Introduction
Tuberculous meningitis (TBM) is an infectious
disease of the central nervous system caused by autologous
Mycobacterium tuberculosis infection, which can affect the
spinal cord in severe cases, thus resulting in high non-cure rate
of the disease (1,2). The early clinical symptoms of TBM are
not obvious, and the patients often cannot receive timely treatment
due to misdiagnosis and errors, thereby missing the best treatment
time (3). At present, the clinical
treatment method of TBM is mainly drug therapy, but the
commonly-used drugs will lead to obvious drug resistance, and thus
to significantly poor prognosis and even death, so it is urgent to
search for new therapeutic target drugs (4). Ní Cheallaigh et al (5) found that the autophagy response in
cerebrospinal fluid in meningitis patients is in an activated
state, and the activation of autophagy can reduce the damage of
inflammatory response to the body and produce a certain protective
effect. Autophagy is a kind of catabolic process involving
lysosomes, which is a bond that links the non-specific immune
system and specific immune system of the body, and plays an
important role in the infection control and immune homeostasis
maintenance. The autophagy response will affect the expression
levels of degradation substrate p62, autophagy constitutive protein
LC3-II and autophagy-associated protein Beclin1 (6–8). At
present, there has been no research on the relationship between TBM
and autophagy, and the correlation of autophagy activation with
TBM-induced inflammatory response remains unclear. The present
study aimed to assist the new mechanism of TBM and provide new
clinical treatment considerations.
Patients and methods
Objects
A total of 60 patients, including 28 males and 32
females aged 42–63 years, treated and diagnosed as TBM in Linyi
People's Hospital (Linyi, China) from March, 2014 to March, 2015
were enrolled in the study, while normal volunteers, including 10
males and 10 females aged 40–60 years, in the same age group were
the controls. The general clinical manifestations of the TBM
patients enrolled were fever, headache, nausea and vomiting;
patients were diagnosed via cerebrospinal fluid, imaging and many
other clinical indexes according to the international expert
diagnosis of tuberculosis in 2010, and suspicious cases were
excluded. Patients with other tuberculosis infections, other
consumptive diseases or a past history of central nervous system
diseases or malignant tumors were eliminated; all the patients
enrolled signed the informed consent and all clinicopathologic data
and treatment programs were saved. The present study was approved
by the Ethics Committee of Linyi People's Hospital.
Instruments and materials
TRIzol kit and Prime Script RT Reagent kit (both
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA);
rabbit monoclonal anti-p62 (cat. no. 23214), rabbit monoclonal
anti-Beclin1 (cat. no. 3495), rabbit monoclonal anti-LC3-II (cat.
no. 3868), rabbit monoclonal anti-glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) (cat. no. 5174) and horseradish
peroxidase-labeled rabbit secondary monoclonal antibody (cat. no.
7074), (dilution, 1:100) (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA) electrochemiluminescence liquid, and developing
powder (both from Invitrogen, Thermo Fisher Scientific, Inc.),
human interleukin-6 (IL-6) enzyme-linked immunosorbent assay
(ELISA) kit, human IL-10 ELISA kit and human tumor necrosis
factor-α (TNF-α) ELISA kit (all from Boster Biological Technology,
Pleasanton, CA, USA); TUNEL kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China); polymerase chain reaction (PCR)
instrument (ABI USA, Vernon, CA, USA); ultraviolet imaging system
(Biometra GmbH, Göttingen, Germany); other relevant equipment and
reagents are described in the relevant parts.
Grouping
A total of 60 TBM patients were selected as the
observation group, while 20 normal volunteers in the same age group
were selected as the control group. The general clinical data of
observation and control group were analyzed, and the results showed
that the differences in age and sex between the two groups were not
statistically significant (P>0.05).
Extraction of cerebrospinal fluid
The cerebrospinal fluid was obtained from patients
and volunteers via lumbar puncture (9): Under the lateral position, the puncture
site was disinfected and covered with surgical towel, followed by
puncture with a puncture needle to extract 5 ml fresh cerebrospinal
fluid; then the cerebrospinal fluid was stored at −80°C for standby
application.
Semi-quantitative RT-PCR
detection
Fresh cerebrospinal fluid (0.5 ml) was taken and
added with 1 ml pre-cooled TRIzol. After standing in ice bath for 5
min, 1 ml chloroform was added and vibrated for 15 sec. After
standing for 3 min, the mixture was centrifuged at 4°C at 9,000 × g
for 10 min. The supernatant was transferred, and the same volume of
isopropyl alcohol was added and vibrated evenly. After standing for
15 min, the mixture was centrifuged at 4°C at 9,000 × g for 10 min.
Then the supernatant was discarded, 75% ethanol was slowly added
along the tube wall and manually turned upside down to wash 5
times, followed by centrifugation at 4°C for 2 min at 10,800 × g.
The above operation was repeated twice and then the ethanol was
discarded. The cover of centrifuge tube was opened and the ethanol
evaporated naturally. After that, 80 µl diethyl pyrocarbonate
(DEPC) water was added to dissolve RNA, and then 2 µl mixture was
taken and diluted with 98 µl DEPC water. The concentration of RNA
and A260/A280 were measured using the nucleic acid protein
quantometer. Whether RNA was degraded was detected via agarose gel
electrophoresis. The results revealed that RNA in each group was of
higher quality and could be used for subsequent experiments. A
total of 4 µl 5× Prime Script Buffer, 1 µl Prime Script RT Enzyme
MixI and 1 µl OligodT Primer were added and the RNA was added until
the total volume was 800 ng according to the concentration of RNA.
Then RNase-free water was added to prepare it into the 20 µl
system, followed by reverse transcription at 37°C for 15 min and
85°C for 5 sec. After cDNA was taken, the primer and PCR Master Mix
were added in proportion for PCR according to the conditions shown
in Table I. The primer sequences are
shown in Table II. After that, the
expression level of each gene was detected via 2% agarose gel
electrophoresis with GAPDH as the internal reference. The
expression levels of different genes in each group were determined
by p62/GAPDH, Beclin1/GAPDH and LC3-II/GAPDH, respectively.
 | Table I.PCR procedures. |
Table I.
PCR procedures.
| Reaction
temperature | Reaction time | Cycles |
|---|
| 95°C | 5 min |
|
| 95°C | 30 sec |
|
| 59°C | 40 sec | 30 cycles |
| 72°C | 1 min |
|
| 72°C | 5 min |
|
 | Table II.PCR primers. |
Table II.
PCR primers.
| Genes | Sequence |
|---|
| p62 | F:
5′-TGCCCCTCTTCTGTCTCATAGT-3′ |
|
| R:
5′-CACTTGTTTTGCTGCCCTAAAT-3′ |
| Beclin1 | F:
5′-CTGGGGACCTTTTTGACATC-3′ |
|
| R:
5′-TTGCGGTTCTTTTCCACGTC-3′ |
| LC3-II | F:
5′-AATCCCGGTGATAATAGAAC-3′ |
|
| R:
5′-TTTCATCCCGAACGTCTCC-3′ |
| GAPDH | F:
5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ |
|
| R:
5′-AAATGAGCCCCAGCCTTCTCCATG-3′ |
Detection of apoptosis in
cerebrospinal fluid via TUNEL
The fresh cerebrospinal fluid was added into the
6-well plate with slides, added with 4% paraformaldehyde and fixed
at 4°C for 30 min. After fixation, it was washed with pre-cooled
phosphate buffered saline (PBS) 3 times (5 min/each time). FJ-B dye
solution in the TUNEL kit was diluted and added into the treated
samples in each group, followed by incubation at 4°C for 90 min and
washing with PBS. Finally, the samples were sealed with DAPI
glycerin, observed and photographed under an inverted fluorescence
microscope (Olympus Corporation, Tokyo, Japan).
Detection of inflammatory factors in
cerebrospinal fluid via ELISA
The standard solution in IL-6, −10 and TNF-α ELISA
kits was diluted to 800, 600, 400, 300, 200 and 100 pg/ml for
determination of standard curve. The biotin-labeled antibodies were
diluted 100 times to prepare it into the working solution. After
100 µl sample or standard substance was added into each well, the
plate was sealed for reaction at 37°C for 60 min. After the
reaction was over, IL-6, −10 and TNF-α antibodies were added
following strictly the instructions of the kit for reaction for 90
min. After the reaction was over, 300 µl buffer was added. After
standing for 1 min, the plate was patted dry and washed 5 times.
Then 100 µl avidin-peroxidase compound was added, followed by plate
sealing and reaction at 37°C for 60 min. The OD value at the
wavelength of 450 nm of each well was measured after the stop
buffer was added. Finally, the sample concentration of each group
was analyzed and calculated using CurveExpert 1.5 software.
Statistical analysis
The data in the present study are presented as mean
± standard deviation (SD) and SPSS 19.0 software (SPSS Inc.,
Chicago, IL, USA) was used for data processing. A t-test was used
for measurement data. Analysis of variance was used for comparison
among groups, t-test was used for comparison of means between the
two groups, and Pearson's test was used for correlation of factors.
P<0.05 was considered to indicate a statistically significant
diference.
Results
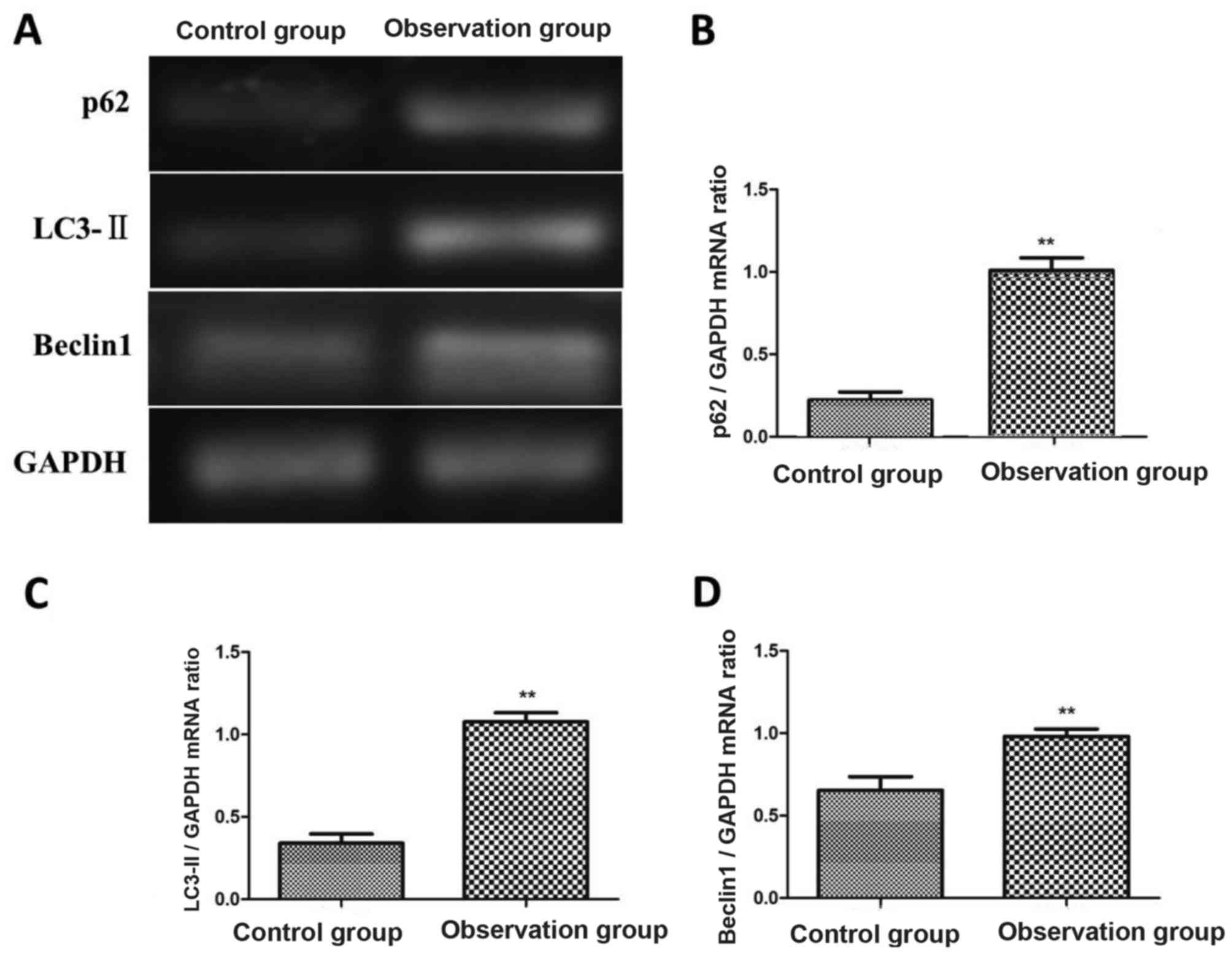
Expression of autophagy-related
genes
The expression levels of p62, Beclin1 and LC3-II in
cerebrospinal fluid of patients in observation group and control
group were detected via semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR). The results
showed that the mRNA levels of p62, Beclin1 and LC3-II in
observation group were significantly higher than those in control
group, and the differences were statistically significant
(P<0.01). Patients in observation group were divided based on
the mRNA expression level of LC3-II: Patients with the mRNA
expression level of LC3-II lower or equal to that in control group
as the low or normal expression group (n=22), and those with the
mRNA expression level of LC3-II higher than that in control group
as the high expression group (n=38) (Fig. 1).
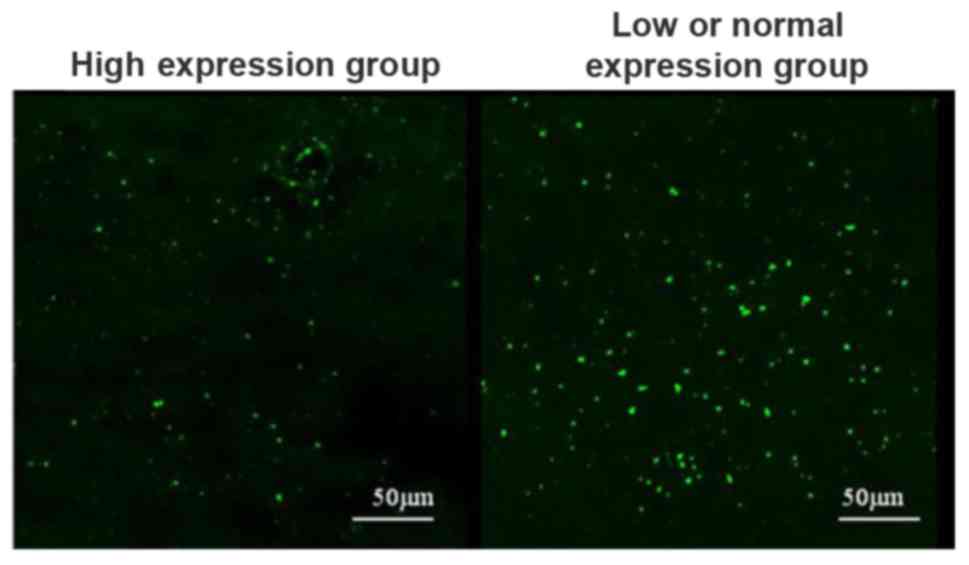
Detection of cell apoptosis in
cerebrospinal fluid via TUNEL
Cell apoptosis in cerebrospinal fluid in high
expression group and low or normal expression group was detected
using the TUNEL kit. The results showed that the number of
apoptotic cells in cerebrospinal fluid in high expression group was
significantly lower than that in low or normal expression groups
(Fig. 2).
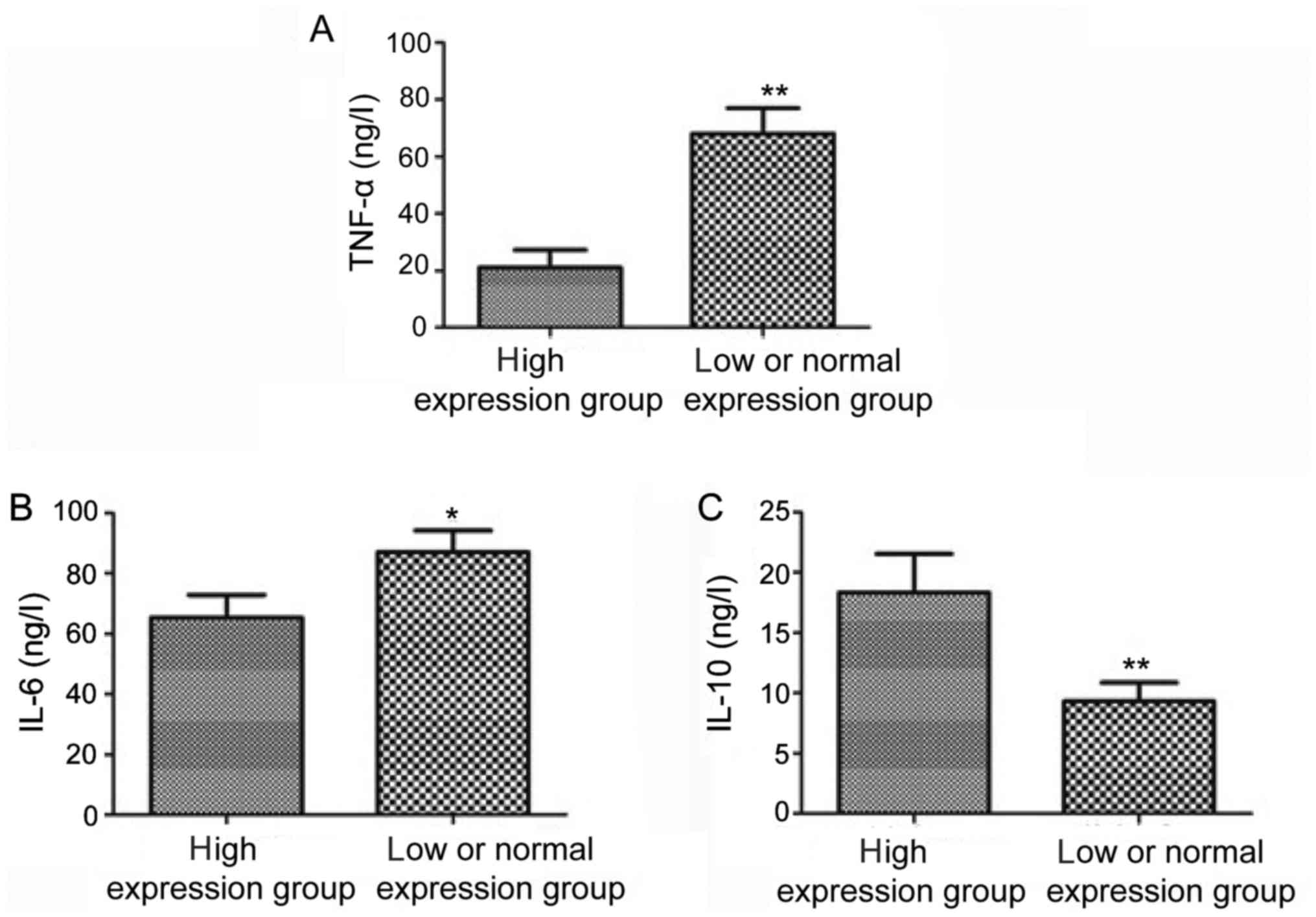
Levels of inflammatory factors in
cerebrospinal fluid
The levels of inflammatory factors in cerebrospinal
fluid in high expression group and low or normal expression group
were detected using the ELISA kit. The results revealed that the
level of IL-10 in cerebrospinal fluid in high expression group was
obviously higher than that in low or normal expression group, but
the levels of IL-6 and TNF-α were obviously lower than those in low
or normal expression group; the differences were statistically
significant (P<0.05, P<0.01) (Fig.
3).
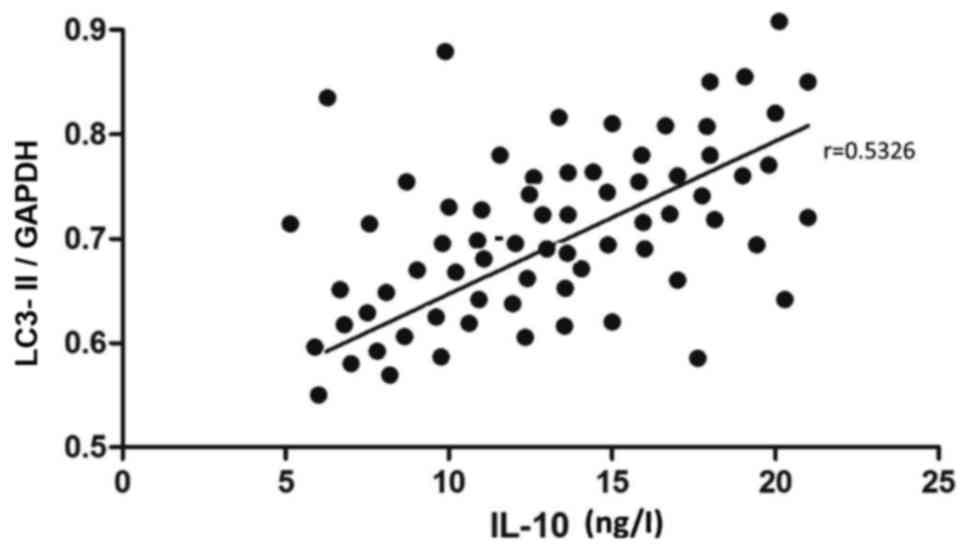
Analysis of correlation between
autophagy-related genes and inflammatory factors
The relative expression level of LC3-II mRNA and the
contents of IL-6, −10 and TNF-α in cerebrospinal fluid in TBM
patients were recorded in detail, followed by correlation analysis.
The mRNA level of LC3-II was positively correlated with the content
of IL-10 (r=0.5326, P<0.05) (Fig.
4). The mRNA level of LC3-II was negatively correlated with the
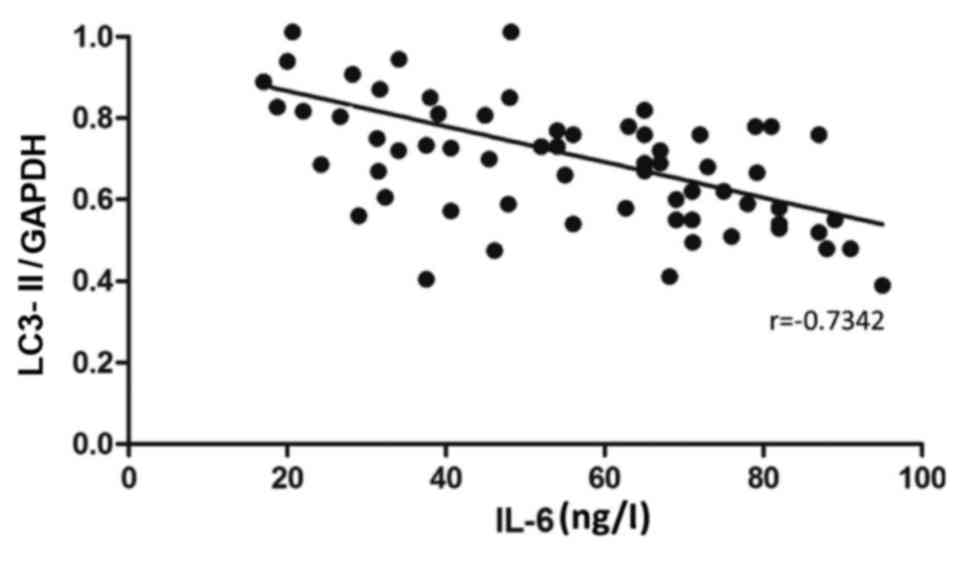
content of IL-6 (r=−0.7342, P<0.05) (Fig. 5). Besides, the mRNA level of LC3-II
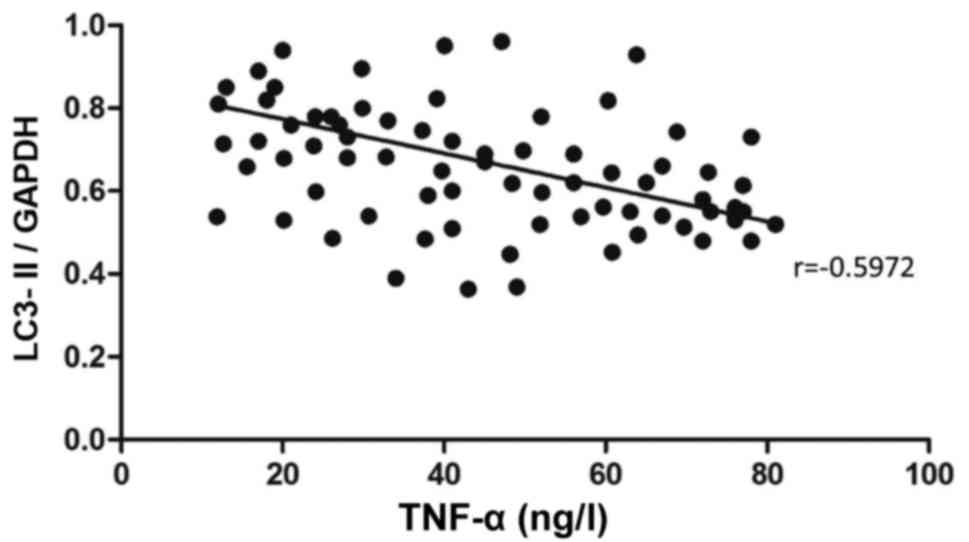
was also negatively correlated with the content of TNF-α
(r=−0.5972, P<0.05) (Fig. 6).
Discussion
TBM is a central inflammatory disease caused by a
single pathogenic bacterium, which can cause death in severe cases.
Therefore, the treatment and prevention of TBM is an urgent problem
(10,11). Inflammatory factors are small
molecule polypeptides that mediate the inflammatory response, which
is synthesized and released by the body after stimulation. The
level of inflammatory factors is closely related to the diagnosis,
treatment and prognosis of inflammation (12,13).
Marais et al (14) revealed
that the levels of inflammatory factors in cerebrospinal fluid in
TBM patients are significantly different from those in normal
people, so the changes in inflammatory factor levels can be used as
auxiliary indexes for TBM diagnosis. The study of Netea-Maier et
al (15) found that LPS-induced
inflammation in mice can activate autophagy in the body, and
autophagy agonists can significantly reduce the increased
pro-inflammatory cytokines caused by inflammatory response, reduce
the inflammatory response and increase the survival rate of mice to
a certain extent. Autophagy is a self-digestive reaction in the
body, and moderate autophagy can protect the body. When autophagy
is activated, a large number of free membrane structures can be
formed, which can wrap the damaged organelles, protein aggregates
and other substances to be degraded. With the gradual maturation,
it can bind with lysosomes to form autophagic lysosomes; moreover,
proteolytic enzymes in lysosomes can effectively degrade its
internal components, and reduce the levels of long-lived protein
and some cytokines in the body (16). Lapaquette et al (17) found that autophagy is closely related
to most cellular stress response pathways, and autophagy affects
multiple parts of the immune system and participates in the
regulation of inflammatory responses.
In the present study, the expression of
autophagy-related genes in cerebrospinal fluid in TBM patients were
investigated, the levels of inflammatory factors in cerebrospinal
fluid in TBM patients were analyzed and the correlation between
contents of inflammatory factors and autophagy-related genes was
also analyzed. It was found in the present study that the mRNA
expression levels of p62, LC3-II and Beclin1 in cerebrospinal fluid
in TBM patients were significantly higher than those in normal
people. The above results indicated that autophagy is in an
activated state in cerebrospinal fluid in TBM patients and involved
in the TBM-induced inflammatory response. Moors et al
(18) found that the level of
autophagy marker protein LC3-II in the striatum of patients with
Parkinson's disease is increased and the autophagic degradation
substrate p62 is also increased, which is consistent with the
results of this experiment. Studies have found that the chronic
inflammation of the nervous system is an important factor of
inducing Parkinson's disease; in other words, the inflammatory
response can activate autophagy to a certain extent. The levels of
inflammatory factors in cerebrospinal fluid in TBM patients were
detected via ELISA. The results revealed that the levels of
pro-inflammatory cytokines, IL-6 and TNF-α, in cerebrospinal fluid
in patients with high expression of LC3-II mRNA were significantly
lower than those in patients with low or normal expression, but the
level of anti-inflammatory factor IL-10 in cerebrospinal fluid in
patients with high expression of LC3-II mRNA was significantly
increased. The activation of autophagy can reduce the levels of
pro-inflammatory cytokines in cerebrospinal fluid in TBM patients
through degradation. When the levels of inflammatory factors in
cerebrospinal fluid are decreased, the levels of anti-inflammatory
factors will be increased to a certain extent under the regulation
of negative feedback mechanism, thus further reducing the
inflammatory response and protecting the body (19). The study by Nakahira et al
(20) found that the relationship
between inflammatory response and autophagy is complex,
inflammatory stimuli can activate autophagy, and autophagy can
further degrade the inflammatory stimuli in the body and reduce the
inflammatory response. In this experiment, the correlation between
LC3-II mRNA level and inflammatory factor levels was analyzed, and
the results revealed that the LC3-II mRNA level was positively
correlated with IL-10, but negatively correlated with IL-6 and
TNF-α. The above results showed that autophagy is closely related
to the inflammatory response of TBM, which may treat TBM through
mediating the smooth autophagy activity in the body to remove waste
accumulated in the body in time under pathological conditions, such
as damaged organelles and protein aggregates. However, there were
some shortcomings in this study. The mechanism of autophagy-related
genes in regulating inflammatory factor levels in cerebrospinal
fluid in TBM patients was not deeply studied, but it will be a
direction in later research.
In conclusion, autophagy is activated in
cerebrospinal fluid in TBM patients, and the mRNA levels of Beclin1
and LC3-II in cerebrospinal fluid are increased, which is
correlated with the inflammatory factor levels in cerebrospinal
fluid, so it can provide reference for the clinical treatment of
TBM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM wrote the manuscript and participated with
statistical analysis. YoZ contributed to conception and design. YaZ
helped with the data of TUNEL. XW and YL conducted
semi-quantitative RT-PCR and ELISA method. AM interpreted the
results. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Linyi People's Hospital (Linyi, China). All the
patients enrolled signed the written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palomo FS, Rivero MGC, Quiles MG, Pinto
FP, Machado AMO and Pignatari Carlos Campos A: Comparison of DNA
extraction protocols and molecular targets to diagnose tuberculous
meningitis. Tuberc Res Treat. 2017:50890462017.PubMed/NCBI
|
|
2
|
Thwaites G, Caws M, Chau TT, D'Sa A, Lan
NT, Huyen MN, Gagneux S, Anh PT, Tho DQ, Torok E, et al:
Relationship between Mycobacterium tuberculosis genotype and
the clinical phenotype of pulmonary and meningeal tuberculosis. J
Clin Microbiol. 46:1363–1368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta R, Garg RK, Jain A, Malhotra HS,
Verma R and Sharma PK: Spinal cord and spinal nerve root
involvement (myeloradiculopathy) in tuberculous meningitis.
Medicine (Baltimore). 94:e4042015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iype T, Pillai AK, Cherian A, Nujum ZT,
Pushpa C, Dae D and Krishnapillai V: Major outcomes of patients
with tuberculous meningitis on directly observed thrice a week
regime. Ann Indian Acad Neurol. 17:281–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheallaigh Ní C, Keane J, Lavelle EC, Hope
JC and Harris J: Autophagy in the immune response to tuberculosis:
Clinical perspectives. Clin Exp Immunol. 164:291–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie Y, Kang R, Sun X, Zhong M, Huang J,
Klionsky DJ and Tang D: Posttranslational modification of
autophagy-related proteins in macroautophagy. Autophagy. 11:28–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou XJ and Zhang H: Autophagy in
immunity: Implications in etiology of autoimmune/autoinflammatory
diseases. Autophagy. 8:1286–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Levine B: Autosis and autophagic
cell death: The dark side of autophagy. Cell Death Differ.
22:367–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Bu H, Hong K, Yin H, Zou Y-L, Geng
S-J, Zheng MM and He JY: Genetic polymorphisms of CCL1 rs2072069
G/A and TLR2 rs3804099 T/C in pulmonary or meningeal tuberculosis
patients. Int J Clin Exp Pathol. 8:12608–12620. 2015.PubMed/NCBI
|
|
10
|
Christensen A-SH, Roed C, Omland LH,
Andersen PH, Obel N and Andersen ÅB: Long-term mortality in
patients with tuberculous meningitis: A Danish nationwide cohort
study. PLoS One. 6:713–725. 2011. View Article : Google Scholar
|
|
11
|
Taheri Sanei M, Karimi MA, Haghighatkhah
H, Pourghorban R, Samadian M and Kasmaei Delavar H: Central nervous
system tuberculosis: An imaging-focused review of a reemerging
disease. Radiol Res Pract. 2015:2028062015.PubMed/NCBI
|
|
12
|
Robb CT, Regan KH, Dorward DA and Rossi
AG: Key mechanisms governing resolution of lung inflammation. Semin
Immunopathol. 38:425–448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slavich GM, Irwin MR, Einaudi S,
Wasniewska M, Miccoli M and Baroncelli GI: From stress to
inflammation and major depressive disorder: A social signal
transduction theory of depression. Psychol Bull. 140:774–815. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marais S, Wilkinson KA, Lesosky M,
Coussens AK, Deffur A, Pepper DJ, Schutz C, Ismail Z, Meintjes G
and Wilkinson RJ: Neutrophil-associated central nervous system
inflammation in tuberculous meningitis immune reconstitution
inflammatory syndrome. Clin Infect Dis. 59:1638–1647. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Netea-Maier RT, Plantinga TS, van de
Veerdonk FL, Smit JW and Netea MG: Modulation of inflammation by
autophagy: Consequences for human disease. Autophagy. 12:245–260.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber TB, Edelstein CL, Hartleben B, Inoki
K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, et
al: Emerging role of autophagy in kidney function, diseases and
aging. Autophagy. 8:1009–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lapaquette P, Guzzo J, Bretillon L and
Bringer M-A: Cellular and molecular connections between autophagy
and inflammation. Mediators Inflamm. 2015:3984832015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moors TE, Hoozemans JJM, Ingrassia A,
Beccari T, Parnetti L, Chartier-Harlin M-C and van de Berg WDJ:
Therapeutic potential of autophagy-enhancing agents in Parkinson's
disease. Mol Neurodegener. 12:266–271. 2017. View Article : Google Scholar
|
|
19
|
Chen Z-H, Wu Y-F, Wang P-L, Wu Y-P, Li
Z-Y, Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, et al: Autophagy is
essential for ultrafine particle-induced inflammation and mucus
hyperproduction in airway epithelium. Autophagy. 12:297–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakahira K, Cloonan SM, Mizumura K, Choi
AM and Ryter SW: Autophagy: A crucial moderator of redox balance,
inflammation, and apoptosis in lung disease. Antioxid Redox Signal.
20:474–494. 2014. View Article : Google Scholar : PubMed/NCBI
|