Introduction
Human rotavirus (HRV) is the most frequent cause of
viral gastroenteritis in young children, with 440,000 estimated
deaths annually worldwide among children <5 years old (1,2).
Rotaviruses are also able to infect other animals, including birds
and companion and livestock animal species, causing extensive
economic losses (3). Therefore, safe
and effective vaccines against rotaviral infection are urgently
required. At present, two new live oral attenuated rotaviral
vaccines, Rotarix® (GlaxoSmithKline, Brentford, England)
and Rotateq® (Merck KGaA, Darmstadt, Germany), have been
approved for distribution. However, both are expensive and require
strict conditions for their production and storage, limiting their
use in developing countries (4,5). No
protective subunit rotavirus vaccine is currently available.
Rotavirus subunit vaccines raise neutralizing antibodies against
the viral outer capsid protein VP7, which contains neutralizing
epitopes that prevent viral attachment to the host cell membrane
(6). A previous study demonstrated
that different vectors that express HRV glycoprotein VP7 are able
to stimulate protective immunity against infection and serve a key
role in protection (7). According to
an amino acid (aa) sequence analysis of rotavirus SA11, the VP7
protein has three major antigenic sites; A (aa 87–99), B (aa
145–150) and C (aa 211–223), which contain the virus neutralization
epitopes (8). VP7 is also the
dominant target protein for rotavirus-specific cytotoxic
T-lymphocyte activity (9). VP7 was
therefore selected as the best candidate protein for subunit
vaccine development.
The use of plants as bioreactors for the production
of foreign proteins has been increasingly directed towards the
generation of experimental immunogens (10). Plants are a potentially inexpensive
source of antigens that can be administered parentally or used as
edible vaccines (11). Oral and
nasal administration of plant-produced vaccines has elicited
mucosal and systemic immune responses in animal and human trials
(12). To date, numerous viral
antigens that are immunogenic in animals have been produced in
transgenic plants (13–18). Several other groups have studied
rotavirus expression in plants, including VP7-RTB fusion (19), a human rotavirus candidate vaccine
expressed in Nicotiana benthamiana (20). However, because proteins are digested
in the intestinal tract, vaccination via the oral route may result
in a significant reduction in the amount of antigen delivered to
antigen-presenting cells (APCs) in gut-associated lymphoid tissues
(21,22). Increasing the production of antigenic
proteins in transformed plants is often problematic; however,
increasing the immunogenicity of plant-produced antigens may
significantly enhance the mucosal immune responses (23).
Cholera toxin B subunit (CTB) is the nontoxic
portion of the cholera toxin molecule that binds to the GM1
ganglioside receptor, a glycolipid expressed on most cells in the
human body, including immune cells (24). Coupling an antigen to CTB increases
the immunogenicity of the antigen as it increases receptor-mediated
uptake and subsequent presentation of the antigen by APCs (25).
Based on these considerations, two plant expression
vectors containing the nucleotide sequence of HRVVP7 or HRVVP7
linked upstream from the CTB subunit sequence were constructed to
test the feasibility of expressing HRVVP7 and an HRVVP7-CTB fusion
protein in transgenic Arabidopsis thaliana. Immunization
with extracts of transgenic Arabidopsis seeds induced an
efficient immune response in mice, which provided passive
protection against infection with rotavirus SA11 in neonatal mice
born to immunized dams. The immunogenic effects of HRVVP7 and
HRVVP7-CTB were compared, with the aim of developing an adjuvant
anti-rotavirus mucosal subunit vaccine.
Materials and methods
Construction of
pPHAP1301-HRVVP7-linker-CTB and pPHAP1301-HRVVP7 expression
vectors
The T-DNA region of the pPHAP1301 plasmid vector
(supplied by the Engineering Research Center of Bioreactor and
Pharmaceutical Development, Ministry of Education, Jilin
Agricultural University, Changchun, China) contains the phaseolin
promoter, phaseolin terminator, 35S promoter, bar gene and
the nopaline synthase gene nos. The HRVVP7 and CTB coding
sequences were retrieved from a patent (patent no.: US 7,285,280B1)
and GenBank (https://www.ncbi.nlm.nih.gov/nuccore/847821/),
respectively and modified by codon optimization based on the codon
usage of plants by Genewiz, Inc. (Suzhou, China). A 15-residue
linker coding sequence (Gly4Ser)3 was used
(26). Restriction sites NcoI
and HindIII were introduced at the 5′ and 3′ ends of the
coding sequence, respectively.
All these genes were then concatenated in the
sequence HRVVP7-linker-CTB by Genewiz, Inc. The pPHAP1301 plasmid
was digested with NcoI and HindIII. The HRVVP7 linker
CTB gene was extracted from PUC19-HRVVP7-linker-CTB (Genewiz, Inc.)
and digested with NcoI and HindIII. This
HRVVP7-linker-CTB fragment was inserted into the cleaved pPHAP1301
plasmid by incubating them with T4 DNA ligase at 4°C for 10 h. The
HRVVP7 sequence was subsequently amplified from the plasmid
pUC19-HRVVP7-linker-CTB (Genewiz, Inc.), using polymerase chain
reaction (2×TransTaq High Fidelity PCR SuperMix kit; TransGen,
Inc., Beijing, China) with the following primers: Forward,
5′-CATGCCATGGGATACATGCCAGAACTCATG-3′ and reverse,
5′-CCCAAGCTTAACTCTATAGTAgAAAGCAGC-3′. The thermal profile of the
PCR program was: Initial denaturation at 94°C for 15 min; 35 cycles
of 92°C for 30 sec, 52°C for 30 sec and 72°C for 1 min; and a final
extension at 72°C for 10 min. The PCR product was cloned into the
pPHAP1301 expression vector to construct the recombinant plasmid
pPHAP1301-HRVVP7. The expression vectors
pPHAP1301-HRVVP7-linker-CTB and pPHAP1301-HRVVP7 were confirmed by
PCR and a restriction enzyme analysis using the aforementioned
protocol. The freeze-thaw method was used to transfer plasmids
pPHAP1301-HRVVP7-linker-CTB and pPHAP1301-HRVVP7 into
Agrobacterium tumefaciens EHA105 (cat. no. AC1010; Shanghai
Weidi Bechnology Co. Ltd., Shanghai, China), and transformation was
confirmed by PCR using the aforementioned protocol.
Transformation and selection of A.
thaliana
A. thaliana ecotype Columbia specimens were
selected for floral dipping when a pot of healthy plants
(Engineering Research Center of Bioreactor and Pharmaceutical
Development, Ministry of Education, Jilin Agricultural University)
contained approximately 25 inflorescences and some maturing
siliques. The siliques are routinely clipped off in the laboratory
(27). The floral-dipping liquid
medium contained 100 g/l sucrose, 1% B5 basal medium (AmyJet
Scientific, Inc., Wuhan, China), 2 mg/l 6-benzylaminopurine, 1 M
sodium hydroxide and 0.04% surfactant Silwet L-77 (GE Healthcare
Life Sciences, Little Chalfont, UK) prepared as previously
described (28). The plants were
inverted and their aerial parts were dipped in Agrobacterium
tumefaciens EHA105-containing floral-dipping medium for 7 min
and wrapped in plastic film to maintain high humidity at 24°C for
20 h. The plastic covers were removed and the plants were grown at
24°C with 40% humidity conditions in a growth chamber until they
were dried and their seeds (T1) were harvested with a sample
bag.
The T1 seeds were grown in sterilized soil until the
majority of the plants had produced six leaves per plant. The
primary transformants were selected using 1% glufosinate
(Sigma-Aldrich; Merck KGaA), which was sprayed onto the plants
three times every 2 days. The cotyledons of the untransformed
plants became chlorotic and bleached within 3–5 days, whereas the
resistant seedlings grew healthy green leaves. The selected,
glufosinate-resistant lines were demonstrated to contain the
pPHAP1301-HRVVP7-linker-CTB or pPHAP1301-HRVVP7 sequence using PCR
with the primers and conditions described above. A large number of
homozygous T3 seeds were obtained with further rounds of plant
reproduction. These T3 seed lines were used for a protein
expression analysis and activity assays.
Total soluble protein (TSP) extract
for oral immunization or immunoassay
The T3 seeds of transgenic A. thaliana were
triturated in liquid nitrogen and mixed with protein isolation
buffer [50 mM/l Tris-Cl (pH 8.0), 10 mmol/l EDTA, 100 mmol/l NaCl,
0.5% Triton X-100, 14 mmol/l mercaptoethanol and 1 mmol/l PMSF].
The mixture was centrifuged at 13,000 × g at 4°C for 22 min. The
TSP content in the supernatant was determined using a Bradford
assay (29). The final TSP samples
were stored at −80°C for subsequent oral immunization or
immunoassay.
Total RNA extraction and reverse
transcription (RT)-PCR analysis of transformed A. thaliana
seeds
Total RNA was extracted from the seeds of the
transformed and wild-type A. thaliana plants using a plant
RNA extraction kit (MiniBEST Plant RNA Extraction kit; cat. no.
9769; Takara Bio, Inc.). The expressed pPHAP1301-HRVVP7-linker-CTB
and pPHAP1301-HRVVP7 mRNA was amplified using an RT-PCR kit
(PrimeScript™; cat. no. RR041A; Takara Bio, Inc.) with
the following primers: Forward,
5′-CATGCCATGGGATACATGCCAGAACTCATG-3′ and reverse,
5′-CCCAAGCTTAGTTAGCCATAGAGATAGCAGCG-3′; and forward,
5′-CATGCCATGGGATACATGCCAGAACTCATG-3′ and reverse,
5′-CCCAAGCTTAAACTCTATAGTAGAAAGCAGC-3′, respectively. The PCR
products were separated by electrophoresis on a 0.8% agarose gel,
stained with ethidium bromide at 25°C for 18 min and visualized
under UV light.
Western blotting analysis and ELISA
quantification of proteins expressed in A. thaliana seeds
TSP (20 µg) from the transgenic and wild type seeds
was separated by 10% SDS-PAGE. The resolved proteins were
subsequently transferred to a polyvinylidene fluoride membrane. The
membrane was blocked with 5% skimmed milk at 25°C for 24 h,
followed by incubation with rabbit polyclonal antibody directed
against rotavirus VP7 (cat. no. LS-C370878; LifeSpan BioSciences,
Seattle, WA, USA; 1:1,000 dilution) at 25°C for 2 h. Following
three washes with TBST (15 min each wash), alkaline
phosphatase-labeled goat anti-rabbit immunoglobulin (Ig)G antibody
(cat. no. ab98505; Abcam, Cambridge, UK; 1:10,000 dilution) was
added and incubated at 25°C for 2 h. Membranes were washed and
antibodies were detected with Western Blue Stabilized Substrate for
Alkaline Phosphatase (Promega Corp., Madison, WI, USA) according to
the manufacturer's protocol. PageRuler Plus Prestained Protein
Ladder (cat. no. 26619 SM1811; Thermon Group Holdings, Sam Marcos,
TX, USA) was used according to the manufacturer's protocol.
To quantify the amount of HRVVP7 expressed, 10 µl of
TSP was diluted in 190 µl of 50 mM carbonate buffer (pH 9.6). A
total of 50 µl/well of each diluted sample was used to coat a
high-binding 96-well plate (Corning Incorporated, Corning, NY USA).
Samples were blocked with 3% (w/v) skimmed milk at 25°C for 1 h,
followed by incubation with the rabbit polyclonal directed against
rotavirus VP7 (1:1,000 dilution) at 25°C for 1 h. Following three
washes with TBST, horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG antibody (cat. no. ab6721; Abcam; 1:20,000
dilution) was added and incubated at 25°C for 1 h.
Tetramethylbenzidine (TMB; 100 µl) was added for 10–15 min at room
temperature, followed by 50 µl 2 M H2SO4 to
stop the reaction. The absorbance was measured at 490 nm using a
microplate reader. Aliquots of samples containing serially diluted
rotavirus VP7 protein (cat. no. 80–1391; Fitzgerald Industries
International, Inc., Concord, MA, USA) were used to construct a
standard curve and the concentrations of VP7 in the samples were
interpolated to determine the quantities of HRVVP7.
Oral immunization and antibody
assessment
A total of 30 female 4–6 weeks old inbred BALB/c
mice (18–20 g) were obtained from the Experimental Animal Center of
Jilin Agricultural University. Mice were acclimatized to a 12 h
light/dark cycle at 22±2°C for 2 weeks with unlimited food and
water in a specific pathogen-free facility. All animal experiments
were approved by the Ethics Committee of the Jilin Agricultural
University Institutional Review Board and were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals (30). Mice were divided
into the HRVVP7-linker-CTB, HRVVP7 and negative control groups (10
mice/group). The groups were gavaged on days 0, 7, 14 and 21 with
TSP from wild-type or T3 transgenic A. thaliana seeds.
Mice in the negative control group were gavaged with
10 mg of TSP from wild type A. thaliana seeds and 0.01 mg
CTB adjuvant. Mice in the HRVVP7 group were gavaged with 8.3 mg of
HRVVP7-containing TSP from transgenic A. thaliana seeds,
which contained 25 µg of HRVVP7 protein from the transgenic seeds
and 0.01 mg CTB adjuvant. Mice in the HRVVP7-linker-CTB group were
gavaged with 6.2 mg of HRVVP7-linker-CTB-containing TSP from the
transgenic A. thaliana seeds, which contained 25 µg of
HRVVP7 protein from the transgenic seeds. Saliva and feces samples
(homogenized in 0.01 M PBS) were collected weekly from for
anti-HRVVP7 IgA analysis. On day 39, serum samples were collected
and tested for their anti-HRVVP7 IgG titers. On day 53, 4 mice in
each group were anesthetized with 2% isoflurane (Sigma-Aldrich;
Merck KGaA) in 98% oxygen and euthanized by cervical dislocation.
Mouse small intestines were subsequently collected and homogenized
in 0.01 M PBS for the analysis of mucosal anti-HRVVP7 IgA. All
antibody titers were evaluated with an indirect ELISA as described
previously (13). In brief, a known
concentration of rotavirus VP7 protein (Fitzgerald Industries
International, Inc.) was probed with mouse serum, saliva,
homogenized feces, or homogenized small intestine and detected
using an HRP-conjugated goat anti-mouse IgG antibody (cat. no.
HS201-01; TransGen Biotech, Beijing, China; 1:15,000 dilution) at
25°C for 1 h. TMB (100 µl) was added at 37°C for 10–15 min,
followed by 50 µl of 2 M H2SO4 to stop the
reaction. The absorbance was measured at 490 nm. Aliquots of
samples containing the serially diluted rabbit polyclonal antibody
directed against rotavirus VP7 (LifeSpan BioSciences) were used to
plot a standard curve and the values for the samples were
interpolated to determine the titers of the serum antibodies. The
antibody titers were determined as the reciprocal of the highest
antibody dilution yielding an absorbance at 490 nm
(A490) that was at least two-fold higher than the mean
A490 for antibody samples from the negative controls.
The results are presented as the mean ± standard deviation.
Neutralization assay
To determine the neutralizing activity of
anti-HRVVP7 antibodies in the serum samples, the best result (as
described above) was mixed with 100 µl 200 PFU of SA11 virus
(provided by Dr. Zhen Lang Sun, Central Hospital of Fengxian
District of Shanghai, Shanghai, China) in a 1:1 volumetric ratio.
Neutralization assays were performed in MA104 cells [originally
thought to originate from Rhesus macaque and later corrected to
originate from African green monkey (31)], which are suitable for rotavirus
research (32,33). The neutralized virus was subsequently
added to the culture medium of MA104 cells and cultured at 37°C in
an atmosphere containing 5% CO2. Following 2 h of culture, MA104
cells were cultured in fresh viral culture medium under same
conditions for 18 h. The cells were then fixed with methanol at
25°C for 30 min. The cells were incubated with 1% bovine serum
albumin and 22.52 mg/ml glycine (both Sigma-Aldrich; Merck KGaA) in
PBS+0.1% Tween20 at 25°C for 30 min to block unspecific binding of
the antibodies. The intracellular rotavirus was detected using the
mouse mAb directed against rotavirus VP7 (cat. no. C01715M;
Meridian Life Science, Inc., Memphis, TN, USA; 1:500 dilution) as
the primary antibody at 25°C for 30 min and
fluorescein-isothiocyanate-labeled goat anti-mouse IgG antibody
(cat. no. ab6785; Abcam) as the secondary antibody (1:500 dilution)
at 37°C for 1 h. The cells were washed three times with PBS at room
temperature (15 min/wash). In the present study, wells containing
no antibody were processed in parallel with the samples as negative
controls. Results were assessed using a fluorescence microscope
(Nikon E800; Nikon Corporation, Tokyo, Japan), at a magnification
of ×125. Neutralization was deemed to have occurred when the number
of fluorescently labeled infected cells was only 60% of the number
in the control wells.
Challenge experiment
The challenge experiment was based on the analysis
of antibodies raised against HRVVP7-CTB and the HRVVP7 without CTB
fusion protein. A total of 3 normal males inbred BALB/c mice (4–6
weeks old, 18–20 g) were obtained from the Experimental Animal
Center of Jilin Agricultural University. Mice were acclimatized to
a 12 h light/dark cycle at 22±2°C for 2 weeks with unlimited food
and water in a specific pathogen-free facility. Two female mice
from each group that showed the best immune response were mated
with normal males and mouse pups were born after a 19–20 day
gestation period. On day 5 post-parturition, 6 pups in each group
were challenged with rotaviral strain SA11. Pups bred from the
control mice gavaged with nontransgenic Arabidopsis seeds were used
as the negative controls (n=6). The pups were challenged using a
method previously described by Yu and Langridge (17). Each pup was challenged with 200 µl
(100 PFU) of simian rotavirus strain SA11. Mice were weighed at
24-h intervals and the anus of each pup was checked three times a
day. The mice from the infected group without watery feces were
classified as ‘non diarrheic’ mice and the others were classed as
‘diarrheic’ mice. The intensity of diarrhea was determined
according to the color of the anus and the amount of adherent
feces. The number of pups showing diarrhea symptoms and the
intensity of their diarrhea symptoms were observed for at least 10
days after challenge.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 for Windows (SPSS, Inc., Chicago, IL, USA). A one-way analysis
of variance with Bonferroni's post-test correction was used to
compare the differences between groups. P<0.001 was considered
to indicate a statistically significant difference.
Results
Genetic and immunoblotting analyses of
HRVVP7-linker-CTB and HRVVP7 from transformed A. thaliana
seeds
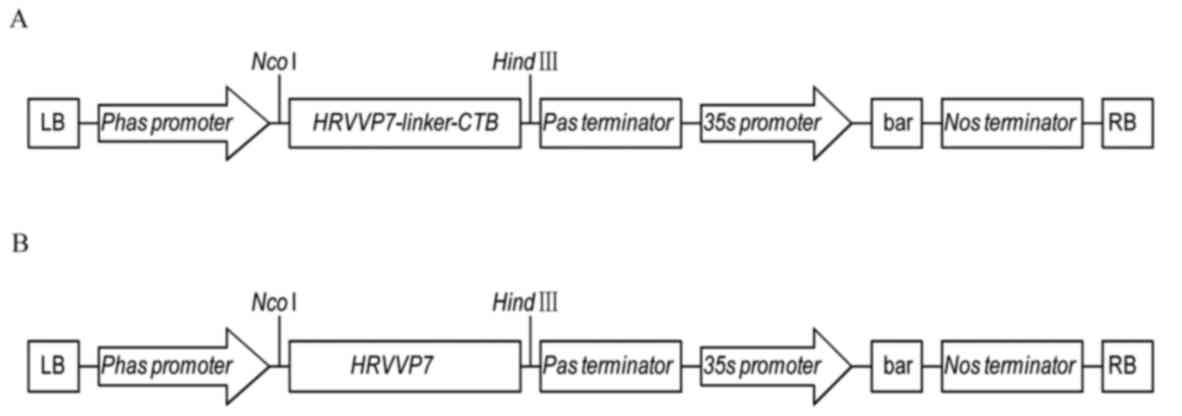
Expression vectors carrying the HRVVP7-linker-CTB or
HRVVP7 sequence were constructed to produce the HRVVP7-linker-CTB
or HRVVP7 protein, respectively (Fig.
1). A. thaliana was transformed with the
pPHAP1301-HRVVP7-CTB or pPHAP1301-HRVVP7 plasmid using the
Agrobacterium tumefaciens transformation methodology with
floral dipping. The presence of the HRVVP7-linker-CTB or HRVVP7
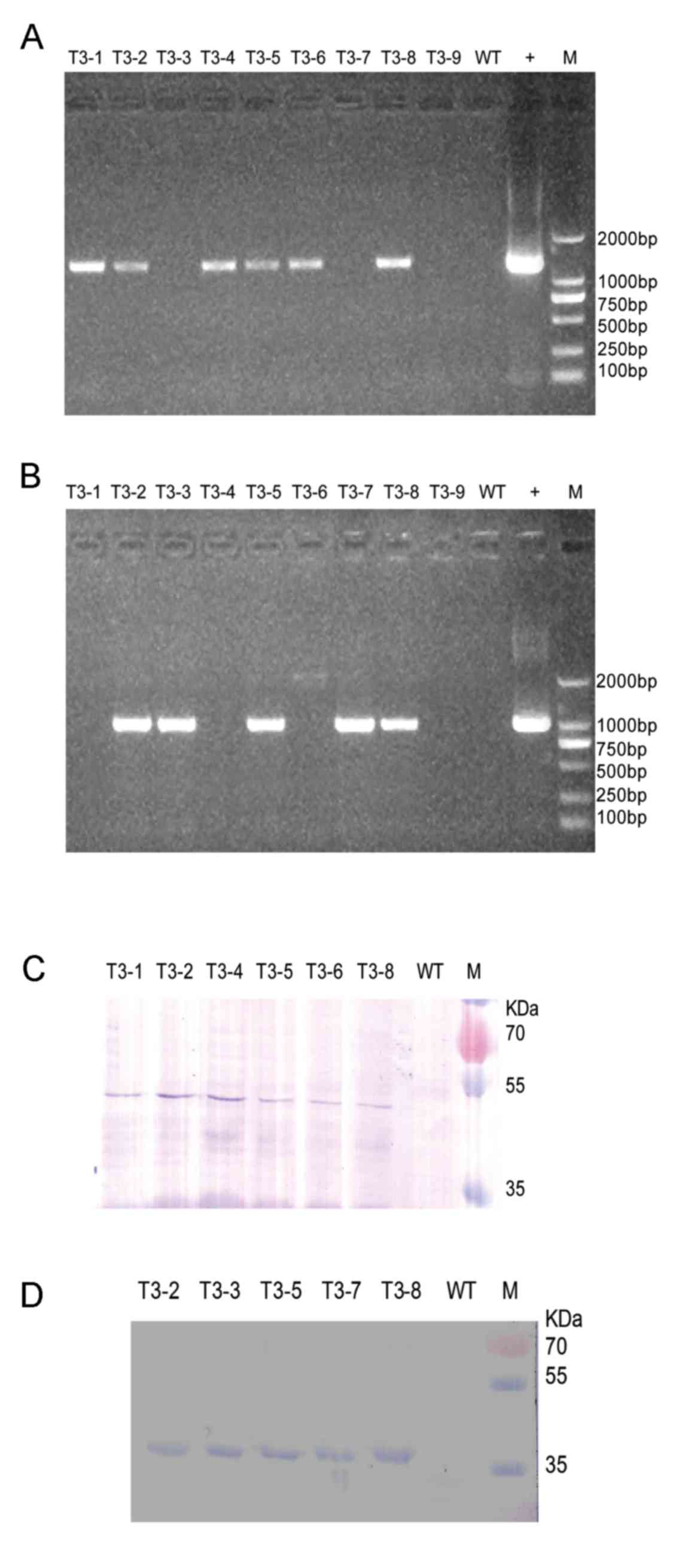
sequence in independent T3 glufosinate-resistant lines was
confirmed using RT-PCR with RNA as the template. Six of nine
glufosinate-resistant lines yielded bands of the expected 1,428 bp
(Fig. 2A) when the HRVVP7-linker-CTB
sequence was specifically primed for PCR. These six independent T3
lines (T3-1, T3-2, T3-4, T3-5, T3-6 and T3-8) were used for
subsequent analyses. Five of nine glufosinate-resistant lines
yielded bands of the expected 1,011 bp (Fig. 2B) when the HRVVP7 sequence was
specific primed for PCR. These five independent T3 lines (T3-2,
T3-3, T3-5, T3-7 and T3-8) were used for subsequent analyses.
Western blotting was used to examine
HRVVP7-linker-CTB and HRVVP7 expression. A 52 or 38 kDa protein was
detectable in the transgenic A. thaliana seeds with an
HRVVP7-specific antibody, indicating that plant-expressed
HRVVP7-linker-CTB and HRVVP7 proteins retained the antigenicity of
the native rotavirus HRVVP7 protein (Fig. 2C and D). Consistent with this, no
bands were present at these positions in the wild type seed samples
(Fig. 2C and D).
An ELISA assay was performed to quantify HRVVP7
expression. The results revealed that levels of HRVVP7 in the A.
thaliana seeds from the HRVVP7-linker-CTB transgenic lines
ranged from 3.00 to 52.65 µg/g and accounted for 0.03–0.39% of TSP.
Line T3-4 had the highest expression, ~52.65 µg of HRVVP7 protein
per gram of seeds. In the HRVVP7 transgenic lines, levels of HRVVP7
in the A. thaliana seeds ranged from 9.00 to 43.3 µg/mg and
accounted for 0.06–0.31% of TSP. Line T3-8 again had the highest
expression, ~43.3 µg of HRVVP7 protein per gram of seeds (data not
shown).
Antibody titers in animals in response
to plant-expressed pPHAP1301-HRVVP7-linker-CTB and
pPHAP1301-HRVVP7
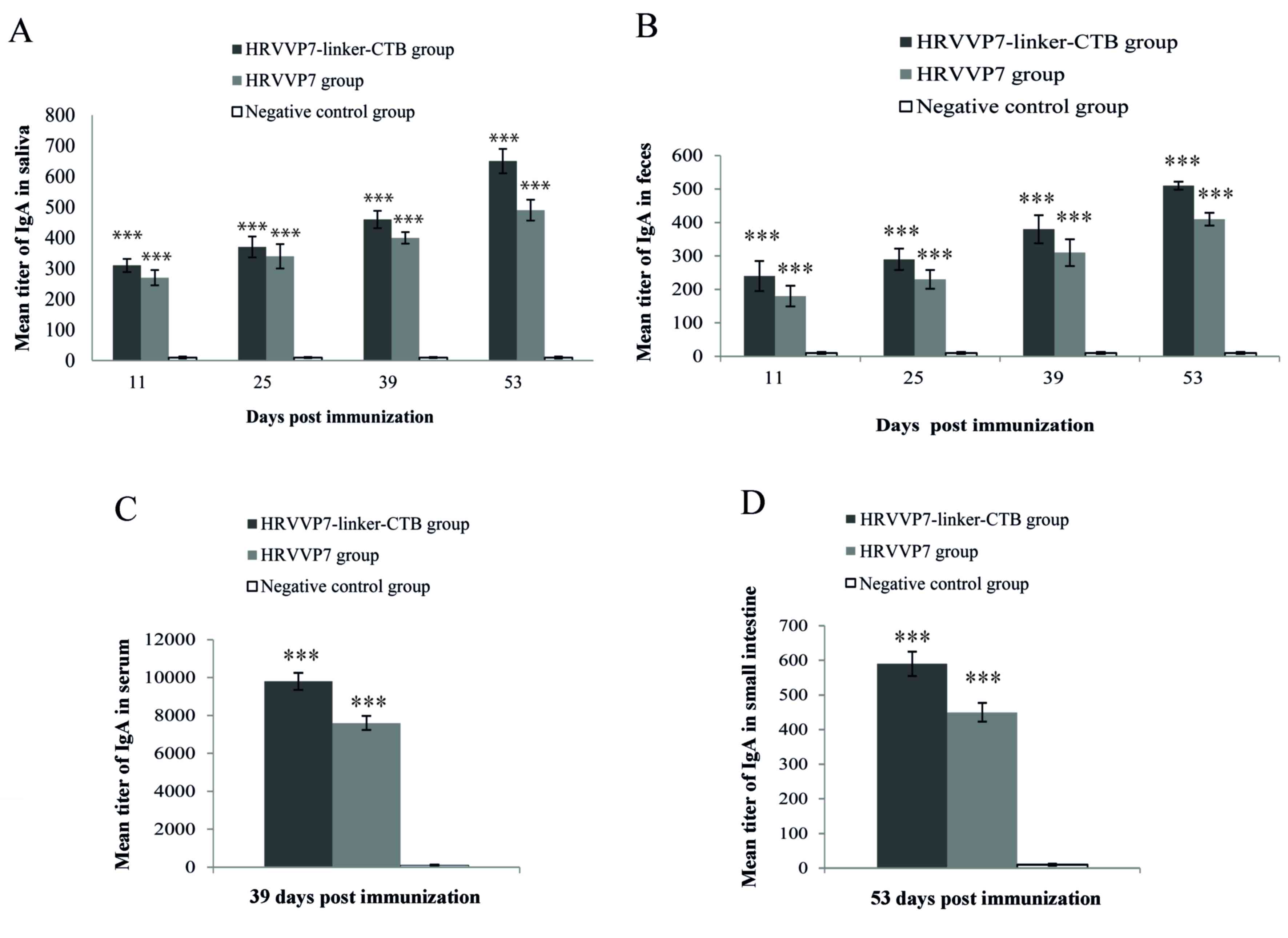
Saliva samples were collected and tested for
HRVVP7-specific IgA titers following immunization on days 11, 25,
39 and 53 (Fig. 3A). Anti-HRVVP7 IgA
antibodies were detected in mouse saliva collected from the
HRVVP7-linker-CTB and HRVVP7 groups from day 11. The anti-HRVVP7
IgA antibody titers increased gradually from day 25 and reached a
maximum on day 53. Anti-HRVVP7 IgA antibody titers in the saliva
samples from the HRVVP7-linker-CTB group ranged from 1:580 to 1:700
and were significantly higher compared with the HRVVP7 group
(P<0.001; Fig. 3A), which ranged
from 1:450 to 1:530. The anti-HRVVP7 IgA antibody titers from feces
followed a similar pattern to the saliva (Fig. 3B). On day 53 following the first
immunization the anti-HRVVP7 IgA antibody titers in the feces
samples ranged from 1:490 to 1:560 in the HRVVP7-linker-CTB group
and 1:380 to 1:450 in the HRVVP7 group. Serum anti-HRVVP7 IgG
antibody titer in the HRVVP7-linker-CTB group ranged from 1:9,600
to 1:10,300, which was significantly higher compared with the
HRVVP7 group (P<0.001; Fig. 3C).
Similarly, the anti-HRVVP7 IgA antibody titer in the small
intestinal mucosa measured at week 8 ranged from 1:560 to 1:620 in
the HRVVP7-linker-CTB group, significantly higher compared with the
HRVVP7 group (P<0.001; Fig. 3D).
No reactivity with HRVVP7 was observed in the sera, feces saliva
and small intestinal mucosa of mice in the negative control group
(Fig. 3A-D).
Effect of serum anti-HPVVP7 IgG in
neutralizing the virus in vitro
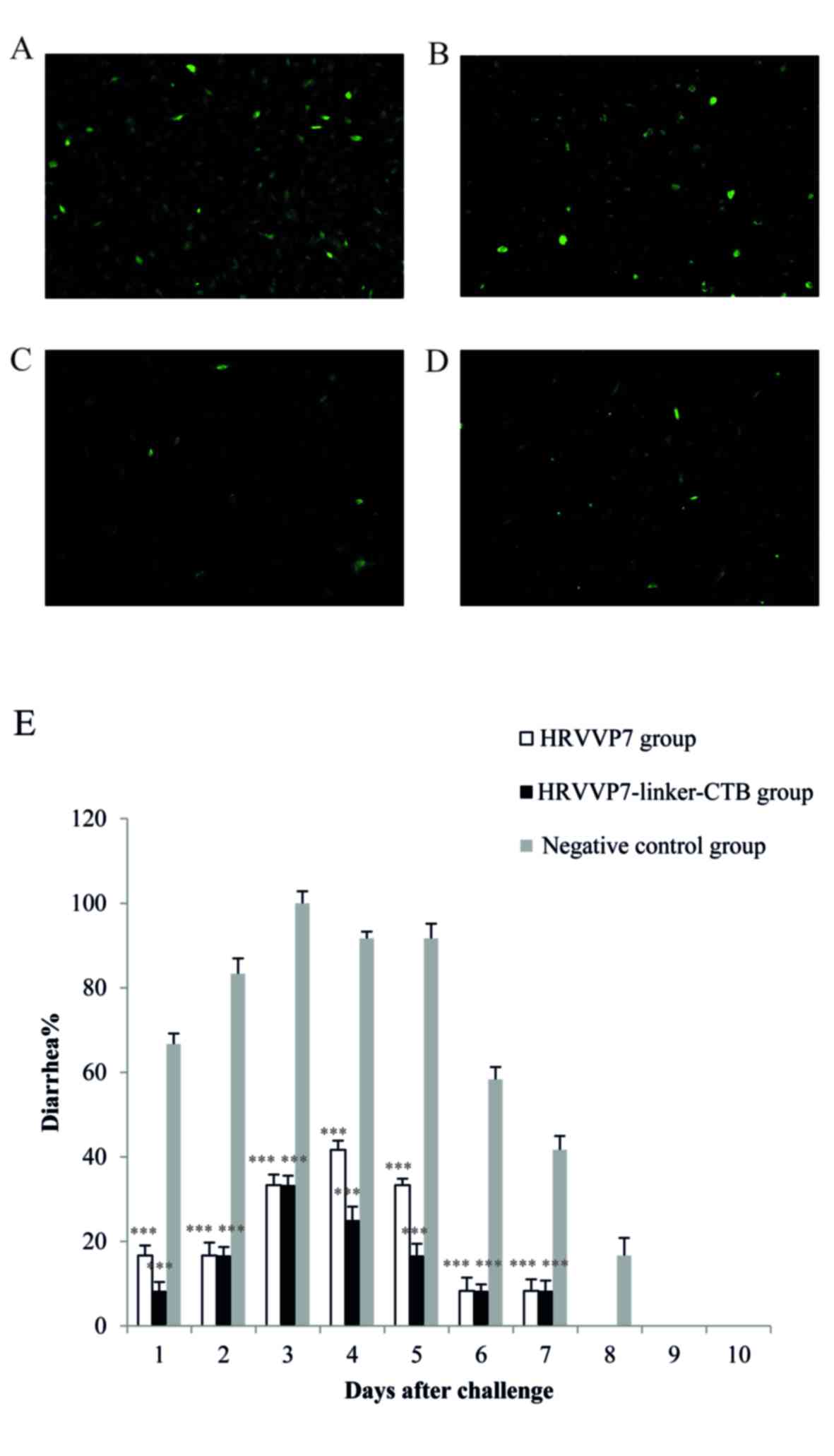
To examine the functional activity of the
anti-rotavirus antibodies detected following the oral
administration of plant-expressed HRVVP7 or HRVVP7-linker-CTB
protein, in vitro rotavirus neutralization assays were
performed using sera collected on day 39 post-immunization
(Fig. 4A-D). The results revealed
the sera from mice immunized with HRVVP7-linker-CTB protein more
efficiently neutralized the virus than the sera from mice immunized
with HRVVP7 protein from transgenic A. thaliana seeds.
Observation and statistical analysis
of mouse pups following viral challenge
To evaluate the protection afforded by the oral
vaccine against rotavirus-induced diarrhea, a viral challenge was
performed in passively immunized suckling mice at 5 days old. The
number of mouse pups with diarrhea following viral challenge was
counted by evaluating the criteria for diarrhea (13). The incidence, severity and duration
of diarrheic symptoms were significantly lower in the HRVVP7 and
HRPPV7-linker-CTB groups compared with the negative control group
from day 1 to day 8 (P<0.001; Fig.
4E). These observations indicated that the mice that received
the plant-expressed HRVVP7 protein effectively provided passive
protection to their offspring from rotaviral infection. These
results suggest that oral delivery of plant-expressed
HRVVP7-linker-CTB or HRVVP7 to female mice provided passive
protection from rotaviral challenge to their suckling neonatal
progeny.
Discussion
In the present study, A. thaliana seeds were
used as a bioreactor to produce recombinant HRVVP7-linker-CTB or
HRVVP7. TSP was extracted from the seeds of transgenic A.
thaliana plants and the immunogenicity of the transgenic
proteins was demonstrated by the production of specific antibodies,
the neutralizing ability of which was tested using SA11 infection
in cell culture and by passive immune protection afforded the
offspring of HRVVP7-immunized mice against SA11 infection.
Rotaviruses are the leading cause of severe
gastroenteritis and dehydrating diarrhea in young children and
animals worldwide (34). A number of
newborn animals are susceptible to rotavirus-induced diarrhea only
during the first weeks of life, making it difficult to actively
immunize the animals prior to exposure to the pathogen (34). Because female mice are able to
passively transfer antibodies to their pups, it is very important
to develop a rotavirus vaccine that can induce efficient passive
protection against viral infection (35,36).
The expression of heterologous proteins in plants to
produce various target antigens and antibodies has previously been
explored (15,37–44).
Although the prokaryotic expression of VP7 has already been
reported (45), recombinant HRVVP7
was successfully expressed in A. thaliana for the first time
in the present study. A strong tissue-specific promoter
(β-phaseolin promoter) was used in the present study to resolve the
common problem of low-level expression in transgenic plant
bioreactors (46). Using this
approach, the production of HRVVP7 reached 0.39% of TSP; however,
this yield is still much lower than that reported in Escherichia
coli (47). Despite the
relatively low yield of HRVVP7, the expression strategy used in the
present study has commercial potential. Furthermore, the shell of
the plant seed is able to protect the exogenous protein from
degradation in the gastrointestinal tract, thus rendering
plant-expressed vaccines suitable for oral application. A previous
study demonstrated that an oral vaccine fused with the mucosal
adjuvant CTB induced a mucosal immune response against
Helicobacter pylori infection in a BALB/c mouse model
(48). A similar approach was used
in the present study, fusing HRVVP7 to CTB and expressing the
fusion protein in A. thaliana plants. In future studies, the
fusion protein should be expressed in edible plants, including
tomato and carrot, to develop an oral HRVVP7 vaccine.
Interestingly, a previous study indicated that the
immune responses of mice fed rotavirus VP7 transgenic plants did
not differ significantly from those of mice fed only transgenic VP7
potatoes or transgenic VP7 potatoes plus bacterially expressed
recombinant CTB, a kind of mucosal adjuvant (49). The reasons for these similar immune
responses may involve the methods used (CTB was dissolved in PBS
and placed on potato tuber slices prior to ingestion. so the CTB
protein may have been digested in the gastrointestinal tract) or
they may have arisen because the transgenic plant itself has a
mucosal adjuvant activity associated with its cell wall. An antigen
co-expressed with CTB is ideal because it has been reported that
the fusion of CTB to pathogenic antigens increases the
adjuvanticity of CTB more than 10,000-fold (50). In the present study, the
immunological responses of mice following the administration of TSP
from transgenic A. thaliana seeds expressing
HRVVP7-linker-CTB were improved compared with the negative control
group. The titers of anti-HRVVP7-linker-CTB IgA in the saliva,
small intestines, feces and serum were higher than those specific
to HRVVP7 in the immunized mice. To investigate the functional
activity of the anti-rotavirus antibodies detected following the
oral administration of plant-expressed HRVVP7-linker-CTB or HRVVP7
protein, rotavirus neutralization assays were performed in
vitro using serum. The results confirmed that HRVVP7-linker-CTB
expressed by transgenic plants induced functionally active
anti-rotavirus serum antibodies more efficiently than the similarly
expressed HRVVP7. Immunization with HRVVP7-linker-CTB-containing
TSP extracts from transgenic A. thaliana seeds also
passively protected the mouse offspring from severe acute diarrhea
after following rotaviral challenge more effectively than
immunization with similarly expressed HRVVP7.
One limitation of the present study is that the
immunological responses to proteins expressed in A. thaliana
seeds were not compared with those expressed in E. coli. Our
group intends to perform such comparative analysis in future
studies. To enhance the versatility and efficacy of the HRVVP7
vaccine, an HRV fusion protein should be made from two or more
rotavirus structural proteins, including VP4, VP6 and VP7. This
allows for further refinement of this rotavirus vaccine.
In conclusion, the results of the present study
demonstrate that A. thaliana may be successfully transformed
with an HRVVP7-encoding plasmid to express the protein in its
seeds. In animal tests, HRVVP7 maintained its immunogenicity and
the neutralizing activity of HRVVP7 against rotavirus was primarily
attributable to IgA and IgG antibodies. Therefore, plants have the
potential to be used as expression systems for the development of
rotavirus vaccines. It was also demonstrated that HRVVP7-CTB fusion
protein exerts a better mucosal adjuvant effect compared with CTB
plus HRVVP7.
Acknowledgements
The authors would like to thank Dr. Zhen Lang Sun
from the Central Hospital of Fengxian District of Shanghai
(Shanghai, China) for providing the virus strain.
Funding
This study was supported by funds from the National
High Technology Research and Development Program (863 program) of
China (2011AA100606), the National Natural Science Foundation of
China (grant nos. 31101172, 31101091 and 31501366), the Science and
Technology Development Project of Jilin Province (grant nos.
20150204027NY, 20140520164JH, 20150104027NY and 20150623024TC-11)
and the Education Department Project of Jilin Province (grant no.
2015382).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL, HL and XiaL conceived and designed the
experiments. YL, LG, XiuL, YG and WL performed the experiments. JY,
FW and XZ analyzed the data. YL, FW and YG wrote the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments complied with the Animal
Research: Reporting In Vivo Experiments Guidelines and were
approved by the Ethics Committee of the Jilin Agricultural
University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parashar UD, Gibson CJ, Bresee JS and
Glass RI: Rotavirus and severe childhood diarrhea. Emerg Infect
Dis. 12:304–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parashar UD, Bresee JS and Glass RI: The
global burden of diarrhoeal disease in children. Bull World Health
Organ. 81:2362003.PubMed/NCBI
|
|
3
|
Charles MD, Holman RC, Curns AT, Parashar
UD, Glass RI and Bresee JS: Hospitalizations associated with
rotavirus gastroenteritis in the United States, 1993–2002. Pediatr
Infect Dis J. 25:489–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madhi SA, Cunliffe NA, Steele D, Witte D,
Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et
al: Effect of human rotavirus vaccine on severe diarrhea in African
infants. N Engl J Med. 362:289–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richardson V, Hernandez-Pichardo J,
Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano
CM, Parashar U and Patel M: Effect of rotavirus vaccination on
death from childhood diarrhea in Mexico. N Engl J Med. 362:299–305.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenberg HB, Valdesuso J, van Wyke K,
Midthun K, Walsh M, McAuliffe V, Wyatt RG, Kalica AR, Flores J and
Hoshino Y: Production and preliminary characterization of
monoclonal antibodies directed at two surface proteins of rhesus
rotavirus. J Virol. 47:267–275. 1983.PubMed/NCBI
|
|
7
|
Andrew ME, Boyle DB, Coupar BE, Reddy D,
Bellamy AR and Both GW: Vaccinia-rotavirus VP7 recombinants protect
mice against rotavirus-induced diarrhoea. Vaccine. 10:185–191.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dyall-Smith ML, Lazdins I, Tregear GW and
Holmes IH: Location of the major antigenic sites involved in
rotavirus serotype-specific neutralization. Proc Natl Acad Sci USA.
83:3465–3468. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Offit PA, Boyle DB, Both GW, Hill NL,
Svoboda YM, Cunningham SL, Jenkins RJ and McCrae MA: Outer capsid
glycoprotein vp7 is recognized by cross-reactive,
rotavirus-specific, cytotoxic T lymphocytes. Virology. 184:563–568.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Łucka M, Kowalczyk T, Szemraj J and
Sakowicz T: Plants as an alternative source of therapeutic
proteins. Postepy Hig Med Dosw (Online). 69:362–373. 2015.(In
Polish). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yusibov V and Streatfield SJ:
Plant-produced microbial vaccines: Alexander V. Karasev, editor:
Current topics in microbiology and immunology 2009; v. 332. Hum
Vaccin. 6:pii: 12006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arntzen CJ: High-tech herbal medicine:
Plant-based vaccines. Nat Biotechnol. 15:221–222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong JL, Liang BG, Jin YS, Zhang WJ and
Wang T: Oral immunization with pBsVP6-transgenic alfalfa protects
mice against rotavirus infection. Virology. 339:153–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou B, Zhang Y, Wang X, Dong J, Wang B,
Han C, Yu J and Li D: Oral administration of plant-based rotavirus
VP6 induces antigen-specific IgAs, IgGs and passive protection in
mice. Vaccine. 28:6021–6027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tacket CO, Mason HS, Losonsky G, Clements
JD, Levine MM and Arntzen CJ: Immunogenicity in humans of a
recombinant bacterial antigen delivered in a transgenic potato. Nat
Med. 4:607–609. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arakawa T, Yu J and Langridge W: Synthesis
of a cholera toxin B subunit-rotavirus NSP4 fusion protein in
potato. Plant Cell Rep. 20:343–348. 2001. View Article : Google Scholar
|
|
17
|
Yu J and Langridge WH: A plant-based
multicomponent vaccine protects mice from enteric diseases. Nat
Biotechnol. 19:548–552. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim TG and Langridge W: Assembly of
cholera toxin B subunit full-length rotavirus NSP4 fusion protein
oligomers in transgenic potato. Plant Cell Rep. 21:884–890.
2003.PubMed/NCBI
|
|
19
|
Choi NW, Estes MK and Langridge WH:
Synthesis of a ricin toxin B subunit-rotavirus VP7 fusion protein
in potato. Mol Biotechnol. 32:117–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pêra FF, Mutepfa DL, Khan AM, Els JH,
Mbewana S, van Dijk AA, Rybicki EP and Hitzeroth II: Engineering
and expression of a human rotavirus candidate vaccine in Nicotiana
benthamiana. Virol J. 12:2052015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mason HS, Tacket CO, Richter LJ and
Arntzen CJ: Subunit vaccines produced and delivered in transgenic
plants as ‘edible vaccines’. Res Immunol. 149:71–74. 1998.
View Article : Google Scholar
|
|
22
|
Yang J, Guan L, Guo Y, Du L, Wang F, Wang
Y, Zhen L, Wang Q, Zou D, Chen W, et al: Expression of biologically
recombinant human acidic fibroblast growth factor in Arabidopsis
thaliana seeds via oleosin fusion technology. Gene. 566:89–94.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mikschofsky H, König P, Keil GM, Hammer M,
Schirrmeier H and Broer I: Cholera toxin B (CTB) is functional as
an adjuvant for cytoplasmatic proteins if directed to the
endoplasmatic reticulum (ER), but not to the cytoplasm of plants.
Plant Sci. 177:35–42. 2009. View Article : Google Scholar
|
|
24
|
Arêas AP, Oliveira ML, Miyaji EN, Leite
LC, Aires KA, Dias WO and Ho PL: Expression and characterization of
cholera toxin B-pneumococcal surface adhesin A fusion protein in
Escherichia coli: Ability of CTB-PsaA to induce humoral immune
response in mice. Biochem Biophys Res Commun. 321:192–196. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo L, Yin R, Liu K, Lv X, Li Y, Duan X,
Chu Y, Xi T and Xing Y: Immunological features and efficacy of a
multi-epitope vaccine CTB-UE against H. pylori in BALB/c mice
model. Appl Microbiol Biotechnol. 98:3495–3507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huston JS, Tai MS, McCartney J, Keck P and
Oppermann H: Antigen recognition and targeted delivery by the
single-chain Fv. Cell Biophys. 22:189–224. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Henriques R, Lin SS, Niu QW and
Chua NH: Agrobacterium-mediated transformation of Arabidopsis
thaliana using the floral dip method. Nat Protoc. 1:641–646. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weigel D and Glazebrook J: ArabidopsisA
Laboratory Manual. Cold Spring Harbor Laboratory Press 165;
2002
|
|
29
|
Bradford MM: A raoid and sensitive method
for the quantification of microgram quantities of protein utilizing
the principle of protein-bye-binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Health Union: Guide for the care and use
of laboratory animals. NIH Publication No. 85–23. 1996.
|
|
31
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR and Freshney RI: Check your cultures! A list of
cross-contaminated or misidentified cell lines. Int J. Cancer.
127:1–8. 2010.
|
|
32
|
Mi K, Ou X, Guo L, Ye J, Wu J, Yi S, Niu
X, Sun X, Li H and Sun M: Comparative analysis of the
immunogenicity of monovalent and multivalent rotavirus immunogens.
PLoS One. 12:e01721562017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rojas-Mancilla E, Oyarce A, Verdugo V,
Morales-Verdejo C, Echeverria C, Velásquez F, Chnaiderman J,
Valiente-Echeverría F and Ramirez-Tagle R: The [Mo(6)Cl14]2-cluster
is biologically secure and has anti-rotavirus activity in vitro.
Molecules. 22:pii: E1108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Ryan M: Rotarix (RIX4414): An oral human
rotavirus vaccine. Expert Rev Vaccines. 6:11–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Offit P and Clark H: Protection against
rotavirus-induced gastroenteritis in a murine model by passively
acquired gastrointestinal but not circulating antibodies. J Virol.
54:58–64. 1985.PubMed/NCBI
|
|
36
|
Filgueira Pérez DM, Mozgovoj M,
Wigdorovitz A, Santos Dus MJ, Parreño V, Trono K, Fernandez FM,
Carrillo C, Babiuk LA, Morris TJ and Borca MV: Passive protection
to bovine rotavirus (BRV) infection induced by a BRV VP8* produced
in plants using a TMV-based vector. Arch Virol. 149:2337–2348.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mason HS, Lam D and Arntzen CJ: Expression
of hepatitis B surface antigen in transgenic plants. Proc Natl Acad
Sci USA. 89:11745–11749. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haq TA, Mason HS, Clements JD and Arntzen
CJ: Oral immunization with a recombinant bacterial antigen produced
in transgenic plants. Science. 268:714–716. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mason HS, Ball JM, Shi JJ, Jiang X, Estes
MK and Arntzen CJ: Expression of Norwalk virus capsid protein in
transgenic tobacco and potato and its oral immunogenicity in mice.
Proc Natl Acad Sci USA. 93:5335–5340. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hiatt A, Caffferkey R and Bowdish K:
Production of antibodies in transgenic plants. Nature. 342:76–78.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma JK, Hiatt A, Hein M, Vine ND, Wang F,
Stabila P, van Dolleweerd C, Mostov K and Lehner T: Generation and
assembly of secretory antibodies in plants. Science. 268:716–719.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kapusta J, Modelska A, Figlerowicz M,
Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H,
Plucienniczak A and Legocki AB: A plant-derived edible vaccine
against hepatitis B virus. FASEB J. 13:1796–1799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fischer R, Stoger E, Schillberg S,
Christou P and Twyman RM: Plant-based production of
biopharmaceuticals. Curr Opin Plant Biol. 7:152–158. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mason HS, Warzecha H, Mor T and Arntzen
CJ: Edible plant vaccines: Applications for prophylactic and
therapeutic molecular medicine. Trends Mol Med. 8:324–329. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perez CA, Eichwald C, Burrone O and
Mendoza D: Rotavirus vp7 antigen produced by Lactococcus lactis
induces neutralizing antibodies in mice. J Appl Microbiol.
99:1158–1164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Perez CA, Eichwald C, Burrone O and
Mendoza D: Rotavirus vp7 antigen produced by Lactococcus lactis
induces neutralizing antibodies in mice. J Appl Microbiol.
99:1158–1164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan LY, Liu Y, Li CH, Sun MS and Dai CB:
Expression in Escherichia coli and immunogenicity of rotavirus VP7.
Sheng Wu Gong Cheng Xue Bao. 17:145–149. 2001.(In Chinese).
PubMed/NCBI
|
|
48
|
Guo L, Liu K, Xu G, Li X, Tu J, Tang F,
Xing Y and Xi T: Prophylactic and therapeutic efficacy of the
epitope vaccine CTB-UA against Helicobacter pylori infection in a
BALB/c mice model. Appl Microbiol Biotechnol. 95:1437–1444. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu YZ, Li JT, Mou ZR, Fei L, Ni B, Geng M,
Jia ZC, Zhou W, Zou LY and Tang Y: Oral immunization with rotavirus
VP7 expressed in transgenic potatoes induced high titers of mucosal
neutralizing IgA. Virology. 313:337–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
George-Chandy A, Eriksson K, Lebens M,
Nordström I, Schön E and Holmgren J: Cholera toxin B subunit as a
carrier molecule promotes antigen presentation and increases CD40
and CD86 expression on antigen-presenting cells. Infect Immun.
69:5716–5725. 2001. View Article : Google Scholar : PubMed/NCBI
|