Introduction
Luting agents have been used in dentistry for over a
century. According to ISO, these materials are categorized as
water-based or polymer-based depending on their principal curing
mechanism (1). In the literature,
the term resin cement is often used to describe polymer-based
luting materials (2).
The use of self-etching, self-adhesive resin cements
has increased in recent years (3).
These materials combine an adhesive and a cement in a single
application, eliminating the need for pre-treatment of the tooth
(4). Due to their ease of use,
self-adhesive resin cements are commonly employed to adhere
restorative materials to teeth (5).
A key component of these cements is an acid methacrylate ester
consisting of a hydrophilic end group, an alkyl chain spacer 3–11
CH2 groups long and a polymerizable methacrylate group.
In order to promote adhesion between the luting agent and the tooth
substrate, carboxylic or phosphoric acid-functionalized monomers,
such as methacrylate monomers, are utilized to achieve enamel and
dentin demineralization (6). The
acidic groups bind to calcium in the hydroxyapatite of the
demineralized smear layer, creating a bond to the resin network
(7). The adhesion mechanism of these
materials is not based on hybrid layer or tag formation, as only a
partial smear layer with dentin demineralization or infiltration of
dentin is generally observed (8).
The concentration of acidic monomers serves a
crucial role in the curing process. The concentration must be high
enough to guarantee proper demineralization and bonding to dentin
and enamel, whilst also being low enough to avoid excessive
hydrophilicity in the cured material (9). As reported by Ferracane et al
(10), hydrophilicity due to low pH
in the cured material may compromise mechanical stability by
promoting excessive water adsorption.
The hydrophilic end group of resin cements is often
acidic, consisting of a phosphoric acid derivative such as a
phosphonate
(H2PO3+/HRPO3+)
or phosphate
(H2PO4−/HRPO4−)
group. These acidic groups simultaneously etch and infiltrate
enamel and dentin and are thought to bond chemically to
Ca2+ in the hydroxyapatite of the tooth and to
superficial oxides on the restorative material (11,12).
In the present study, the pH of three self-adhesive
resin cements was measured and the effect of phosphoric ester type
(phosphonate or phosphate) on bond strength was assessed. The null
hypothesis was that pH and acidic behavior of self-adhesive resin
cements would have no influence on the long-term mechanical
properties of bonds between human dentin and dental materials.
Materials and methods
Materials
The self-adhesive resin cements used were RelyX
Unicem (3M ESPE AG, St. Paul, MN, USA), Maxcem (Kerr-Hawe, Orange,
CA, USA), and Multilink Sprint (Ivoclar Vivadent, Schaan,
Liechtenstein). The materials and their compositions are described
in Table I.
 | Table I.Composition and pH of resin
cements. |
Table I.
Composition and pH of resin
cements.
| Cement | Composition
(%) | pH |
|---|
| RelyX Unicem | Methacrylated
phosphoric acid esters (15–25) TEGDMA (10–20) Fillers (55–75) | 3.78 |
| Maxcem | GPDM (20–35)
Bis-GMA, TEGDMA and fillers (67) | 1.78 |
| Multilink
Sprint | Dimethacrylate
(24–26) Methacrylated phosphoric acid ester (5) Fillers (71) | 3.42 |
Preparation of dentin specimens
The dentin specimens were obtained from 120
caries-free third human third molars within 3 months of extraction
between September 2014 and November 2015. All patients provided
prior written informed consent and the study protocol was approved
by the Ethics Committee at the First Hospital of Jilin University
(Changchu, China). The teeth were stored in an aqueous 1%
chloramine T solution (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 4°C until use. As previously described (13), the teeth were embedded in self-curing
acrylic resin (Plexil A6; Escil, Chassieu, France) in a cylindrical
mold (30 mm in diameter and 15 mm in height). Following
polymerization of the resin, the occlusal enamel each tooth was
removed perpendicular to the long axis of the tooth using a
low-speed diamond disk saw (IsoMet; Buehler, Lake Bluff, IL, USA).
Prior to bonding, the specimens were randomly divided into 12
groups of 10 teeth and stored in distilled water at 37°C.
The restorative materials included Ni-Cr-based alloy
(10% Cr, 4% Mo; Goodfellow, Lille, France), micro-filled veneering
composite resin (Adoro; Ivoclar-Vivadent, Schaan, Liechtenstein),
glass-ceramic (E-Max; Ivoclar-Vivadent), and sintered
Yttria-tetragonal zirconia polycrystalline ceramicY-TZP zirconium
ceramic; (HTI, Decines, France). The materials were appropriately
tooled, molded, cast or sintered to obtain cylindrical samples 5×5
mm. The bonding surfaces of each cylinder were ground using
800-grit SiC paper under running tap water T 16°C for 1–3 min
depending on the hardness of the materials. The composites and
alloy samples were then sandblasted using 50 µm
Al2O3 powder under 0.4 MPa pressure for 10
sec, while the zirconia and E-Max surfaces were kept in the
polished state. The experimental groups and treatments are listed
in Table II. Each sample was washed
with acetone prior to priming or bonding.
 | Table II.Experimental groups and treatment
protocols. |
Table II.
Experimental groups and treatment
protocols.
| Group | Substrate | Substrate
pretreatment | Resin cement | Curing mode |
|---|
| 1 | Ni-Cr |
Al2O3
sandblasting | Rely X Unicem | Auto-cured |
| 2 | Ni-Cr |
Al2O3
sandblasting | Maxcem | Auto-cured |
| 3 | Ni-Cr |
Al2O3
sandblasting | Multilink
Sprint | Auto-cured |
| 4 | Composite |
Al2O3
sandblasting | Rely X Unicem | Dual-cured |
| 5 | Composite |
Al2O3
sandblasting | Maxcem | Dual-cured |
| 6 | Composite |
Al2O3
sandblasting | Multilink
Sprint | Dual-cured |
| 7 | E-Max | #800 SiC | Rely X Unicem | Dual-cured |
| 8 | E-Max | #800 SiC | Maxcem | Dual-cured |
| 9 | E-Max | #800 SiC | Multilink
Sprint | Dual-cured |
| 10 |
ZrO2 | #800 SiC | Rely X Unicem | Dual-cured |
| 11 |
ZrO2 | #800 SiC | Maxcem | Dual-cured |
| 12 |
ZrO2 | #800 SiC | Multilink
Sprint | Dual-cured |
Bonding procedure and
measurements
The self-adhesive cements were mixed and directly
applied as a thin layer to the substrates and dentin surfaces. The
samples were positioned in a fixture to align the cylinders on each
dentin surface and subsequently fixed in position with screws
tightened to exert a pressure of ~20 N. Excess cement was carefully
removed. The luting cements of the composite, glass-ceramic and
zirconium groups were light-cured on opposite sides for 20 sec each
side. The cement joints of the alloy group were not
light-polymerized in order to simulate clinical conditions. Each
bonded assembly was maintained under constant pressure for 10 min
in the alignment device in air at room temperature. The specimens
were then stored in 100% relative humidity at 37°C for 1 day
(Fig 1). Bond strength was measured
in shear mode using a universal testing machine (LRX; JJ Lloyd
Instruments, Fareham, UK) at a crosshead speed of 0.5 mm/min. The
failure load in N was divided by the bond area in mm to obtain the
SBS in MPa. The results of the bond strength tests were analyzed
using the descriptive and inferential statistics of the
Kruskal-Wallis and Games-Howell tests. In order to compare the
average bond strength of substrates in various groups of luting
cements Kruskal-Wallis analysis was used. The Games-Howell post hoc
test was employed to characterize the average bond strengths of the
luting cements between substrate and dentin. P≤0.05 was considered
to indicate a statistically significant difference.
pH measurements
pH measurements were obtained using a pH meter (PHM
MeterLab 210; Hach Company, Loveland, CO, USA). Because the
adhesive formulations did not contain water, the contact surface of
the electrode was wetted with distilled water containing a neutral
pH buffer. The results of the pH measurements are located in
Table I.
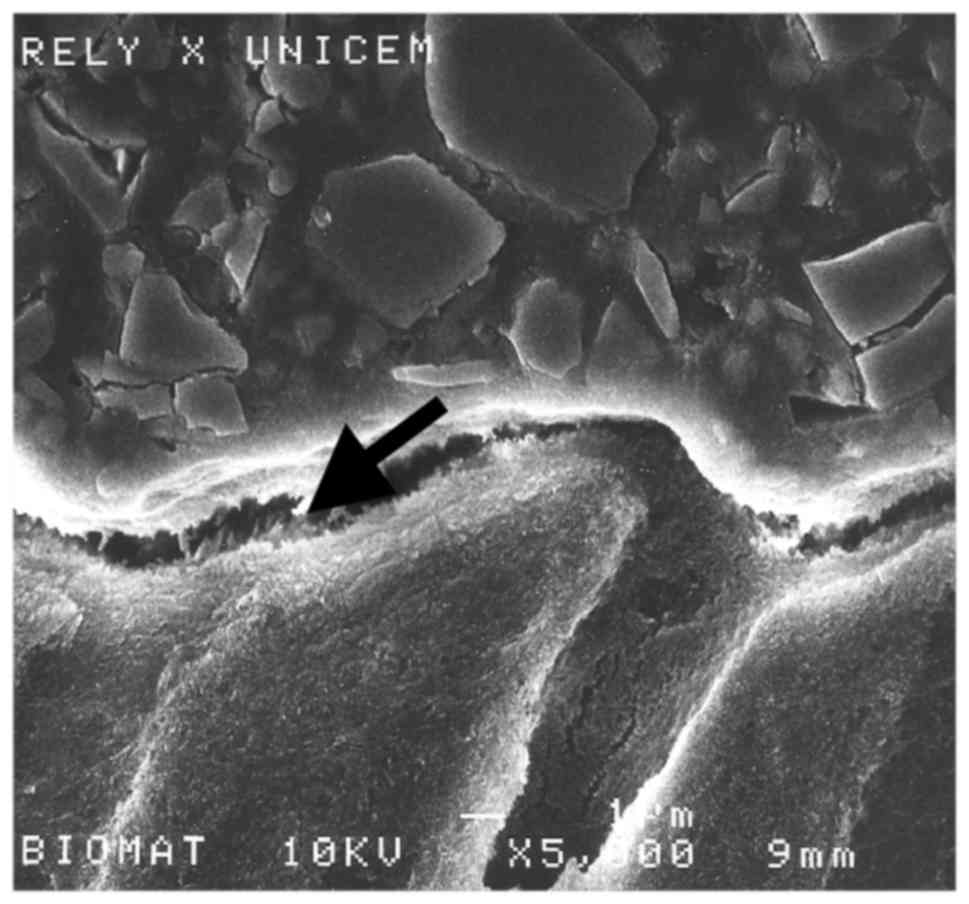
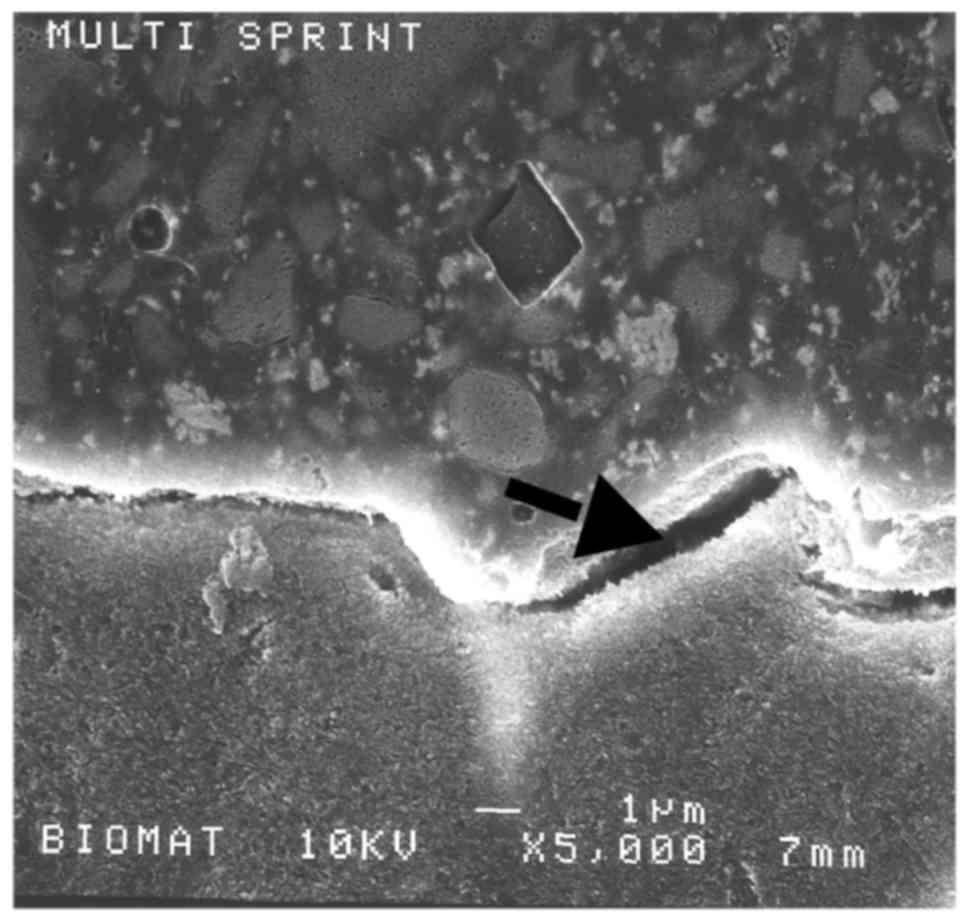
Scanning electron microscopy
Representative samples were sectioned perpendicular
to the bonded surface using successively finer diamond abrasives up
to 4,000-grit (Isomet; Buehler, Lake Bluff, IL, USA) under water.
The samples were coated with gold using an SC 500 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) coater and the
restorative/dentin interface was examined using a JSM 6400 scanning
electron microscope (Jeol, Tokyo, Japan) at 15–20 kV with a beam
intensity of 10−11 A (Figs.
2 and 3).
Results
Shear bond strength
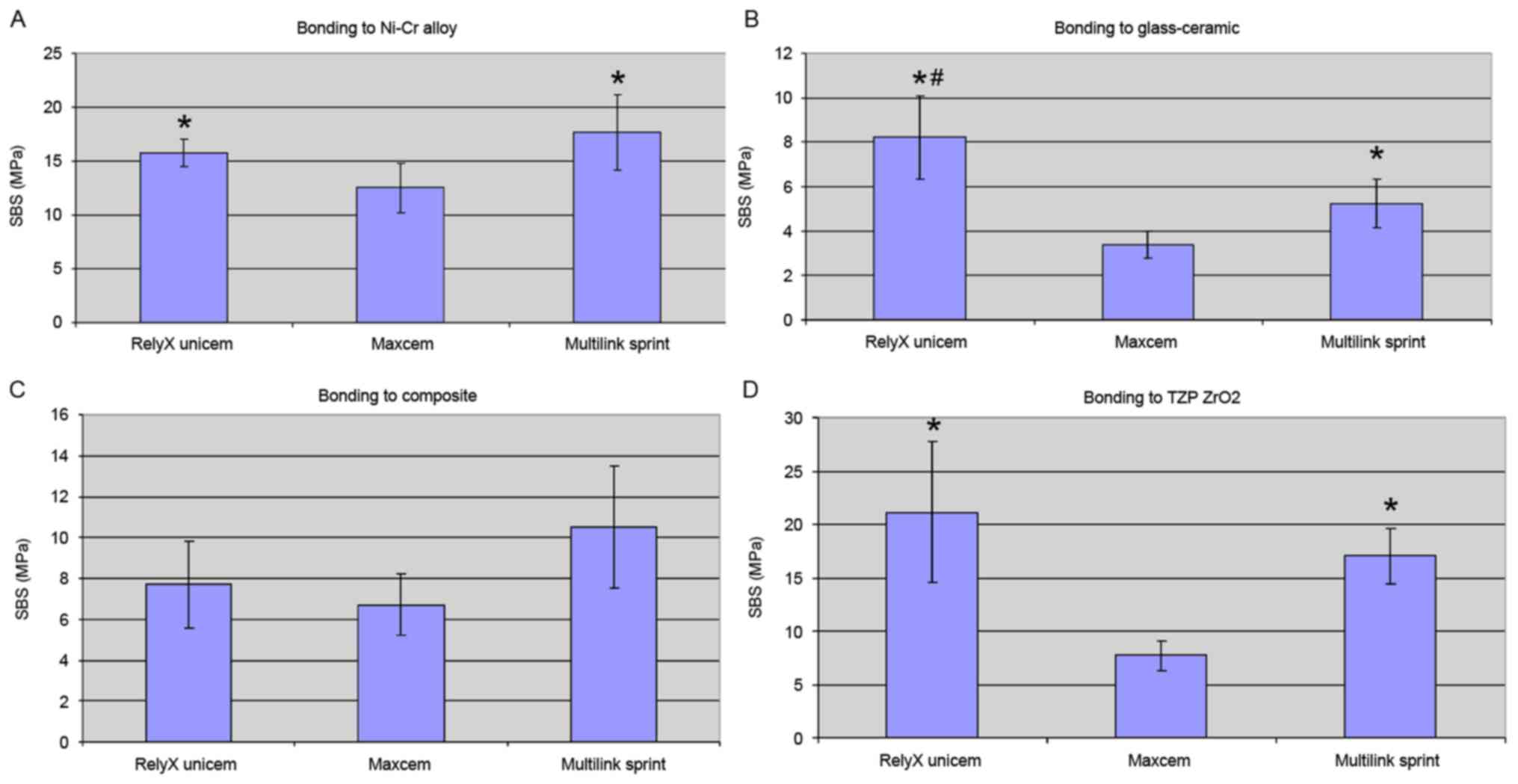
The bond strength test results are presented in
Table III and Fig. 4. Regardless of the restorative
material used, the weakest bonds occurred in samples prepared using
Maxcem (P<0.05).
 | Table III.Results of shear bond strength tests
(n=10). |
Table III.
Results of shear bond strength tests
(n=10).
|
| Ni-Cr alloy | Glass-ceramic | Composite | Y-TZP
ZrO2 |
|---|
|
|
|
|
|
|
|---|
| Substrates | SBS (MPa) | SD | SBS (MPa) | SD | SBS (MPa) | SD | SBS (MPa) | SD |
|---|
| RelyX Unicem | 15.75a | 1.3 | 8.21a,b | 1.9 | 7.7 | 2.1 | 21.11a | 6.6 |
| Maxcem | 12.52c | 2.3 | 3.37c | 0.6 | 6.7 | 1.5 | 7.76c | 1.4 |
| Multilink
Sprint | 17.63 | 3.5 | 5.23 | 1.1 | 10.5 | 3 | 17.01 | 2.6 |
Bonding to Ni-Cr alloy
Multilink Sprint and RelyX Unicem provided similar
bond strengths, and performed significantly better than Maxcem
(P<0.05; Fig. 4A).
Bonding to glass-ceramic
The mean bond strengths were relatively low
(3.4–10.4 MPa). Nevertheless, a significant difference was observed
between the cements, with RelyX Unicem (8.2 MPa) being stronger
than Multilink Sprint (5.2 MPa), which in turn was stronger than
Maxcem (3.4 MPa; P<0.05; Fig.
4B).
Bonding to composite
The highest bond strength occurred in samples
prepared using Multilink Sprint (10.5 MPa); however, no significant
difference was observed between this value and that of RelyX Unicem
(7.7 MPa; Fig. 4C). Maxcem (6.7 MPa)
had the lowest bond strength to composite. There was no significant
difference between the 3 cements (P>0.05).
Bonding to Y-TZP ZrO2
RelyX Unicem provided higher mean bond strengths
when bonding to Y-TZP ZrO2, however no significant
differences were observed when compared with the Multilink Sprint
samples (Fig. 4D). RelyX Unicem and
Multilink Sprint formed significantly stronger bonds to Y-TZP
ZrO2 compared with Maxcem (P<0.05; Fig. 4D).
Discussion
Substrate materials used in the present study
included Ni-Cr alloy, composite, glass-ceramic and Y-TZP
ZrO2. The glass-ceramic and Y-TZP ZrO2
surfaces were ground using 800-grit SiC paper; the Ni-Cr alloy and
composite surfaces were sandblasted using 50 µm alumina
(Al2O3). Prior to bonding, the surfaces were
thoroughly washed with acetone to eliminate
Al2O3 particles and other residues. Ni-Cr
alloy specimens abraded using 50 µm Al2O3
particles exhibited higher bond strength to composite resin
compared with samples abraded using 250 µm particles (13). Based on surface energy and topography
measurements of Ni-Cr alloy specimens treated using 4,000 grit SiC
paper, or sandblasted using 50 or 250 µm
Al2O3, sandblasting increases the polar
contributions to surface energy (particularly in the case of 50 µm
particles) and is able to promote acid-base interactions with
adhesives (13).
Debonding at the composite-adhesive interface is a
major problem for indirect composite restorations (14). Several studies have indicated that
composite surfaces treated by air-abrasion with
Al2O3 exhibit an increase in bond strength
between the indirect composite and resin cement (14,15). In
particular, air abrasion with 50 µm Al2O3 and
tribochemical silica followed by application of the bonding agent
has been reported to produce the highest repair shear bond strength
(16).
Due to its fracture toughness and chemical
durability, Y-TZP ZrO2 is one of the most commonly used
all-ceramic core materials (17).
Several techniques, including airborne particle abrasion with
alumina, have been reported to mechanically enhance the bond
strength between resin cement and Y-TZP ceramic (18–20).
Other researchers have reported that sandblasting induces
transformation of the tetragonal phase to monoclinic, resulting in
long-term catastrophic failure (21). The duration of treatment and the
particle size used during the abrasion process affect the roughness
and phase transformation of Y-TZP; longer treatment times with
larger particles may result in material degradation (22).
A recent study indicated that particle abrasion
results in matrix erosion and exposure of lithium disilicate
crystals in glass ceramic surfaces, whereas hydrofluoric acid
etching produced a microroughened surface (23). In the present study, Y-TZP
ZrO2 and glass ceramic samples were not sandblasted and
were polished using 800-grit SiC instead.
All of the self-adhesive resin cements used in the
present study contained some type of phosphoric ester-based
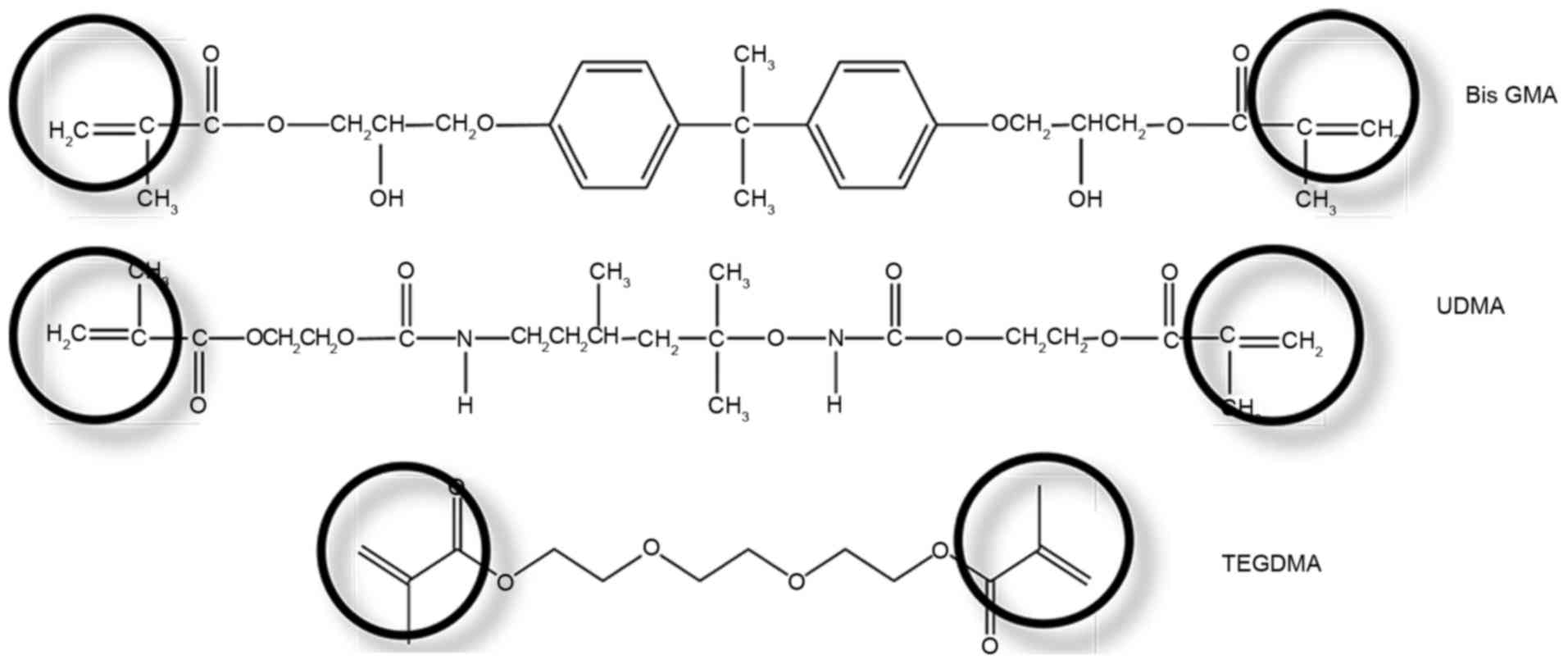
monomer. Multifunctional phosphoric acid methacrylate monomers
incorporate two setting reactions: A polymerizable group that is
able to react with other monomers, as well as the restorative
material, and an acid adhesive group capable of etching dental hard
tissues and interacting with the tooth substance (Fig. 5). In polymer-based self-adhesive
resin cements, ionization occurs in situ, utilizing water
associated with the dentin or produced during neutralization of the
phosphate monomers by basic filler materials (24). Bonding primarily occurs within an
interfacial layer incorporating partially dissolved smear particles
(25) and possibly involves local
formation of a nanohybrid layer (26).
Regardless of the restorative substrate, the weakest
bonds observed in the present study were in samples prepared using
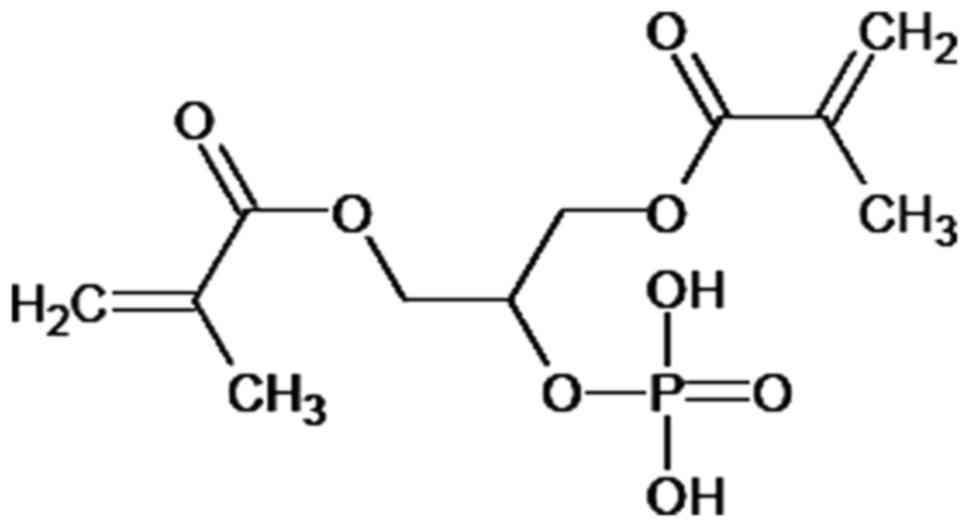
Maxcem. The principal component of Maxcem is glycerol
dimethacrylate dihydrogen phosphate (Fig. 6), a glycerol dimethacrylate ester of
phosphoric acid that was one of the first dental materials
specifically employed to improve bonding to dentin (27).
The significant differences in bond strength
observed between samples prepared using RelyX Unicem and Multilink
Sprint, which contain phosphonate groups, and those prepared using
Maxcem, which contains phosphate groups, may be attributable to the
composition of the adhesive monomer. Bonding occurs between the Ca
ions associated with the hydroxyapatite component of dentin and the
ionized phosphoric acid groups of the monomer mixture, creating
Ca3(P5+O4)2 in
phosphate-based cements and CaHO3P3+ in
phosphonate-based cements.
In the case of acidic methacrylate phosphates, an
additional instability results from hydrolysis of the methacrylate
ester bond in the presence of water, which is frequently used as a
co-solvent in self-etching adhesives (28). Hydrolysis is catalyzed by the
hydrogen ions of the phosphoric acid group (29). The phosphoric acid ester bonds in
diesters are less hydrolytically stable than monoesters under
acidic conditions, including the low pH environment in resin
cements (30). Maxcem exhibits a
relatively low bonding ability, irrespective of the tooth substrate
(31).
Cross-linking dimethacrylates are used in
enamel-dentin adhesives to generate a polymer network, which
provides a number of favorable effects. Firstly, the polymerization
rate increases due to the gel effect. Secondly, the mechanical
properties of a polymer network are improved compared to linear
polymers (32). Finally, the
cross-linked material is less water soluble and the degree of
swelling decreases with increasing polymer network density
(33).
A recent study reported that RelyX Unicem is unable
to completely dissolve the smear layer. The remaining layer
represents a weak zone in the dentin-adhesive interface (7) and explains why the bond strengths
achieved using self-adhesive cements are limited regardless of the
associated restorative materials. Other researchers have observed
that the conventional dual-cured luting cements Multilink Sprint
and Multilink Automix provide higher bond strengths compared with
self-adhesive resin cements (13).
As resin cements do not contain water, these
materials must be applied to slightly wet surfaces for proper
adhesion. The pH values of RelyX Unicem, Maxcem and Multilink
Sprintare were 3.78, 1.78 and 3.42, respectively. Conventional
etching-rinse adhesives employ 37% phosphoric acid, which has a pH
of ~0.7 (34). The acidity of these
self-adhesive resin cements is insufficient to achieve optimal
demineralization and infiltration of dentin. As reported by
Ferracane et al (10), a
hydrophilic character and low pH in the cured material may
compromise mechanical stability by promoting excessive water
adsorption. Some of the differences observed in the pH measurements
may be a result of the testing method, as the outcome of pH
measurements is dependent on the experimental configuration
(2).
In the present study, the surfaces of the zirconia
ceramic samples were polished and cleaned prior to bonding. Bonding
to flat and smooth surfaces enables a more accurate assessment of
the chemical aspects of adhesion. For the bonding of conventional
glass ceramics, pretreatment with hydrofluoric acid etching or
silanization enhances the resin bond (35). Unlike glass ceramics, zirconia is not
susceptible to hydrofluoric acid etching due to its polycrystalline
and glass-free nature. However, the phosphoric acid methacrylate
monomers of RelyX Unicem have been reported to be particularly
effective on zirconia (36).
Nevertheless, in the Ni-Cr alloy samples, the phosphonate groups of
Multilink Sprint were more effective than the phosphonate groups of
RelyX Unicem. Depending on the nature of the multifunctional
monomer they contain, self-adhesive resin cements are potentially
capable of specific adhesion to selected restorative
substrates.
Some limitations of the present study require
consideration. Notably, only phosphoric acid derivatives, including
phosphonates
(H2PO3+/HRPO3+)
and phosphates
(H2PO4−/HRPO4−)
were studied. Furthermore, some of the differences observed in the
pH measurements may be due to an artifact of the testing method. In
future research, more hydrolytically stable esters, including
carbamides, could be studied (37),
as well as the effectiveness in improving the bond strength by
increasing the length of the acidic monomer spacer group.
In conclusion, the performance of an adhesive
material depends on the chemical structure of the functional
monomer. The pH of acidic functional monomers containing
phosphonate or phosphate groups influences the bond strength
between dentin and restorative materials. Further studies are
warranted to investigate whether modifying the acidic monomer or
changing the monomer spacer length may improve the bond
strength.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WL, ZS and RJ performed the study. HM performed the
data analyses. CAD interpreted the data. CZ conceived the study.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Hospital of Jilin University (Changchun,
China) and was performed in accordance with the Declaration of
Helsinki of the World Medical Association. All enrolled patients
provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pameijer CH: A review of luting agents.
Int J Dent. 2012:7528612012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zorzin J, Petschelt A, Ebert J and
Lohbauer U: pH neutralization and influence on mechanical strength
in self-adhesive resin luting agents. Dent Mater. 28:672–679. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petropoulou A, Vrochari AD, Hellwig E,
Stampf S and Polydorou O: Water sorption and water solubility of
self-etching and self-adhesive resin cements. J Prosthet Dent.
114:674–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radovic I, Monticelli F, Goracci C,
Vulicevic ZR and Ferrari M: Self-adhesive resin cements: A
literature review. J Adhes Dent. 10:251–258. 2008.PubMed/NCBI
|
|
5
|
Piwowarczyk A, Bender R, Ottl P and Lauer
HC: Long-term bond between dual-polymerizing cementing agents and
human hard dental tissue. Dent Mater. 23:211–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ikemura K, Kadoma Y and Endo T: A review
of the developments of self-etching primers and adhesives -effects
of acidic adhesive monomers and polymerization initiators on
bonding to ground, smear layer-covered teeth. Dent Mater J.
30:769–789. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Assaf K, Chakmakchi M, Palaghias G,
Karanika-Kouma A and Eliades G: Interfacial characteristics of
adhesive luting resins and composites with dentine. Dent Mater.
23:829–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monticelli F, Osorio R, Mazzitelli C,
Ferrari M and Toledano M: Limited decalcification/diffusion of
self-adhesive cements into dentin. J Dent Res. 87:974–979. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zorzin J, Petschelt A, Ebert J and
Lohbauer U: pH neutralization and influence on mechanical strength
in self-adhesive resin luting agents. Dent Mater. 28:672–679. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferracane JL, Stansbury JW and Burke FJ:
Self-adhesive resin cements - chemistry, properties and clinical
considerations. J Oral Rehabil. 38:295–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vrochari AD, Eliades G, Hellwig E and
Wrbas KT: Curing efficiency of four self-etching, self-adhesive
resin cements. Dent Mater. 25:1104–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerth HU, Dammaschke T, Züchner H and
Schäfer E: Chemical analysis and bonding reaction of RelyX Unicem
and Bifix composites-a comparative study. Dent Mater. 22:934–941.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C and Degrange M: Shear bond
strengths of self-adhesive luting resins fixing dentine to
different restorative materials. J Biomater Sci Polym Ed.
21:593–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirmali O, Barutcugil C, Harorli O, Kapdan
A and Er K: Resin cement to indirect composite resin bonding:
Effect of various surface treatments. Scanning. 37:89–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Spitznagel FA, Horvath SD, Guess PC and
Blatz MB: Resin bond to indirect composite and new ceramic/polymer
materials: A review of the literature. J Esthet Restor Dent.
26:382–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho SD, Rajitrangson P, Matis BA and Platt
JA: Effect of Er, Cr:YSGG laser, air abrasion, and silane
application on repaired shear bond strength of composites. Oper
Dent. 38:E1–E9. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piconi C and Maccauro G: Zirconia as a
ceramic biomaterial. Biomaterials. 20:1–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozcan M and Vallittu PK: Effect of surface
conditioning methods on the bond strength of luting cement to
ceramics. Dent Mater. 19:725–731. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsukakoshi M and Shinya A, Gomi H, Lassila
LV, Vallittu PK and Shinya A: Effects of dental adhesive cement and
surface treatment on bond strength and leakage of zirconium oxide
ceramics. Dent Mater J. 27:159–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blatz MB, Chiche G, Holst S and Sadan A:
Influence of surface treatment and simulated aging on bond
strengths of luting agents to zirconia. Quintessence Int.
38:745–753. 2007.PubMed/NCBI
|
|
21
|
Denry IL and Holloway JA: Microstructural
and crystallographic surface changes after grinding zirconia-based
dental ceramics. J Biomed Mater Res B Appl Biomater. 76:440–448.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turp V, Sen D, Tuncelli B, Goller G and
Özcan M: Evaluation of air-particle abrasion of Y-TZP with
different particles using microstructural analysis. Aust Dent J.
58:183–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aboushelib MN and Sleem D: Microtensile
bond strength of lithium disilicate ceramics to resin adhesives. J
Adhes Dent. 16:547–552. 2014.PubMed/NCBI
|
|
24
|
Catel Y, Besse V, Zulauf A, Marchatc D,
Pfunda E, Phama TN, Bernache-Assolantc D, Degrangeb M, Lequeuxa T,
Madeca PJ and Pluart LL: Synthesis and evaluation of new
phosphonic, bisphosphonic and difluoromethylphosphonic acid
monomers for dental application. Eur Polym J. 48:318–330. 2012.
View Article : Google Scholar
|
|
25
|
Pedreira AP, Pegoraro LF, de Góes MF,
Pegoraro TA and Carvalho RM: Microhardness of resin cements in the
intraradicular environment: Effects of water storage and softening
treament. Dent Mater. 25:868–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Landuyt KL, Snauwaert J, De Munck J,
Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts
P and Van Meerbeek B: Systematic review of the chemical composition
of contemporary dental adhesives. Biomaterials. 28:3757–3785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stona P, Borges GA, Montes MA, Júnior LH,
Weber JB and Spohr AM: Effect of polyacrylic acid on the interface
and bond strength of self-adhesive resin cements to dentin. J Adhes
Dent. 15:221–227. 2013.PubMed/NCBI
|
|
28
|
Yoshihara K, Nagaoka N, Okihara T,
Kuroboshi M, Hayakawa S, Maruo Y, Nishigawa G, De Munck J, Yoshida
Y and Van Meerbeek B: Functional monomer impurity affects adhesive
performance. Dent Mater. 31:1493–1501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moszner N, Zeuner F, Angermann J, Fischer
UK and Rheinberger V: Monomers for adhesive polymers, 4. Macromol
Mater Eng. 288:621–628. 2003. View Article : Google Scholar
|
|
30
|
Cal E, Türkün LS, Türkün M, Toman M and
Toksavul S: Effect of an antibacterial adhesive on the bond
strength of three different luting resin composites. J Dent.
34:372–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goracci C, Cury AH, Cantoro A, Papacchini
F, Tay FR and Ferrari M: Microtensile bond strength and interfacial
properties of self-etching and self-adhesive resin cements used to
lute composite onlays under different seating forces. J Adhes Dent.
8:327–335. 2006.PubMed/NCBI
|
|
32
|
Yamasaki LC, De Vito Moraes AG, Barros M,
Lewis S, Francci C, Stansbury JW and Pfeifer CS: Polymerization
development of ‘low-shrink’ resin composites: Reaction kinetics,
polymerization stress and quality of network. Dent Mater.
29:e169–e179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moszner N, Salz U and Zimmermann J:
Chemical aspects of self-etching enamel-dentin adhesives: A
systematic review. Dent Mater. 21:895–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peumans M, De Munck J, Mine A and Van
Meerbeek B: Clinical effectiveness of contemporary adhesives for
the restoration of non-carious cervical lesions. A systematic
review. Dent Mater. 30:1089–1103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oyagüe RC, Monticelli F, Toledano M,
Osorio E, Ferrari M and Osorio R: Effect of water aging on
microtensile bond strength of dual-cured resin cements to
pre-treated sintered zirconium-oxide ceramics. Dent Mater.
25:392–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kilambi H, Beckel ER, Berchtold KA,
Stansbury JW and Bowman CN: Influence of molecular dipole on
monoacrylate monomer reactivity. Polymer. 46:4735–4742. 2005.
View Article : Google Scholar
|
|
37
|
Cakir FY, Korkmaz Y, Firat E, Oztas SS and
Gurgan S: Chemical analysis of enamel and dentin following the
application of three different at-home bleaching systems. Oper
Dent. 36:529–536. 2011. View Article : Google Scholar : PubMed/NCBI
|