Introduction
Surgical staging is an important and critical value
to determine the presence of occult disease, the prognosis, and the
need for adjuvant chemotherapy in gynecological cancers. In early
ovarian cancer (FIGO I/II) proper surgical staging lead to
upstaging in one third to one half of these patients (1,2) and is
considered as an independent prognostic factor for survival
(3,4). In contrast, in more advanced disease
(FIGO stage III/IV), where 75% of patients affected with ovarian
cancer present at the time of diagnosis (5,6),
cytoreductive surgery is of greater value because the most
significant postoperative factor for prognosis is the residual
tumor (7).
Today routine measures in abdominal staging of
gynecological malignancies include hysterectomy and bilateral
salpingo-oophorectomy (if no fertility preservation required),
peritoneal washing (PW), peritoneal biopsies (PB), omentum biopsies
or omentectomy and lymphonodectomie. PW and PB (including omental
biopsies) are routinely employed to assess microscopic tumor spread
to the peritoneal surface including the diaphragm.
Intraoperative PW cytology was introduced in 1956 by
Keetle and Elkin (8). For ovarian
cancer the PW results were included in the FIGO classification
system in 1975, and consequently, normal cytologic findings
resulted in a significantly increased overall survival regardless
of other factors (9). For
endometrial cancer, however, the role of PW cytology remained
controversy and led to the exclusion of PW cytology in the revised
FIGO staging system of 2009: Some studies suggested a prognostic
value while others did not (10,11).
Peritoneal smears (PS) and specifically
diaphragmatic smears (DS) have been introduced on the basis that
the area of examination is larger and this technique thus more
sensitive. PS showed sensitivities similar to those of PW but a
better specimen quality in one ovarian cancer study (12) but superior to those of PB (97 vs.
59%) in another (13). On the other
hand, DS showed limited reliability: 15% were unsatisfactory due to
too little cellular material or to air-drying artifact, and only 3
of 142 smears were positive for malignant cells (14).
Although in ovarian cancer the diaphragm is the
third most affected organ of occult disease after the peritoneum
and the colon (15), PS and in
particular DS are not commonly recommended and routinely taken.
However, some studies favor a cytology via scrape or diaphragmatic
wash, or even blind biopsies even in absence of an obvious
macroscopic diaphragmatic disease (16–18), and
the National Comprehensive Cancer Network (NCCN) Guidelines for
surgical treatment of ovarian cancer suggest blind diaphragmatic
biopsies or, alternatively, scraping (19).
Upstaging can occur as a result of presence of
occult microscopic disease spread and impacts the treatment and
accuracy of risk profile and prognosis (3,20,21), in
ovarian and endometrial cancer patients. Owing the lack of
respective data, we retrospectively addressed the question as to
whether DS provide an additional diagnostic benefit over PW and PB
and necessitates upstaging.
Materials and methods
Study cohort
Files of all patients who underwent laparotomy for
suspected gynecological cancer between June 2009 and April 2015
were reviewed using the Gynecological Tumor Database, a
computerized oncological database of the Department of Gynecology
and Gynecological Oncology, University Hospital Basel, Switzerland.
Patients were included from whom DS together with either PB, PW or
ascites or all of them have been taken as part as the staging
procedure. Women with benign findings were excluded. The study was
approved by the local Ethics Committee ‘Ethikkomission Nordwest-und
Zentralschweiz’, EKNZ BASEC 2015-408, in Switzerland in November
2015. Neither written nor verbal informed consent is necessary for
this retrospective study.
Sample collection and preparation
Sample collection and preparation was performed the
same way for all patients. Upon entering the peritoneal cavity,
ascites was aspired. In absence of ascites PW were collected prior
to the smears or biopsies by instilling approximately 100–500 ml of
warmed-up isotonic saline in the Douglas and paracolic spaces and
washing it around. Ascites and PW were collectively referred to as
PW and were centrifuged and smears were made in a timely matter at
the Department of Pathology, University Hospital Basel.
The DS were always collected by the same surgeon
(RZD) using a cervical cytobrush. DS were taken from both the left
and right diaphragm, were immediately preserved in a BD
SurePath™ vial, and processed by BD
PrepStain™ (Becton Dickinson AG, Allschwil, Switzerland)
according to the manufacturer's protocol. DS and PW smears were
stained using the Papanicolaou method.
PB were usually taken in the upper and lower left
and right quadrant, in some cases in the pelvis or Douglas or in
the most suspicious areas. PB were fixed in formalin, imbedded into
paraffin, and stained with hematoxylin/eosin.
Specimen assessment
All cytological specimens were assessed by two
gynecological pathologists at the University Hospital Basel. PB
results were considered positive, when at least 1 sample was
positive. Positive omental biopsies were also counted PB-positive.
DS results were considered positive when at least one out of two
samples was positive. Results were negative when DS or PB samples
were negative. Sensitivity and specificity were calculated for the
comparison of DS vs. PB and PW together (defined as ‘gold standard’
or reference for evidence of presence of peritoneal disease) to
determine the diagnostic value of the measures.
Results
Patient cohort: Histology and baseline
characteristics
A total of 43 patients from whom DS had been taken
in order to evaluate occult gynecological disease have been
identified in our database between June 2009 and April 2015. Of
these, 4 (9.3%) presented with benign findings (3 cystadenomas of
the ovary, 1 uterine leiomyoma) and hence were excluded from the
study. The remaining 39 patients presented with 41 malignancies: 2
patients had two tumors simultaneously and their results hence
counted twice in all calculations (unless a differentiation was
made between the two cancers). The 41 malignancies comprised 27
(65.8%) ovarian cancers, 10 (24.4%) endometrial cancers, 2 (4.9%)
primary peritoneal cancers, and 2 (4.9%) ‘other’ cancers (Table I). The two patients with two
simultaneous tumors presented with endometrial and ovarian cancers.
The two ‘other’ cancers were an endometrioid adenocarcinoma of the
vaginal stump and a peritoneal cancer originated from the pancreas
and both ovaries. The recorded cytological data include cases of
ovarian, peritoneal and endometrial cancers. There is no case of
cervical cancer, because the cytology is not part of the standard
staging procedure. Table I lists the
histological subtypes of these cancers.
 | Table I.Histology of the patient cohort. |
Table I.
Histology of the patient cohort.
| Histology | Number of cases
(n=41) |
|---|
| Ovarian cancer | 27 |
|
Serous | 9 |
|
Endometrioid | 5 |
|
Mucinous | 4 |
| Clear
cell | 1 |
|
Borderline | 3 |
|
Other | 5 |
| Endometrial
cancer | 10 |
|
Endometrioid | 5 |
|
Serous-papillary | 2 |
| Clear
cell | 1 |
|
Other | 2 |
| Peritoneal
cancer | 2 |
|
Endometrioid | 1 |
|
Serous | 1 |
| Other | 2 |
The baseline characteristics of the patients (mean
age and range, Ca-125) and the malignancies (residual disease,
nodal status, grading, and International Federation of Gynecology
and Obstetrics (FIGO) classification details) are summarized in
Table II. Among the 41
malignancies, 18 cases were FIGO stage I/II (43,9%), 20 were FIGO
stage III/IV (48.8%), and 3 (7.3%) were of unknown FIGO stage.
Table II also summarizes the FIGO
stage details for each cancer separately. They show that 11 of the
27 ovarian cancer cases (40.8%) were FIGO stage I/II, 14 (51.8%)
were FIGO stage III/IV, and 2 were of unknown FIGO stage. Among the
10 endometrial cancers one half was FIGO stage I/II and the other
FIGO stage III/IV, and one peritoneal cancer was FIGO stage I and
the other of unknown FIGO stage.
 | Table II.Baseline characteristics of the
patient cohort. |
Table II.
Baseline characteristics of the
patient cohort.
|
Characteristics | All (n=41) | Ovarian (n=27) | Endometrial
(n=10) | Peritoneal
(n=2) | Other (n=2) |
|---|
| Age (years), mean
(range) | 57.0 (16–85) | 56.3 (16–85) | 56.9 (38–75) | 67.5 | 52.0 |
| Ca-125 (U/ml) | 626.3 | 658 | 580.7 | 330 | n/a |
| Residual
disease |
| R
0 | 31 | 17 | 7 | 2 | 1 |
| R 0–1
cm | 2 | 1 | 0 | – | – |
| R >1
cm | 1 | 4 | 1 | – | – |
| R
X | 7 | 4 | 2 | – | 1 |
| Nodal status |
| N0 | 25 | 18 | 6 | – | 1 |
| N1 | 6 | 5 | 1 | – | – |
| NX | 10 | 4 | 3 | 2 | 1 |
| Grade |
| G1 | 5 | 3 | 1 | 1 |
|
| G2 | 6 | 3 | 3 | – | – |
| G3 | 16 | 11 | 5 | – | – |
| G
unknown | 14 | 10 | 2 | 1 | 1 |
| FIGO I | 13 (31.7%) | 8 (29.7%) | 4 (40%) | 1 (50%) |
|
| FIGO II | 5 (12.2%) | 3 (11.1%) | 1 (10%) | – | 1 (50%) |
| FIGO III | 13 (31.6%) | 9 (33.3%) | 4 (40%) | – | – |
| FIGO IV | 7 (17.1%) | 5 (18.5%) | 1 (10%) | – | 1 (50%) |
| Unknown | 3 (7.3%) | 2 (7.4%) | – | 1 (50%) | – |
Relationship between the
cytological/histopathological results and the FIGO stage
We assigned the cytological/histopathological
results (positive or negative findings) for each of the three
diagnostic measures (DS, PW/ascites, and PB) to the respective FIGO
stages for all 41 malignancies (Fig.
1A) and for each cancer (ovarian, endometrial, peritoneal, and
‘other’) separately (Fig. 1B). The
following data were obtained and presented in more detail for each
of the three diagnostic measures.
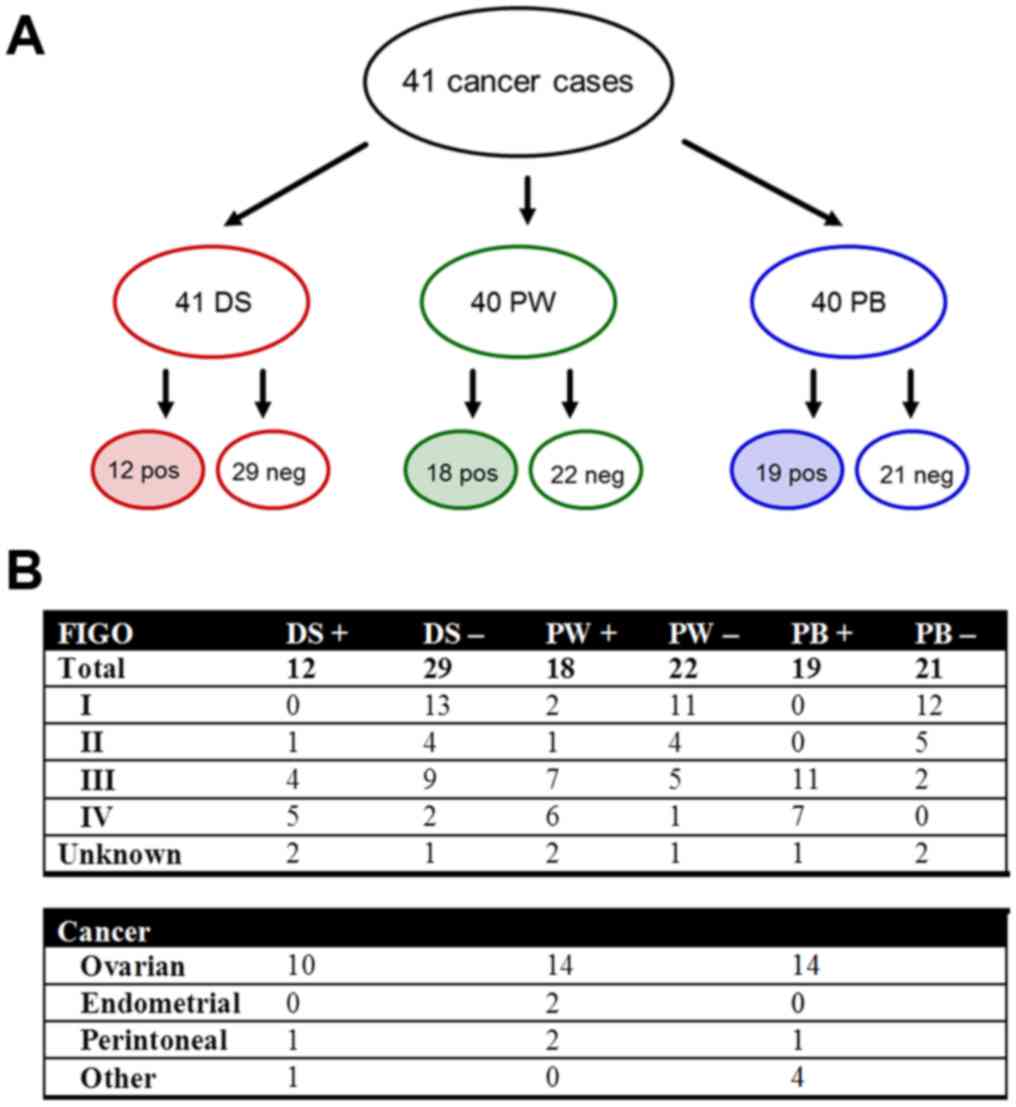 | Figure 1.(A) Cytological and histopathological
results for DS, PW/ascites (collectively PW), and PB showing the
positive or negative results for the presence of peritoneal
disease. (B) Distribution of the results among the FIGO stages for
all malignancies and among each type of cancer (ovarian,
endometrial, peritoneal and ‘other’). Positive results are shaded.
PB, peritoneal biopsies; PW, peritoneal washing; DS, diaphragmatic
smears; FIGO, International Federation of Gynecology and
Obstetrics; pos/+, positive; neg/-, negative. |
PW/ascites: Either PW cytology or ascites cytology
(collectively referred to as PW) was available in 40 of 41 cases,
whereof 18 (45%) were positive, i.e., contained malignant cells in
the abdominal fluid either through positive PW (8/28) or through
positive ascites (10/12). Three of these 18 positive cases (16.7%)
were FIGO I/II and 13 (72.2%) were FIGO III/IV. The majority
(14/18; 77.8%) were ovarian cancers, 2 were endometrial cancer, and
2 peritoneal cancers.
PB: PB were available in 40 cases, where of 19
(47.5%) were positive and were, apart from one unknown case, FIGO
III/IV. Four cases were solely positive through positive omentum
biopsies. All 21 negative cases were negative through at least 2 PB
except for 2 cases, in which only the omentum was biopsied and
negative. Positive cases subdivided into 14 ovarian (73.7%), 1
peritoneal (5.3%), and 4 (21%) other cancers.
DS: DS from all 41 cases were available, taken from
each the left and the right hemidiaphragm (82 DS samples in total).
Positive DS were found in 12 cases (10 ovarian cancers, 1
peritoneal, and 1 ‘other’): 10 cases with positivity on either side
of the diaphragm and 2 cases with positivity only on one side. Nine
of the 12 (75%) positive cases were FIGO III/IV, whereof 6 cases
had positive ascites cytology. One case with positive DS was FIGO
II (serous ovarian borderline tumor) and 2 positive cases were of
unknown FIGO stage. One out of the 29 negative cases was negative
owing unsatisfactory amount of material. Interestingly, in 3 cases
the diaphragm was clinically involved: 2 showed positive and 1
negative DS. The latter was obviously a false negative case,
because the right diaphragm was highly suspicious and the removed
adjacent peritoneum was histologically positive. She was a patient
with primary debulking by advanced endometrial cancer (FIGO IIIB).
In that same case the rest of PB and PW was positive.
Detection of peritoneal disease by PB,
PW, and DS
Our data show that among the 41 peritoneal diseases
19 were identified by PB, 18 by PW, and 12 by DS. In order to
evaluate whether taking DS in addition to PW and PB is of any
benefit in identifying peritoneal disease, i.e., identifies occult
diseases not detected by PW and PB, a Venn diagram was calculated
(Fig. 2). This diagram presents the
number of positive findings for each intersection of the three
diagnostic measures DS, PB, and PW. This is to determine which
measure alone or combination of measures identifies the presence of
peritoneal disease and in particular whether one specific measure
such as DS identifies a disease that is missed by either one or the
other or both other measures. The Venn diagram again displays the
positive results for DS (12, red circle), PB (18, blue circle), and
PW (19, green circle). The most important finding is that no single
case of peritoneal disease was identified solely based on positive
DS result (red shaded area), i.e., when both PB and PW were
negative. In addition, 9 PB-positive and 7 PW-positive cases were
DS-negative (5 of these DS-negative cases are both PB- and
PW-positive), 9 cases were positive for all three measures, 2
DS-positive cases were also PW-positive but PB-negative, and 1
DS-positive cases was PW-negative but PB-positive. Interestingly, 4
(2+2) positive cases (17.4%) were missed based solely on positive
PB results and 5 (1+4) positive cases (21.7%) solely on positive PW
results. Only 9 of 23 (39.1%) positive cases were positive in all
three measures.
Taken together, these data demonstrate that all 23
positive cases were detected by PB and PW together with a
sensitivity of 100% and specificity of 62%, indicating that
additional DS are not of benefit and hence not useful.
DS and upstaging
We evaluated whether patients were upstaged based on
PW and particular on DS. In none of the cases an upstaging was
indicated solely based on positive DS. One patient with left-sided
ovarian cancer was upstaged from FIGO IA to IC owing of positive PW
and possible intraoperative rupturing of the ovarian capsule.
Discussion
The present retrospective study addressed the
question as to whether DS provide an additional benefit in
detecting peritoneal disease that otherwise would be missed by the
today routine measures (PB and PW/ascites) in abdominal staging.
Our results show that i) no single case of peritoneal disease was
identified solely based on positive DS results, i.e., all cases
were detected by the combination of PB and PW/ascites with 100%
sensitivity, and that ii) no case of upstaging was indicated on
this basis. We may conclude that DS is not of any additional (to PB
and PW) benefit in diagnosing peritoneal spread in gynecological
cancers.
The first major finding of this study is that
positive DS results did not reveal peritoneal diseases left
undetected by PB and PW, meaning that these two measures together
detected all positive cases of peritoneal disease and that hence
additional DS were not of additional diagnostic value. This is
largely consistent with an earlier study (14) that evaluated the utility of DS as a
diagnostic measure and considered it limited: DS were occasionally
of insufficient quality and of low specimen yield, and identified
only few as positive cases. Unfortunately the study also did not
report on direct comparison between DS and PW, leaving open whether
the number of positive DS cases was perhaps underestimated. Owing
the scarcity and the inconclusiveness of data, DS like PS are not
commonly recommended and routinely performed in most
institutions.
However, cytology via scrape or diaphragmatic wash
or even blind biopsies (16–18) are recommended when macroscopic
diaphragmatic disease is not obvious. Blind diaphragmatic biopsies
or alternatively diaphragmatic scraping is suggested by NCCN
Guidelines for surgical treatment of ovarian cancer (19). Generally, diaphragmatic cytology is
of considerable significance. On the one hand, the diaphragm is
following the peritoneum and the colon the third most common
localization of spread ovarian cancer in almost half (44%) of the
patients (15). The diaphragm is
affected in 7% of the stage I ovarian cancer patients (1,22) and in
most cases (80%) not only the left but both hemidiaphragms were
affected (23), possibly owing the
clockwise transportation of peritoneal fluid. On the other hand, an
ovarian cancer patient with positive PW is commonly upstaged from
FIGO IA to IC, but will be upstaged according to the new 2013 FIGO
classification to FIGO IIIA2 (presence of microscopic peritoneal
metastasis beyond the pelvis) in case of a positive diaphragmatic
result and given confirmed histological evidence.
Interestingly, a considerable number of positive
cases (up to about 20%) were missed when only either PB or
PW/ascites was taken. Alike, the combination of either PB or
PW/ascites with positive DS results only lowered but not reduced to
zero the number of these missed cases, meaning that positive DS
results can in particular circumstances, for instance when only
either positive PB or PW/ascites results are available, be helpful
in identifying additional positive findings.
The second major finding is that no case upstaged by
positive DS results only, suggesting that positive diaphragmatic
cytology and in particular DS are not significant and hence are not
of benefit in this context. An earlier study, however, showed the
utility of diaphragmatic cytology to detect occult metastasis, as
6.5% of stage I ovarian cancer patients were upstaged to IIIA based
on positive diaphragmatic cytology (24).
Upstaging as a result of presence of occult
microscopic abdominal disease routinely detected either by biopsies
from the omentum, diaphragm, and random sites in the peritoneum or
in PW is common and according to International Guideline and has an
impact on adjuvant treatment, chemotherapy type to be selected, and
accuracy of risk profile and prognosis (3,20,21).
Complete and accurate surgical staging and adherence to the
guideline has indeed been shown to improve survival outcome in
early ovarian cancer (25). On the
other hand, incomplete and inaccurate staging, insufficient
specimen quality, and guideline incompliance may limit the value of
staging as diagnostic and prognostic measures (1,20,26) and
may lead to inadequate treatment decisions despite accurate
upstaging (27).
The utility regarding the prognostic significance of
PW in gynecological neoplasms is also controversial. PW cytology
only poorly detected peritoneal implants and predicted clinical
outcome analysis of ovarian serous tumors of low malignant
potential in one study (28) but was
considered as a useful procedure for staging malignant genital
tract neoplasms in another (29). A
recent retrospective study evaluating the utility of cytology in
tumor staging in ovarian and fallopian tube neoplasms (30) reported upstaging based on positive PW
results in 20% of the patients.
In our study, 5 (out of 23) positive cases were
missed based on solely positive PW results. In contrast, positive
PW results led to an upstaging in three cases (out of 18: 16.7%)
but not to changes in postoperative treatment. This is consistent
with two previous studies reporting 85% sensitivity and 95%
specificity (31) and 87%
sensitivity and 79% specificity (32) for the detection of malignant cells in
the peritoneal cavity in In FIGO stage I and II ovarian cancer. One
of these three patients had left-sided ovarian cancer and was
upstaged from FIGO IA to IC, but it was unclear whether upstaging
occurred as a consequence of intraoperative rupturing of the
ovarian capsule.
Likewise, the value of PW cytology in endometrial
cancer also remains ambiguous. Positive PW and poor survival were
associated in a 1971 study (9) and
positive PW cytology reported to be an independent risk factor for
disease to spread to lymph nodes in endometrial cancer in a 2013
study (33). Conversely,
contradicting data and low positive cytology rates were reported in
other studies (34–38). This led in 2009 to the exclusion of
PW-positivity as criterion in the FIGO classification system
(39), although taking PW cytology
still remains usual in the clinical practice. In our study, only 2
positive PW, 4 positive PB results, and no case of diaphragmatic
involvement were found in endometrial cancer.
The occasionally insufficient quality of DS reported
in other studies was, apart from one inconclusive result due to
poor amount of specimen, not observed in our study. Likewise, PB
and PW were qualitatively unproblematic and did not deliver
inconclusive results, a fact owing to an experienced team including
a gynecological oncological surgeon and trained pathologist.
In summary, DS is not of any additional benefit for
detection of occult peritoneal disease in ovarian and endometrial
cancers and hence is not recommended as a routine measure in
clinical practice.
Glossary
Abbreviations
Abbreviations:
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
NCCN
|
National Comprehensive Cancer
Network
|
References
|
1
|
Young RC, Decker DG, Wharton JT, Piver MS,
Sindelar WF, Edwards BK and Smith JP: Staging laparotomy in early
ovarian cancer. JAMA. 250:3072–3076. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grabowski JP, Harter P, Buhrmann C, Lorenz
D, Hils R, Kommoss S, Traut A and du Bois A: Re-operation outcome
in patients referred to a gynecologic oncology center with presumed
ovarian cancer FIGO I–IIIA after sub-standard initial surgery. Surg
Oncol. 21:31–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trimbos JB, Vergote I, Bolis G, Vermorken
JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone
G, et al: Impact of adjuvant chemotherapy and surgical staging in
early-stage ovarian carcinoma: European organisation for research
and treatment of cancer-adjuvant chemotherapy in ovarian neoplasm
trial. J Natl Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timmers PJ, Zwinderman AH, Coens C,
Vergote I and Trimbos JB: Understanding the problem of inadequately
staging early ovarian cancer. Eur J Cancer. 46:880–884. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO committee
on gynecologic oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the Arbeitsgemeinschaft Gynaekologische
Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe
d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keetle WC and Elkin HB: Experience with
radioactive colloidal gold in the treatment of ovarian carcinoma.
Am J Obstet Gynecol. 71:553–568. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Creasman WT and Rutlidge F: The prognostic
value peritoneal cytology in gynecologic malignant disease. Am J
Obstet Gynecol. 110:773–781. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garg G, Gao F, Wright JD, Hagemann AR,
Mutch DG and Powell MA: Positive peritoneal cytology is an
independent risk-factor in early stage endometrial cancer. Gynecol
Oncol. 128:77–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fadare O, Mariappan MR, Hileeto D, Wang S,
McAlpine JN and Rimm DL: Upstaging based solely on positive
peritoneal washing does not affect outcome in endometrial cancer.
Mod Pathol. 18:673–680. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luesley DM, Williams DR, Ward K, Redman CR
and Lawton FG: Prospective comparative cytologic study of direct
peritoneal smears and lavage fluids in patients with epithelial
ovarian cancer and benign gynecologic disease. Acta Cytol.
34:539–544. 1990.PubMed/NCBI
|
|
13
|
Jadhon ME, Morgan MA, Kelsten ML, Carlson
JA Jr and Mikuta JJ: Cytologic smears of peritoneal surfaces as a
sampling technique in epithelial ovarian carcinoma. Obstet Gynecol.
75:102–105. 1990.PubMed/NCBI
|
|
14
|
Jacques SM and Selvaggi SM: Multiple
peritoneal cytologies collected during laparotomy for gynecologic
malignancy. Diagn Cytopthol. 7:482–486. 1991. View Article : Google Scholar
|
|
15
|
Sehouli J, Senyuva F, Fotopoulou C,
Neumann U, Denkert C, Werner L and Gülten OO: Intra-Abdominal tumor
dissemination pattern and surgical outcome in 214 Patients with
primary ovarian cancer. J Surg Oncol. 99:424–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soper JT: Management of early-stage
epithelial ovarian cancer. Clin Obstet Gynecol. 37:423–438. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozols RF, Rubin SC and Thomas G:
Epithelial ovarian cancerHoskins WJ, Perez CA and Young RC:
Principles and practice of gynecologic oncology. 4th ed.
Philadelphia: Lippincott & Wilkins; pp. 895–988. 2005
|
|
18
|
Wheeless CR Jr and Roenneburg ML: Staging
of Gynecologic Oncology Patients With Exploratory Laparotomy. Atlas
of pelvic surgery (On-Line Edition). http://www.atlasofpelvicsurgery.com/10MalignantDisease/1StagingofGynecologicOncologyPatientsWithExploratoryLaparotomy/cha10sec1.html
|
|
19
|
NCCN: Epithelial Ovarian Cancer (including
Fallopian Tube Cancer and Primary Peritoneal Cancer). Version 2.
2013.https://www.nccn.org/
|
|
20
|
Lee JY, Kim HS, Chung HH, Kim JW, Park NH
and Song YS: The role of omentectomy and random peritoneal biopsies
as part of comprehensive surgical staging in apparent early-stage
epithelial ovarian cancer. Ann Surg Oncol. 21:2762–2766. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paik ES, Lee YY, Lee EJ, Choi CH, Kim TJ,
Lee JW, Bae DS and Kim BG: Survival analysis of revised 2013 FIGO
staging classification of epithelial ovarian cancer and comparison
with previous FIGO staging classification. Obstet Gynecol Sci.
58:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Piver MS, Barlow JJ and Lele SB: Incidence
of subclinical metastases in stage I and II ovarian carcinoma.
Obstet Gynecol. 52:100–104. 1978.PubMed/NCBI
|
|
23
|
Einenkel J, Ott R, Handzel R, Braumann DU
and Horn LC: Characteristics and management of diaphragm
involvement in patients with primary advanced stage ovarian,
fallopian tube, or peritoneal cancer. Int J Gynecol Cancer.
19:1288–1297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eltabbakh GH and Mount SL: Comparison of
diaphragmatic wash and scrape specimens in staging of women with
ovarian cancer. Gynecol Oncol. 81:461–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JY, Kim TH, Suh DH, Kim JW, Kim HS,
Chung HH, Park NH, Song YS and Kang SB: Impact of guideline
adherence on patient outcomes in early-stage epithelial ovarian
cancer. Eur J Surg Oncol. 41:585–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Buchsbaum HJ and Lifshitz S: Staging and
surgical evaluation of ovarian cancer. Semin Oncol. 11:227–237.
1984.PubMed/NCBI
|
|
27
|
Powless CA, Bakkum-Gamez JN, Aletti GD and
Cliby WA: Random peritoneal biopsies have limited value in staging
of apparent early stage epithelial ovarian cancer after thorough
exploration. Gynecol Oncol. 115:86–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sneige N, Thomison JB, Malpica A, Gong Y,
Ensor J and Silva EG: Peritoneal washing cytologic analysis of
ovarian serous tumors of low malignant potential to detect
peritoneal implants and predict clinical outcome. Cancer
Cytopathol. 120:238–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giordano G, Varotti E, Brigati F and
Berretta R: The value of peritoneal washing cytology during
intra-abdominal surgery for female genital tract neoplasms. Clin
Genitourin Cancer. 12:e95–e101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Davidson W, Madan R, O'Neil M, Tawfik OW
and Fan F: Utility of peritoneal washing cytology in staging and
prognosis of ovarian and fallopian tube neoplasms: A 10-year
retrospective analysis. Ann Diagn Pathol. 22:54–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anastasiadis PG, Romanidis KN,
Polichronidis A, Koutlaki NG, Tamiolakis D and Simopoulos K: The
contribution of rapid intraoperative cytology to the improvement of
ovarian cancer staging. Gynecol Oncol. 86:244–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Binesh F, Akhavan A, Behniafard N, Zabihi
S and Hosseinizadeh E: Prognostic value of peritoneal washing
cytology in gynecologic malignancies: A controversial issue. Asian
Pac J Cancer Prev. 15:9405–9410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garg G, Gao F, Wright JD, Hagemann AR,
Zighelboim I, Mutch DG and Powell MA: The risk of lymph-node
metastasis with positive peritoneal cytology in endometrial cancer.
Int J Gynecol Cancer. 23:90–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kashimura M, Sugihara K, Toki N, Matsuura
Y, Kawagoe T, Kamura T, Kaku T, Tsuruchi N, Nakashima H and Sakai
H: The significance of peritoneal cytology in uterine cervix and
endometrial cancer. Gynecol Oncol. 67:285–290. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Havrilesky LJ, Cragun JM, Calingaert B,
Secord Alvarez A, Valea FA, Clarke-Pearson DL, Berchuck A and Soper
JT: The prognostic significance of positive peritoneal cytology and
adnexal/serosal metastasis in stage IIIA endometrial cancer.
Gynecol Oncol. 104:401–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wong FC, Pang CP, Tang SK, Tung SY, Leung
TW, Sze WK and Cheung KB: Treatment results of endometrial
carcinoma with positive peritoneal washing, adnexal involvement and
serosal involvement. Clin Oncol (R Coll Radiol). 16:350–355. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zuna RE and Behrens A: Peritoneal washing
cytology in gynecologic cancers: Long term follow up of 355
patients. J Natl Cancer Inst. 88:980–987. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mathew S and Erozan YS: Significance of
peritoneal washings in gynecologic oncology. The experience with
901 intraoperative washings at an academic medical center. Arch
Pathol Lab Med. 121:604–606. 1997.PubMed/NCBI
|
|
39
|
Lewin SN, Herzog TJ, Medel Barrena NI,
Deutsch I, Burke WM, Sun X and Wright J: Comparative performance of
the 2009 international Federation of gynecology and obstetrics'
staging system for uterine corpus cancer. Obstet Gynecol.
116:1141–1149. 2010. View Article : Google Scholar : PubMed/NCBI
|
















