Introduction
Liver cancer remains a major health issue because it
is the sixth most common malignancy and the third principal cause
of cancer deaths worldwide (1).
Despite the progress of potentially curative treatments, liver
transplantation and surgical resection remain the first choice for
liver cancer patients (2). Natural
compounds have been widely used for cancer prevention and treatment
because of its multi-level, multi-target and coordinated
intervention effects (3). The
significant anticancer effects against liver cancer have also been
identified, such as curcumin and baicalein, indicating that these
natural compounds represent an important medical and pharmaceutical
resource for the development of new treatments for liver cancer
(4,5).
Cinchonine
(C19H22N2O) is a natural compound
which has been effectively used as antimalarial drug along with
quinine, quinidine and cinchonidine and all these agents were
separated from Cinchona bark (6).
Interestingly, all these agents have been found with antitumor
effects and among which cinchonine has much lower toxicity and
higher activity (7). Cinchonine
exerted multidrug resistance (MDR) reversal activity and
synergistic apoptotic effect with paclitaxel (TAX) in MES-SA/DX5
uterine sarcoma cells as a potent MDR-reversal and combined therapy
agent with TAX (8). A very recent
study found that cinchonine could reduce proliferation and induce
apoptosis in both HeLa and A549 cells. Their computational modeling
predicted that cinchonine could target the RING domain of TRAF6
protein, leading to the disruption of the binding with Ubc13
protein, its natural ligand, and inhibiting the downstream events
including AKT and TAK1 activations (9). However, whether cinchonine could be
used as an antitumor agent against liver cancer remains
elusive.
Endoplasmic reticulum (ER) stress is triggered by
perturbations in ER function, called unfolded protein response
(UPR) (10). There are three
signaling pathways of eukaryotic cells responding to ER stress and
are initiated by ER stress sensors, PERK, IRE1, and ATF6,
respectively (11). PERK
phosphorylates eIF2-alpha to reduce the overall rate of
translational initiation, and activates the downstream
transcriptional factor CHOP (12,13).
CHOP mediates cell death in a number of ER stress models and
induced the expression of Bim (14),
which initiates the mitochondrial pathway of apoptosis, ultimately
resulting in caspase-9 and caspase-3 activation.
In the present study, we found that cinchonine
elicited inhibition of cell viability and promotes apoptosis in
liver cancer cells by promoting endoplasmic-reticulum (ER)
stress.
Materials and methods
Cell culture
Liver cancer cell lines Bel-7402, MHCC97H, HepG2,
Hep3B and SMCC7721 were obtained from The Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). All
cell lines were grown in Dulbecco's modified Eagle's medium (DMEM;
Gibco, Shanghai, China) supplemented with 10% fetal bovine serum
(Gibco), 100 IU/ml penicillin as well as 100 mg/ml streptomycin.
Cells were maintained at 37°C in a humidified atmosphere with 5%
CO2. 3-(4,
5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay.
Cells were seeded into 96-well plate. Different
concentrations of cinchonine were added to these wells. After
treatment, cells were incubated with 20 µl MTT (5 mg/ml) for 4 h at
37°C. The MTT solution was discarded and formazan was dissolved in
150 µl DMSO. The absorbance of each well was read using a
Microplate reader at 490 nm.
Flow cytometry
Apoptosis of the cells were determined using the
Annexin V-FITC Apoptosis Detection kit (Becton-Dickinson, Franklin
Lakes, NJ, USA) according to the manufacturer's protocol. Cells
were seeded into six-well plate with cinchonine administration.
Cells were then washed twice in cold PBS and resuspended in binging
buffer. Five µl Annexin V-FITC and PI were added into 100 µl cell
suspension. The mixture was incubated for 15 min at room
temperature in the dark. Results were immediately analyzed with a
flow cytometry (Becton-Dickinson) and all data were analyzed by
ModFit software (Verity Software House, Inc., Topsham, ME,
USA).
Western blot analysis
Cells were harvested and lysed with ice-cold lysis
buffer (50 mM Tris-HCl, pH 6.8, 100 mM 2-mercaptoethanol, 2% SDS
and 10% glycerol). After centrifugation at 15,000 × g for 15 min at
4°C, proteins in the supernatants were quantified and separated by
10–12% SDS PAGE. Western blot assay was performed using the
following antibodies: Anti-GRP78 and poly (ADP-Ribose) polymerase
(PARP) 1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
eIF2a, P-eIF2a, PERK, P-PERK and Δ Caspase-3 (Cell Signaling
Technology, Inc., CA, USA). Protein levels were normalized to total
GAPDH, using a mouse anti-GAPDH antibody (Santa Cruz Biotechnology,
Inc.).
Tumor growth assay
Male BALB/c nude mice aged 4 weeks were purchased
from Shanghai Laboratory Animal Company (Shanghai, China). Mice
were first subcutaneously inoculated with cancer cells
(5×106 cell/ml) to establish transplanted mode of liver
cancer for 3 weeks. The mice were observed over 5 weeks for tumor
formation. After the mice were sacrificed, the tumors were
recovered and the wet weights of each tumor were determined. The
present study was approved by the Animal Ethical and Welfare
Committee of the Second Clinical Medical College, Yangtze
University (Jingzhou, China).
Statistical analysis
The results are expressed as the mean ± SD.
Statistical significance was calculated using one-way ANOVA,
followed by Duncan's multiple range tests. All the statistical
analyses were performed with Graphpad prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indiciate a statistically significant difference.
Results
Cinchonine inhibited cell growth and
promoted apoptosis in human liver cancer cells
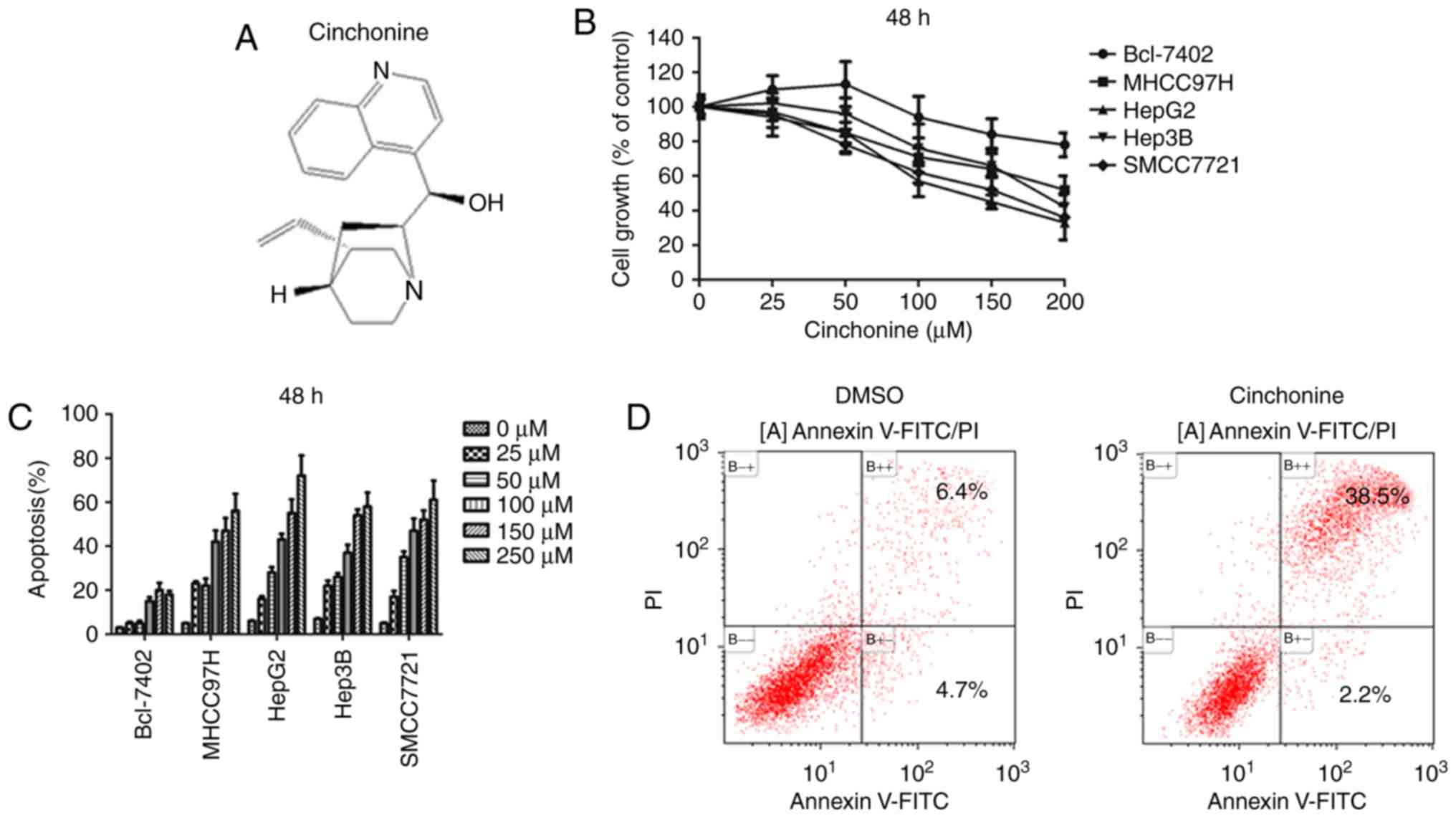
Cinchonine is a natural compound of Cinchona bark
(Fig. 1A). Previous studies have
reported that cinchonine is an inhibitor of human platelet
aggregation and has antitumor effect in MES-SA/DX5 uterine sarcoma
cells (8,15). However, the antitumor effect of
cinchonine in liver cancer cells has never been studied. To test
whether cinchonine could inhibit liver cancer cells proliferation,
five liver cancer cell lines were enrolled in this study, including
Bel-7402, MHCC97H, HepG2, Hep3B and SMCC7721 cells. To determine
the effect of cinchonine on the viability of liver cancer cells,
these cells were treated with various concentrations of cinchonine,
and the cell viability was then measured with an MTT assay. We
found that, cinchonine significantly suppressed the viability of
the four out of five liver cancer cell lines we used in a
dose-dependent after 48 h of treatment (Fig. 1). Cinchonine treatment also caused
decreased viability of Bel-7402 cells but with less effective when
compared with other four liver cancer cell lines. A recent study
suggested that 50 µM cinchonine is stable and sufficient to induce
apoptosis in in HeLa and A549 cells in vitro. In consistent
with these data, our flow cytometry assay with Annexin V-FITC/PI
labeling showed that 50 µM cinchonine is sufficient to induce
apoptosis in four out of five liver cancer cell lines after 48 h of
treatment. In consistent with MTT data, Bel-7402 cells were also
less sensitive to cinchonine treatment when compared with other
four liver cancer cell lines. Taken together, these data suggested
that cinchonine inhibited cell growth and promoted apoptosis in
most human liver cancer cells.
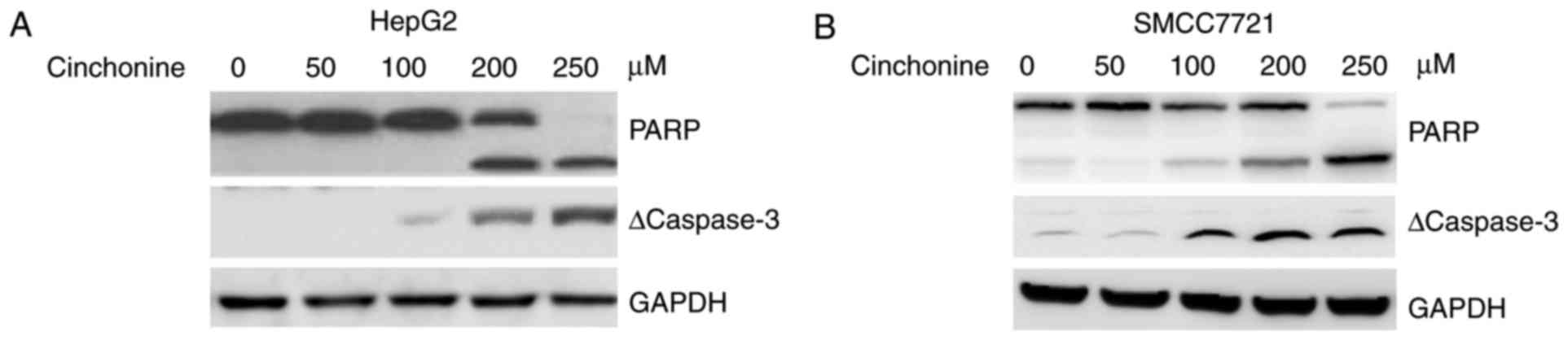
Cinchonine promoted caspase-3
activation and PARP1 cleavage in human liver cancer cells
Then the expression of apoptosis-related proteins in
the cell lysates of the two liver cancer cell lines was measured by
western blot 48 h after cinchonine treatment. Our results showed
that cinchonine activated promoted caspase-3 activation and PARP1
cleavage in both HepG2 and SMCC7721 in a dose-dependent manner
(Fig. 2A and B). These data
suggested that cinchonine inhibited liver cancer cells
proliferation by activating caspase-3-dependent apoptosis.
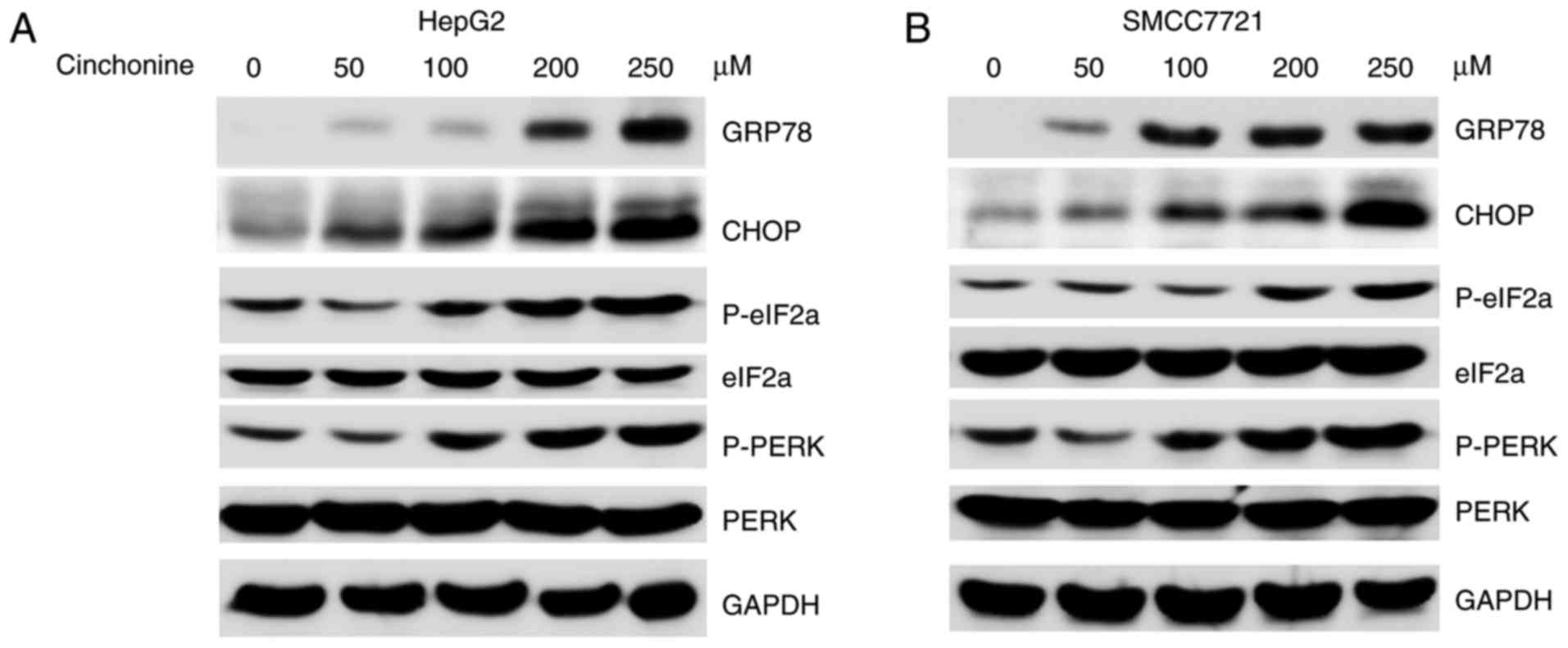
Cinchonine activated endoplasmic
reticulum stress in human liver cancer cells
Prolonged ER stress could trigger cellular apoptosis
(16,17). We then asked whether cinchonine could
activate ER stress to induce apoptosis. The markers for ER stress,
such as phospho-PKR-like ER kinase (p-PERK), phospho-eukaryotic
initiation factor-2α (p-eIF2α) and GRP78 were investigated. To this
end, HepG2 and SMCC7721 cells were treated with increased dose of
cinchonine for 12 h. We found that cinchonine markedly induced the
expressions of ER stress makers in liver cancer cells in a dose
dependent manner (Fig. 3A and B).
Taken together, these data suggested that cinchonine efficiently
activated ER stress in liver cancer, and eventually leading to
cells apoptosis.
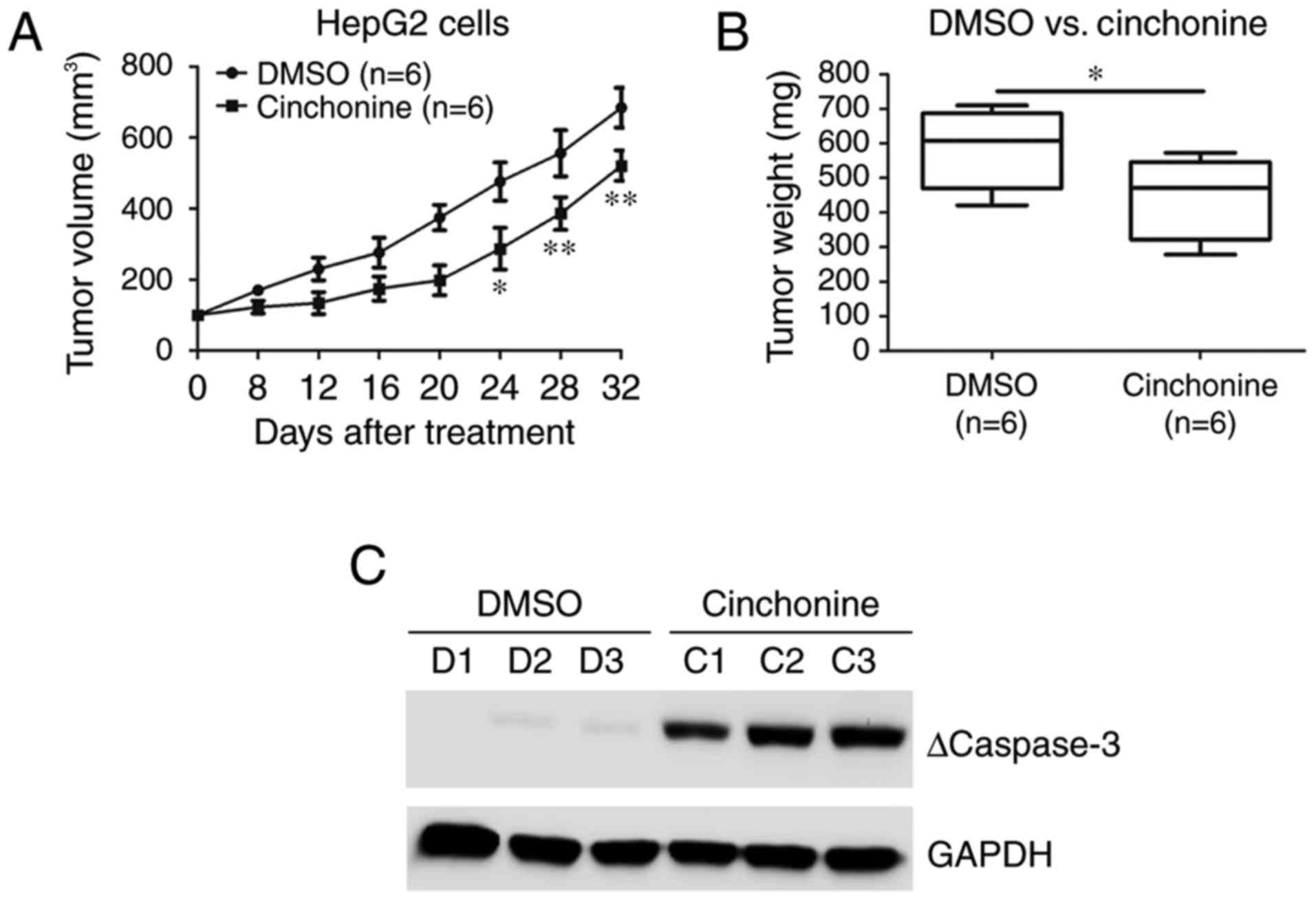
Cinchonine inhibited liver cancer
cells growth in vivo
We then evaluated the antitumor effects of
cinchonine in vivo using a xenograft mouse model. HepG2
cells were injected subcutaneously into the flanks of Balb/c-nude
mice. After the tumors reached about 150 mm3 in volume,
these mice were treated with DMSO or cinchonine (0.5 mg/Kg) by
intraperitoneal injection once per day for 32 days. From about
three weeks after treatment, the tumor growth was markedly
inhibited in the cinchonine-treated group than in DMSO group
(Fig. 4A). At the end of the
treatment, the tumors in the cinchonine-treated group were
significantly lighter than those in the control group (Fig. 4B). We examined the protein expression
levels of cleaved caspase-3 in xenograft tumor samples from both
groups. We try to confirm our in vitro findings concerning
the associations between caspase-3 activation and tumor growth
inhibition induced by cinchonine treatment. Western blot data
revealed increased cleaved caspase-3 levels after cinchonine
treatment in HepG2 tumor tissues (Fig.
4C), indicating that enhanced cell apoptosis contributed to
cinchonine-induced tumor growth inhibition.
Discussion
Previous studies have reported that cinchonine is an
inhibitor of human platelet aggregation through the inhibition of
Ca2+ influx and PKC pathways in platelets (15). Cinchonine has been shown to possess a
suppressive effect on adipogenesis and it also attenuates
inflammation in the adipose tissue of mice fed on the High-Fat-Diet
(18). A recent study found that
cinchonine bound to TRAF6 in HeLa and A549 cells to induce
apoptosis of these cancer cells, suggesting the potential antitumor
effect of cinchonine. However, whether cinchonine is effective
against liver cancer is still unknown.
In the present study, we found that cinchonine
inhibits most liver cancer cells proliferation and promotes
apoptosis in a dose-dependent manner. Perturbation of the ER
environment, such as increased improperly folded proteins in ER,
can cause ER stress and trigger the unfolded protein response. High
levels of misfolded proteins can disrupt cellular homeostasis,
induce ER stress, and might eventually lead to apoptosis (10). We found that cinchonine dramatically
increased GRP78/CHOP protein levels and promoted PERK and eIF2a
phosphorylation, suggesting that cinchonine activated ER stress
response. We further observed that prolonged cinchonine exposure
promoted caspase-3 activation and PARP1 cleavage in liver cancer
cells, suggesting that cinchonine activate ER stress-induced
apoptosis in liver cancer cells. Moreover, we also observed that
cinchonine significantly suppressed HepG2 xenograft tumors growth
in mice and also activated caspase-3 activation in vivo.
Bel-7402 cell was not very sensitive to cinchonine treatment, and
perhaps it contains less amount of GRP78 or CHOP protein levels,
which should be test in the future.
In conclusion, cinchonine promotes ER-stress in
liver cancer which in turn activating caspase-3-dependent apoptosis
both in vitro and in vivo. These results suggest
cinchonine might be a potential antitumor drug in the treatment of
human liver cancer.
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cabibbo G and Craxì A: Epidemiology, risk
factors and surveillance of hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 14:352–355. 2010.PubMed/NCBI
|
|
3
|
Mann J: Natural products in cancer
chemotherapy: Past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: A review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang RR, Zhang S, Qi JA, Wang ZD, Li J,
Liu PJ, Huang C, Le XF, Yang J and Li ZF: Preferential inhibition
of hepatocellular carcinoma by the flavonoid Baicalein through
blocking MEK-ERK signaling. Int J Oncol. 41:969–978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh P, Singh IN, Mondal SC, Singh L and
Garg VK: Platelet-activating factor (PAF)-antagonists of natural
origin. Fitoterapia. 84:180–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nobili S, Landini I, Giglioni B and Mini
E: Pharmacological strategies for overcoming multidrug resistance.
Curr Drug Targets. 7:861–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SY, Rhee YH, Jeong SJ, Lee HJ, Lee HJ,
Jung MH and Kim SH, Lee EO, Ahn KS, Ahn KS and Kim SH:
Hydrocinchonine, cinchonine, and quinidine potentiate
paclitaxel-induced cytotoxicity and apoptosis via multidrug
resistance reversal in MES-SA/DX5 uterine sarcoma cells. Environ
Toxicol. 26:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen P, Wang K, Guo W, Liu X, Liu Y and Li
C: Enantioselective reactions of 2-sulfonylalkyl phenols with
allenic esters: Dynamic kinetic resolution and [4+2] cycloaddition
involving ortho-Quinone methide intermediates. Angew Chem Int Ed
Engl. 56:3689–3693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryoo HD: Long and short (timeframe) of
endoplasmic reticulum stress-induced cell death. FEBS J.
283:3718–3722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palam LR, Baird TD and Wek RC:
Phosphorylation of eIF2 facilitates ribosomal bypass of an
inhibitory upstream ORF to enhance CHOP translation. J Biol Chem.
286:10939–10949. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harding HP, Novoa I, Zhang Y, Zeng H, Wek
R, Schapira M and Ron D: Regulated translation initiation controls
stress-induced gene expression in mammalian cells. Mol cell.
6:1099–1108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah BH, Nawaz Z, Virani SS, Ali IQ, Saeed
SA and Gilani AH: The inhibitory effect of cinchonine on human
platelet aggregation due to blockade of calcium influx. Biochem
Pharmacol. 56:955–960. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lipson KL, Fonseca SG and Urano F:
Endoplasmic reticulum stress-induced apoptosis and auto-immunity in
diabetes. Curr Mol Med. 6:71–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung SA, Choi M, Kim S, Yu R and Park T:
Cinchonine prevents high-fat-diet-induced obesity through
downregulation of adipogenesis and adipose inflammation. PPAR Res.
2012:5412042012. View Article : Google Scholar : PubMed/NCBI
|