Introduction
Spontaneous intracerebral hemorrhage (ICH) is a
particularly devastating cerebral vascular disease with a high
mortality rate. Complications, including enlarged hematoma volumes,
edema exacerbation and secondary brain injury, often develop
following ICH. Craniotomy to clear blood clots, antioxidants,
antithrombin, neutrophil infiltration inhibitors and heme oxygenase
inhibitors are used to treat ICH (1–5).
However, there are still few effective methods able to prevent
secondary brain injury following ICH.
Resveratrol (RESV) is a natural, non-flavonoid,
polyphenol compound found in grapes and other berries that can
induce pleiotropic effects in vertebrates (6–9). It has
been demonstrated that resveratrol exhibits a neuroprotective
function, as it ameliorates kainate-induced excitotoxicity
(10). Previous studies have
revealed that RESV improves neurological functions in various
diseases of the central nervous system, including cerebral
ischemia/reperfusion (11), acute
and secondary spinal injury (12),
neurodegenerative diseases (13) and
depression (14). It has been
suggested that the protective effects of RESV may be mediated
through the sirtuin 1, adenosine 5′-phosphate-activated kinase and
nuclear factor erythroid 2-related factor pathways (15–17).
The autologous blood perfusion model is used in
physiological, pathomorphological and therapeutic research into
ICH, as it mimics hypertensive cerebral hemorrhage (18). Therefore the present study
investigated the neuroprotective function of RESV using autologous
blood perfusion in a rat model of ICH. Rat motor disturbance and
neural damage/inflammation were assessed using behavioral tests and
immunohistochemistry, respectively.
Materials and methods
Animals
A total of 30 male Sprague-Dawley rats, weighing
300–350 g and aged 55 days, were purchased from the Guangdong
Medical Laboratory Animal Center (Guangzhou, China). Rats were
cared for in accordance with the Guideline for the Care and Use of
Laboratory Animals published by the National Institutes of Health
(NIH Publications no. 8023, revised 1978) (19). Rats were housed conventionally (room
temperature, 20±2°C; humidity, 55%; 15 air changes per h and a 12-h
light-dark cycle) in polycarbonate cages on hardwood bedding and
acclimatized for at least 7 days prior to study initiation. Rats
were provided with tap water and rodent chow ad libitum. All
experimental procedures were approved by the Animal Care and Use
Committee of Peking University Shenzhen Graduate School (Shenzhen,
China).
Establishing a hypertensive cerebral
hemorrhage rat model
Rats were randomly divided into 3 groups: A
sham+dimethyl sulphoxide (DMSO) group, an ICH+DMSO group and an
ICH+RESV group (all n=10). ICH was induced using the autologous
blood perfusion model. Rats were anesthetized with 1% sodium
pentobarbital (60 mg/kg, intraperitoneally; Shanghai Longsheng
Chemical Co., Ltd., Shanghai, China) and were immobilized in a
stereotactic apparatus frame (RWD Life Science Co., Ltd., Shenzhen,
China). A 1-mm bur hole was punctured into rat skulls (1 mm
anterior and 3 mm lateral to bregma). Fresh blood (100 µl) was
drawn from rat caudal arteries using a microsyringe and was
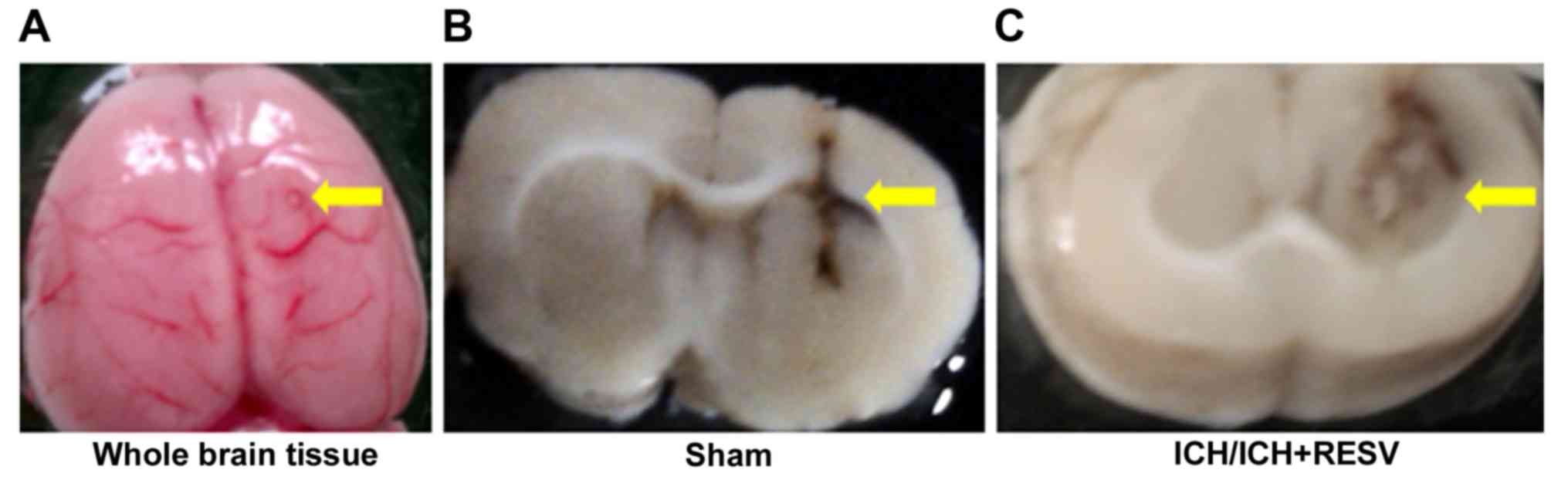
injected into the caudate putamen (5.5 mm deep to bregma; Fig. 1A). Autologous blood was infused over
10 min using a microinfusion pump (RWD Life Science Co., Ltd.). The
needle was slowly withdrawn 40 min following injection to prevent
the backflow of infused blood and to allow for hematoma formation.
The burr hole was then sealed with bone wax and the wound was
sutured. The needle of an empty syringe was inserted into rats in
the sham group. Rat body temperature was maintained at 36±0.5°C
using a feedback-controlled heating pad. Following the cessation of
anesthesia, rats in the ICH+RESV group were treated with 100 mg/kg
RESV in 5% DMSO (15 mg/ml), administered intraperitoneally once per
day. Sham and ICH groups were administered with the same volume of
5% DMSO once per day for 14 days. All rats were sacrificed for
histopathological staining following competition of all
neurological behavior tests. Brains were subsequently removed from
the skull and fixed in 10% neutral formalin buffer (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at room temperature for 24 h. Brain
damage following surgery to induce ICH was confirmed using 2–3
mm-thick horizontal sections (Fig. 1B
and C).
Rotarod test
Rats underwent a rotarod test on the ZB-200 (Chengdu
Techman Software Co., Ltd., Chengdu, China) at a velocity of 5 rpm,
which was subsequently increased to 60 rpm over 55 sec. All rats
were trained 20 times with a 20 sec interval. The mean duration of
the 20 trials was recorded to assess the daily performance of rats.
Rats were trained for 8 days prior to ICH surgery. The performance
of rats on the final 3 days was recorded and the mean duration was
used as a baseline value. The recovery of motor impairment was
examined 3, 7 and 14 days following ICH surgery.
Open field test
Rats were exposed to a circular arena (100×40 cm)
constructed from black plywood and the floor was divided into 25
sections. Rats were individually placed in the center of the
apparatus and their behavior was recorded for 10 min using a
digital camera situated above their head. Tests were performed 1, 7
and 14 days following surgery. The total distance travelled,
average speed, number of total rotations and line crossings were
analyzed using ANY Maze software (version 4.84; Stoelting Co., Wood
Dale, IL, USA).
Tissue processing
Rats were euthanized 14 days following surgery with
an intraperitoneal injection of 120–150 mg/kg sodium pentobarbital
and then were perfused intracardially with 4% paraformaldehyde in
PBS. Brains were removed and fixed in 10% neutral formalin buffer
at room temperature for 24 h. Following dehydration with graded
ethanol and xylene, brains were embedded in paraffin wax. A rotary
mictrotome (RM2255; Leica Microsystems GmbH, Wetzlar, Germany) was
utilized to cut 5-µm tissue sections, which were subsequently
stored at 4°C.
Nissl staining
To quantify brain injuries induced via ICH, Nissl
staining was performed following the manufacturer's protocol
(Beyotime Institute of Biotechnology, Shanghai, China).
Paraffin-embedded sections were dewaxed, rehydrated and stained
with Cresyl violet (C0117; Beyotime Institute of Biotechnology).
Images were captured using an Olympus fluorescence microscope
(CKX41; Olympus Corporation, Tokyo, Japan) at a magnification of
×400 or ×100 and a cooled charge-coupled camera (QICAM 12-bit), and
were processed using the QCapture Pro 6.0 program (both QI imaging,
Surrey, Canada). To assess whether the number of living neurons
differed significantly between the groups, cells in three
independent microscopic fields were examined. The mean ratio of
normal neurons was calculated from three independent counts and
plotted with error bars representing standard deviation. Neurons
with round and pale staining nuclei were regarded as surviving,
while shrunken neurons with condensed nuclei were regarded as
damaged (20).
Immunostaining
To determine the anti-inflammatory effects of RESV,
immunofluorescence with anti-ionized calcium binding adaptor
molecule 1 (Iba-1) antibodies was utilized to assess microglial
activation in the cortex as previously described (21). Slides were deparaffinized, rehydrated
(100, 90, 80, 70 and 50% ethanol, and ddH2O) and
immersed in 3% H2O2/methanol for 10 min at
room temperature to inactivate endogenous peroxidase. Antigens were
heat-retrieved in sodium citrate buffer (10 mM sodium citrate;
0.05% Tween-20; pH 6.0) at 100°C for 8 min. Following blocking in
3% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 20 min at
room temperature, tissues were incubated with rabbit anti-Iba-1
antibodies (cat. no. 019-19741; 1:500; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) overnight at 4°C. Sections were
washed in PBS and incubated with a fluorescein
isothiocyanate-conjugated secondary goat anti-rabbit Immunoglobulin
G antibody (cat. no. 111-095-003; 1:50; Jackson ImmunoResearch
Europe, Ltd., Newmarket, UK) at room temperature for 1 h.
Subsequently, sections were mounted in mowiol mounting medium
containing 1 µg/ml DAPI for DNA staining. Images were captured
using a fluorescence microscope at a magnification of ×400 or ×100
and a cooled charge-coupled camera, and processed using the
QCapture Pro 6.0 software. The images were analyzed using ImageJ
1.42q software (32-bit; National Institutes of Health, Bethesda,
MD, USA). To quantify neuroinflammation, cortex Iba-1 positive
cells were counted and compared between different groups.
Statistical analysis
The results are presented as the mean ± standard
deviation and all experimental data were analyzed using Prism 5.0
Software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical
differences among sham, ICH and ICH+RESV groups were assessed using
one-way analysis of variance followed by the Tukey's test for the
comparison of multiple groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
RESV improves motor ability following
ICH
To investigate the neuroprotective effects of RESV
following ICH, the recovery of rat motor abilities were assessed
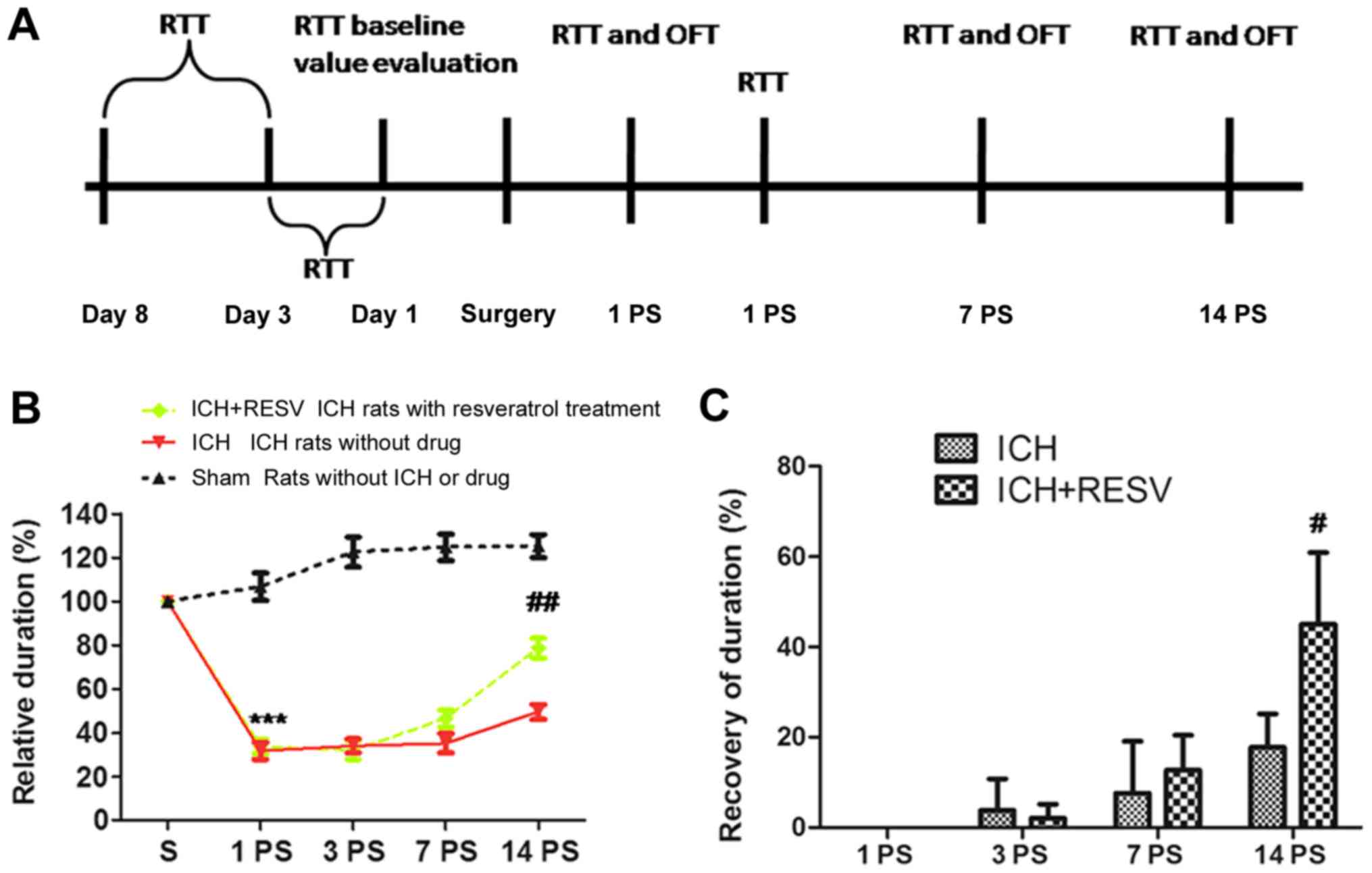
following ICH (Fig. 2A).
Rotarod tests
The time spent on the rod during the rotarod test
was markedly decreased 1 day post-surgery in the ICH and ICH+RESV
groups, compared with their performance prior to surgery (Fig. 2B). No significant differences in
performance were identified between the ICH and ICH+RESV groups on
day 1. A recovery was observed 7 and 14 days post-surgery in the
ICH+RESV and ICH groups (Fig. 2B).
Sham rats exhibited a small increase in relative duration 1 day
post-surgery but subsequently remained steady between 3 and 14 days
post-surgery. Although the ICH and ICH+RESV groups exhibited a
significant recovery 14 days post-surgery, the ICH+RESV group
exhibited a significant increase in relative duration compared with
the ICH group (P<0.05; Fig. 2C).
The results indicate that RESV accelerates the recovery of rat
motor abilities following ICH.
Open field test
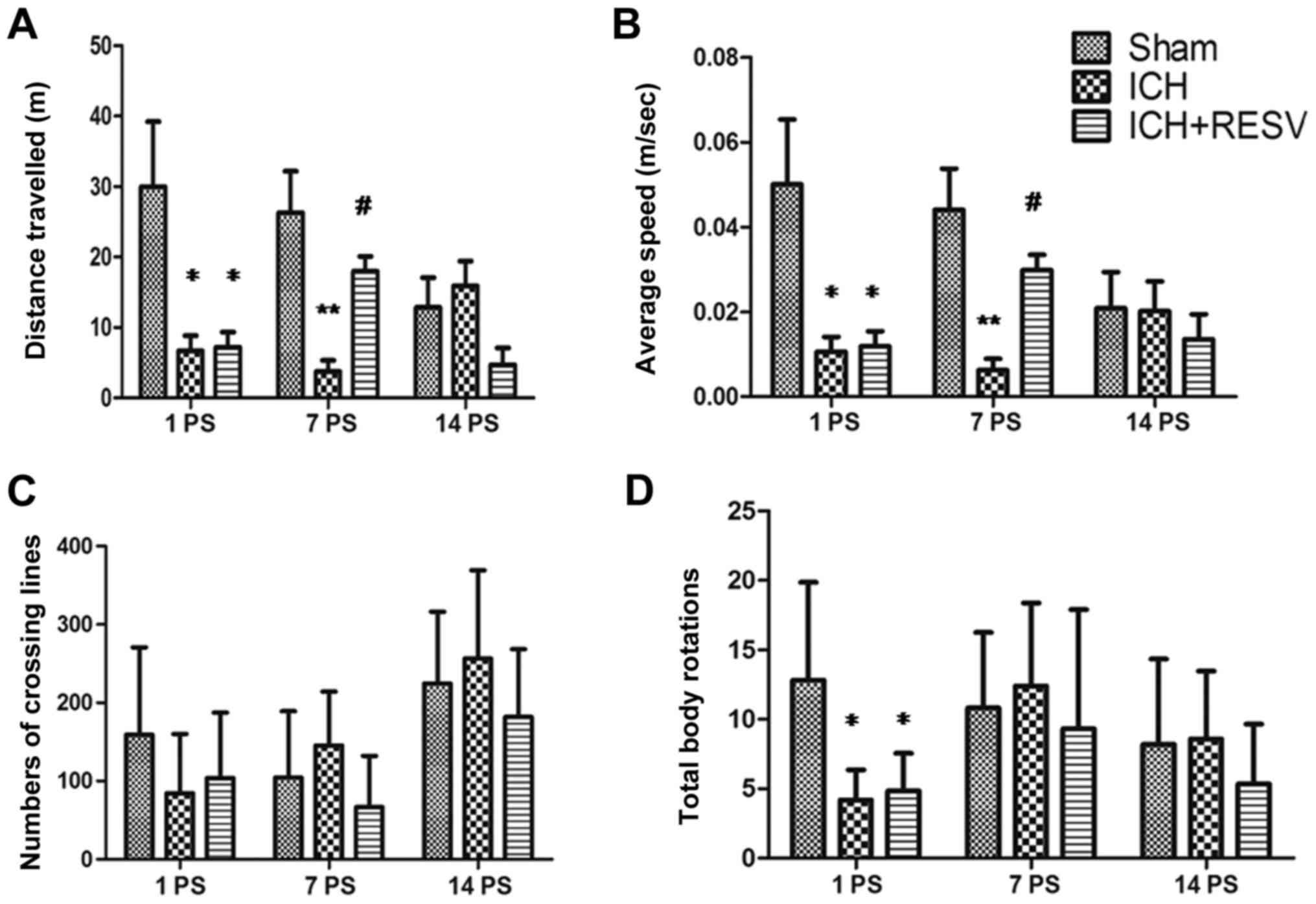
Parameters, including the total distance traveled
(Fig. 3A), average speed (Fig. 3B), number of lines crossed (Fig. 3C) and total body rotations (Fig. 3D) were evaluated in an open field
test 1, 7 and 14 days following ICH and RESV treatment. Compared
with the sham group, the distance traveled, average speed and total
body rotations were significantly decreased 1 day post-surgery in
the ICH and ICH+RESV groups (all P<0.05; Fig. 3A, B and D). Rats in the ICH group
travelled shorter distances and moved at slower speeds than the
sham group 1–7 days post-surgery (P<0.05; Fig 3A and B). No significant differences
between the ICH and ICH+RESV groups were identified in any of the
tests conducted 1 day post-surgery. In addition, no significant
differences in the total number of lines crossed were identified
among any of the groups (Fig. 1C).
These data indicate that the motor ability of rats is significantly
decreased following ICH.
Following RESV administration for 7 days
post-surgery, the total distance travelled and average speed of
rats in the ICH+RESV group were significantly increased compared
with ICH rats (P<0.05; Fig. 3A and
B). However, no significant differences between these groups
were identified in the number of lines crossed and total body
rotations. These results indicated that the motor abilities of rats
had partially recovered following RESV treatment. However, no
significant differences were identified in any groups 14 days
post-surgery. This may have been due to the habituation of rats to
the experimental environment following test repetition. These data
demonstrate that RESV treatment may improve the motor abilities of
rats following ICH.
RESV alleviates damage to neurons in
the hippocampus
Nissl staining was performed to evaluate the
morphological changes of neurons in the hippocampus following ICH.
Integrated bluish-violet neuronal cells with an articulate
structure of mottled nuclei and cytoplasm were observed in the sham
group, while condensed and irregular cytons of injured neurons,
combined with few normal neurons, were identified in the ICH groups
(Fig. 4A). The number of normal
neurons significantly decreased following ICH surgery compared with
the sham group (P<0.001; Fig.
4B). Following RESV treatment, the number of normal neurons
significantly increased (P<0.001; Fig. 4B). These results suggest that RESV
treatment alleviates the neuronal damage induced by ICH.
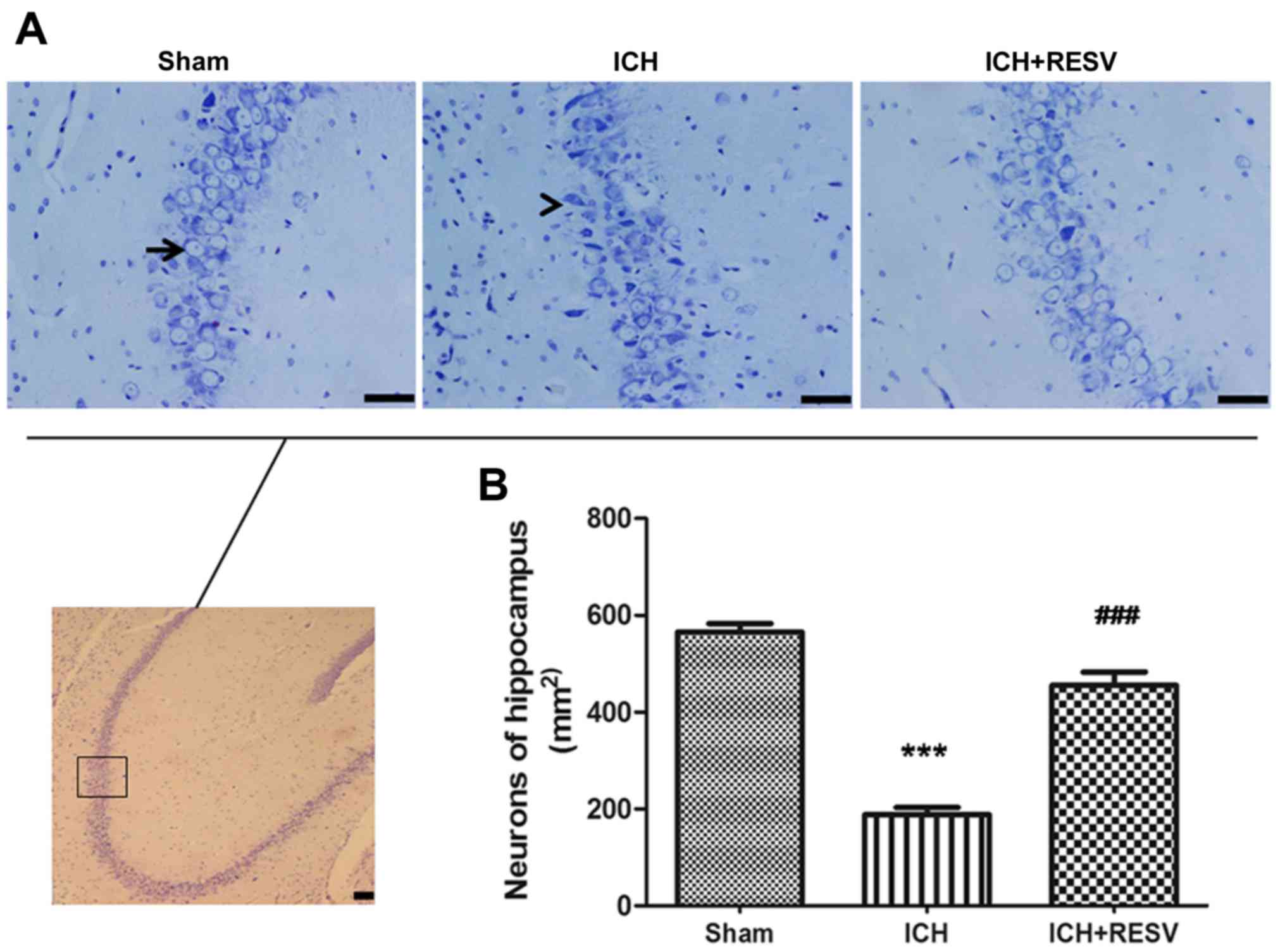 | Figure 4.RESV alleviated damage to neurons in
the hippocampus region. (A) Neurons in the hippocampus were stained
with Nissl 14 days following ICH and microscopic images were
captured; magnification, ×400. The image in the black square of
lower panel is in the same region as the images in the upper three
panels; magnification, ×100. The image in the lower panel is an
example image of the area. Blue neuronal cells with an articulate
structure, exhibiting mottled nuclei and cytoplasm, were detected
in the control group (black arrow), while injured neurons with
condensed and irregular cytons, alongside surviving neurons were
identified in the ICH group (black arrowhead). (B) Quantitative
analysis of neuronal cells. The number of neurons was decreased in
the ICH group, compared with the sham group. The survival rate of
neurons in the ICH+RESV group was greater compared with the ICH
group. Scale bar, 50 µm. Data are presented as the mean ± standard
deviation. ***P<0.001 vs. sham group, ###P<0.001
vs. ICH group. RESV, resveratrol; ICH, intracerebral
hemorrhage. |
Neuroinflammatory responses are
ameliorated by RESV following ICH
Microglia are the resident macrophages of the
central nervous system and are the primary form of active immune
defense available (22). Following
activation by neuroinflammation, the overall size and soma of
microglia increase and the thickness of axons increases. Iba-1, a
biomarker of microglia, stains microglia green color and revealed
that the neurons comprise of a soma and several axons (23). Furthermore, Iba-1 identifies the
disperse axons involved in nerve conduction (24). The morphological and proliferative
changes of microglia following ICH were determined using Iba-1 to
assess whether the neuroprotective effects of RESV were mediated by
the anti-inflammatory response of glial cells. Iba-1-stained
microglia were activated by the formation of an intracerebral
hematoma following ICH (Fig. 5A).
The number of microglia and length of microglial axons were
significantly increased following ICH (P<0.01; Fig. 5B). However, the administration of
RESV post-surgery significantly reversed the upregulation of ICH
induced Iba-1 (P<0.05; Fig. 5B).
These data indicate that the neuroinflammatory response induced by
ICH is ameliorated following RESV treatment.
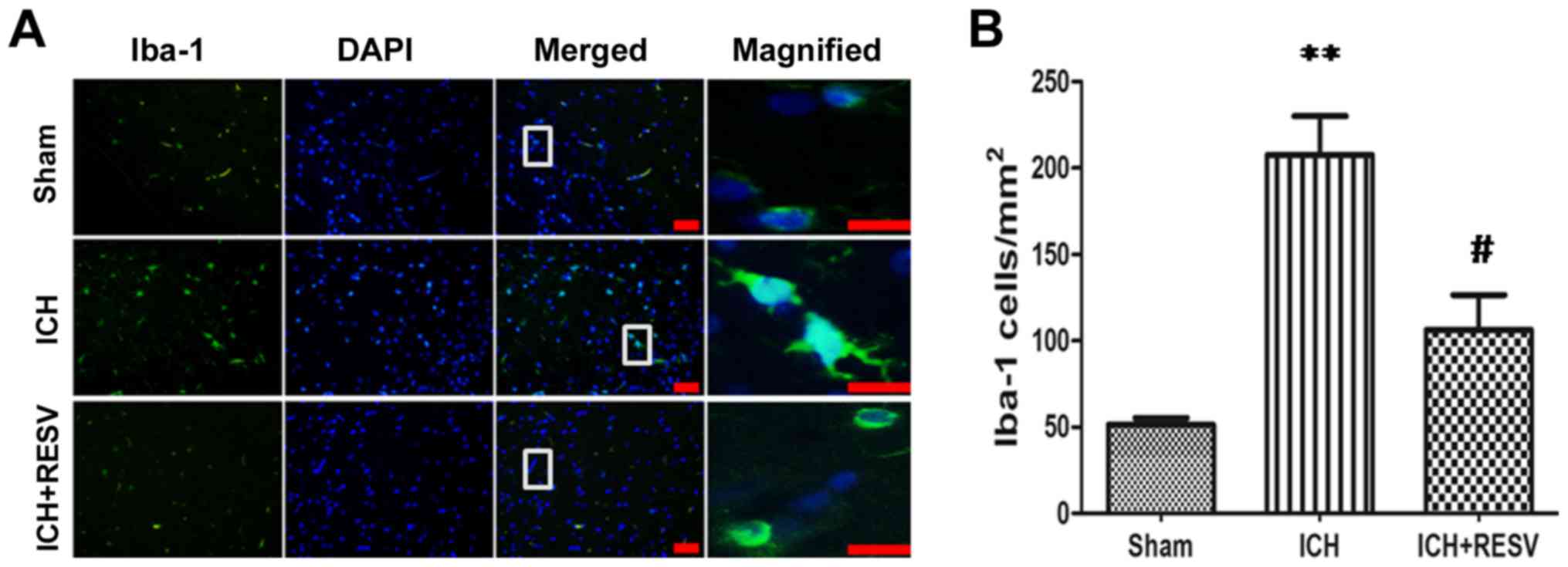 | Figure 5.Microglial activation, induced by
ICH, was attenuated by RESV. (A) Immunofluorescence staining of
Iba-1 in the cortex 14 days post-ICH (magnification, ×100). White
squares on merged images were magnified (magnification, ×400) and
presented in the right panel. DNA and Iba-1 were stained blue and
green, respectively. (B) Quantification of Iba-1 positive cells.
Scale bar, 50 µm. Data are presented as the mean ± standard
deviation. **P<0.01 vs. sham group, #P<0.05 vs.
ICH group. ICH, intracerebral hemorrhage; RESV, resveratrol; Iba-1,
ionized calcium binding adaptor molecule 1. |
Discussion
Previous studies have demonstrated that secondary
brain injury, rather than primary mechanical injury, contributes to
the serious complications that occur following ICH (25,26).
Excessive bleeding of blood vessels within the brain activates the
coagulation cascade leading to the production of thrombin, which
may induce the release of pro-inflammatory cytokines, including
interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α). This
may, in turn, lead to the activation of microglia and other
pro-inflammatory molecules (27).
The inflammatory response may therefore exacerbate neuronal
impairment within the brain. Thus, novel therapeutic strategies to
treat the secondary brain injury that occurs following ICH are
required to improve the mortality and disability rates of patients
with ICH (28).
It has been demonstrated that RESV may attenuate
neurological deficits in certain diseases that results in brain
injury (29–32). However, to the best of our knowledge,
no studies have assessed the effects of RESV on brain injury
induced by ICH. Thus, the present study examined the
neuroprotective function of RESV on ICH in rats.
The rotarod test used in the present study indicated
that 100 mg/kg RESV administered intraperitoneally for 2 weeks
stimulated the recovery of motor abilities following ICH. The
recovery time with RESV administration was decreased when compared
with a previous study (33). This
unexpected result may have been due to higher doses of RESV being
used in the present study. In the open field test, ICH rats treated
with RESV were more active than those in the ICH group.
Furthermore, rats in the ICH+RESV group travelled a greater
distance and at a higher speed than rats in the ICH group 7 days
post-surgery. This indicates that RESV treatment exhibits a
positive effect on the recovery of rat motor abilities following
ICH. However, a decrease in the total distance traveled and average
speed was observed 14 days post-surgery among all groups, which may
have been due to the habituation of rats to the experimental
environment over repeated exposure to the open field test, which
was also documented in a previous study (34). Thus, future studies may require more
behavioral tests, including the water maze, balance beam and
contralateral hindlimb retraction tests to assess motor functions,
to limit the likelihood of habituation.
In addition, the present study demonstrated that the
number of Nissl-stained neurons in the hippocampus was
significantly increased following 2 weeks RESV treatment. This was
consistent with the results of previous studies, which demonstrated
that RESV reduces cell loss, inhibits blood brain barrier
disruption and decreases edema following brain injury (29,35). The
behavioral performances of rats were also improved following an
increase in neural cell survival rate. This may help to explain the
results of the present study. In addition, the number of activated
microglia decreased following RESV treatment post-ICH, which was in
accordance with the anti-inflammatory function of RESV identified
in previous studies (36,37). The RESV-induced downregulation of
brain immune cell activation via the inhibition of transcriptional
factors, including peroxisome proliferator-activated receptor α
(38) and nuclear factor-κB have
also been identified (39). However,
it remains unclear whether the expression of certain downstream
inflammatory factors, including matrix metalloproteinase 9, IL-1β
and TNF-α, are regulated by RESV. The expression of astrocytes
should also be examined in order to compare the function of these
immune cells during neuroinflammation. Further studies are
therefore required to elucidate the exact molecular mechanism of
RESV.
Clinical trials have demonstrated that RESV is safe
to use (40), however, side effects
associated with high doses of RESV remains a challenge. For this
reason, RESV has not yet been approved by the food and drug
association for use in the treatment of ICH. Therefore, the
toxicological properties of RESV should be investigated in future
studies. However, clinical trials have demonstrated that RESV has a
greater effect than cattle encephalon glycoside and ignotin
(41), monosialotetrahexosyl
ganglioside (41) and human neural
stem cells expressing brain-derived neurotrophic factor (42) in the treatment of ICH (Table I). Thus, RSEV may be an appropriate
candidate to treat patients with ICH.
 | Table I.Comparison of drugs used to treat
ICH. |
Table I.
Comparison of drugs used to treat
ICH.
| Treatment | CEGI | GM-1 | BNDF | RESV |
|---|
| Neurological
outcome | 4 ml/kg CEGI
improves neurobehavioral outcomes 7 days PS (modified Garcia scale)
(41) | GM-1 improves
neurobehavioral outcomes 14 days PS (corner turn test) (41) | F3. BDNF improves
neurobehavioral outcomes 8 days PS (mouse ICH model, rota-rod)
(42) | RESV improves
neurobehavioral outcomes 7 days PS (open field)/14 days PS
(Rota-rod) |
In conclusion, the present study demonstrated that
RESV improves rat motor abilities and deactivates the
neuroinflammatory response following ICH. These results indicate
that treatment with 100 mg/kg RESV attenuates the neurological
deficit caused by ICH and may be used as a novel therapeutic agent
to treat ICH.
Acknowledgements
The authors would like to thank Dr Feng Yin (Key
Laboratory of Chemical Genomics, School of Chemical Biology and
Biotechnology, Peking University Shenzhen Graduate School) for the
critical reading of the manuscript and Dr Jun Ju (Key Laboratory of
Chemical Genomics, School of Chemical Biology and Biotechnology,
Peking University Shenzhen Graduate School) for the assistance in
image processing.
Funding
The present study was supported by the Shandong
Provincial Medical and Health Development Projects of China (grant
no. 2014WS0012) and the Natural Science Foundation of Guangdong
Province (grant. no. 2014A030313779). The funders had no role in
study design, data collection and interpretation, or the decision
to submit the work for publication.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the regulations of
the researched funding but are available from the corresponding
author on reasonable request.
Authors' contributions
JCC, WL and FL proposed the hypothesis, analyzed the
results, and wrote the manuscript. JCC designed and executed the
majority of the experiments. WBK, XXZ, PM, CXL and YW assisted in
the execution of some experiments. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Animal Care and Use Committee of Peking University Shenzhen
Graduate School (Shenzhen, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rojas H, Lekic T, Chen W, Jadhav V, Titova
E, Martin RD, Tang J and Zhang J: The antioxidant effects of
melatonin after intracerebral hemorrhage in rats. Acta Neurochir
Suppl. 105:19–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu CH, Yang RL, Huang SY, Li HZ, Wang KY,
Yang DH, Yan XH, Xue XH, Wu SY, Wang JM, et al: Analysis of
thrombin-antithrombin complex contents in plasma and hematoma fluid
of hypertensive intracerebral hemorrhage patients after clot
removal. Eur J Neurol. 18:1060–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitaoka T, Hua Y, Xi G, Hoff JT and Keep
RF: Delayed argatroban treatment reduces edema in a rat model of
intracerebral hemorrhage. Stroke. 33:3012–3018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu Y, Gong Y, Liu WQ, Keep RF, Xi G and
Hua Y: Zinc protoporphyrin attenuates white matter injury after
intracerebral hemorrhage. Acta Neurochir Suppl. 121:199–202. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fung C, Murek M, Klinger-Gratz PP,
Fiechter M, Z'Graggen WJ, Gautschi OP, El-Koussy M, Gralla J,
Schaller K, Zbinden M, et al: Effect of decompressive craniectomy
on perihematomal edema in patients with intracerebral hemorrhage.
PLoS One. 11:e01491692016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dragone T, Cianciulli A, Calvello R, Porro
C, Trotta T and Panaro MA: Resveratrol counteracts
lipopolysaccharide-mediated microglial inflammation by modulating a
SOCS-1 dependent signaling pathway. Toxicol In Vitro. 28:1126–1135.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bobermin LD, Wartchow KM, Flores MP, Leite
MC, Quincozes-Santos A and Goncalves CA: Ammonia-induced oxidative
damage in neurons is prevented by resveratrol and lipoic acid with
participation of heme oxygenase 1. Neurotoxicology. 49:28–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang T, Gao D, Jiang X, Hu S, Zhang L and
Fei Z: Resveratrol inhibits oxygen-glucose deprivation-induced
MMP-3 expression and cell apoptosis in primary cortical cells via
the NF-κB pathway. Mol Med Rep. 10:1065–1071. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen JK, Jorfi M, Buchanan KL, Park DJ,
Foster EJ, Tyler DJ, Rowan SJ, Weder C and Capadona JR: Influence
of resveratrol release on the tissue response to mechanically
adaptive cortical implants. Acta Biomater. 29:81–93. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Virgili M and Contestabile A: Partial
neuroprotection of in vivo excitotoxic brain damage by chronic
administration of the red wine antioxidant agent, trans-resveratrol
in rats. Neurosci Lett. 281:123–126. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren J, Fan C, Chen N, Huang J and Yang Q:
Resveratrol pretreatment attenuates cerebral ischemic injury by
upregulating expression of transcription factor Nrf2 and HO-1 in
rats. Neurochem Res. 36:2352–2362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Shi Z, Fan L, Zhang C, Wang K and
Wang B: Resveratrol improves neuron protection and functional
recovery in rat model of spinal cord injury. Brain Res.
1374:100–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davinelli S, Sapere N, Zella D, Bracale R,
Intrieri M and Scapagnini G: Pleiotropic protective effects of
phytochemicals in Alzheimer's disease. Oxid Med Cell Longev.
2012:3865272012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Wang R, Chen C, Du X, Ruan L, Sun J,
Li J, Zhang L, O'Donnell JM, Pan J and Xu Y: Antidepressant-like
effect of trans-resveratrol in chronic stress model: Behavioral and
neurochemical evidences. J Psychiatr Res. 47:315–322. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Della-Morte D, Dave KR, DeFazio RA, Bao
YC, Raval AP and Perez-Pinzon MA: Resveratrol pretreatment protects
rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling
protein 2 pathway. Neuroscience. 159:993–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ungvari Z, Bagi Z, Feher A, Recchia FA,
Sonntag WE, Pearson K, de Cabo R and Csiszar A: Resveratrol confers
endothelial protection via activation of the antioxidant
transcription factor Nrf2. Am J Physiol Heart Circ Physiol.
299:H18–H24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deinsberger W, Vogel J, Kuschinsky W, Auer
LM and Böker DK: Experimental intracerebral hemorrhage: Description
of a double injection model in rats. Neurol Res. 18:475–477. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Acadamies
Press; Washington, DC: 2011
|
|
20
|
Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q,
Zhao Q, Zhang J and Sheng J: Hydrogen-rich saline attenuated
subarachnoid hemorrhage-induced early brain injury in rats by
suppressing inflammatory response: Possible involvement of NF-κB
pathway and NLRP3 inflammasome. Mol Neurobiol. 53:3462–3476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HI, Park JH, Park MY, Kim NG, Park KJ,
Choi BT, Shin YI and Shin HK: Pre-conditioning with transcranial
low-level light therapy reduces neuroinflammation and protects
blood-brain barrier after focal cerebral ischemia in mice. Restor
Neurol Neurosci. 34:201–214. 2016.PubMed/NCBI
|
|
22
|
Kettenmann H, Hanisch UK, Noda M and
Verkhratsky A: Physiology of microglia. Physiol Rev. 91:461–553.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tischer J, Krueger M, Mueller W,
Staszewski O, Prinz M, Streit WJ and Bechmann I: Inhomogeneous
distribution of Iba-1 characterizes microglial pathology in
Alzheimer's disease. Glia. 64:1562–1572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed Z, Shaw G, Sharma VP, Yang C,
McGowan E and Dickson DW: Actin-binding proteins coronin-1a and
IBA-1 are effective microglial markers for immunohistochemistry. J
Histochem Cytochem. 55:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ducruet AF, Zacharia BE, Hickman ZL,
Grobelny BT, Yeh ML, Sosunov SA and Connolly ES Jr: The complement
cascade as a therapeutic target in intracerebral hemorrhage. Exp
Neurol. 219:398–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiex R and Tsirka SE: Brain edema after
intracerebral hemorrhage: Mechanisms, treatment options, management
strategies, and operative indications. Neurosurg Focus. 22:E62007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT
and Xi G: Tumor necrosis factor-alpha increases in the brain after
intracerebral hemorrhage and thrombin stimulation. Neurosurgery.
58:542–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xi G, Keep RF and Hoff JT: Mechanisms of
brain injury after intracerebral haemorrhage. Lancet Neurol.
5:53–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao AW, Wu HJ, Chen S, Ammar AB, Zhang JM
and Hong Y: Resveratrol attenuates early brain injury after
subarachnoid hemorrhage through inhibition of NF-κB-dependent
inflammatory/MMP-9 pathway. CNS Neurosci Ther. 20:182–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koronowski KB, Dave KR, Saul I, Camarena
V, Thompson JW, Neumann JT, Young JI and Perez-Pinzon MA:
Resveratrol preconditioning induces a novel extended window of
ischemic tolerance in the mouse brain. Stroke. 46:2293–2298. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CJ, Chen TH, Yang LY and Shih CM:
Resveratrol protects astrocytes against traumatic brain injury
through inhibiting apoptotic and autophagic cell death. Cell Death
Dis. 5:e11472014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghaiad HR, Nooh MM, El-Sawalhi MM and
Shaheen AA: Resveratrol promotes remyelination in cuprizone model
of multiple sclerosis: Biochemical and histological study. Mol
Neurobiol. 54:3219–3229. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu L, Wang S, Xu R, Zheng J, Tang J, Tang
X and Zhang D: Experimental intracerebral haemorrhage: Description
of a semi-coagulated autologous blood model in rats. Neurol Res.
37:874–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paylor R, Spencer CM, Yuva-Paylor LA and
Pieke-Dahl S: The use of behavioral test batteries, II: Effect of
test interval. Physiol Behav. 87:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arteaga O, Revuelta M, Urigüen L, Álvarez
A, Montalvo H and Hilario E: Pretreatment with resveratrol prevents
neuronal injury and cognitive deficits induced by perinatal
hypoxia-ischemia in rats. PLoS One. 10:e01424242015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W,
Lv S, Zhou M, Zhang X and Hang C: Resveratrol attenuates early
brain injury after experimental subarachnoid hemorrhage via
inhibition of NLRP3 inflammasome activation. Front Neurosci.
11:6112017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang XS, Li W, Wu Q, Wu LY, Ye ZN, Liu
JP, Zhuang Z, Zhou ML, Zhang X and Hang CH: Resveratrol attenuates
acute inflammatory injury in experimental subarachnoid hemorrhage
in rats via inhibition of TLR4 pathway. Int J Mol Sci. 17:pii:
E13312016. View Article : Google Scholar
|
|
38
|
Ayub A, Poulose N and Raju R: Resveratrol
improves survival and prolongs life following hemorrhagic shock.
Mol Med. 21:305–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q, Yuan L, Zhang Q, Gao Y, Liu G,
Xiu M, Wei X, Wang Z and Liu D: Resveratrol attenuates
hypoxia-induced neurotoxicity through inhibiting microglial
activation. Int Immunopharmacol. 28:578–587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turner RS, Thomas RG, Craft S, van Dyck
CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R, Aisen
PS, et al: A randomized, double-blind, placebo-controlled trial of
resveratrol for Alzheimer disease. Neurology. 85:1383–1391. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li R, Ma K, Zhao H, Feng Z, Yang Y, Ge H,
Zhang X, Tang J, Yin Y, Liu X, et al: Cattle encephalon glycoside
and ignotin reduced white matter injury and prevented
post-hemorrhagic hydrocephalus in a rat model of intracerebral
hemorrhage. Sci Rep. 6:359232016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee HJ, Lim IJ, Lee MC and Kim SU: Human
neural stem cells genetically modified to overexpress brain-derived
neurotrophic factor promote functional recovery and neuroprotection
in a mouse stroke model. J Neurosci Res. 88:3282–3294. 2010.
View Article : Google Scholar : PubMed/NCBI
|