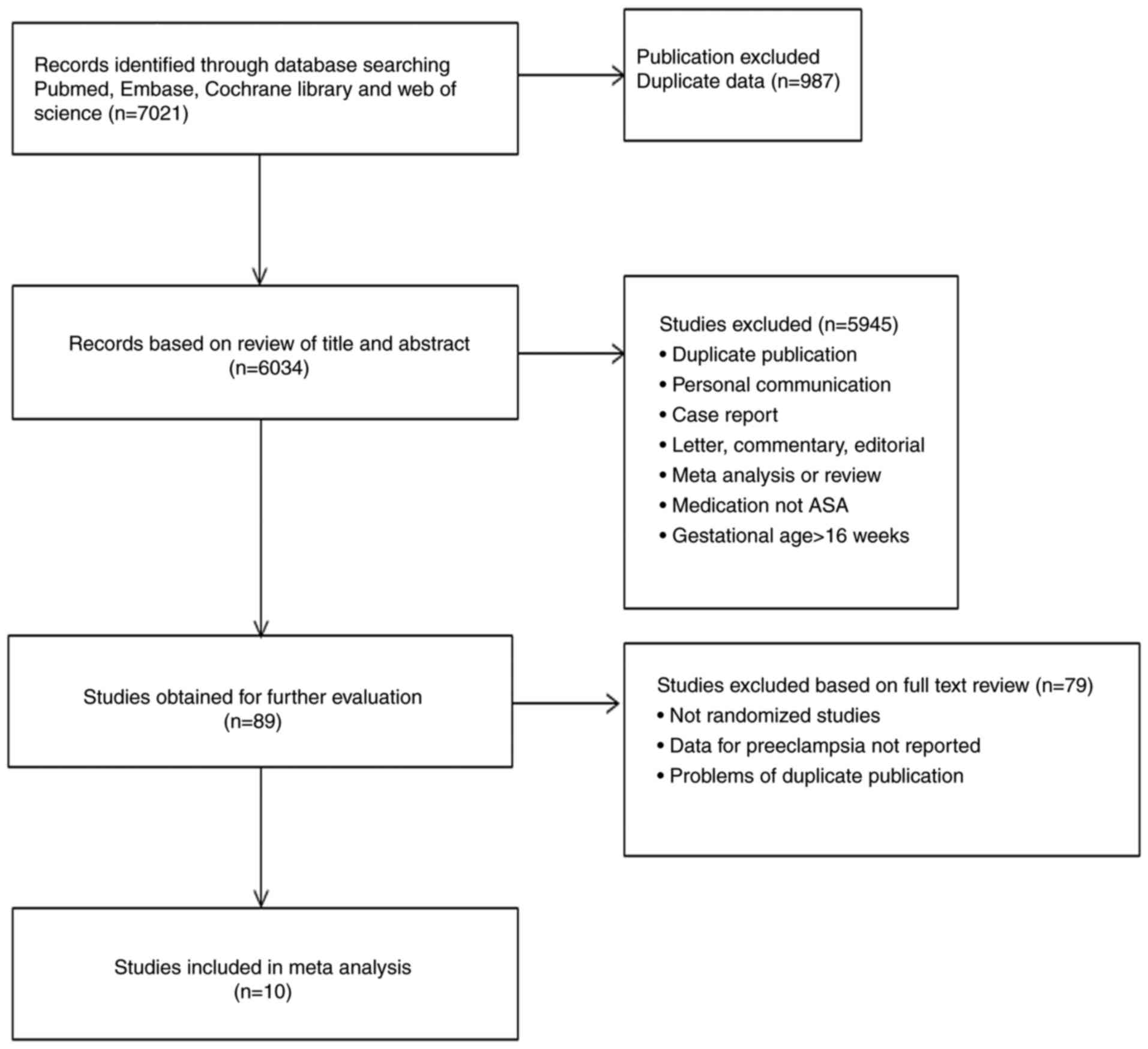
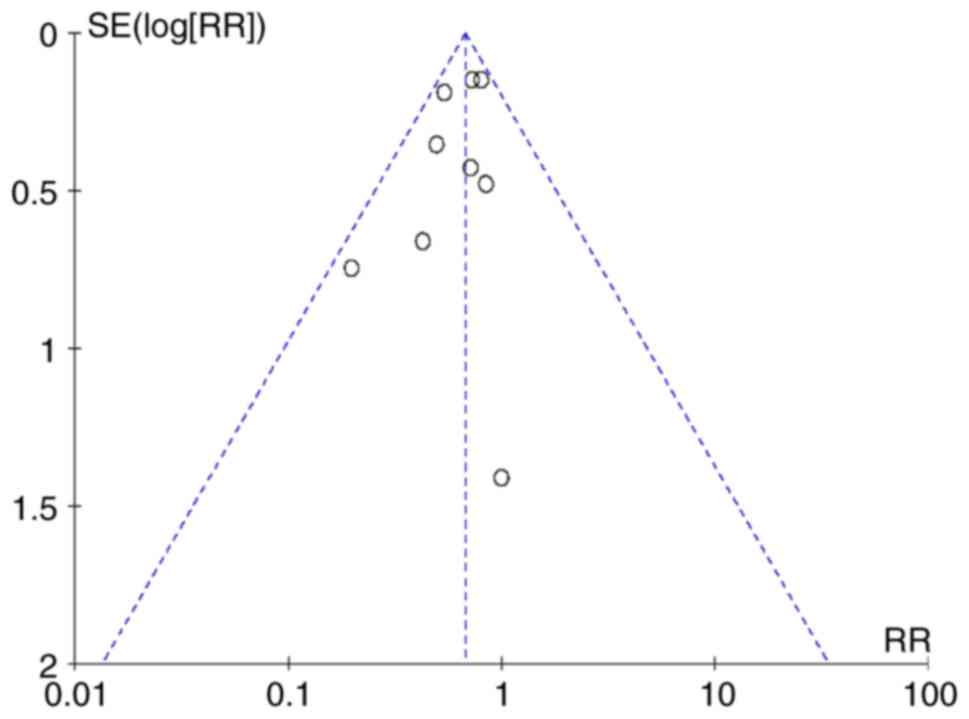
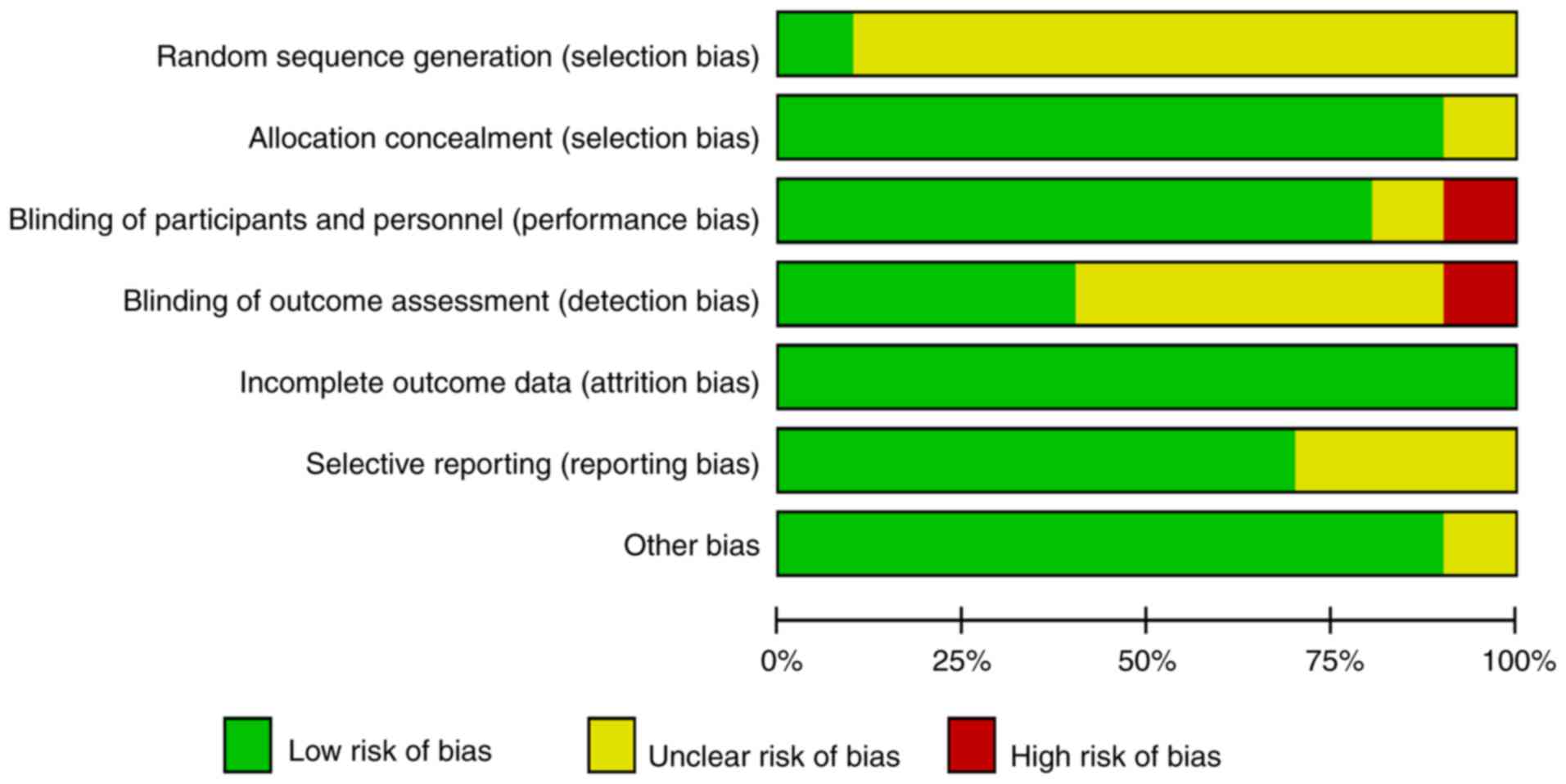
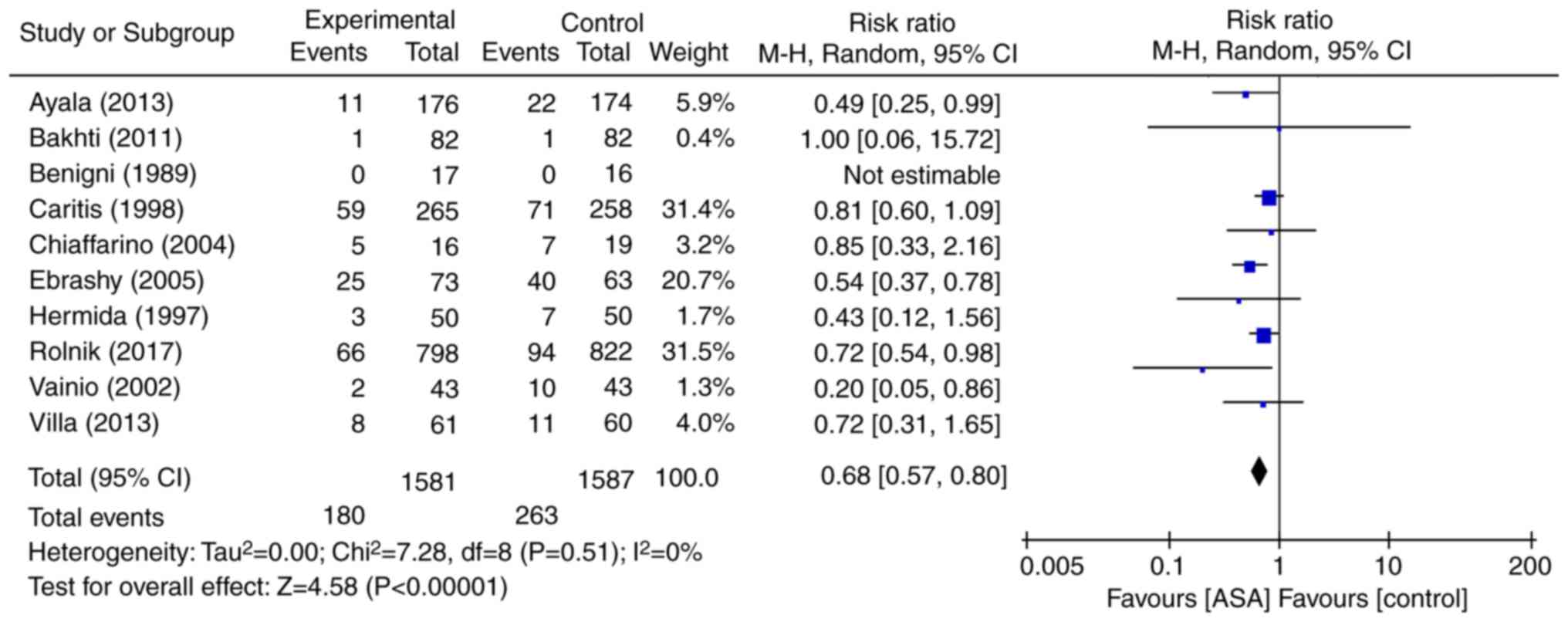
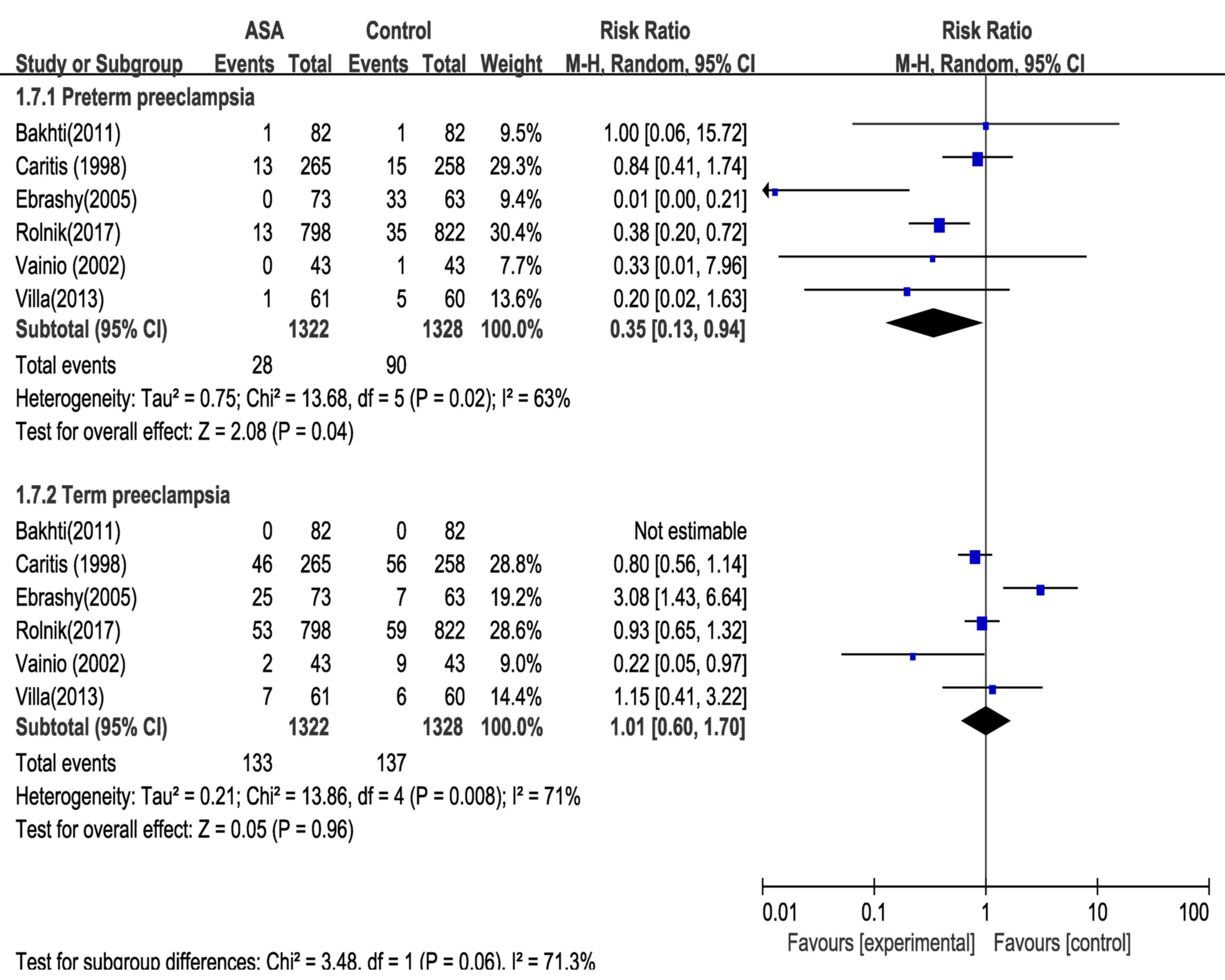
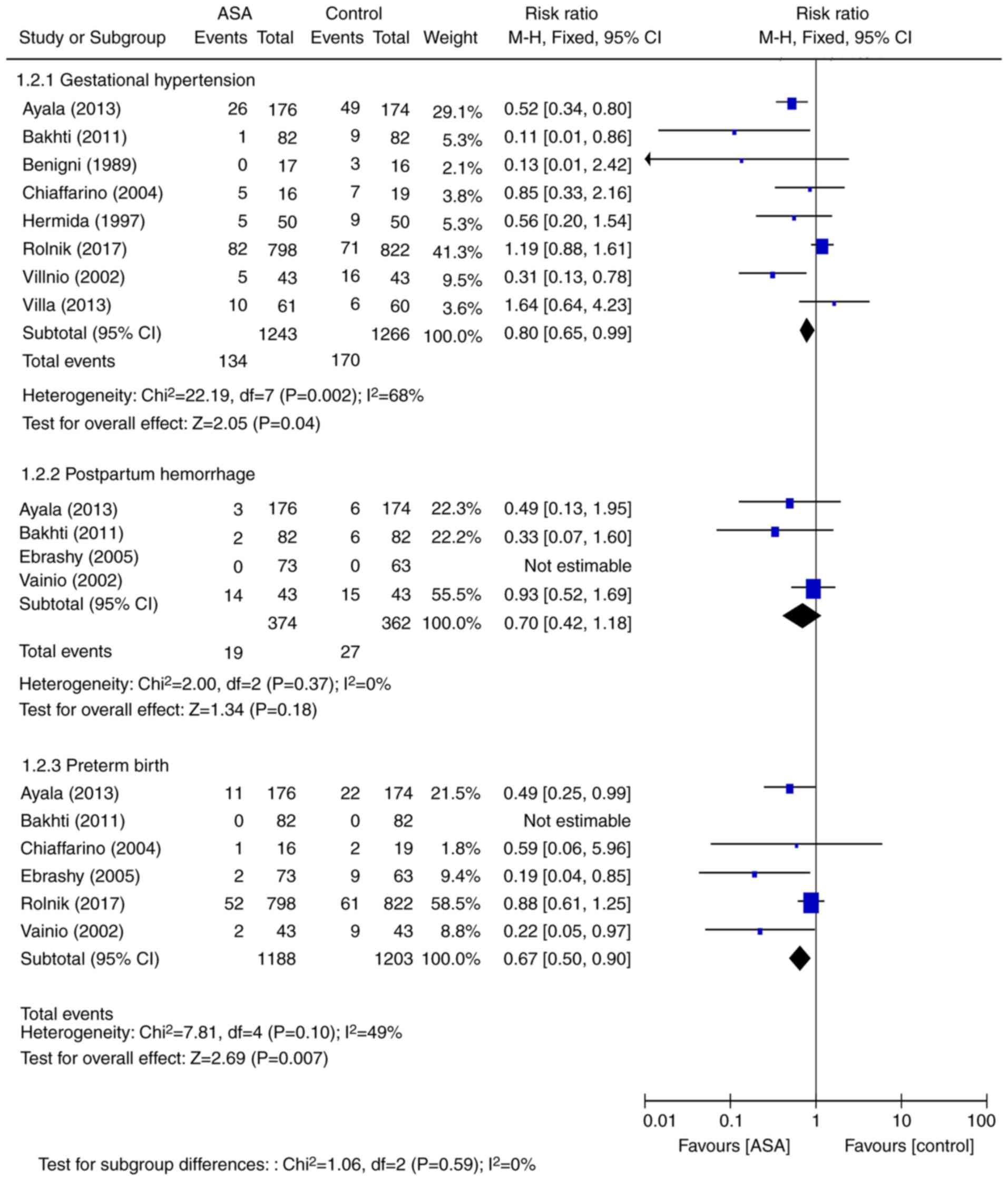
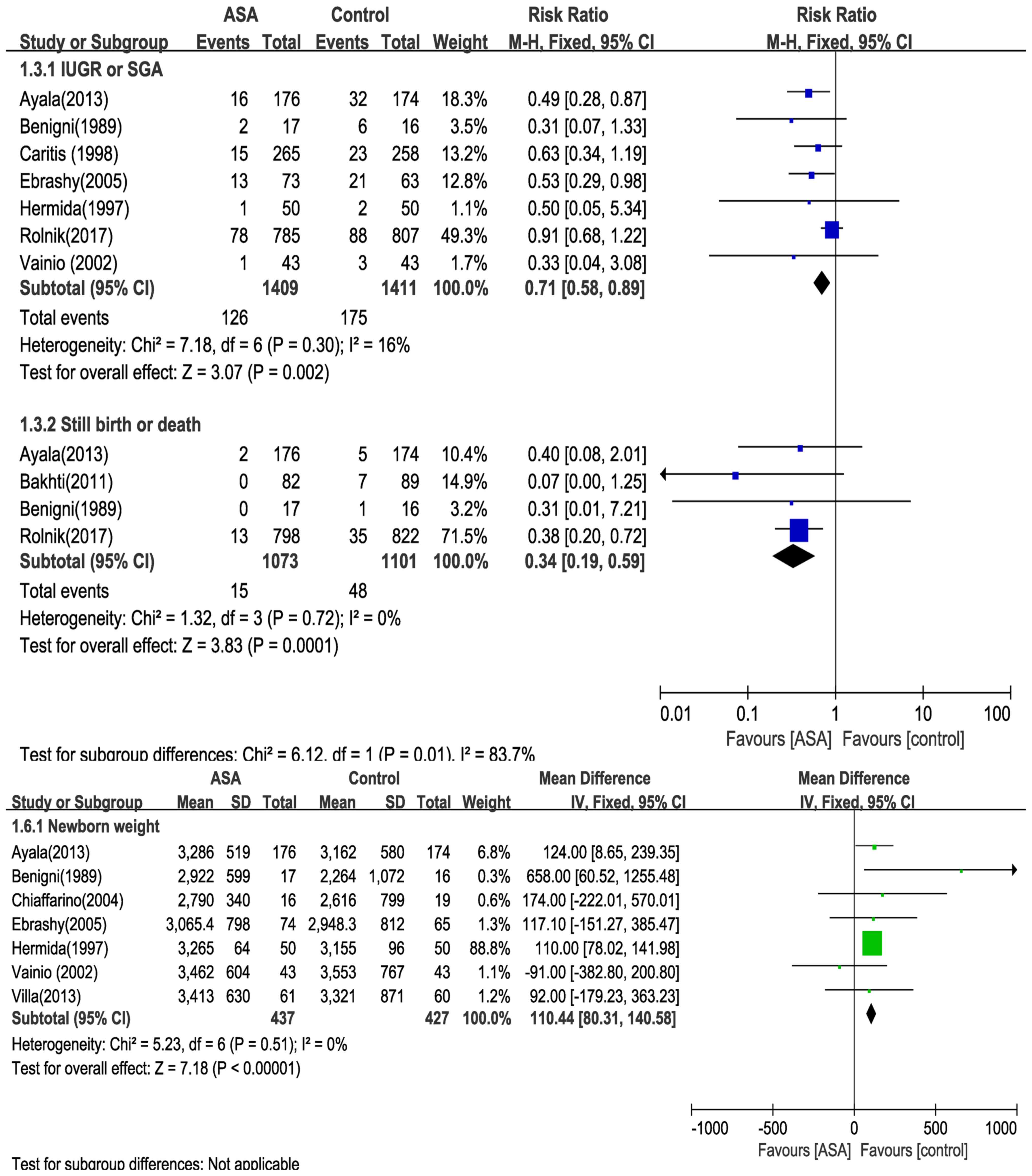
|
1
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tooher J, Thornton C, Makris A, Ogle R,
Korda A, Horvath J and Hennessy A: Hypertension in pregnancy and
long-term cardiovascular mortality: A retrospective cohort study.
Am J Obstet Gynecol. 214:722.e1–6. 2016. View Article : Google Scholar
|
|
3
|
Crandon AJ and Isherwood DM: Effect of
aspirin on incidence of pre-eclampsia. Lancet. 1:13561979.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Askie LM, Duley L, Hendersonsmart DJ and
Stewart LA: PARIS Collaborative Group: Antiplatelet agents for
prevention of pre-eclampsia: A meta-analysis of individual patient
data. Lancet. 369:1791–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Groeneveld E, Lambers MJ, Lambalk CB,
Broeze KA, Haapsamo M, de Sutter P, Schoot BC, Schats R, Mol BW and
Hompes PG: Preconceptional low-dose aspirin for the prevention of
hypertensive pregnancy complications and preterm delivery after
IVF: A meta-analysis with individual patient data. Hum Reprod.
28:1480–1488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
CLASP: A randomized trial of low-dose
aspirin for the prevention and treatment of pre eclampsia among
9364 pregnant-women. CLASP (Collaborative Low-dose Aspirin Study in
Pregnancy) Collaborative Group. Lancet. 343:619–629. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rolnik DL, Wright D, Poon LC, O'Gorman N,
Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D,
Singh M, et al: Aspirin versus placebo in pregnancies at high risk
for preterm preeclampsia. N Engl J Med. 377:613–622. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
World Health Organization (WHO): WHO
recommendations for prevention and treatment of pre-eclampsia and
eclampsia. WHO; Geneva: 2011
|
|
9
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green S: Cochrane Handbook for Systematic
Reviews of Interventions: Cochrane Book Series. Wiley-Blackwell;
2008, View Article : Google Scholar
|
|
11
|
Roberge S, Nicolaides K, Demers S, Hyett
J, Chaillet N and Bujold E: The role of aspirin dose on the
prevention of preeclampsia and fetal growth restriction: Systematic
review and meta-analysis. Am J Obstet Gynecol. 216:110–120.e6.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberge S, Nicolaides KH, Demers S, Villa
P and Bujold E: Prevention of perinatal death and adverse perinatal
outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet
Gynecol. 41:491–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henderson JT, Whitlock EP, O'Conner E,
Senger CA, Thompson JH and Rowland MG: Low-dose aspirin for
prevention of morbidity and mortality from preeclampsia: A
systematic evidence review for the U.S. preventive services task
force. Ann Intern Med. 160:695–703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roberge S, Sibai B, McCaw-Binns A and
Bujold E: Low-dose aspirin in early gestation for prevention of
preeclampsia and small-for-gestational-age neonates: Meta-analysis
of large randomized trials. Am J Perinatol. 33:781–785. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergeron TS, Roberge S, Carpentier C,
Sibai B, Mccaw-Binns A and Bujold E: Prevention of preeclampsia
with aspirin in multiple gestations: A systematic review and
meta-analysis. Am J Perinatol. 33:605–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meher S and Alfirevic Z: Aspirin for
pre-eclampsia: Beware of subgroup meta-analysis. Ultrasound Obstet
Gynecol. 41:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abalos E, Duley L, Steyn DW and
Henderson-Smart DJ: Antihypertensive drug therapy for mild to
moderate hypertension during pregnancy. Cochrane Database Syst Rev:
Cd002252. 2007. View Article : Google Scholar
|
|
18
|
Levine RJ, Hauth JC, Curet LB, Sibai BM,
Catalano PM, Morris CD, DerSimonian R, Esterlitz JR, Raymond EG,
Bild DE, et al: Trial of calcium to prevent preeclampsia. N Engl J
Med. 337:69–76. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Conde-Agudelo A, Romero R, Kusanovic JP
and Hassan SS: Supplementation with vitamins C and E during
pregnancy for the prevention of preeclampsia and other adverse
maternal and perinatal outcomes: a systematic review and
metaanalysis. Am J Obstet Gynecol. 204:503.e1–12. 2011. View Article : Google Scholar
|
|
20
|
Costantine MM, Cleary K, Hebert MF, Ahmed
MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis
SN, et al: Safety and pharmacokinetics of pravastatin used for the
prevention of preeclampsia in high-risk pregnant women: a pilot
randomized controlled trial. Am J Obstet Gynecol.
214:720.e1–720.e17. 2016. View Article : Google Scholar
|
|
21
|
Knöfler M and Pollheimer J: IFPA award in
placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33 Suppl:S55–S62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quyyumi AA: Effects of aspirin on
endothelial dysfunction in atherosclerosis. Am J Cardiol.
82:31S–33S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarim E, Bal N, Kilicdag E, Kayaselcuk F,
Bağiş T and Kuscu E: Effects of aspirin on placenta and perinatal
outcomes in patients with poor obstetric history. Arch Gynecol
Obstet. 274:209–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bujold E, Roberge S, Lacasse Y, Bureau M,
Audibert F, Marcoux S, Forest JC and Giguère Y: Prevention of
preeclampsia and intrauterine growth restriction with aspirin
started in early pregnancy: A meta-analysis. Obstet Gynecol.
116:402–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meher S, Duley L, Hunter K and Askie L:
Antiplatelet therapy before or after 16 weeks' gestation for
preventing preeclampsia: an individual participant data
meta-analysis. Am J Obstet Gynecol. 216:121–128.e2. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borenstein M, Hedges LV, Higgins JPT and
Rothstein HR: Introduction to meta-analysis. John Wiley & Sons;
Hoboken, NJ: 2009, View Article : Google Scholar
|
|
27
|
Ayala DE, Ucieda R and Hermida RC:
Chronotherapy with low-dose aspirin for prevention of complications
in pregnancy. Chronobiol Int. 30:260–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bakhti A and Vaiman D: Prevention of
gravidic endothelial hypertension by aspirin treatment administered
from the 8th week of gestation. Hypertens Res.
34:1116–1120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Benigni A, Gregorini G, Frusca T,
Chiabrando C, Ballerini S, Valcamonico A, Orisio S, Piccinelli A,
Pinciroli V, Fanelli R, et al: Effect of low-dose aspirin on fetal
and maternal generation of thromboxane by platelets in women at
risk for pregnancy-induced hypertension. N Engl J Med. 321:357–362.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caritis S, Sibai B, Hauth J, Lindheimer
MD, Klebanoff M, Thom E, VanDorsten P, Landon M, Paul R, Miodovnik
M, et al: Low-dose aspirin to prevent preeclampsia in women at high
risk National Institute of Child Health and Human Development
Network of Maternal-Fetal Medicine Units. N Engl J Med.
338:701–705. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chiaffarino F, Parazzini F, Paladini D,
Acaia B, Ossola W, Marozio L, Facchinetti F and Del Giudice A: A
small randomised trial of low-dose aspirin in women at high risk of
pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 112:142–144. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ebrashy A, Ibrahim M, Marzook A and Yousef
D: Usefulness of aspirin therapy in high-risk pregnant women with
abnormal uterine artery Doppler ultrasound at 14–16 weeks
pregnancy: Randomized controlled clinical trial. Croat Med J.
46:826–831. 2005.PubMed/NCBI
|
|
33
|
Hermida RC, Ayala DE, Iglesias M, Mojón A,
Silva I, Ucieda R and Fernández JR: Time-dependent effects of
low-dose aspirin administration on blood pressure in pregnant
women. Hypertension. 30:589–595. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vainio M, Kujansuu E, Iso-Mustajärvi M and
Mäenpää J: Low dose acetylsalicylic acid in prevention of
pregnancy-induced hypertension and intrauterine growth retardation
in women with bilateral uterine artery notches. BJOG. 109:161–167.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villa PM, Kajantie E, Räikkönen K, Pesonen
AK, Hämäläinen E, Vainio M, Taipale P and Laivuori H: PREDO Study
group: Aspirin in the prevention of pre-eclampsia in high-risk
women: A randomised placebo-controlled PREDO Trial and a
meta-analysis of randomised trials. BJOG. 120:64–74. 2013.
View Article : Google Scholar : PubMed/NCBI
|