Introduction
Musculoskeletal tumors are frequent recording an
incidence of 3/1,000 in USA, but only 0.67% are malignant, that
pose a serious public health problem in the modern world (1). Malignant musculoskeletal tumors are
associated with high mortality and disability rates (2–4).
Although it is not possible to determine the exact cost of the
burden of malignant musculoskeletal tumors, a good example comes
from a model for the productivity costs of cancer mortality that
projected the annual expenses for USA at approximately $115.8
billion in the year 2000, and $147.6 billion for 2020 (5). It is very important to raise global
awareness of the growing burden of malignant musculoskeletal tumor.
Benign and malignant musculoskeletal tumours are notoriously
difficult to differentiate and represent a clinical, radiological
and histological challenge. Meanwhile, accurate prediction of the
grade of tumor cell before surgical removal could affect the type
of surgery and need for adjuvant therapy, but it is not always
possible with the current imaging methods. Typical imaging
characteristics exist for common benign lesions such as lipomas and
hemangiomas. In contrast, no specific imaging features and clinical
manifestations exist for certain musculoskeletal tumors. Therefore,
distinguishing between malignant tumors and benign tumors is
difficult using common methods (6,7). At
present, pathological inspection is clearly the ‘gold standard’ for
identifying pathological changes, and histopathology is the most
objective method because it involves the direct examination of
pathological changes. However, the inherent shortcoming of
histopathology is that it involves invasive inspection. Therefore,
there is an urgent need for clinicians to identify non-invasive
approaches that are comparable to biopsies to qualitatively
describe lesions.
Functional magnetic resonance imaging has become
commonly used worldwide and has great potential for characterizing
pathological changes (8,9). Functional magnetic resonance imaging
includes perfusion-weighted imaging, diffusion-weighted imaging and
magnetic resonance spectroscopy.
Tumor angiogenesis is the formation of new blood
vessels by budding or sprouting from the existing vasculature
(10). Angiogenesis plays a key role
in the tumorigenesis, invasion and metastasis of solid tumors
(11,12) and is considered a sign of tumor
invasion because the resulting rich vascular network provides tumor
cells with sufficient oxygen and nutrients and contributes to tumor
metastasis (13). The microvessel
density (MVD) count is the standard method used to analyze tumor
angiogenesis (14). Dynamic
observations of angiogenesis and the functional status in living
organisms cannot be performed due to the invasiveness and in
vitro of such procedures.
Three-dimensional arterial spin labeling (3D-ASL)
can perform using a pseudo-continuous arterial spin labeling
sequence that uses blood as an endogenous contrast agent, allowing
noninvasive perfusion measurements to be performed without
gadolinium administration. The objective of our study is to
evaluate 3D-ASL perfusion imaging in discriminating between benign,
intermediate and malignant musculoskeletal tumors as well as to
analyze the correlation between tumor blood flow (TBF) and MVD.
Patients and methods
Patients and MR imaging
3D-ASL was performed on 44 patients (20 males and 24
females with a median age of 27 years and an age range of 9 to 57
years) from March 2013 to January 2014. The patients were selected
according to the following inclusion criteria: i) The presence of
low extremity or pelvic musculoskeletal tumors; ii) surgical
resection performed and a definite pathologic diagnosis obtained
within two weeks; and exclusion criteria: i) the general condition
cannot tolerate MR examination; ii) undergo radiotherapy or
chemotherapy treatment prior to 3D-ASL; and iii) the presence of
vascular stenosis in extremity or pelvis.
The patients were divided into benign, intermediate
and malignant groups according to the International Classification
of Diseases for Oncology (15).
All subjects were scanned on a 3.0-T magnetic
resonance (MR) system (Discovery 750; GE Healthcare, Milwaukee, WI,
USA) with use of a 32-element phased-array abdominal coil (GE
Healthcare), an ADW4.6 workstation and Functool software. The
examination protocol comprised axial T1-weighted image with
fat-suppressed, T2-weighted images with fat saturation and 3D-ASL.
The T1-weighted, T2-weighted scan parameters were as follows:
repetition time (TR) 250/4,000 msec; echo time (TE) 25/76 msec;
slice thickness/gap 4/0 mm; number of excitations (NEX) 2.0; matrix
320×256; and field of view (FOV) 30×30-40×40 cm (selected to
accommodate the size of the tumor). For 3D-ASL, calibration was
performed before scanning using the same slice thickness, gap,
matrix and FOV as T2-WI and with TE 10.4 msec and TR 4,552 msec,
post label delaey (PLD) 1,525 msec, NEX=2.0.
This study was approved by the Committee for the
Protection of Human Subjects of the Institutional Review Board of
Ningbo Medical Treatment Center Lihuili Hospital, and informed
consent was obtained from all subjects.
Image processing and data
analysis
After exporting the raw ASL data, post-processing
was performed to obtain the TBF maps using the GE ADW4.6
workstation, followed by slice-by-slice registration and fusion
with the T2-WI images by radiologists. The brightly colored regions
in TBF images represent areas of high perfusion. Regions of
interest (ROIs) for quantitative analysis were manually placed on
the highest perfusion slice at TBF maps independently by two
radiologists (more than 15 years of experience) who were dedicated
to musculoskeletal tumor study in our hospital. Necrosis, cystic
areas, hemorrhage and calcification were excluded by T1-weighted,
T2-weighted. In principle, the area of the ROI should be 20–35
mm2. Three ROIs were measured, and the mean value was
calculated.
Pathologic examination
CD34 (cat. no. MAB-0034 QBEnd/10) and DAB kit (cat.
no. Kit-0014; MaxVision Biosciences Inc., Fuzhou, China) was used
for immunohistochemical staining of tumor specimens. CD34
monoclonal antibody was used to label vessel endothelial cell. The
CD34 monoclonal antibody and the DBA kit were both purchased from
Maxin Biotech Co., Ltd. (Fuzhou, China). The staining was conducted
according to manufacturer's instruction. MVD counts were performed
according to Weidner's method (14).
We found neovascular ‘hotspots’ by scanning the tumor sections at
low power (×40). Individual microvessel counts were then performed
on a ×400 field within the neovascular hotspot. Any individual
endothelial cell or endothelial cell cluster that was positive for
CD34 (observed as brown staining) and was clearly separate from an
adjacent cluster was considered to be a single, countable
microvessel. Microvessels larger than 8 erythrocyte diameters were
not considered in the vessel counts. Any counts were performed by
two investigators using a double-headed light microscope
simultaneously, both had to agree on what constituted a single
microvessel before any vessel was included in the count. Each count
was expressed as the highest number of microvessels identified
within any ×400 field.
Statistical analysis
All statistical analyses were performed using the
SPSS 21.0 package for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. An intra-class correlation coefficient test was used to
determine if there was good inter-observer agreement in the TBF
values measured independently by two experienced radiologists.
Group differences in TBF were assessed using one-way analysis of
variance followed by the Least Significant Difference test to
compare TBF among three groups. A receiver operating characteristic
(ROC) curve was computed to display the sensitivity and specificity
of TBF in predicting the nature of musculoskeletal tumors. The area
under the receiver operating characteristic curve (AUC) of TBF was
calculated, and the cut-off value was then calculated to achieve
the maximum accuracy percentage. Correlations between TBF and MVD
were assessed by Pearson's correlation analysis.
Results
There were 12 subjects in the benign group, 10 in
the intermediate group and 22 in the malignant group. Detailed
diagnoses are reported in Table I.
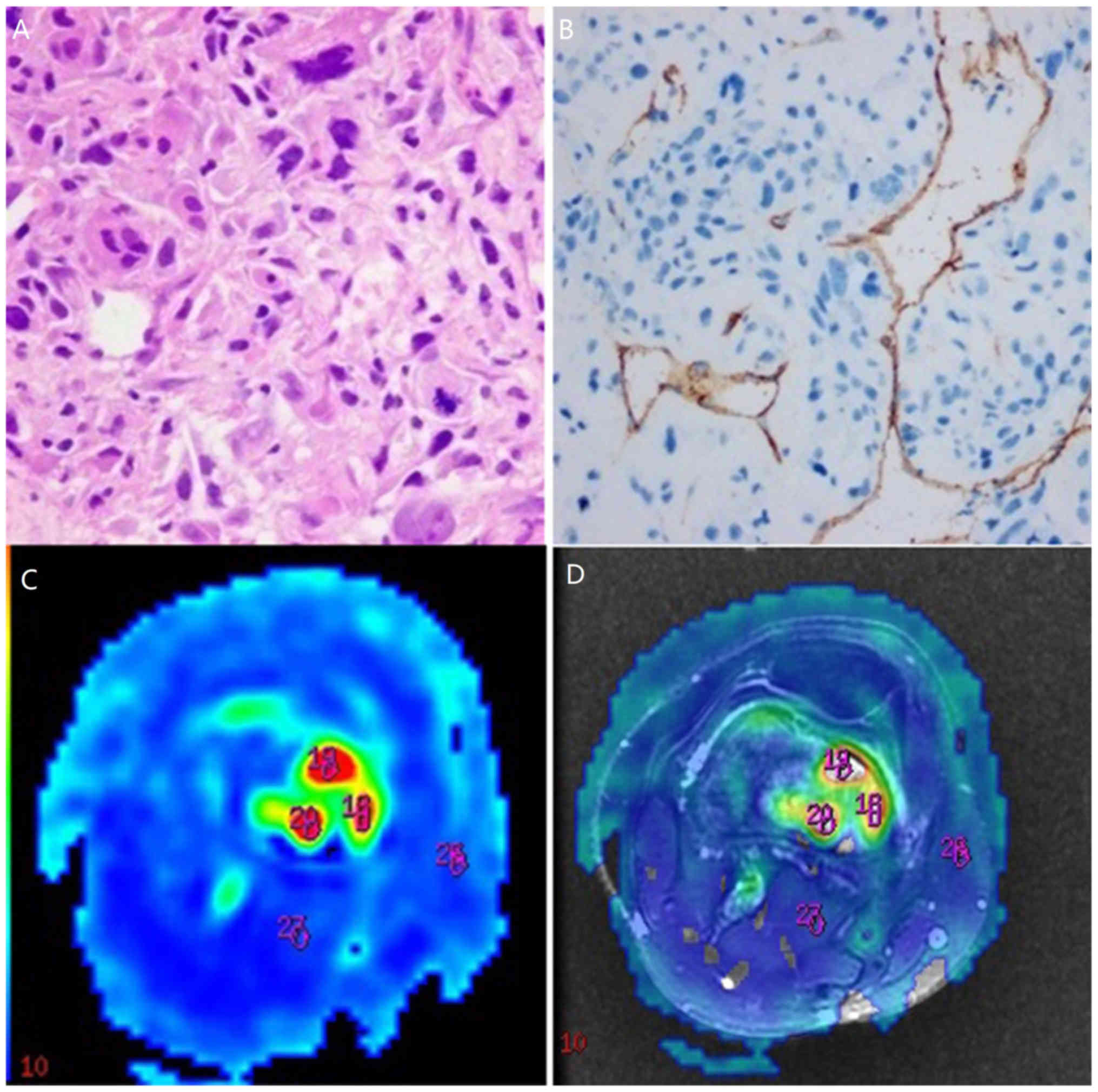
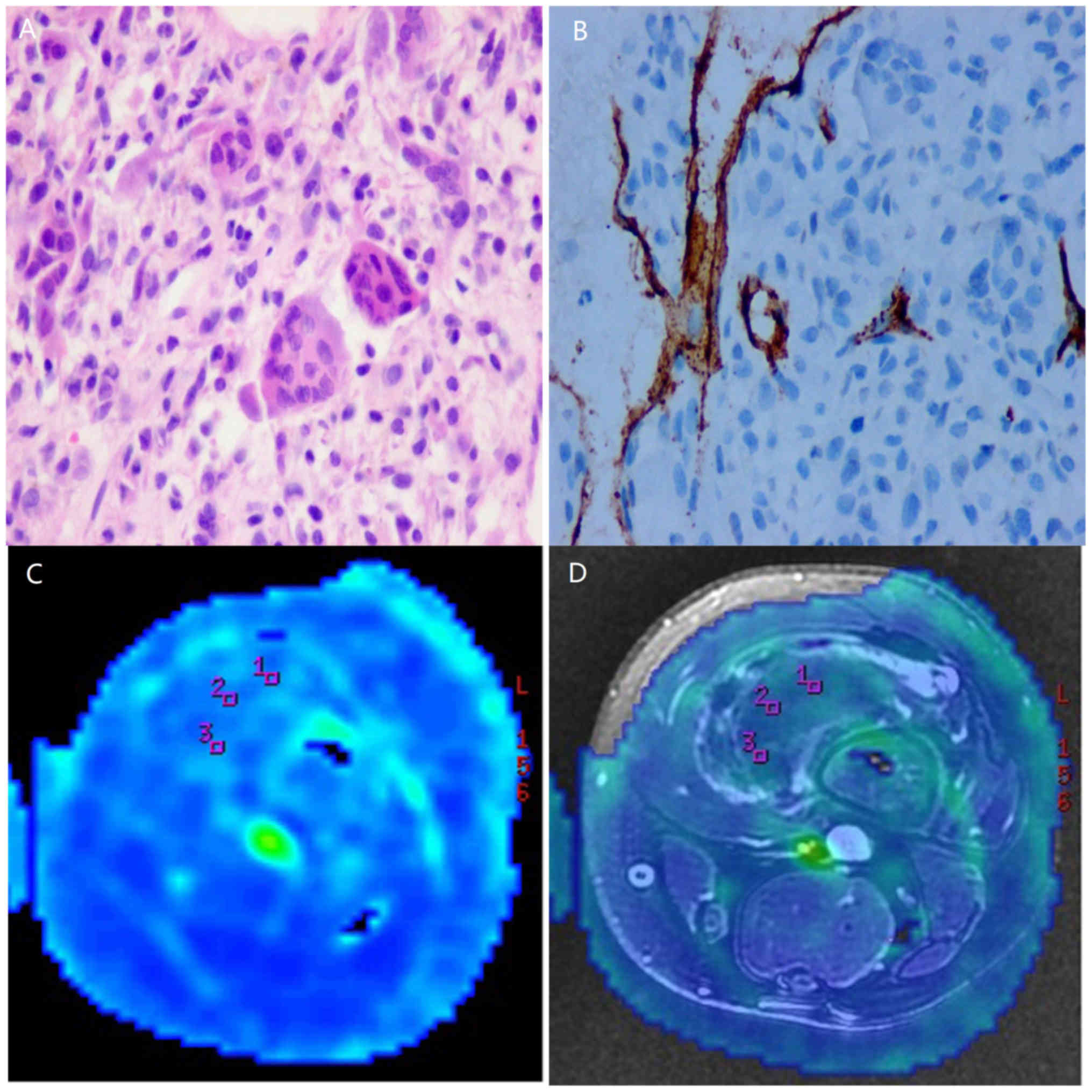
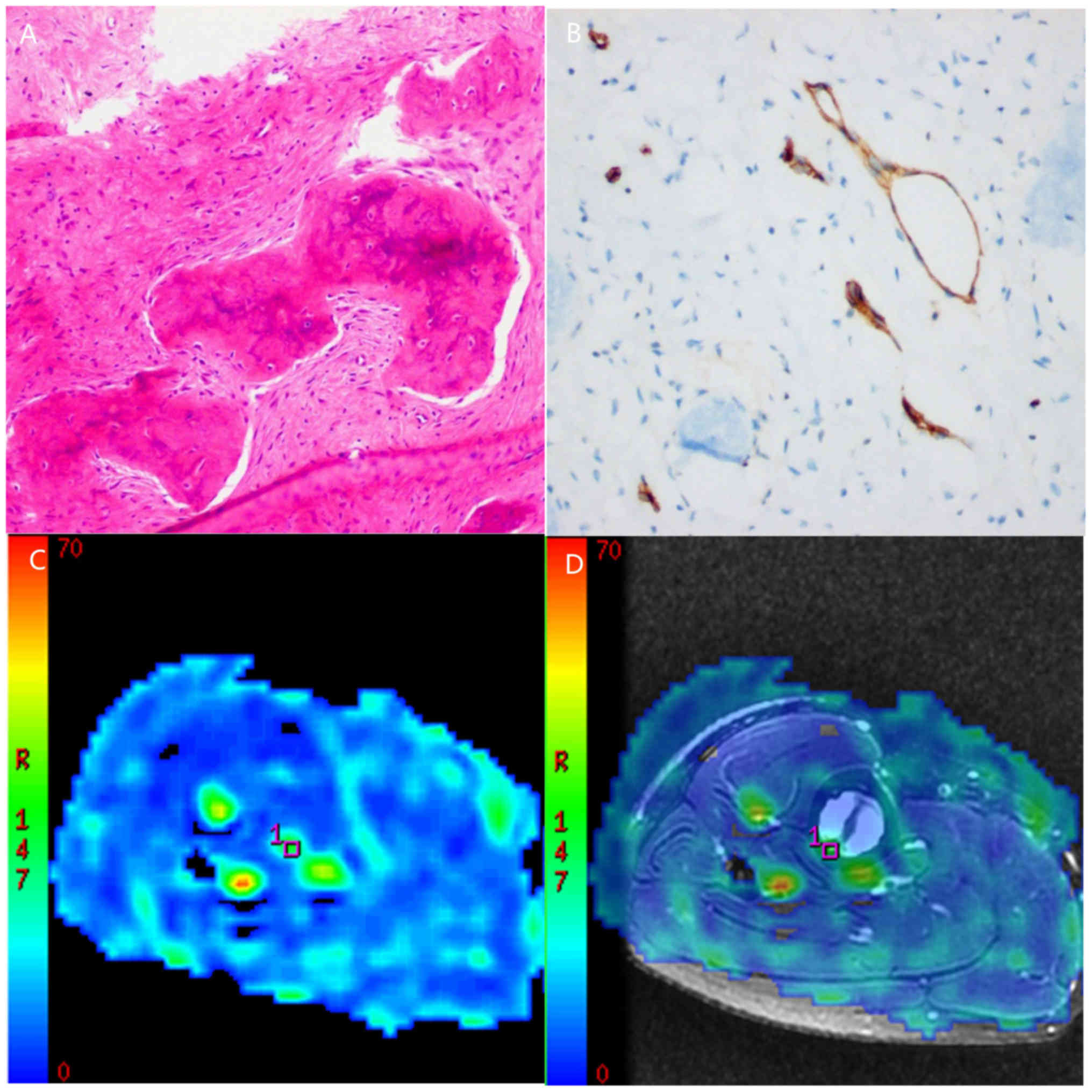
Routine pathological sections were obtained in all subjects
(Figs. 1A, 2A and 3A).
All tumor sections were positive for CD34 (Figs. 1B, 2B
and 3B). The MVD counts were
10.38±4.58 and 14.64±6.69 in the benign and intermediate groups,
respectively, and 32.97±11.61 in the malignant group.
 | Table I.Histopathological description of tumor
and subtypes of 44 cases included in the present study. |
Table I.
Histopathological description of tumor
and subtypes of 44 cases included in the present study.
| Subtype | No. of patients |
|---|
| Benign |
|
|
Chondroblastoma | 2 |
|
Hemangioma | 1 |
|
Osteochondroma | 1 |
|
Enchondroma | 3 |
|
Fibroma | 2 |
|
Aneurismal bone cyst | 2 |
| Intermediate |
|
| Desmoid
type fibromatosis | 2 |
| Giant
cell tumor of bone | 5 |
| Solitary
fibrous tumor | 2 |
| Malignant |
|
|
Osteosarcoma | 11 |
|
Metastases involving bone | 3 |
|
Liposarcoma | 1 |
| Primitive
neuroectodermal tumor | 1 |
| Plasma
cell myeloma | 1 |
|
Pleomorphic undifferentiated
sarcoma | 1 |
|
Epithelioid
hemangioendothlioma | 1 |
| Ewing's
sarcoma | 1 |
|
Sclerosing epithelioid
fibrosarcoma | 1 |
Good inter-observer agreement was found for the TBF
measurements made by the two observers as assessed by the
intra-class correlation coefficient test (ICC=0.891, P<0.05).
The brightly colored regions in TBF images obtained by 3D-ASL
represent areas of high perfusion. The solid-appearing parts of
malignant tumors were brighter in color than the benign and
intermediate tumors (Figs. 1C,
2C and 3C), which can be observed more obviously in
fusion images (Figs. 1D, 2D and 3D).
Additionally, necrotic or cystic areas demonstrated perfusion
defects. Significant differences were found in the MVD and TBF
among the three groups (F=28.33, P<0.05, F=32.34, P<0.05,
respectively). The MVD and TBF results are displayed in Table II. The MVD and TBF values were
higher for malignant tumors than for benign and intermediate tumors
(P<0.05), and TBF was not significantly different between benign
and intermediate tumors.
 | Table II.MVD and TBF of the musculoskeletal
tumors of the three groups. |
Table II.
MVD and TBF of the musculoskeletal
tumors of the three groups.
| Group | No. | MVD (Pieces/HPF) | TBF (ml·min |
|---|
| Benign | 12 | 10±4a |
30±10a |
| Intermediate | 10 | 15±6b |
30±12b |
| Malignant | 22 |
33±11 | 84±29 |
| F-value |
| 28.33 | 32.34 |
| P-value |
| <0.05 | <0.05 |
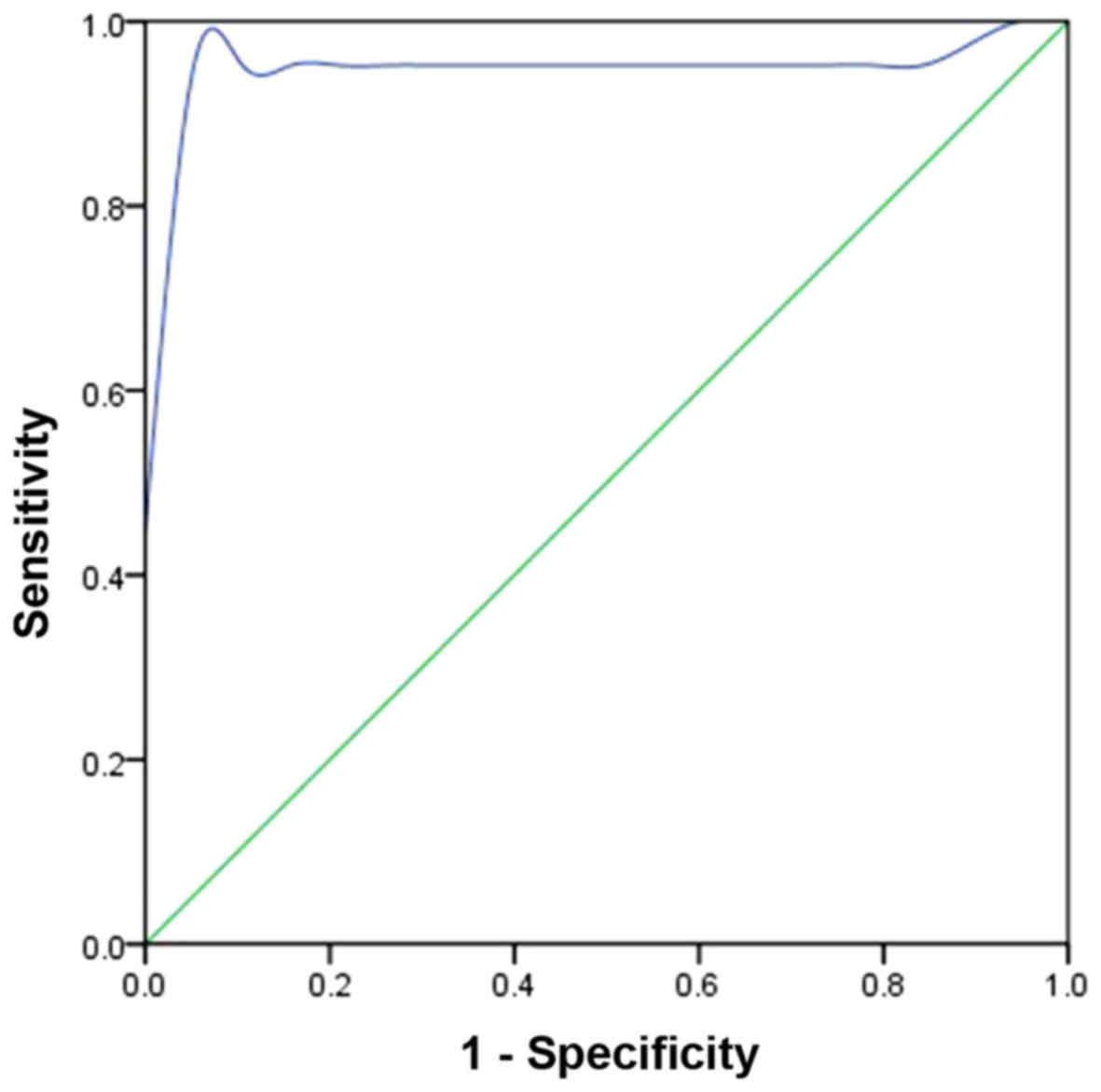
The ROC curve of malignant musculoskeletal tumors
plotted in Fig. 4 demonstrates that
the TBF AUCROC is 0.951 and that the ROC cut-off value
of TBF is 45 ml·min−1·100 g−1 (P<0.05).
The diagnostic sensitivity and specificity are 90.5% and 100%,
respectively.
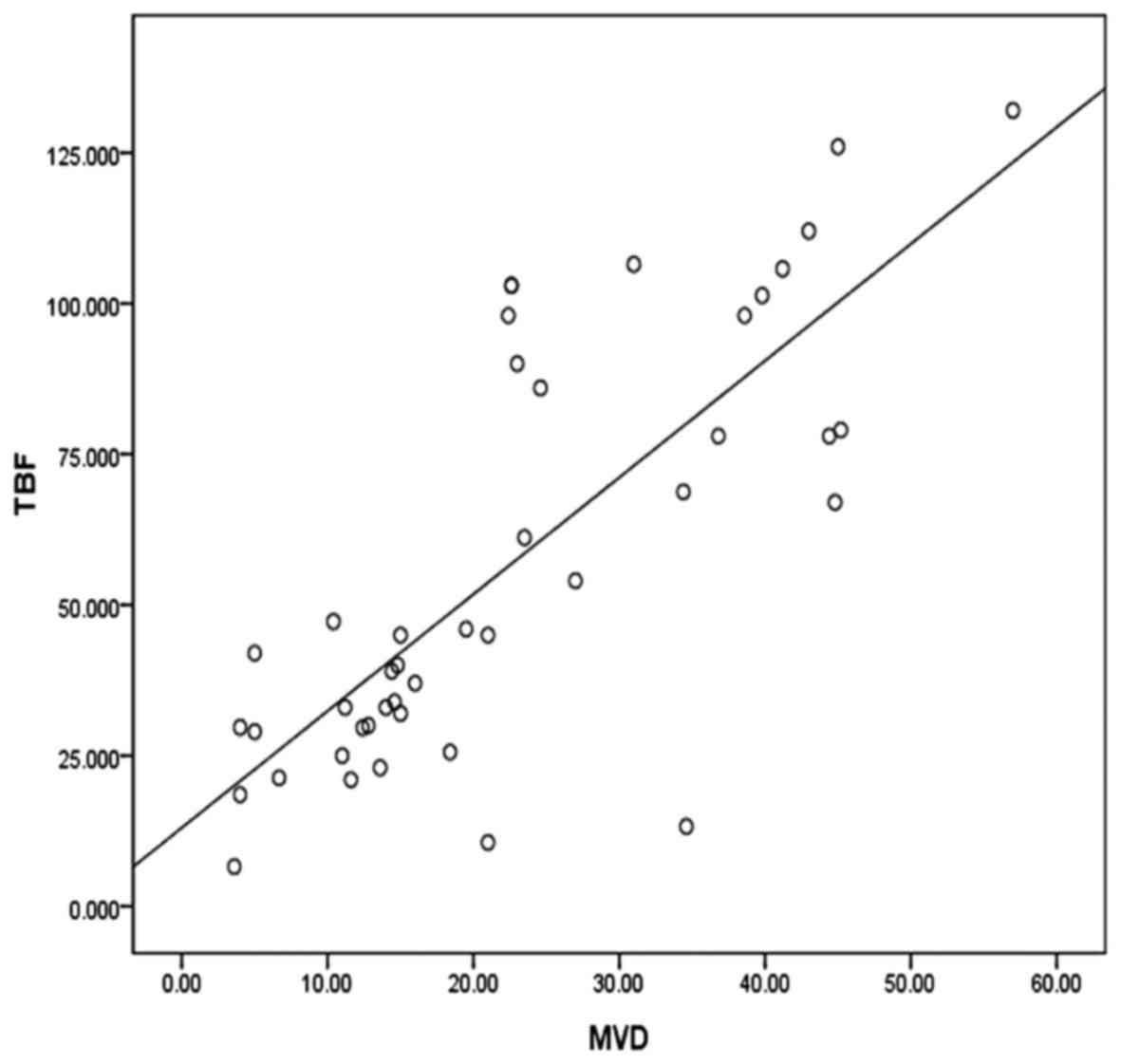
According to Pearson's correlation analysis, a
significant positive correlation was found between TBF and MVD
(r=0.784, P<0.05), as demonstrated by the approximately linear
positive correlation shown in Fig
5.
Discussion
Angiogenesis is a dynamic process that is related to
many factors. Under physiological conditions, angiogenesis is
strictly controlled by various factors to maintain the delicate
balance between angiogenic and anti-angiogenic factors (16). Under pathological conditions, in
which this regulation is out of balance, angiogenic factors surpass
anti-angiogenic factors, and control is lost (17). The presence of many immature new
blood vessels is an important feature of malignant tumors.
Angiogenesis in tumors is closely related to the tumor growth rate,
the grade of malignancy, the possibility of invasion or metastasis
and the prognosis (18–20). Quantitatively analyzing angiogenesis
is therefore critical. Since Weidner et al (14) first used MVD to evaluate tumor
angiogenesis, many scholars (21,22) have
investigated and analyzed tumor angiogenesis in a variety of solid
tumors using the MVD. At present, the MVD is the ‘gold standard’
for assessing tumor angiogenesis because it is an objective and
sensitive measure. In the present study, differences in the MVD
between the benign and malignant groups and between the
intermediate and malignant groups were statistically significant
(P<0.05). Thus, the MVD may reflect the nature and the degree of
differentiation of musculoskeletal tumors to a certain extent.
Luczynska and colleagues reached a similar conclusion in their
study of prostate tumors (23). In
our study, no significant difference in the MVD was found between
the benign and intermediate groups. There are a couple possible
explanations for this result. First, although we investigated
various musculoskeletal tumor types, the sampling capacity of the
study was limited. Second, tumor angiogenesis is not only related
to the number of capillaries but also to the function of
capillaries, which includes vascular endothelial cell and basement
membrane integrity, blood vessel permeability and capillary flow.
Therefore, further research on the subject is warranted. Although
we agree that the MVD is the ‘gold standard’ for identifying
angiogenesis in tumors, MVD counts cannot be extensively performed
due to invasiveness as well as to in vitro assay defects
(24).
Functional imaging can evaluate tumor angiogenesis
based on the level of perfusion. CT perfusion imaging (25) and MR perfusion-weighted imaging
(26) can both measure perfusion,
but both modalities require the intravenous injection of contrast
medium, which may be associated with contrast medium allergies or
side effects to the viscera. CT examination is also associated with
a risk of radiation exposure. Loizides et al (27) showed by Contrast Enhanced Ultrasound
that the enhancement curves of benign and malignant tumors are
different. This difference may contribute to the ability to
distinguish between benign and malignant tumors of a similar size
and location. However, the results were based on the analysis of
semi-quantitative data; therefore, the reliability was not very
good.
In this study, 3D-ASL was performed using a
pseudo-continuous arterial spin labeling sequence that uses blood
as an endogenous contrast agent, allowing noninvasive perfusion
measurements to be performed without gadolinium administration.
Inflowing blood is selectively labeled with the opposite
magnetization of the destination tissue. The difference between a
labeled image (tag) and an unlabeled image (control) can be used to
calculate tissue perfusion. 3D-ASL uses a spin echo sequence as an
acquisition approach to obtain a wide range of perfusion with a low
specific absorption rate (SAR) and a high signal-to-noise ratio and
tag rate. 3D-ASL, first applied clinically to examine the brain,
has recently demonstrated some clinical value in body examinations
(28). To date, ASL perfusion
weighted-imaging has rarely been performed on musculoskeletal
tumors in humans. However, a few studies using a rabbit VX2 tumor
model have used 3D-ASL to evaluate tumor angiogenesis (29). In the present study, the TBF values
of benign and intermediate tumors were lower than the TBF value of
malignant tumors; this finding is consistent with the MVD results.
Not only the organ injury risk but also the higher cost and poor
repeatability due to intravenous injection limit the use of MR
perfusion-weighted imaging with contrast agents, although CT
perfusion imaging and MR perfusion-weighted imaging have achieved
similar positive results (30,31).
Although both TBF and MVD relate to angiogenesis, how close the
relationship is between them remains unknown. According to our
research, TBF is significantly positively correlated with MVD. This
conclusion strengthens the theory that TBF indicates angiogenesis
by reflecting tissue perfusion (32). Therefore, we believe that TBF
obtained from 3D-ASL is an effective biomarker of tumor
angiogenesis.
According to the ROC analysis, TBF has good
sensitivity and specificity in the characterization musculoskeletal
tumors. We consider 3D-ASL an important sequence in discriminating
between benign, intermediate and malignant musculoskeletal tumors
used in combination with routine imaging methods. Additionally, TBF
imaging combined with traditional structural imaging can provide
direct tumor perfusion information for future operation or further
treatment.
This paper has following limits: The tumors are very
heterogenous. Many of these are represented by a single case.
Furthermore, 3D-ASL is operator-dependent as it is subjective the
choice of ROIs in the quantitative analysis. Another problem is
that TBF image cannot match the structure image perfectly due to
technique reasons, that will cause the deviation in measurement.
Nevertheless, we believe that the inclusion of 3D-ASL in the
work-up of musculoskeletal masses and the Correlation with MVD can
serve as a new, fast-forward diagnostic tool for distinguishing
malignant musculoskeletal tumors from their benign counterparts,
the more so as 3D-ASL is a broadly available, non-invasive
technique compared with contrast enhanced MRI and CT perfusion, for
example.
In conclusion, the MVD represents musculoskeletal
tumor angiogenesis and may reflects their biological behavior.
Therefore, the MVD has clinical value in discriminating between
types of tumors. Furthermore, a correlation exists between TBF and
the MVD. 3D-ASL could be useful for pathological examination to a
certain extent and is promising as a repeatable, completely
non-invasive technique to evaluate musculoskeletal tumor
angiogenesis in vivo. It has some application to
determination of malignancy, but that it is premature to state that
it can be used as a sole, or even dominant criterion. At least, the
inclusion of 3D-ASL in established perfusion-based assessment
protocols improves the evaluation of musculoskeletal masses. By the
analysis of tumour perfusion, an important aspect of tumour
pathophysiology-which is beyond the scope of grey-scale and
conventional contrast enhanced MRI-becomes assessable. This has
practical consequences for patient management: Better detection of
malignant tumours by 3D-ASL and thus timely, fast-forward
institution of further patient work-up, biopsy and treatment.
For the near future, a multicenter study is hoped in
order to increase the number of the subject and the type of the
musculoskeletal tumor, that will help the persuasion of the
research.
Acknowledgements
This study was supported by the Science and
Technology Program for Public Wellbeing of Ningbo municipal Bureau
of Science and Technology (grant no. 2015C50022).
Glossary
Abbreviations
Abbreviations:
|
3D-ASL
|
three-dimensional arterial spin
labeling
|
|
TBF
|
tumor blood flow
|
|
MVD
|
microvessel density
|
|
ROI
|
regions of interest
|
|
SAR
|
absorption rate
|
References
|
1
|
Campanacci M: Bone and soft tissue
tumours. New York: Springer-Verlag; pp. 199–232. 1999
|
|
2
|
Weeden S, Grimer RJ, Cannon SR, Taminiau
AH and Uscinska BM: European Osteosarcoma, Intergroup: The effect
of local recurrence on survival in resected osteosarcoma. Eur J
Cancer. 37:39–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marulanda GA, Henderson ER, Johnson DA,
Letson GD and Cheong D: Orthopaedic surgery options for the
treatment of primary osteosarcoma. Cancer Control. 15:13–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grimer RJ, Taminiau AM and Cannon SR:
Surgical Subcommitte of the European Osteosarcoma Intergroup:
Surgical outcomes in osteosarcoma. J Bone Joint Surg Br.
84:395–400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bradley CJ, Yabroff KR, Dahman B, Feuer
EJ, Mariotto A and Brown ML: Productivity costs of cancer mortality
in the United States: 2000–2020. J Natl Cancer Inst. 100:1763–1770.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kransdorf MJ and Murphey MD: Radiologic
evaluation of soft-tissue masses: A current perspective. AJR Am J
Roentgenol. 175:575–587. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel MJ: Magnetic resonance imaging of
musculoskeletal soft tissue masses. Radiol Clin North Am.
39:701–720. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang CK, Li CW, Hsieh TJ, Chien SH, Liu GC
and Tsai KB: Characteriztion of bone and soft tissue tumors with in
vivo 1H-MR spectroscopy: Initial results. Radiology. 232:599–605.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johansen R, Jensen LR, Rydland J, Goa PE,
Kvistad KA, Bathen TF, Axelson DE, Lundgren S and Gribbestad IS:
Predicting survival and early clinical response to primary
chemotherapy for patients with locally advanced breast cancer using
DCE-MRI. J Magn Reson Imaging. 29:1300–1307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Hu T, He L, Huang X, Tian X, Zhang
H, He L, Pu W, Zhang L, Sun H, et al: Genetic targeting of
sprouting angiogenesis using Apln-CreER. Nat Commun. 6:60202015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukumura D and Jain RK: Imaging
angiogenesis and the microenvironment. APMIS. 116:695–715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rak JW, St Croix BD and Kerbel RS:
Consequences of angiogenesis for tumor progression, metastasis and
cancer therapy. Anticancer Drugs. 6:3–18. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weidner N, Folkma J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flecher CD, Unni KK and Merten F: World
Health Organization classification of tumousPathology and genetics
of tumors of soft tissue and bone. IARC Press; Lyon: pp. 2–10.
2002
|
|
16
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukumura D, Duda DG, Munn LL and Jain RK:
Tumor microvasculature and microenvironment: Novel insights through
intravital imaging in pre-clinical models. Microcirculation.
17:206–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rak JW, St Croix BD and Kerbel RS:
Consequences of angiogenesis for tumor progression, metastasis and
cancer therapy. Anticancer Drugs. 6:3–18. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bose S, Lesser ML, Norton L and Rosen PP:
Immunophenotype of intraductal carcinoma. Arch Pathol Lab Med.
120:81–85. 1996.PubMed/NCBI
|
|
20
|
Gilles R, Zafrani B, Guinebretière JM,
Meunier M, Lucidarme O, Tardivon AA, Rochard F, Vanel D,
Neuenschwander S and Arriagada R: Ductal carcinoma in situ: MR
imaging-histopathologic correlation. Radiology. 196:415–419. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlemmer HP, Merkle J, Grobholz R, Jaeger
T, Michel MS, Werner A, Rabe J and van Kaick G: Can pre-operative
contrast-enhanced dynamic MR imaging for prostate cancer predict
microvessel density in prostatectomy specimens? Eur Radiol.
14:309–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marković O, Marisavljević D, Cemerikić V,
Vidović A, Perunicić M, Todorović M, Elezović I and Colović M:
Expression of VEGF and microvessel density in patients with
multiple myeloma: Clinical and prognostic significance. Med Oncol.
25:451–457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luczynska E, Gasinska A, Blencharz P,
Stelmach A, Jereczek-Fossa BA and Reinfuss M: Value of perfusion CT
parameters, microvessl density and VEGF expression in
differentiation of benign and malignant prostate tumours. Pol J
Pathol. 65:229–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tozer GM: Measuring tumour vascular
response to antivascular and antiangiogenic drugs. Br J Radiol.
76:S23–S35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Yang ZG, Chen TW, Chen HJ, Sun JY
and Lu YR: Peripheral lung carcinoma: Correlation of angiogenesis
and first-pass perfusion parameters of 64-detector row CT. Lung
Cancer. 61:44–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gordon Y, Partovi S, Müller-Eschner M,
Amarteifio E, Bäuerle T, Weber MA, Kauczor HU and Rengier F:
Dynamic contrast-enhanced magnetic resonance imaging: Fundamentals
and application to the evaluation of the peripheral perfusion.
Cardiovasc Diagn Ther. 4:147–164. 2014.PubMed/NCBI
|
|
27
|
Loizides A, Peer S, Plaikner M, Djurdjevic
T and Gruber H: Perfusion pattern of musculoskeletal masses using
contrast-enhanced ultrasound: A helpful tool for characterisation?
Eur Radiol. 22:1803–1811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu WC, Wang J, Detre JA, Ratcliffe SJ and
Floyd TF: Transit delay and flow quantification in muscle with
continuous arterial spin labeling perfusion-MRI. J Magn Reson
Imaging. 28:445–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Meng Q, Gao Z, Cai H, Ye Z and Wu
H: Evaluation of angiogenesis of VX2 soft tissue tumor by arterial
spin labeling perfusion imaging. Chin J Radiol. 10:1084–1088.
2010.
|
|
30
|
Bivard A, Krishnamurthy V, Stanwell P,
Levi C, Spratt NJ, Davis S and Parsons M: Arterial spin labeling
versus bolus-tracking perfusion in hyperacute stroke. Stroke.
45:127–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wolf RL, Wang J, Wang S, Melhem ER,
O'Rourke DM, Judy KD and Detre JA: Grading of CNS neoplasms using
continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J
Magn Reson Imaging. 22:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koizumi S, Sakai N, Kawaji H, Takehara Y,
Yamashita S, Sakahara H, Baba S, Hiramatsu H, Sameshima T and Namba
H: Pseudo-continuous arterial spin labeling reflects vascular
density and differentiates angiomatous meningiomas from
non-angiomatous meningiomas. J Neuroonncol. 121:549–556. 2015.
View Article : Google Scholar
|