Introduction
Bone marrow-derived mesenchymal stem cells (BMSCs)
maybe readily obtained from bone marrow aspirates (1). The isolation of mesenchymal stem cells
(MSCs) from the bone marrow of rats, cats, dogs, baboons, rabbits,
pigs, goats and sheep has been standardized (2–4). Leptin
is an adipocyte-derived hormone that regulates food intake, body
weight and glucose homeostasis (5).
Leptin regulates the release of insulin and glucagon, key hormones
that regulate glucose homeostasis, by direct action on the β- and
α-cells of the pancreatic islets, respectively (6). It has therefore been suggested that the
adipo-insular axis is crucial for maintaining nutrient balance and
that dysregulation of this axis contributes to obesity and diabetes
(7).
Leptin acts on cells in the hypothalamus to reduce
the production of orexigenic neuropeptides and reciprocally
enhances the secretion of anorectic peptides, there by controlling
food intake (8). In addition to its
role as a satiety indicator, leptin has a wide range of biological
functions, affecting reproduction, immunity, angiogenesis and
anti-apoptotic effects (9).
Previous studies have focused on the effect of
leptin on neural and embryonic stem cell growth (10); however, few studies have examined the
effect of leptin on MSCs. In the present study, leptin was used to
investigate the underlying molecular mechanisms affecting the
growth potential of rabbit (r)BMSCs. The results revealed that
leptin triggers the growth of rBMSCs via the extracellular
signal-regulated kinase (ERK)1/2 signaling pathway.
Materials and methods
Chemicals and reagents
Unless otherwise specified, all chemicals and
reagents were purchased from the Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Antibodies against Anti-Mouse immunoglobulin
(Ig)G (cat. no. ab6789), GAPDH (cat. no. ab8245), cluster of
differentiation (CD)44 (cat. no. ab119348), CD34 (cat. no. ab8158),
inhibitor of phosphorylated (p)-ERK1/2 (U0126; cat. no. ab120241),
ERK1/2 (cat. no. ab54230), c-Jun N-terminal kinases (JNK; cat. no.
ab201624), p38 (cat. no. ab31828), p-p38 (cat. no. 45381), the
ribosomal s6 kinase p90rsk (cat. no. ab32114), p-ERK1/2
(cat. no. ab50011), p-JNK (cat. no. ab46821), anti-leptin receptors
(cat. no. ab104403) and p-p90rsk (cat. no. ab32413) were
purchased from Abcam (Cambridge, MA, USA). MTT was purchased from
Beyotime Institute of Biotechnology (Haimen, China). Opti-MEM I
medium was purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA).
Isolation and culture of rBMSCs
Bone marrow was obtained from a male neonatal New
Zealand white rabbit (0.75 kg, 1 month old; Vital River Laboratory
Animal Technology Co., Ltd. Beijing, China). The rabbit was
provided with free access to food and water and housed at 25°C with
the humidity of 50–60% and a regular day-night cycle (12 h
light/dark cycle). The present study was carried out in strict
accordance with the Guidelines on the Care and Use of Laboratory
Animals issued by the Chinese Council on Animal Research and the
Guidelines of Animal Care (11). The
rabbit was anesthetized via intraperitoneal injection with
pentobarbital sodium (35 mg/kg; Sigma-Aldrich; Merck KGaA) prior to
the retrieval of bone marrow and sacrifice. Bone marrow was flushed
from the femur with low glucose Dulbecco's modified Eagle's medium
(DMEM) as previously described (12). The harvested cells were cultured in
an incubator at 37°C in an atmosphere containing 5% CO2.
Cell growth was monitored on days 2, 4 and 8 under an inverted
phase contrast microscope (Nikon Corporation, Tokyo, Japan). The
animal protocol was approved by The Inner Mongolia Medical
University Experimental Animal Management Committee (Hohhot,
China).
MTT assay
rBMSCs were seeded in 96-well plates at a density of
1×103 cells/well in DMEM supplemented with leptin at
increasing concentrations (0, 10, 102, 103 or
104 ng/ml). The control cells were cultured in DMEM
containing 0.1% dimethyl sulfoxide (DMSO). MTT (20 µl with a final
concentration of 0.5%) was added to each well once daily for 9 days
following treatment with leptin at 37°C. Cells were subsequently
incubated for 4 h at 37°C in the dark and 150 µl DMSO was added to
each well for 10 min to dissolve the formazan crystals. The
absorbance was detected using a microplate reader (EXL800;
Cole-Parmer, Vernon Hills, IL, USA) at 490 nm. All experiments were
repeated five times. The viability of leptin treated cells was
expressed as the percentage of population growth plus the standard
error of the mean relative to untransfected control cells. Cell
growth was calculated as follows: Viability=[(mean experimental
absorbance-mean control absorbance)/mean control absorbance]
×100.
Immunofluorescence
Cells were fixed and permeabilized with 0.5% Triton
X-100 for 15 min at room temperature and were subsequently blocked
with 10% goat serum (Gibco; Thermo Fisher Scientific, Inc.) at 4°C
overnight. Cells were incubated with anti-leptin receptors (1:400),
CD44 (1:500) and CD34 (1:800) primary antibodies diluted in PBS at
37°C for 2 h. The primary antibodies were detected by
horseradish-conjugated (H+L) secondary antibodies (cat. no. 7074;
1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA) at 37°C
for 1 h. DAPI (1 µg/ml) was used to stain for 15 min at 37°C. The
fluorescence was captured using a fluorescence microscope at a
magnification of ×200. The experiments were performed five
times.
Western blot analysis
Protein was isolated from rBMSCs treated with leptin
with 103 ng/ml using a Beyotime Cell Protein Extraction
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The total concentration of protein was
determined using the bicinchoninic acid assay method. Proteins (20
µg/ml; 0.6 µg/lane) were separated by 12% SDS-PAGE and transferred
to nitrocellulose membranes. The membranes were blocked for 30 min
with 5% fat-free milk at room temperature and incubated with the
following primary antibodies: Anti-ERK1/2 (1:1,000), anti-p-ERK1/2
(1:800), anti-JNK (1:1,500), anti-p-JNK (1:1,000), anti-p38
(1:500), anti-p-p38 (1:800), anti-p90rsk (1:1,000) and
anti-p-p90rsk (1:1,000) for a minimum of 1 h at 37°C.
Membranes were subsequently incubated with horseradish-conjugated
(H+L) secondary antibodies (1:4,000) at 37°C and developed using a
Pierce™ enhanced chemiluminescence plus western blotting substrate
(cat. no. 32132; Thermo Fisher Scientific, Inc.). UVP VisionWorksLS
software (UVP, LLC; DBA Analytik Jena US, Upland, CA, USA).
Leptin inhibition of rBMSCs
The phosphorylation status of the mitogen-activated
protein kinases (MAPKs) ERK1/2, JNK and p38 were detected at 0, 20,
40 and 60 min following treatment with 103 ng/ml leptin.
The phosphorylation status = the phosphorylation protein gray
level/the protein gray level.
U0126 assay
Cells were incubated with U0126 (40 ng/ml), a
p-ERK1/2 incubator, for 12 h at 37°C. Leptin at a concentration of
103 ng/ml or 200 µl DMEM with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) was then added and
cell viability was measured at 0, 20, 40 and 60 min. The MTT assay
measured cell viability.
RNA interference and cell
transfection
To confirm the role of leptin receptors in rBMSCs,
leptin receptor short hairpin RNA (shRNA) was used to silence the
leptin receptor gene expression. The leptin receptor shRNA (sense,
5′-GGCUCUGUCUCCUUGAUAA-3′; and antisense,
3′-UUAUCAAGGAGACAGAGCC-5′) was synthesized by Shanghai GeneChem
Co., Ltd. (Shanghai, China) and transfected into rBMSCs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 1×105 cells were seeded into 6-well plates
containing an antibiotic-free Opti-MEM™ I and incubated overnight
at 37°C. In each well, 5 µl shRNA was mixed with 125 µl Opti-MEM™
I. The mixture was then combined with a solution of 5 µl
Lipofectamine® 2000 in 125 µl Opti-MEM I. Following a 20
min incubation period at room temperature, the mixture was applied
to cells in an appropriate volume of Opti-MEM I to achieve a final
concentration of 100 nmol/l for each shRNA. Following incubation
for 6 h at 37°C, Roswell Park Memorial Institute-1640 supplemented
with fetal bovine serum (FBS; both Gibco; Thermo Fisher Scientific,
Inc.) was added to the wells. Cells were cultured for an additional
24 h at 37°C prior to analysis.
Statistical analysis
Statistically significant differences among groups
were determined by one-way analysis of variance and GraphPad Prism
version 5 software (GraphPad Software, Inc., La Jolla, CA, USA) was
used to analyze the data. When the overall F test result of
analysis of variance was significant, a multiple-comparison Tukey's
post hoc test was used. Student's t-test was used in two-mean
comparisons. Five independent replicates were performed for all
experiments. P<0.05 was considered to indicate a statistically
significant difference. Data are presented as the mean ± standard
deviation.
Results
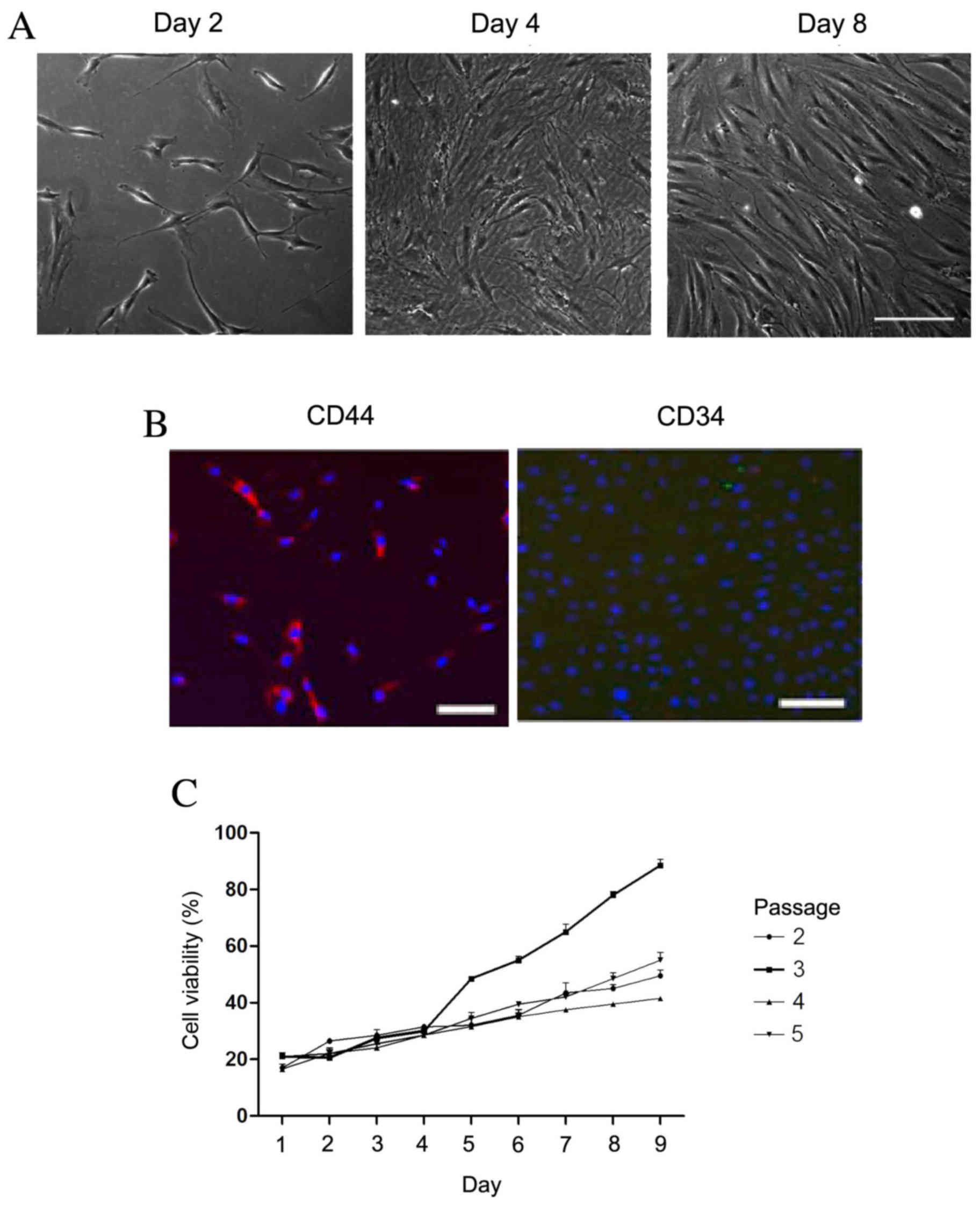
Identification of rBMSCs
The morphology of rBMSCs was determined by
visualizing the cells under a microscope (Fig. 1A). Cell density increased with
duration of culturing. On day 2, the cells reached 10% confluence.
On day 8, the cells displayed a uniform spindle shape and reached
80% confluence. To further identify the rBMSCs, CD34 and CD44 cell
markers were detected using immunofluorescence. The cultured cells
were positive for CD44 (red fluorescence; Fig. 1B); however, CD34 was not observed.
Fig. 1C revealed the cell viability
during the third passage was higher than that of other passages day
5. Therefore, the third passage of cells had highest cell
viability.
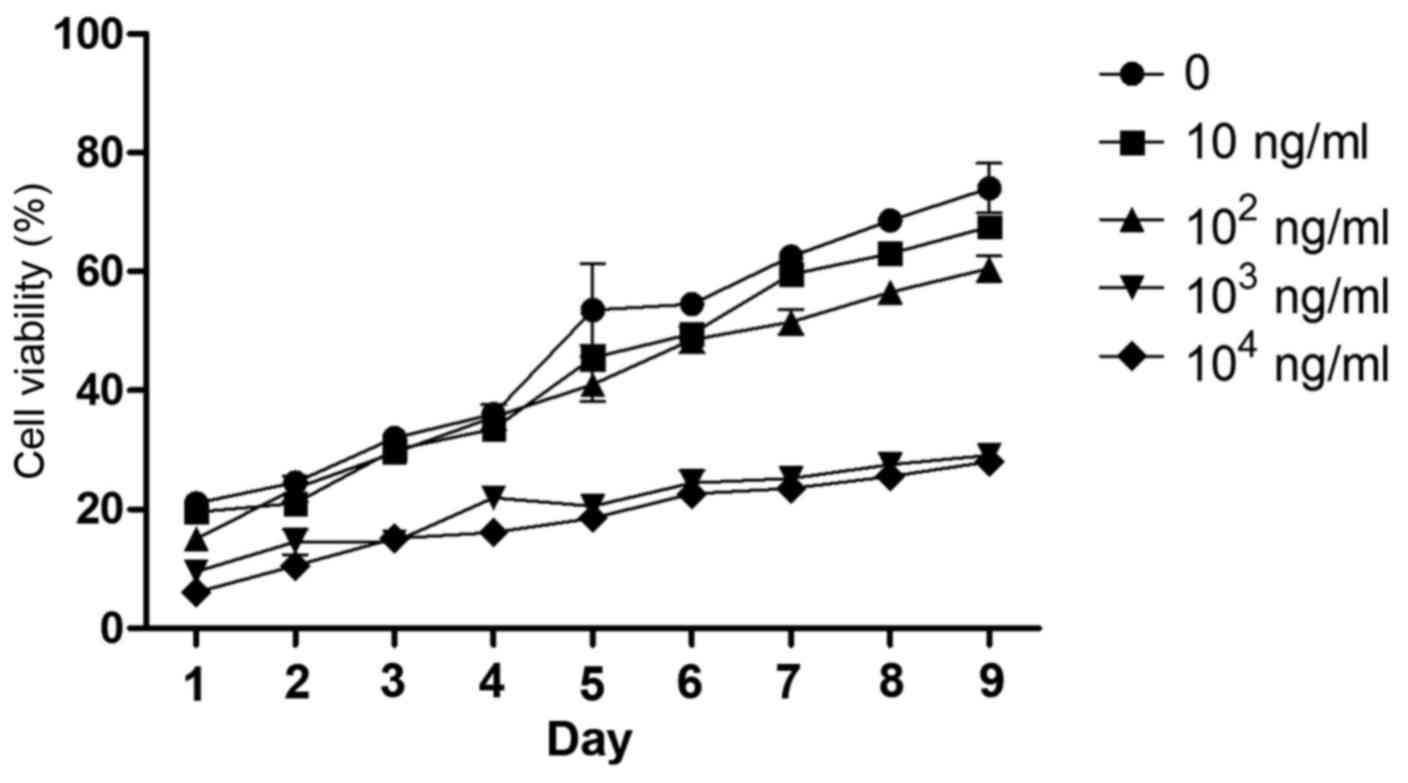
Optimal concentration and time for
leptin-induced effects on rBMSCs
The cell viability of rBMSCs was determined for 1–9
days following the addition of leptin (0, 10, 102,
103, or 104 ng/ml) to the culture medium from
the fourth cell passage (Fig. 2).
Leptin caused minimum viability rate at 103 ng/ml on the
fifth day of treatment. Although leptin at 104 and
103 ng/ml caused the greatest decrease in cell viability
at all time points, the lower concentration leptin was selected for
subsequent experiments as this reduced cost and material usage.
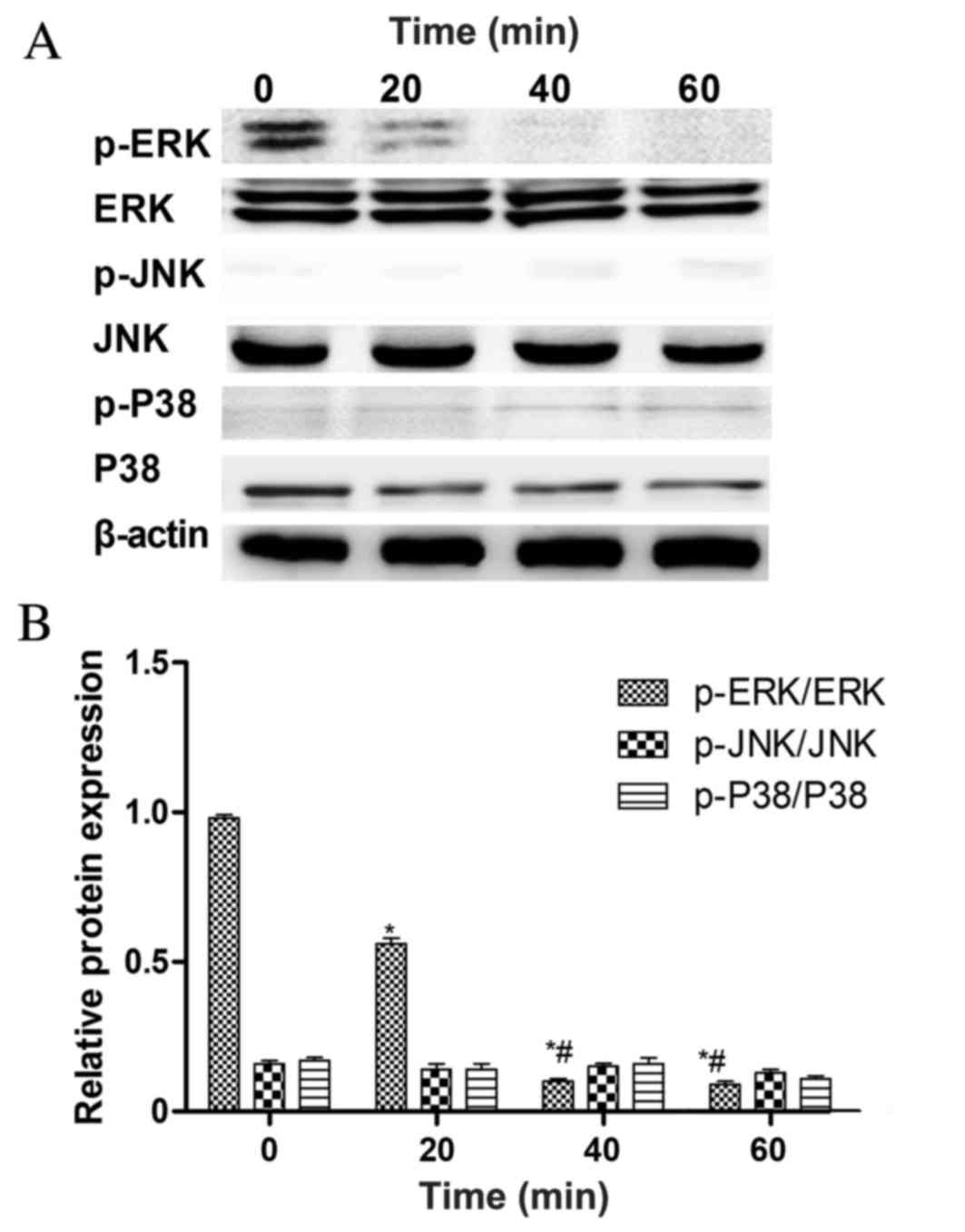
Leptin inhibits the growth of rBMSCs
via the ERK1/2 signaling pathway
To determine which pathway mediates the
leptin-induced inhibition of rMSC growth, the phosphorylation
states of the MAPKs, ERK1/2, JNK and p38 were detected at 0, 20, 40
and 60 min following treatment with 103 ng/ml of leptin
(Fig. 3A). ERK1/2 phosphorylation
was significantly decreased at 40 min compared with the 0 and 20
min groups, whereas JNK and p38 phosphorylation was not (Fig. 3B). This suggests that the inhibition
of ERK1/2 phosphorylation serves a key role in the ability of
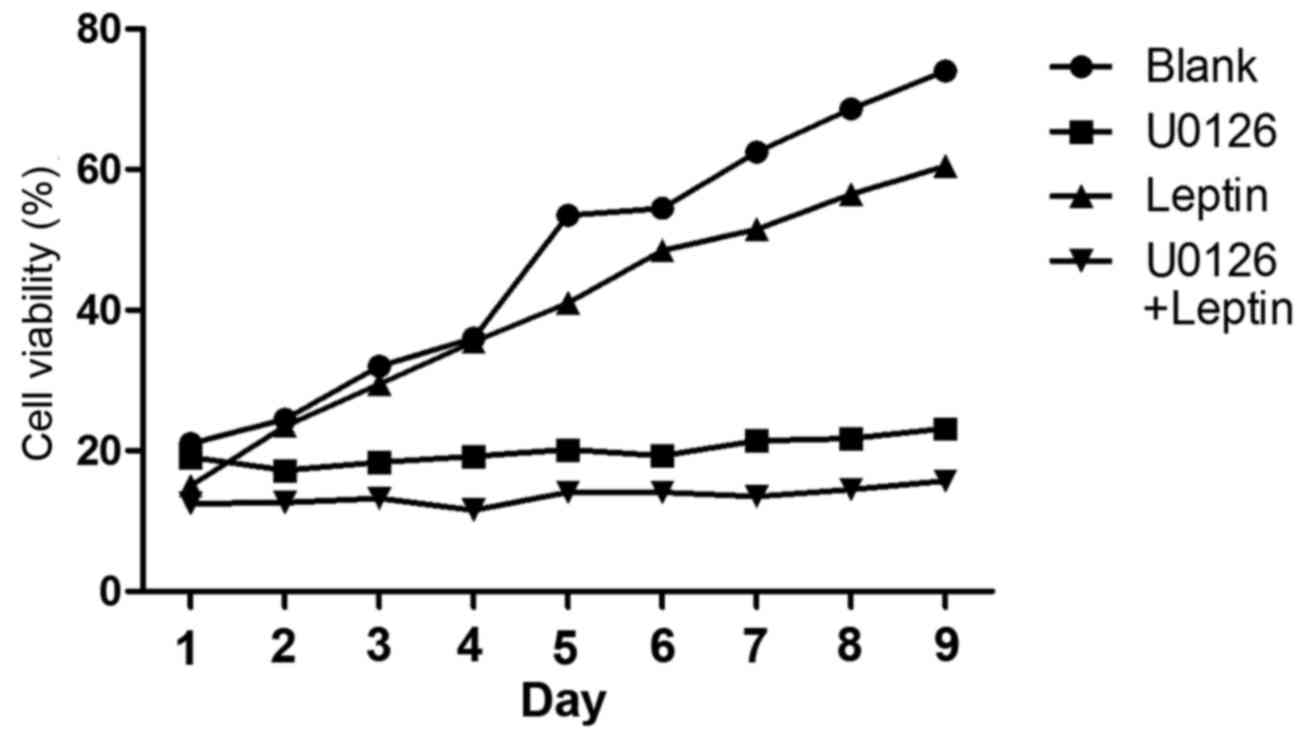
leptin to inhibit the growth of rBMSCs. To test this hypothesis,
U0126 (a specific inhibitor of ERK1/2 phosphorylation) was used to
blockERK1/2 expression. It was revealed that, in the presence of
U0126, leptin (103 ng/ml) inhibits the growth of rBMSCs
(Fig. 4).
Effect of silencing the leptin
receptor in rBMSCs
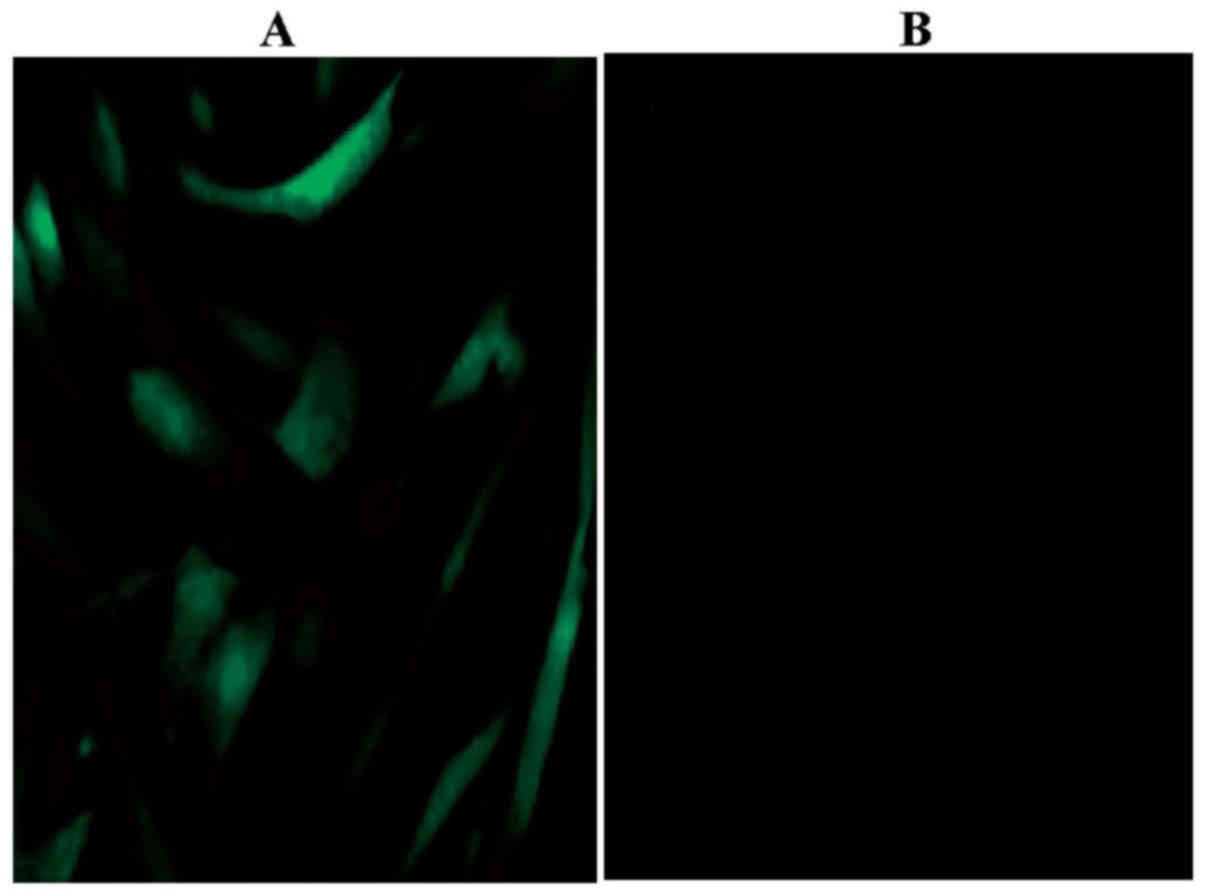
Using immunocytochemistry, the expression of the
leptin receptor (green) was detected in rBMSCs (Fig. 5). The leptin receptor was not
detected in the blank control. To determine whether the effects of
leptin were mediated by the receptor, leptin receptor gene
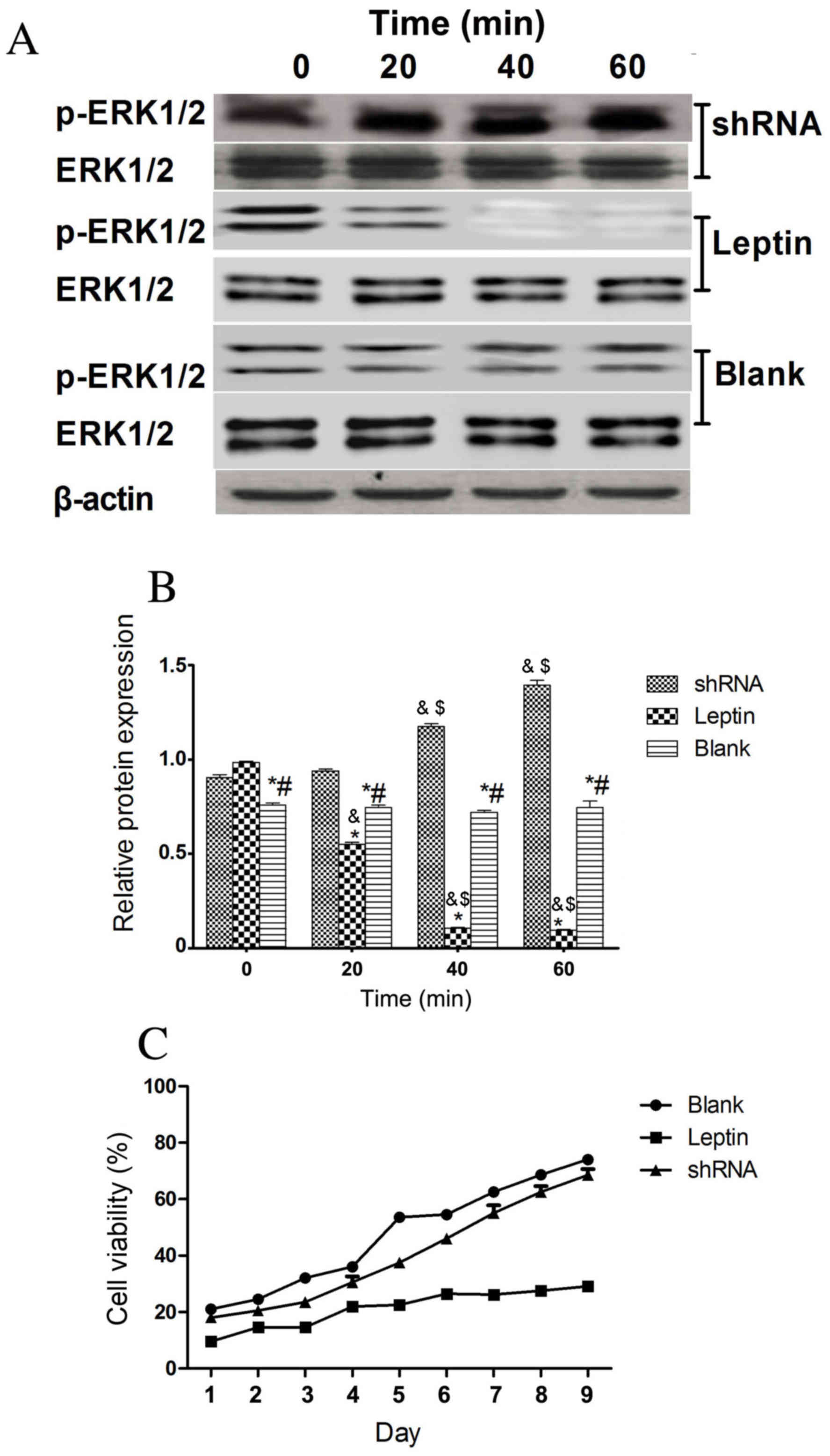
expression was silenced using shRNA. Fig. 6A reveals that ERK1/2 was
phosphorylated in rBMSCs with silenced leptin receptor genes and
Fig. 6B demonstrates that there was
no significant difference in ERK1/2 phosphorylation between each
time point of the shRNA group. However, the cell viability of
rBMSCs on days 1–9 was not significantly different in the shRNA
knockdown group compared with the blank control group (Fig. 6C). This indicates that leptin
receptors mediate the leptin-mediated inhibition of cell
growth.
Discussion
Leptin is a member of the proinflammatory
interleukin-6 family of cytokines (13). In keeping with its immune regulatory
role, the long form of the leptin receptor is expressed in immune
cells, including monocytes, T cells, dendritic cells, eosinophils,
and B cells (14). Leptin receptors
are also localized on adipose tissue cells (15,16).
Leptin binding to the leptin receptors stimulates stem cell
proliferation, differentiation and cytokine secretion from adipose
tissue (5).
In the present study, it was observed that leptin
slows the growth of rBMSCs by inhibiting phosphorylation of the
ERK1/2 branch of the MAPK signaling pathway. The optimal leptin
concentration for inhibiting growth was 103 ng/ml. The
effect of leptin was blocked by shRNA knockdown of the leptin
receptors, indicating that the leptin-induced inhibition of growth
in rBMSCs was mediated by the leptin receptors, which were
expressed at high levels in rBMSCs.
To further investigate the mechanism by which leptin
inhibits rMSC growth, the activity of the downstream signaling
pathways of the leptin receptor were evaluated. MAPKs are a
superfamily of serine/threonine kinases that includes ERK, JNK and
p38 (16). These kinases are
primarily associated with the activation of nuclear transcription
factors that control cell proliferation, differentiation and
apoptosis (17). The results of the
present study suggest that leptin inhibits the growth of rBMSCs via
the ERK signaling pathway. At 20–60 min following treatment with
leptin the phosphorylation of ERK was inhibited, which indicates
that inhibition is time-dependent.
In conclusion, the results of the present study
indicate that leptin inhibits rMSC growth primarily through an
ERK-dependent signaling pathway. These findings suggest that leptin
maybe useful for inhibiting the number of rBMSCs. It may be useful
to reduce the concentration of leptin in a clinical setting to
promote rBMSCs proliferation. The present study only performed
in vitro studies, further study is required and it may be
useless to also performed in vivo studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of this study are
available from Dr HW, but restrictions apply to the availability of
these data, which were used under license for the current study,
and so are not publicly available. Data are however available from
the authors upon reasonable request and with permission of Dr
HW.
Authors' contributions
LS and QQ performed all experiments. RL and HW
performed statistical analysis of the data.
Ethics approval and consent to
participate
The animal protocol was approved by The Inner
Mongolia Medical University Experimental Animal Management
Committee (Hohhot, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang Y and Wang W: Effects of bone marrow
mesenchymal stem cell transplantation on light-damaged retina.
Invest Ophthalmol Vis Sci. 51:3742–3748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng H, Martin JA, Duwayri Y, Falcon G
and Buckwalter JA: Impact of aging on rat bone marrow-derived stem
cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 62:136–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Marion AM, Thiele J, Kvasnicka HM and
van den Tweel JG: Morphology of the bone marrow after stem cell
transplantation. Histopathology. 48:329–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B, Zhang J, Brewer E, Tu Q, Yu L, Tang
J, Krebsbach P, Wieland M and Chen J: Osterix enhances
BMSC-associated osseointegration of implants. J Dent Res.
88:1003–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng Q, Banaszak L, Fracci S, Basali D,
Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger
NA, et al: Leptin receptor maintains cancer stem-like properties in
triple negative breast cancer cells. Endocr Relat Cancer.
20:797–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng B, Jiang J, Luo K, Liu L, Lin M,
Chen Y and Yan F: Increased osteogenesis in osteoporotic bone
marrow stromal cells by overexpression of leptin. Cell Tissue Res.
36:845–856. 2015. View Article : Google Scholar
|
|
7
|
Tse HF, Yiu KH and Lau CP: Bone marrow
stem cell therapy for myocardial angiogenesis. Curr Vasc Pharmacol.
5:103–112. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noda T, Kikugawa T, Tanji N, Miura N, Asai
S, Higashiyama S and Yokoyama M: Long-term exposure to leptin
enhances the growth of prostate cancer cells. Int J Oncol.
46:1535–1542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saucillo DC, Gerriets VA, Sheng J,
Rathmell JC and Maciver NJ: Leptin metabolically licenses T cells
for activation to link nutrition and immunity. J Immunol.
192:136–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurtovic S, Ng TT, Gupta A, Arumugaswami
V, Chaiboonma KL, Aminzadeh MA, Makkar R, Dafoe DC and
Talavera-Adame D: Leptin enhances endothelial cell differentiation
and angiogenesis in murine embryonic stem cells. Microvasc Res.
97:65–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang WZ, Yao XD, Huang XJ, Li JQ and Xu H:
Effects of TGF-β1 and alginate on the differentiation of rabbit
bone marrow-derived mesenchymal stem cells into a chondrocyte cell
lineage. Exp Ther Med. 10:995–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nokhbehsaim M, Keser S, Nogueira AV, Jäger
A, Jepsen S, Cirelli JA, Bourauel C, Eick S and Deschner J: Leptin
effects on the regenerative capacity of human periodontal cells.
Int J Endocrinol. 2014:1803042014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cassano S, Pucino V, La Rocca C,
Procaccini C, De Rosa V, Marone G and Matarese G: Leptin modulates
autophagy in human CD4+CD25-conventional T cells. Metabolism.
63:1272–1279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reis BS, Lee K, Fanok MH, Mascaraque C,
Amoury M, Cohn LB, Rogoz A, Dallner OS, Moraes-Vieira PM, Domingos
AI and Mucida D: Leptin receptor signaling in T cells is required
for Th17 differentiation. J Immunol. 194:5253–5260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dupuis L, Schuermann Y, Cohen T, Siddappa
D, Kalaiselvanraja A, Pansera M, Bordignon V and Duggavathi R: Role
of leptin receptors in granulosa cells during ovulation.
Reproduction. 147:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Réus GZ, Vieira FG, Abelaira HM, Michels
M, Tomaz DB, dos Santos MA, Carlessi AS, Neotti MV, Matias BI, Luz
JR, et al: MAPK signaling correlates with the antidepressant
effects of ketamine. J Psychiatr Res. 55:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delhanty PJ, van der Eerden BC, van der
Velde M, Gauna C, Pols HA, Jahr H, Chiba H, van der Lely AJ and van
Leeuwen JP: Ghrelin and unacylated ghrelin stimulate human
osteoblast growth via mitogen-activated protein kinase
(MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of
GHS-R1a. J Endocrinol. 188:37–47. 2006. View Article : Google Scholar : PubMed/NCBI
|