Introduction
Ovarian cancer is a lethal gynecological malignancy,
the incidence of which is gradually increasing (1). Notably, survival rates differ depending
on the clinical stage at diagnosis. In particular, the 5-year
survival rate for patients at all stages of ovarian cancer is
estimated to be 40% following surgery and paclitaxel/cisplatin
chemotherapy. This rate increases to 70% if patients are diagnosed
at an early stage (2,3), indicating the importance of early
diagnosis. However, only 15% patients are diagnosed at an early
stage of ovarian cancer, whereas approximately 80% cases are
diagnosed at an advanced stage (stage III or IV) with extensive
abdominal dissemination or distant metastasis (4).
Epithelial ovarian cancer, which constitutes 70–90%
of all ovarian cancers, represents the predominant pathological
type of this disease (5). The
biomarker most widely used to monitor epithelial ovarian cancer is
serum cancer antigen 125 (CA 125), although this marker exhibits
limited sensitivity and specificity in early detection of ovarian
cancer (2,6,7). A large
randomized controlled trial using a combination of serum CA 125
measurement and ultrasonography for screening ovarian cancer in
asymptomatic women showed no benefit in terms of decreased
mortality (8). It appears that the
only population that may benefit from such ovarian cancer screening
may be women with BRCA1 and BRCA2 mutations with or
without a family history of ovarian cancer (9,10). Thus,
reliable methods for screening specific and sensitive markers for
early detection of ovarian cancer are required.
Chloride intracellular channel 1 (CLIC1) was first
described in the human mononuclear cell line U937 by Valenzuela
et al in 1997 (11). Since
its discovery, CLIC1 has been identified to have a role in various
fundamental biological processes, including maintenance of cell
volume, ion homeostasis, trans-epithelial transport, and pH
regulation. Studies have shown that CLIC1 regulates the cell cycle,
as well as cell proliferation, apoptosis, and differentiation
(12,13). Recently, CLIC1 was shown to be
upregulated in gastric, pancreatic, and liver cancers (14–16).
However, although CLIC1 has been associated with
several cancers, its clinical significance in ovarian cancer has
not yet been determined. In this study, we investigated CLIC1
expression in subjects with ovarian cancer and in healthy controls
to elucidate the association between CLIC1 levels and pathological
features in patients with epithelial ovarian cancer. All cases were
followed-up to assess the prognostic value of CLIC1 based on
progression-free survival (PFS) and overall survival (OS).
Materials and methods
Patients and tissues
Clinical samples were obtained between 2007 and 2016
from patients undergoing surgery in Beijing Chao-Yang Hospital,
Capital Medical University. The study protocol was approved by the
Ethics Committee Board of Beijing Chao-Yang Hospital, and informed
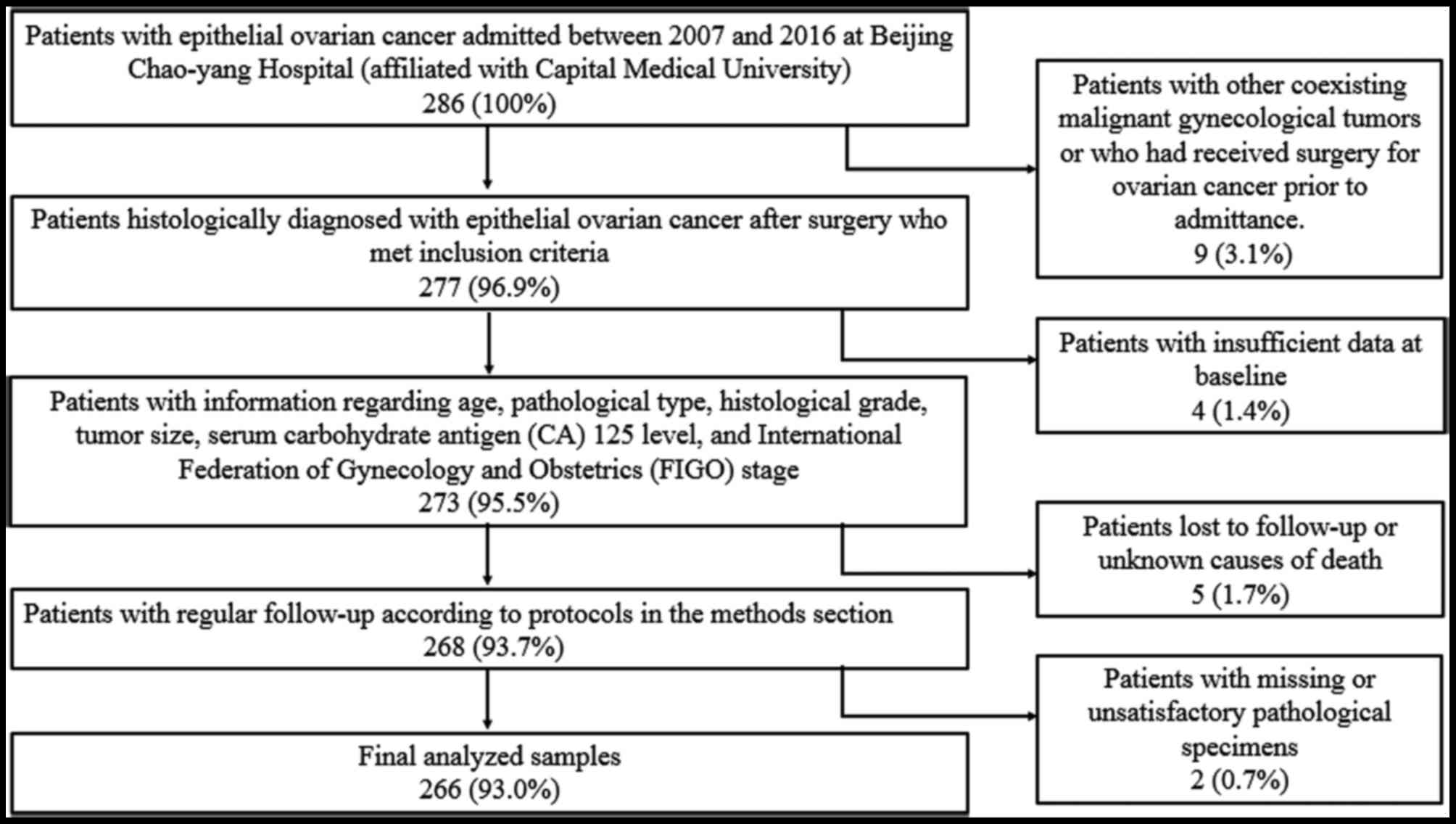
consent was obtained from all participating patients. In total, 266
patients with epithelial ovarian cancer were included in this
study. All tissues were formalin-fixed and embedded in paraffin
blocks. All diagnoses were confirmed by pathological examination.
Patients with additional confirmed malignant gynecological tumors
or those who received surgical treatments for ovarian cancer were
also excluded. Patients with insufficient data or those who died of
unknown causes were excluded from follow-up (Fig. 1).
Clinicopathological data, including age,
pathological type, histological grade, tumor size, serum cancer
antigen (CA) 125 level and International Federation of Gynecology
and Obstetrics (FIGO) stage, were retrospectively reviewed. Patient
age ranged from 35 to 74 years, with a mean age of 54.3±9.2 years.
Among the 266 patients with epithelial ovarian cancer, 188 (70.7%)
were diagnosed with serous adenocarcinoma, 26 (9.8%) had
endometrioid adenocarcinoma, 22 (8.3%) had clear cell
adenocarcinoma, and 30 (11.2%) had mucous adenocarcinoma. Of these
cases, 72 were in stages I–II, whereas 194 were in stages III–IV.
All cases were followed-up and assessed in outpatient clinics or
over the telephone at 3-month intervals for the first two years,
4–6 month intervals for the following three years and at yearly
intervals if the patients were disease-free for five years. All
patients were followed-up until end of 2017 or until death, with an
average follow-up of 4.4 years.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total tissue RNA was extracted using TRIzol (Takara
Bio, Inc., Otsu, Japan) according to the manufacturer's
instructions. The RT reaction was conducted in a 20-µl reaction
volume using a One Step PrimeScript RT-PCR Kit (Takara Bio, Inc.)
on an ABI Prism 7500 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The RT reaction
was performed at 42°C for 5 min and 95°C for 10 sec. The PCR
amplification was performed at 95°C for 5 sec and 60°C for 34 sec
for 40 consecutive cycles. Human β-actin was used as an endogenous
control. Relative mRNA levels were calculated using the
2−ΔΔCq method (17). The
following primers were used: CLIC1 forward,
5′-GTTGACACCAAAAGGCGGAC-3′, and reverse,
5′-GGGCTTTCAGGAGTCCCTTC-3′; β-actin forward,
5′-CCTGGCACCCAGCACAAT-3′, and reverse: 5′-GCTGATCCACATCTGCTGGAA-3′.
All primers were synthesized by GenePharma (Shanghai, China).
Western blot analysis
Western blotting was performed on cancer and healthy
samples. The tissues were stored at −80°C. Total protein was
extracted by ristocetin-induced platelet aggregation (Beyotime
Institute of Biotechnology, Jiangsu, China) in the presence of
phosphatase and protease inhibitors according to the manufacturer's
instructions (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Protein concentrations were determined using a
bicinchoninic acid (BCA) protein assay kit according to the
manufacturer's instructions (Boster Biological Technology Co.,
Ltd., Wuhan, China). Thirty micrograms of protein per lane were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred to 0.45 µm nitrocellulose filter
membrane (EMD Millipore, Billerica, MA, USA), followed by blocking
with skim milk at room temperature for 2 h. Subsequently, the
membranes were incubated with mouse anti-human CLIC1 antibody
(ab77214; 1:750 dilution; Abcam, Cambridge, UK) or mouse anti-human
β-actin (1:2,000 dilution; Boster Biological Technology,
Pleasanton, CA, USA) overnight at 4°C. The membranes were incubated
with horseradish peroxidase-conjugated goat anti-mouse antibody
(1:2,500 dilution; Boster Biological Technology) for 1 h and then
subjected to semi-quantification by electrochemiluminescence
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical staining of tissue
microarrays
CLIC1 levels in epithelial ovarian cancer tissues
were evaluated by immunohistochemical staining of issue microarray
samples. All specimens were obtained from the Pathology Center of
the Beijing Chao-Yang Hospital, Capital Medical University. A total
of 36 ovarian samples were included in the study as healthy
controls. Briefly, formalin-fixed and paraffin-embedded samples
were cut into 4-µm-thick sections and placed on slides. The tissues
were dewaxed by baking at 70°C for 60 min. Sections were
deparaffinized with xylene (10 min each for 3 times) and rehydrated
in graded alcohol (5 min each). For antigen retrieval, tissue
sections were heated in an autoclave with citrate buffer solution
(10 mM, pH 6.0) for 20 min. Blocking of endogenous peroxidase
activity was performed using 3% hydrogen peroxide, followed by
rinsing thrice in phosphate-buffered saline. The slides were
incubated in normal goat serum at room temperature for 60 min and
then labeled with mouse anti-human CLIC1 antibody (ab77214; Abcam)
at a 1:200 dilution overnight at 4°C. A horseradish
peroxidase-conjugated goat anti-mouse antibody (Zhongshan Golden
Bridge Biotechnology, Beijing, China) was added as the secondary
antibody for 60 min at room temperature, followed by
diaminobenzidine coloration, hematoxylin counterstaining, and
neutral resin sealing. Ovarian cancer cell line A2780 was used as
the positive control. Mouse nonimmune serum and phosphate-buffered
saline with no visible staining were used as the negative and blank
controls, respectively.
Evaluation of immunohistochemical
staining
All samples were independently examined and
evaluated by two senior pathologists who had no knowledge of
patient information. Positivity was determined by the presence of
yellow or brown staining in the cytoplasmic membrane or nucleus.
For semi-quantification, an H-score-based assessment that combined
both the percentage (0–100%) of stained cells and staining
intensity (0, none; 1, weak; 2, moderate; 3, strong) was performed
using the following formula: H-score=∑ percentage × intensity. A
digital pathological section scanner KF-PRO-005 (KFBIO, Ningbo,
China) was used to score tissues to avoid subjectivity.
Statistical analysis
Statistical analyses were conducted using the SPSS
statistical package for Windows (version 21.0; IBM Corp., Armonk,
NY, USA). Chi-square or Kruskal-Wallis tests were performed to
compare categorical variables. Associations between clinical
features and CLIC1 expression were examined by Student's t-test and
one-way analysis of variance with Fisher's Least Significant
Difference post hoc test. Univariate survival analyses were
estimated using the Kaplan-Meier method and compared by log-rank
testing. Cox regression models were used in multivariate analyses.
All experiments were repeated thrice. A two-sided P<0.05 was
considered to indicate a statistically significant difference.
Results
Western blotting and RT-qPCR analyses
of CLIC1 expression
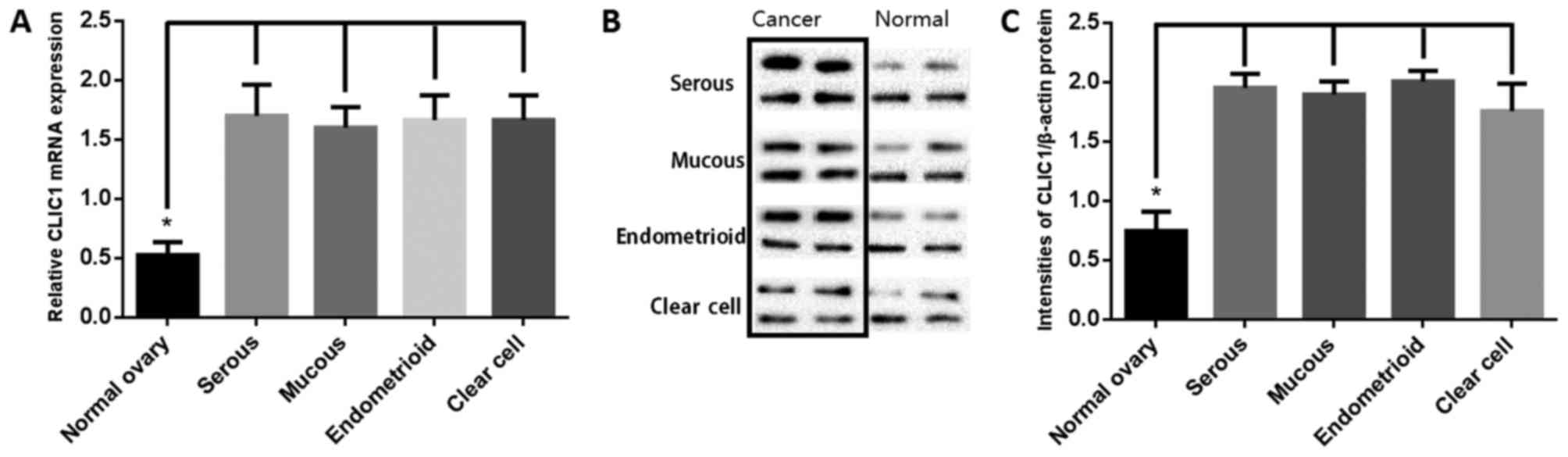
CLIC1 mRNA and protein levels were analyzed by
RT-qPCR and western blotting of 16 each of ovarian cancer tissues
(four each of serous, mucous, endometrioid, and clear cell
adenocarcinoma) and paired healthy ovarian tissues. As shown in
Fig. 2A, CLIC1 transcript levels
were significantly higher in cancer samples than in healthy ovarian
tissues. Western blotting was performed to determine the CLIC1
levels (Fig. 2B). Results of
semi-quantitative western blotting indicated that CLIC1 levels in
ovarian cancer tissues was significantly higher than that in
healthy ovarian tissues, which is consistent with the RT-qPCR
results (Fig. 2C).
Immunohistochemical assessment of
CLIC1 levels in epithelial ovarian cancer
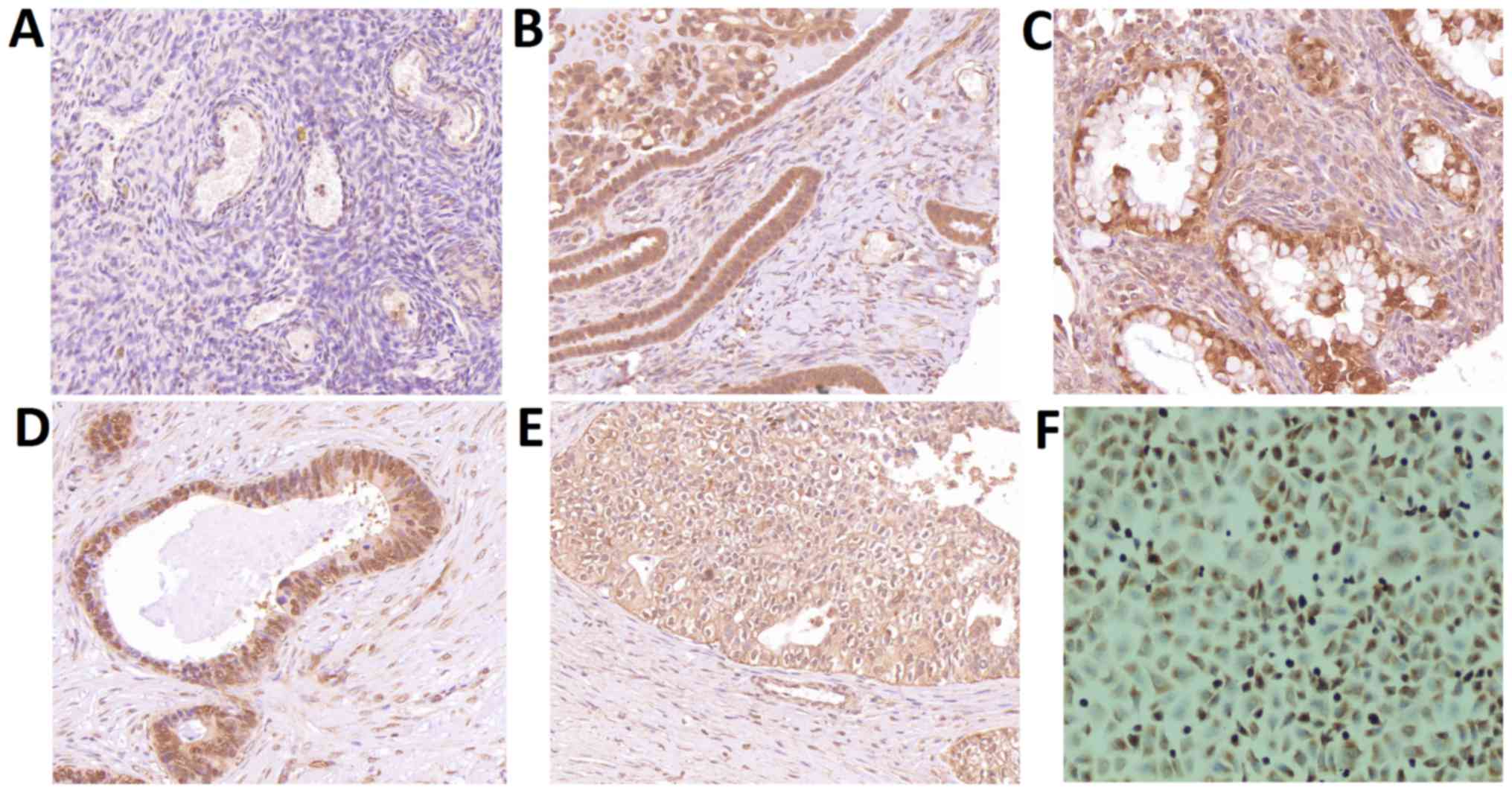
Immunohistochemical staining showed negative
staining in healthy ovarian tissues and paracancerous tissues,
while positive CLIC1 signals were observed in the four types of
epithelial ovarian cancer cells (Fig.
3). CLIC1 staining signals were significantly higher in the 266
samples from patients with epithelial ovarian cancer (P<0.001)
than that observed in healthy controls. There was no difference
between different pathological types.
Correlations between CLIC1 expression
and clinicopathological features
The relationship between CLIC1 expression and
clinicopathological parameters in patients with epithelial ovarian
cancer, including age, pathological type, histological grade, tumor
size, serum CA-125 levels, FIGO stage, and cisplatin resistance,
were evaluated. However, there were no significant associations
between CLIC1 levels and patient age, tumor type, FIGO stage, or
cisplatin resistance in patients with epithelial ovarian cancer
(Table I), wherein cisplatin
resistance was associated with a PFS interval less than six months
after cisplatin therapy. For the histological grade, a markedly
higher level of CLIC1 was observed in patients with high-grade
tumors than those with low-grade tumors. Furthermore, patients with
cisplatin resistance had higher levels of CLIC1 than those
sensitive to cisplatin therapy (P<0.001).
 | Table I.Association between CLIC1 expression
and clinicopathological features in patients with epithelial
ovarian cancer. |
Table I.
Association between CLIC1 expression
and clinicopathological features in patients with epithelial
ovarian cancer.
| Feature | No. of cases | H-score | P-value |
|---|
| Age (years) |
|
| 0.692 |
|
<55 | 139 | 177.8±21.3 |
|
| ≥55 | 127 | 178.9±22.4 |
|
| Tumor diameter
(cm) |
|
| 0.397 |
|
<5 | 81 | 180.1±22.3 |
|
| ≥5 | 185 | 177.6±21.6 |
|
| Histological
type |
|
| 0.085 |
|
Serous | 188 | 180.5±20.9 |
|
|
Mucus | 30 | 174.7±26.5 |
|
|
Endometrioid | 26 | 172.5±20.7 |
|
| Clear
cell | 22 | 171.7±22.6 |
|
| Tumor Grade |
|
| <0.001 |
|
Low | 28 | 162.6±31.9 |
|
|
Moderate | 73 | 177.6±17.8 |
|
|
High | 165 | 181.3±20.3 |
|
| Serum CA-125 level
(U/ml) |
|
| 0.979 |
|
<35 | 78 | 178.3±20.2 |
|
|
≥35 | 188 | 178.4±22.5 |
|
| FIGO stage |
|
| 0.539 |
| Early
(I–II) | 72 | 177.0±23.1 |
|
|
Advanced (III–IV) | 194 | 178.8±21.4 |
|
| Cisplatin
resistance |
|
| <0.001 |
|
Yes | 90 | 185.0±20.1 |
|
| No | 176 | 172.9±22.0 |
|
Relationship between CLIC1 expression
and prognosis
In subsequent analysis, we determined the
relationship between CLIC1 expression and patient prognosis using a
Kaplan-Meier survival curve and Cox proportional hazards model.
During the follow-up period, 41.4% (110/266) patients died, whereas
28.6% (76/266) remained progression-free. The shortest PFS interval
was 1 month, and the longest was 118 months, with a mean PFS period
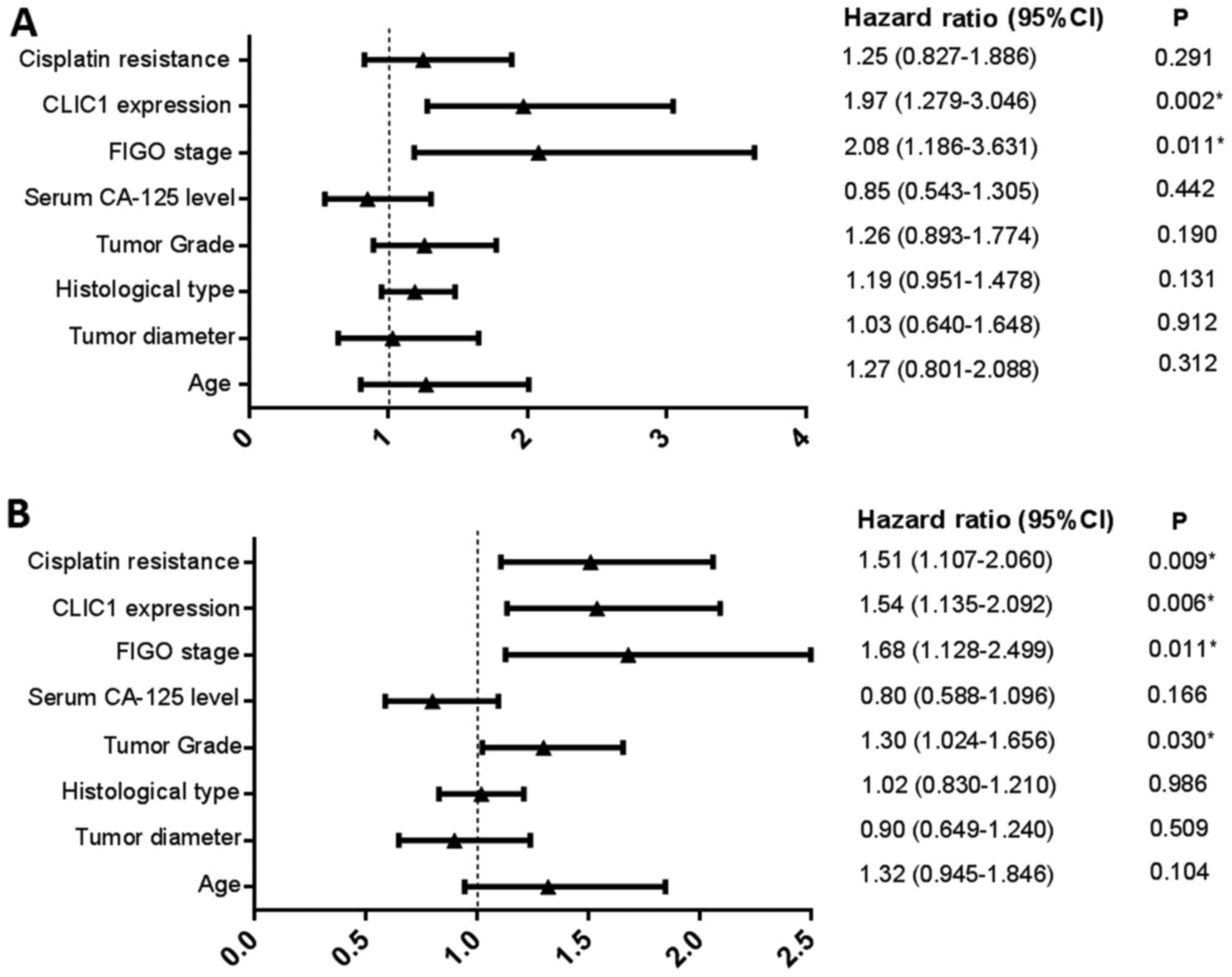
of 21.4±15.7 months. In the multivariate analysis, we identified
FIGO stage (P=0.011) and CLIC1 expression (P=0.002) as independent
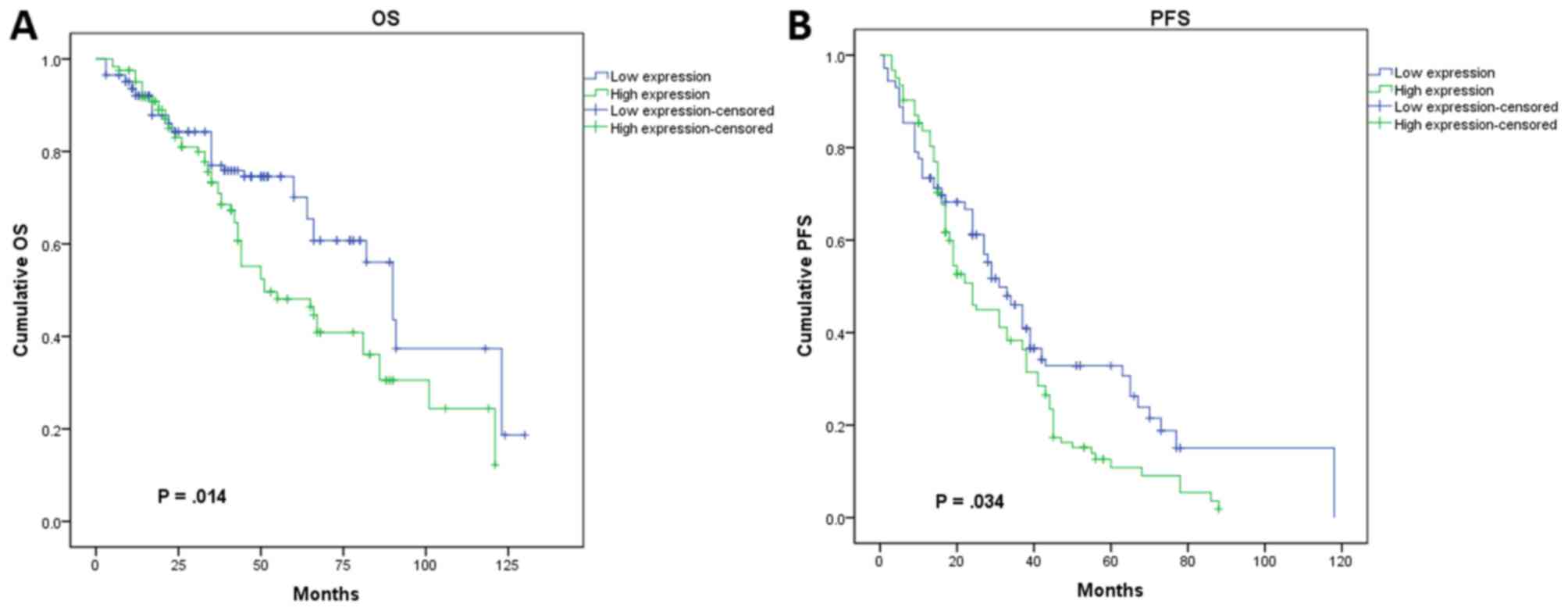
risk factors affecting OS in all cases (Fig. 4A). Patients who had higher CLIC1
levels also had poorer OS. Investigation of factors affecting PFS
by Cox regression analysis showed that tumor grade (P=0.030), FIGO
stage (P=0.011), CLIC1 expression (P=0.006), and cisplatin
resistance (P=0.009) were significant independent prognostic
factors (Fig. 4B). In the univariate
analysis using Kaplan-Meier survival curves, higher CLIC1 levels
led to poorer prognosis in terms of both PFS (P=0.034) and OS
(P=0.014; Fig. 5), which is
consistent with the results of the Cox model. These results
suggested that CLIC1 overexpression is associated with poor
prognosis of patients with epithelial ovarian cancer.
Discussion
In the current study, we evaluated the ability of
CLIC1 to serve as a biomarker for epithelial ovarian cancer.
Results of RT-qPCR and western blotting showed that CLIC1 mRNA and
protein levels were significantly upregulated in ovarian cancer
tissues than in healthy controls. We also evaluated the expression
of CLIC1 in 266 patients with ovarian cancer by immunohistochemical
staining, which showed that CLIC1 level was significantly higher in
ovarian cancer tissues than in healthy ovarian tissues.
All seven members of the CLIC protein family
(CLIC1-6 and p64) are components of mammalian ion channels
(18–21) with highly conserved carboxyl termini.
As a member of the CLIC family, CLIC1 is widely expressed in
various species and exists in both plasma-soluble and
membrane-binding forms. Notably, CLIC1 can auto-transit from the
cytoplasm to the plasma membrane without transport vesicles during
cell proliferation (22). A study in
Chinese hamster ovary cells showed that chloride conductance
differed based on the cell cycle phase and that CLIC1 largely
relocated to plasma membranes in the G2/M phase. The application of
a chloride ion channel blocker led to the arrest of cells in the
G2/M phase, indicating that CLIC1 was involved in cell cycle
regulation (12). Peretti et
al (23) and Gurski et al
(24) showed that CLIC1 was strongly
overexpressed in certain tumor types and that it translocated from
the cytoplasm to the membrane to act as a signal transducer during
cell cycle progression in pathological conditions and neoplastic
transformation, indicating that it might be a novel therapeutic
target for cancer.
CLIC1 regulates anti-apoptosis signaling pathways
and cellular transformation (25).
Further analyses of the relationship between CLIC1 expression and
clinical features showed that CLIC1 expression was more intense in
poorly differentiated than in well-differentiated ovarian
carcinoma. In addition, we showed in a previous study that
CLIC1 knockdown slowed the growth and proliferation of
ovarian cancer cells (A2780) via cell cycle arrest in the G1 phase
in vitro, suggesting that upregulated CLIC1 promoted cell
transformation (26). Similar
results were also reported by Li et al (15), who observed that CLIC1 contributed to
the proliferation of Hca-F hepatic carcinoma cells and
overexpression of CLIC1 modulated cell division and resulted in
cellular transformation. Furthermore, we observed that patients
with lower CLIC1 levels showed increased sensitivity to cisplatin
treatment in 266 patients with ovarian cancer. This was possibly
because CLIC1 participates in the regulation of anti-apoptosis
signaling pathways in response to chemotherapy. Similarly, Kang
observed that application of a chloride channel blocker to a human
glioblastoma multiforme cell line promoted the killing effect of
chemotherapeutic drugs and accelerate cell apoptosis.
Several studies have also indicated that CLIC1 is
involved in tumor angiogenesis, possibly by regulating the
expression of integrins on endothelial cell surfaces (27). Integrins are important surface
molecules that mediate cell migration and invasion, cellular
attachment, adhesion to the extracellular matrix, intracellular
signaling, and cell growth and proliferation (28). Tung and Kitajewski (29) reported that CLIC1 knockdown
increased the expression of integrin subtypes on endothelial cell
surfaces, leading to a noticeably decreased density of capillary
sprouting and branching compared to that observed in the control
groups, indicating that lower levels of CLIC1 can moderate tumor
cell migration and capillary network formation by regulating the
expression of endothelial integrins. High-grade cancer exhibits
strong invasion and migration ability, mainly via blood and
lymphatic metastases. In the present study, we observed that
patients with high-grade ovarian cancer had higher CLIC1 levels
than patients with low-grade tumors. Shorter PFS and OS were
expected in patients harboring such tumors.
This study has some limitations. There could have
been selection bias owing to the retrospective nature of the
analysis. Since our hospital is a tertiary referral institution, it
is possible that patients with serious conditions are more likely
to be admitted to our center because of referral bias. Their
outcomes may differ from those of mild cases who were not
transferred to our center.
Taken together, our observations indicate that the
CLIC1 is significantly overexpressed in epithelial ovarian cancer
tissues. Overexpressed CLIC1 may promote malignant transformation
and increase cisplatin resistance, indicating a poor prognosis for
patients with ovarian cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by International Science
and Technology Cooperation Program of China (grant no.
2012DFR30490).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY, CL and HD conceived and designed the study; WY,
RC, HQ and CL acquired, analyzed and interpreted the raw data; HD
and ZZ revised it critically for important intellectual content. ZZ
also obtained the raw data and gave final approval of the version
to be published.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee Board of Beijing Chao-Yang Hospital (reference no.
2008L01498), and informed consent was obtained from all
participating patients.
Consent for publication
The patient, or parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CA 125
|
cancer antigen 125
|
|
CLIC1
|
chloride intracellular channel 1
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
References
|
1
|
Webb PM and Jordan SJ: Epidemiology of
epithelial ovarian cancer. Best Prac Res Clin Obste Gynaecol.
41:3–14. 2017. View Article : Google Scholar
|
|
2
|
Mills K and Fuh K: Recent advances in
understanding, diagnosing, and treating ovarian cancer. F1000Res.
6:842017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oronsky B, Ray CM, Spira AI, Trepel JB,
Carter CA and Cottrill HM: A brief review of the management of
platinum-resistant-platinum-refractory ovarian cancer. Med Oncol.
34:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jessmon P, Boulanger T, Zhou W and
Patwardhan P: Epidemiology and treatment patterns of epithelial
ovarian cancer. Expert Rev Anticancer Ther. 17:427–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang WL, Lu Z and Bast RC Jr: The role of
biomarkers in the management of epithelial ovarian cancer. Expert
Rev Mol Diagn. 17:577–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ebell MH, Culp MB and Radke TJ: A
systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buys SS, Partridge E, Black A, Johnson CC,
Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B,
et al: Effect of screening on ovarian cancer mortality: The
prostate, lung, colorectal and ovarian (PLCO) cancer screening
randomized controlled trial. JAMA. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Futreal PA, Liu Q, Shattuck-Eidens D,
Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A,
Swensen J, Miki Y, et al: BRCA1 mutations in primary breast and
ovarian carcinomas. Science. 266:120–122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bayraktar S and Arun B: BRCA mutation
genetic testing implications in the United States. Breast.
31:224–232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valenzuela SM, Martin DK, Por SB, Robbins
JM, Warton K, Bootcov MR, Schofield PR, Campbell TJ and Breit SN:
Molecular cloning and expression of a chloride ion channel of cell
nuclei. J Biol Chem. 272:12575–12582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valenzuela SM, Mazzanti M, Tonini R, Qiu
MR, Warton K, Musgrove EA, Campbell TJ and Breit SN: The nuclear
chloride ion channel NCC27 is involved in regulation of the cell
cycle. J Physiol. 529:541–552. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang
F, Song X, Gao G, Mu J, Wang Z, et al: Chloride intracellular
channel 1 (CLIC1) is activated and functions as an oncogene in
pancreatic cancer. Med Oncol. 32:6162015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia N, Dong S, Zhao G, Gao H, Li X and
Zhang H: CLIC1 overexpression is associated with poor prognosis in
pancreatic ductal adenocarcinomas. J Cancer Res Ther. 12:892–896.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li RK, Zhang J, Zhang YH, Li ML, Wang M
and Tang JW: Chloride intracellular channel 1 is an important
factor in the lymphatic metastasis of hepatocarcinoma. Biomed
Pharmacother. 66:167–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma PF, Chen JQ, Wang Z, Liu JL and Li BP:
Function of chloride intracellular channel 1 in gastric cancer
cells. World J Gastroenterol. 18:3070–3080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Averaimo S, Milton RH, Duchen MR and
Mazzanti M: Chloride intracellular channel 1 (CLIC1): Sensor and
effector during oxidative stress. FEBS Lett. 584:2076–2084. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ulmasov B, Bruno J, Woost PG and Edwards
JC: Tissue and subcellular distribution of CLIC1. BMC Cell Biol.
8:82007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Littler DR, Harrop SJ, Brown LJ, Pankhurst
GJ, Mynott AV, Luciani P, Mandyam RA, Mazzanti M, Tanda S, Berryman
MA, et al: Comparison of vertebrate and invertebrate CLIC proteins:
The crystal structures of Caenorhabditis elegans EXC-4 and
Drosophila melanogaster DmCLIC. Proteins. 71:364–378. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashley RH: Challenging accepted ion
channel biology: p64 and the CLIC family of putative intracellular
anion channel proteins (Review). Mol Membr Biol. 20:1–11. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goodchild SC, Howell MW, Cordina NM,
Littler DR, Breit SN, Curmi PM and Brown LJ: Oxidation promotes
insertion of the CLIC1 chloride intracellular channel into the
membrane. Eur Biophys J. 39:129–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peretti M, Angelini M, Savalli N, Florio
T, Yuspa SH and Mazzanti M: Chloride channels in cancer: Focus on
chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins
in tumor development and as novel therapeutic targets. Biochim
Biophys Acta. 1848:2523–2531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gurski LA, Knowles LM, Basse PH, Maranchie
JK, Watkins SC and Pilch J: Relocation of CLIC1 promotes tumor cell
invasion and colonization of fibrin. Mol Cancer Res. 13:273–280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang MK and Kang SK: Pharmacologic
blockade of chloride channel synergistically enhances apoptosis of
chemotherapeutic drug-resistant cancer stem cells. Biochem Biophys
Res Commun. 373:539–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu H, Chen Y, Cao G, Liu C, Xu J, Deng H
and Zhang Z: Identification and validation of differentially
expressed proteins in epithelial ovarian cancers using quantitative
proteomics. Oncotarget. 7:83187–83199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Biasiotta A, D'Arcangelo D, Passarelli F,
Nicodemi EM and Facchiano A: Ion channels expression and function
are strongly modified in solid tumors and vascular malformations. J
Transl Med. 14:2852016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tung JJ and Kitajewski J: Chloride
intracellular channel 1 functions in endothelial cell growth and
migration. J Angiogenes Res. 2:232010. View Article : Google Scholar : PubMed/NCBI
|