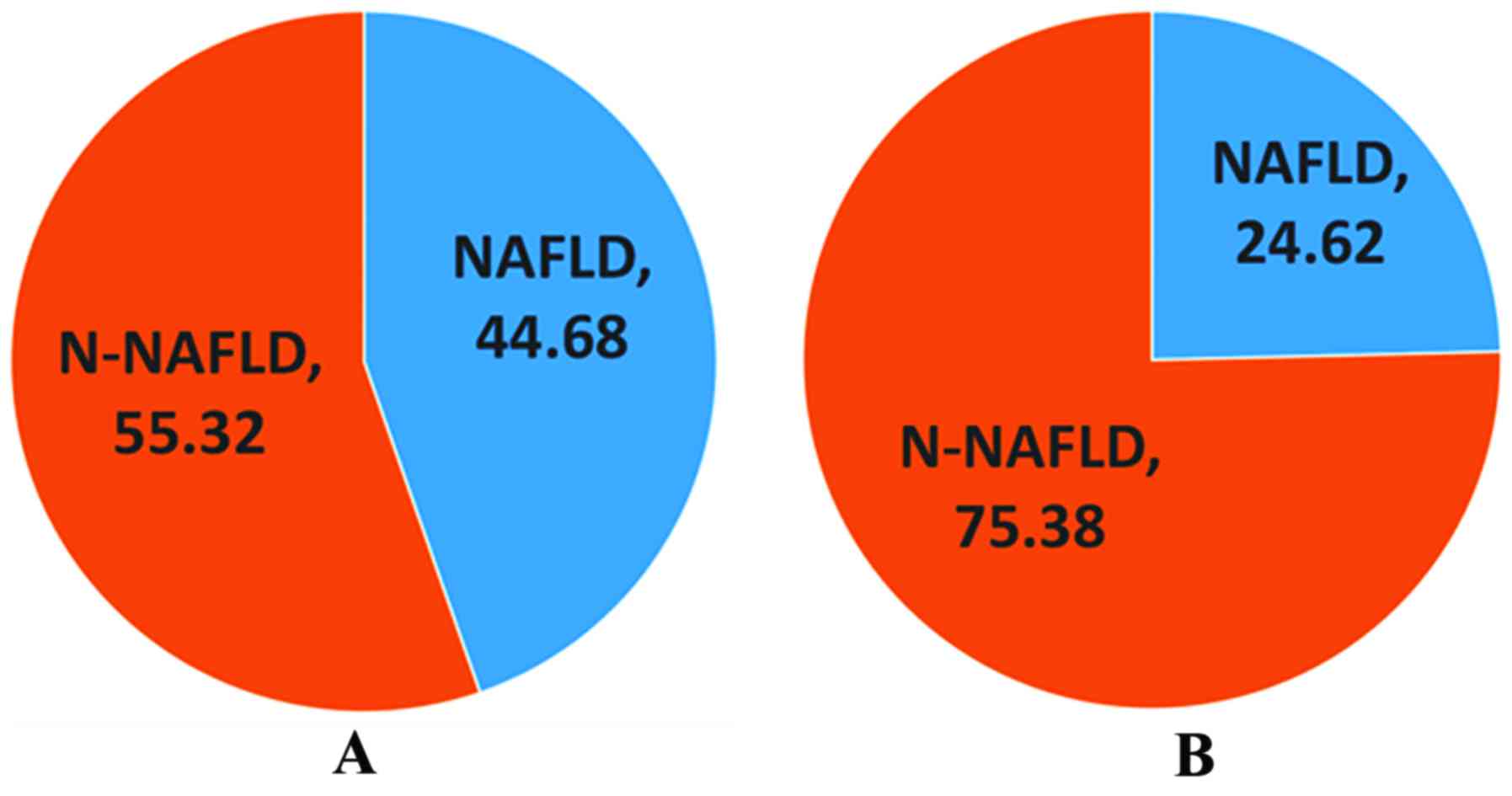
Comparison of prevalence rate of NAFLD
between normal control group and PCOS group
There were 16 cases (24.62%) of NAFLD in normal
group, and 84 cases (44.68%) of NAFLD in PCOS group. The prevalence
rate of NAFLD in PCOS group was significantly higher than that in
normal control group (P<0.05) (Fig.
1).
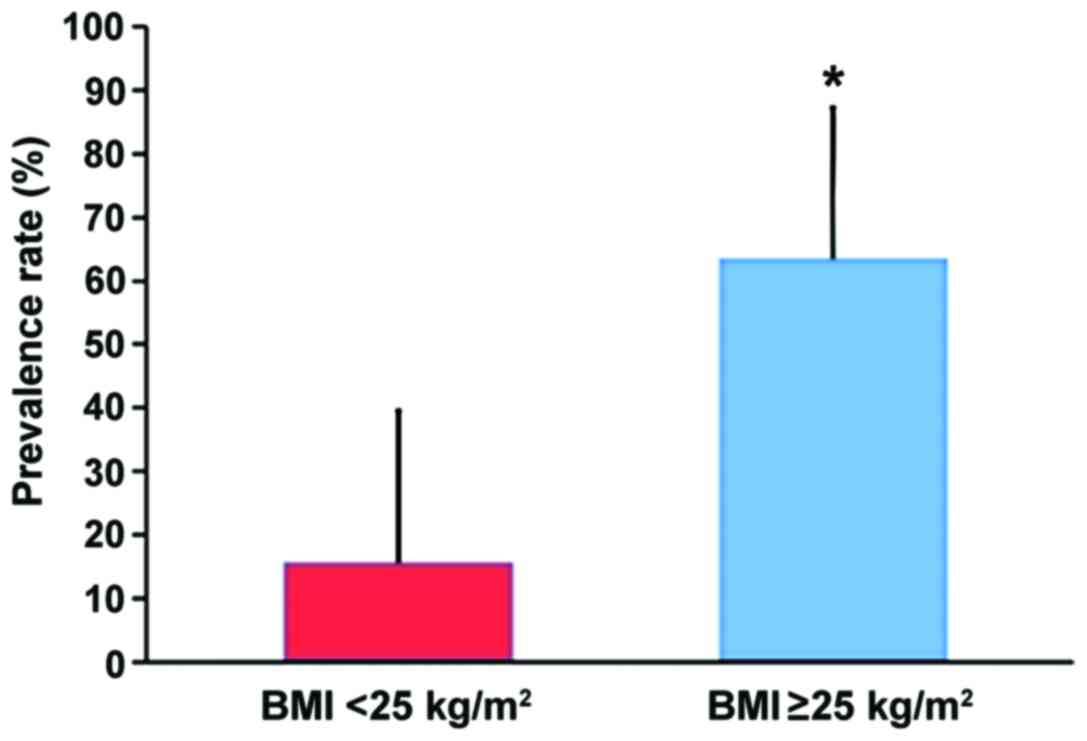
Comparison of prevalence rate of NAFLD
in patients with different BMI in PCOS group
Among 188 PCOS patients, there were 74 cases with
BMI ≥25 (obesity group) and 114 cases with BMI <25 (non-obesity
group). The prevalence rate of NAFLD in PCOS patients in obesity
group (47/74, 63.51%) was higher than that in PCOS patients in
non-obesity group (18/114, 15.79%) (χ2=30.12) (Fig. 2).
Comparisons of clinical data between
NAFLD group and N-NAFLD group in PCOS group
NAFLD group had higher age, BMI and WHR than N-NAFLD
group (P<0.05); the FAI level in NAFLD group was higher than
that in N-NAFLD group, but the SHBG level in NAFLD group was lower
than that in N-NAFLD group (P<0.05); there were no statistically
significant differences in comparisons of T, LH, FSH and LH/FSH
between the two groups (P>0.05); the levels of FBG, FINS and
HOMA-IR index in NAFLD group were higher than those in N-NAFLD
group (P<0.05); the levels of TC and TG in NAFLD group were
higher than those in N-NAFLD group (P<0.05), but the level of
HDL was lower than that in N-NAFLD group (P<0.05); the level of
ALT in NAFLD group was higher than that in N-NAFLD group
(P<0.05) (Table II).
 | Table II.Comparisons of clinical data between
NAFLD group and N-NAFLD group in PCOS group. |
Table II.
Comparisons of clinical data between
NAFLD group and N-NAFLD group in PCOS group.
| Index | N-NAFLD group
(n=104) | NAFLD group
(n=84) | P-value |
|---|
| Age (years) |
25.16±5.07 |
27.32±6.15 | 0.007 |
| BMI
(kg/m2) |
23.25±3.18 |
28.61±4.27 | >0.05 |
| WHR |
0.83±0.09 |
0.94±0.08 | >0.05 |
| LH (IU/l) |
8.41±5.36 |
10.56±9.05 | 0.061 |
| FSH (IU/l) |
7.36±2.79 |
7.13±3.09 | 0.523 |
| LH/FSH |
1.23±0.82 |
1.51±1.14 | 0.109 |
| T (nmol/l) |
2.19±0.78 |
2.17±0.89 | 0.513 |
| TG (mmol/l) |
0.93±0.14 |
1.66±0.22 | >0.05 |
| TC (mmol/l) |
4.38±1.04 |
4.78±0.91 | 0.029 |
| HDL (mmol/l) |
1.54±0.39 |
1.14±0.21 | >0.05 |
| DHEAs (µg/dl) |
228.56±111.84 |
219.75±98.46 | 0.478 |
| SHBG
(nmol/l)a | 49.21
(40.47–57.69) | 24.16
(18.98–29.34) | >0.05 |
| FAI | 6.71 (5.35–7.98) | 13.97
(11.61–16.37) | >0.05 |
| FBG (mmol/l) |
4.74±0.43 |
5.02±0.46 | 0.002 |
| FINS
(mIU/l)a | 8.79
(7.45–10.14) | 19.71
(16.56–22.82) | >0.05 |
| HOMA-IR |
1.90±0.32 |
4.83±0.84 | >0.05 |
| ALT
(U/l)a | 13.37
(11.26–15.48) | 42.12
(35.04–49.20) | >0.05 |
Comparisons of clinical data between
PCOS group complicated with NAFLD and control group complicated
with NAFLD
There were no statistically significant differences
in comparisons of age, BMI and WHR between PCOS group complicated
with NAFLD and control group complicated with NAFLD (P>0.05); LH
and LH/FSH in PCOS group complicated with NAFLD were higher than
those in control group complicated with NAFLD (P<0.05), but
there was no statistically significant difference in the FSH level
between the two groups (P>0.05); compared with those in control
group complicated with NAFLD, FINS and HOMA-IR index in PCOS group
complicated with NAFLD were higher (P<0.05), but there was no
statistically significant difference in the FBG level between the
two groups (P>0.05) (Table
III).
 | Table III.Comparisons of clinical data between
PCOS group complicated with NAFLD and control group complicated
with NAFLD. |
Table III.
Comparisons of clinical data between
PCOS group complicated with NAFLD and control group complicated
with NAFLD.
| Index | Control group
complicated with NAFLD (n=16) | PCOS group
complicated with NAFLD (n=84) | P-value |
|---|
| Age (years) |
27.46±4.49 |
27.32±6.15 | 0.057 |
| BMI
(kg/m2) |
28.92±3.68 |
28.61±4.27 | 0.192 |
| WHR |
0.91±0.07 |
0.94±0.08 | 0.936 |
| LH (IU/l) |
8.41±5.36 |
10.56±9.05 | 0.061 |
| FSH (IU/l) |
7.36±2.79 |
7.13±3.09 | 0.523 |
| LH/FSH |
1.23±0.82 |
1.51±1.14 | 0.109 |
| T (nmol/l) |
1.66±0.93 |
2.17±0.89 | >0.05 |
| DHEAs (µg/dl) |
217.43±115.31 |
219.75±98.46 | 0.962 |
| SHBG
(nmol/l)a | 39.89
(22.65–43.13) | 24.16
(18.98–29.34) | 0.002 |
| FAIa | 6.35
(4.31–8.39) | 13.97
(11.61–16.37) | >0.05 |
| FBG (mmol/l) |
4.84±0.43 |
5.02±0.46 | 0.831 |
| FINS
(mIU/l)a | 13.21
(11.05–15.38) | 19.71
(16.56–22.82) | 0.027 |
| HOMA-IR |
2.30±0.62 |
4.83±0.84 | >0.05 |
Logistic regression analyses of
influencing factors of PCOS complicated with NAFLD
HOMA-IR index [odds ratio (OR)=28.903], BMI
(OR=21.542), WHR (OR=1.712) and TG (OR=1.685) were risk factors for
PCOS complicated with NAFLD. HOMA-IR had the highest correlation
with PCOS complicated with NAFLD, followed by BMI, WHR and TG. The
higher the HOMA-IR index, BMI, WHR and TG levels were, the higher
the risk of PCOS in NAFLD patients would be (Table IV).
 | Table IV.Logistic regression analyses of
influencing factors of PCOS complicated with NAFLD. |
Table IV.
Logistic regression analyses of
influencing factors of PCOS complicated with NAFLD.
| Factors | b-value | Wald χ2
value | P-value | OR (95% CI) |
|---|
| HOMA-IR | 3.109 | 32.324 | 0.001 | 28.903
(7.835–65.641) |
| BMI | 2.184 | 16.672 | 0.002 | 21.542
(4.352–47.436) |
| TG | 0.523 | 4.839 | 0.026 | 1.685
(1.055–2.693) |
| WHR | 0.531 | 1.643 | 0.045 | 1.712
(0.006–2.894) |
Discussion
Our results showed that the prevalence rate of PCOS
complicated with NAFLD was 44.68%, which is consistent with
previous reports (7); it was
significantly higher than the prevalence rate of NAFLD in normal
people (24.62%, P<0.05). It has been shown that obesity is the
most important risk factor for NAFLD (8). Obesity is also the most common clinical
manifestation of PCOS patients, and approximately 2/3-4/5 PCOS
patients suffer from obesity (9). It
was also found in this study that NAFLD patients in PCOS group had
higher BMI than N-NAFLD patients, which is consistent with the
results of the study by Kauffman et al (10). BMI ≥25 indicates obesity; the
prevalence rate of NAFLD in obesity patients with PCOS (63.51%) was
significantly higher than that in non-obesity patients (15.79%)
(P<0.05), suggesting that obesity is closely related to PCOS
complicated with NAFLD. In the present study the incidence rate of
transaminase disorder in PCOS patients complicated with NAFLD was
higher. Besides, studies have shown that liver cells with fatty
degeneration more or less leads to impaired liver function
(11), but another study showed that
more than 70% people with normal liver enzyme have been in various
stages of NAFLD, among which 1/3 people showed fibrosis, and 1/10
people show liver cirrhosis (12). B
ultrasound is simple, economical and non-invasive with good
reproducibility, good sensitivity and high specificity, so it is
more widely accepted than pathological examination of liver tissues
(13,14), and has become an important method for
the diagnosis and monitoring of NAFLD. PCOS patients with normal
liver function need to be paid close attention to. Serum ALT is a
sensitive index of liver injury and liver fibrosis, and an
important basis of NAFLD clinical typing (15). Clinically, PCOS patients should
receive liver ultrasound examination regularly, so as to realize
the early detection and dynamic monitoring of fatty liver. In
particular, the examination of obesity patients needs to be
strengthened; liver function examination is of significance in the
active treatment of NAFLD.
At present, it is generally accepted that the
mechanism of NAFLD is the ‘second-strike’ hypothesis (16). The IR-induced lipid accumulation in
hepatocytes is the ‘first strike’ against the liver, and the
oxidative stress-induced inflammation and apoptosis is the ‘second
strike’, resulting in gene regulation imbalance of intrahepatic
lipid synthesis and fatty acid oxidation. IR is defined as a
biological effect of lower insulin than normal level after a
certain amount of insulin specifically binds to receptors. NAFLD
and IR interact as both cause and effect; on the one hand, NAFLD
significantly aggravates the liver IR, increasing risk of type 2
diabetes mellitus (17); on the
other hand, a study showed (18)
that NAFLD is a result of IR-induced degeneration and necrosis of
hepatocytes. It is reported that non-alcoholic steatohepatitis is
found in liver biopsy of PCOS patients complicated with IR, and the
pathological features of liver tissues are also improved after
patients undergo life intervention therapy (19). Increased FINS and HOMA-IR are
important indexes of IR. In the present study, the related
pathogenic factors to NAFLD in PCOS patients were analyzed. Results
showed that compared with those in N-NAFLD patients, BMI, HOMA-IR
index, TG, TC, LDL and FAI levels were higher in NAFLD patients in
PCOS group, but HDL and SHBG levels were lower (P<0.05), which
is consistent with the conclusion in literature that obesity
(20), IR, high TC and low HDL
(21) are predisposing factors of
NAFLD. IR and androgen excess are two major pathological features
of PCOS. This study found that there was a positive correlation
between FBG level and androgen level in PCOS patients, and NAFLD
patients had more obvious disorders of glucose metabolism indexes
(high FBG, FINS and HOMA-IR index) than N-NAFLD patients in PCOS
group, and FAI was increased, but SHBG was decreased (P<0.05);
the above findings are similar to the results of Vassilatou et
al (22).
In conclusion, analyses of risk factors for PCOS
complicated with NAFLD show that HOMA-IR index, BMI, WHR and TG are
independent risk factors for PCOS complicated with NAFLD,
suggesting that blocking the high risk factors through weight
control, avoidance of excessive intake of high-fat diet,
improvement of IR, increase of insulin sensitivity, is an important
link to prevent the occurrence of NAFLD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the study and drafted the manuscript. JZ
and WW revised the manuscript critically for important intellectual
content. JH, CZ and WW acquired the data, analyzed and interpreted
statistical analysis. YJ and XK analyzed the data. All authors read
and approved the final manuscript and agree to be accountable for
all aspects of the study.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shengli Oilfield Central Hospital (Dongying, China). Patients who
participated in this research signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joham AE, Teede HJ, Ranasinha S, Zoungas S
and Boyle J: Prevalence of infertility and use of fertility
treatment in women with polycystic ovary syndrome: Data from a
large community-based cohort study. J Womens Health (Larchmt).
24:299–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunt EM: Nonalcoholic steatohepatitis:
Definition and pathology. Semin Liver Dis. 21:3–16. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kelley CE, Brown AJ, Diehl AM and Setji
TL: Review of nonalcoholic fatty liver disease in women with
polycystic ovary syndrome. World J Gastroenterol. 20:14172–14184.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gambarin-Gelwan M, Kinkhabwala SV, Schiano
TD, Bodian C, Yeh HC and Futterweit W: Prevalence of nonalcoholic
fatty liver disease in women with polycystic ovary syndrome. Clin
Gastroenterol Hepatol. 5:496–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brzozowska MM, Ostapowicz G and Weltman
MD: An association between non-alcoholic fatty liver disease and
polycystic ovarian syndrome. J Gastroenterol Hepatol. 24:243–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ortiz-Lopez C, Lomonaco R, Orsak B, Finch
J, Chang Z, Kochunov VG, Hardies J and Cusi K: Prevalence of
prediabetes and diabetes and metabolic profile of patients with
nonalcoholic fatty liver disease (NAFLD). Diabetes Care.
35:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teede HJ, Meyer C, Hutchison SK, Zoungas
S, McGrath BP and Moran LJ: Endothelial function and insulin
resistance in polycystic ovary syndrome: The effects of medical
therapy. Fertil Steril. 93:184–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kauffman RP, Baker TE, Baker V, Kauffman
MM and Castracane VD: Endocrine factors associated with
non-alcoholic fatty liver disease in women with polycystic ovary
syndrome: Do androgens play a role? Gynecol Endocrinol. 26:39–46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Setji TL, Holland ND, Sanders LL, Pereira
KC, Diehl AM and Brown AJ: Nonalcoholic steatohepatitis and
nonalcoholic Fatty liver disease in young women with polycystic
ovary syndrome. J Clin Endocrinol Metab. 91:1741–1747. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sorrentino P, Tarantino G, Conca P,
Perrella A, Terracciano ML, Vecchione R, Gargiulo G, Gennarelli N
and Lobello R: Silent non-alcoholic fatty liver disease-a
clinical-histological study. J Hepatol. 41:751–757. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omagari K, Kadokawa Y, Masuda J, Egawa I,
Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K, et al:
Fatty liver in non-alcoholic non-overweight Japanese adults:
Incidence and clinical characteristics. J Gastroenterol Hepatol.
17:1098–1105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia MF, Yan HM, He WY, Li XM, Li CL, Yao
XZ, Li RK, Zeng MS and Gao X: Standardized ultrasound hepatic/renal
ratio and hepatic attenuation rate to quantify liver fat content:
An improvement method. Obesity (Silver Spring). 20:444–452. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gramlich T, Kleiner DE, McCullough AJ,
Matteoni CA, Boparai N and Younossi ZM: Pathologic features
associated with fibrosis in nonalcoholic fatty liver disease. Hum
Pathol. 35:196–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menzaghi C, Trischitta V and Doria A:
Genetic influences of adiponectin on insulin resistance, type 2
diabetes, and cardiovascular disease. Diabetes. 56:1198–1209. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi JH, Rhee EJ, Bae JC, Park SE, Park
CY, Cho YK, Oh KW, Park SW and Lee WY: Increased risk of type 2
diabetes in subjects with both elevated liver enzymes and
ultrasonographically diagnosed nonalcoholic fatty liver disease: A
4-year longitudinal study. Arch Med Res. 44:115–120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marchesini G, Avagnina S, Barantani EG,
Ciccarone AM, Corica F, DallAglio E, Dalle Grave R, Morpurgo PS,
Tomasi F and Vitacolonna E: Aminotransferase and
gamma-glutamyltranspeptidase levels in obesity are associated with
insulin resistance and the metabolic syndrome. J Endocrinol Invest.
28:333–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown AJ, Tendler DA, McMurray RG and
Setji TL: Polycystic ovary syndrome and severe nonalcoholic
steatohepatitis: Beneficial effect of modest weight loss and
exercise on liver biopsy findings. Endocr Pract. 11:319–324. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee S, Jin Kim Y, Yong Jeon T, Hoi Kim H,
Woo Oh S, Park Y and Soo Kim S: Obesity is the only independent
factor associated with ultrasound-diagnosed non-alcoholic fatty
liver disease: A cross-sectional case-control study. Scand J
Gastroenterol. 41:566–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohashi K, Ouchi N, Kihara S, Funahashi T,
Nakamura T, Sumitsuji S, Kawamoto T, Matsumoto S, Nagaretani H,
Kumada M, et al: Adiponectin I164T mutation is associated with the
metabolic syndrome and coronary artery disease. J Am Coll Cardiol.
43:1195–1200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vassilatou E, Lafoyianni S, Vryonidou A,
Ioannidis D, Kosma L, Katsoulis K, Papavassiliou E and Tzavara I:
Increased androgen bioavailability is associated with non-alcoholic
fatty liver disease in women with polycystic ovary syndrome. Hum
Reprod. 25:212–220. 2010. View Article : Google Scholar : PubMed/NCBI
|