Introduction
Diabetes mellitus (DM) is a type of metabolic
disease caused by insulin secretion dysfunction and/or abnormal
insulin action, characterized by chronic hyperglycemia,
carbohydrate, fat and protein metabolic disorders (1). DM causes a series of physiological and
pathological changes in the body and chronic lesions in lung,
heart, brain, kidney, nerve and other organs and even leads to
functional defects and failure (2).
Diabetic nephropathy (DN) is one of the potentially
destructive complications of DM, its incidence is high and ~20% of
patients with type 2 diabetes are likely to be eventually
complicated by DN (3). DN is the
leading cause of end-stage renal disease, accounting for ~50% of
the total of end-stage renal disease (4). High renal perfusion and high
filtration, thickening of glomerular basement membrane and
extracellular matrix accumulation dominated by mesangial area leads
to diffuse and nodular glomerulosclerosis, which is clinically
manifested as increased blood pressure, proteinuria, renal
insufficiency and other symptoms with a great risk of
cardiovascular death (5).
AMP-activated protein kinase (AMPK) is the main sensor and
modulator of a cellular energy state. In metabolic stress, AMPK
inhibits anabolism and promotes the catabolic processes to restore
the energy homeostasis (6), of which
α subunits (AMPKα) are catalytically active and play important
roles in liver glucose and lipid metabolism (7). At present, studies have shown that
hypersensitive C-reactive protein (hs-CRP), as a risk factor of DN,
can predict the risk of DN in patients with type 2 diabetes to a
certain extent, pro-inflammatory immune FcγR is the Fc receptor in
IgG constant region and involved in the inflammatory process of DN,
which is closely related to DN development and progression
(8).
This study clarified the effects of baicalein on the
DN rats by detecting the levels of AMPKα, hs-CRP and FcγR.
Materials and methods
Experimental materials, apparatus and
reagents
A total of 60 healthy adult Sprague-Dawley male rats
(approximately 200 g) were provided by Liaoning Changsheng
Biotechnology Co., Ltd. (Benxi, China). Experimental apparatus and
reagents included: Microtome produced by Leica Biosystems (Wetzlar,
Germany), centrifugal machine manufactured by Beijing Guangan
Medical Instrument Factory (Beijing, China), electronic balance
manufactured by Changzhou Hongheng Electronic Equipment (Changzhou,
China), UV-2000 UV analyzer produced by Shanghai Scientific
Instrument Factory (Shanghai, China), microplate reader (Jiangsu
Potebio Co., Ltd., Jiangsu, China), electrophoresis tank
manufactured by Beijing Liuyi Instrument Factory (Beijing, China),
streptozotocin (STZ; Sigma-Aldrich, Darmstadt, Germany), Astragalus
injection manufactured by Gaoyou subsidiary of New Asia
Pharmaceutical Co., Ltd. (Shanghai, China), rabbit anti-rat AMPKα
(Cell Signaling Technology, Danvers, MA, USA), horseradish
peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology,
Dallas, TX, USA), polyvinylidene fluoride membrane (PVDF; Roche,
Indianapolis, IN, USA), western blotting luminescence reagent
(Santa Cruz Biotechnology), agarose (Promega, Madison, WI, USA),
TRIzol kit (Invitrogen Life Technologies, Carlsbad, CA, USA),
hs-CRP and FcγR kits were provided by Shanghai Yueyan Biotechnology
Co., Ltd. (Shanghai, China) and the primers were provided by
Shanghai Yingjun Biotechnology Co., Ltd. (Shanghai, China).
Methods
Model preparation and grouping
All the rats had access to food and water ad
libitum for one week and were then divided into the observation
(n=30) and control (n=30) groups using the random number table
method. The control group was fed normally, while the observation
group was fed with high-fat and high-sugar diet and a single
injection of STZ (25 mg/kg). After 4 weeks, fasting blood glucose
was detected and the level ≥7.8 mmol/l was regarded as the DM rat
model. After 8 weeks, the DM rat model with 24 h urine microalbumin
(24 h U-ALB) value of 30 and 300 mg was regarded as the DN rat
model. After the establishment of DN model, 1 ml Astragalus
injection was mixed into 5 ml normal saline for the gavage
administration (400 mg/kg) of observation group for 8 consecutive
weeks, and the control group was treated with the gavage
administration with 3 ml distilled water.
Morphological observation of
kidney
After 8 weeks of administration, the rats were
laparotomized and the kidney tissues were taken as the samples,
followed by fixation and dehydration and 70, 80, 90 and 95% ethanol
was added in turn for treatment during dehydration, followed by
soaking via xylene and embedding via paraffin. Microtome was used
to cut the sample into 5 µm sections, which were stained with
H&E and sealed by neutral balsam, followed by observation of
renal pathological changes under the microscope (Olympus
Corporation, Tokyo, Japan). Ethics approval was obtained from
Nanjing University of Chinese Medicine (Nanjing, China).
Detection of AMPKα in renal
tissues
The mRNA expression in AMPKα in renal tissues was
detected via RT-PCR: i) After 8 weeks of drug administration, 100
mg renal tissues were taken from the rats in each group and stored
at −80°C; ii) the total RNA was extracted strictly according to the
instructions of TRIzol kit; the concentration and purity of RNA
were detected and the concentration ratio was required to be
between 1.8 and 2.0; iii) primer design: The experimental primers
were designed and synthesized by Shanghai Yingjun Biotechnology
(primer sequences are shown in Table
I); iv) access RT-qPCR system (Promega) was used to amplify the
total RNA into target DNA fragment; amplification conditions:
Degeneration at 95°C for 2 min, 94°C for 30 sec, 60°C for 30 sec,
72°C for 1 min, a total of 35 cycles, extension at 72°C for 5 min;
and v) after EB staining and agarose gel electrophoresis, PCR
products were observed and analyzed quantitatively and the relative
expression level of AMPKα mRNA was expressed by the gray level
ratio of AMPKα mRNA to β-actin.
 | Table I.AMPKα and β-actin primer
sequences. |
Table I.
AMPKα and β-actin primer
sequences.
| Item | Sequence |
|---|
| AMPKα | F:
5′-GTTCTACCTCGCCTCCAGTC-3′ |
|
| R:
5′-TGCTCCACCACCTCATCATC-3′ |
| β-actin | F:
5′-ACTGGCATTGTGATGGACTC-3′ |
|
| R:
5′-AGGAAGGAAGGCTGGAAGAG-3′ |
The protein expression in AMPKα in renal tissues was
detected by western blot analysis: i) Iced cell lysis buffer (300
ml) was added into 100 ml renal tissues and then the solution
received ultrasound examination 3 times (5 sec/time) after 30 min
and was centrifuged at 8,600 × g for 60 min at 4°C and the
supernatant was removed; ii) protein quantification was performed
using Lorry method; total protein (40 µg) was dissolved in the
isopyknic buffer solution and boiled for 10 min, followed by
polyacrylamide gel electrophoresis to separate the protein; iii)
the protein was transferred onto the PVDF membrane; and iv) the
PVDF membrane was placed into the blocking solution (dilution,
1:5,000), sealed for 30 min and placed overnight at 4°C; on the
second day, the blocking solution was removed and rabbit anti-rat
AMPKα monoclonal antibody (dilution, 1:5,000; cat. no. 5831; Cell
Signaling Technology, Inc.) was added to incubate for 30 min at
37°C, followed by rinsing 3 times (5 min/time). Horseradish
peroxidase-labeled goat anti-rabbit IgG polyclonal antibody
(dilution 1:10,000; cat. no. 7074; Cell Signaling Technology, Inc.)
was then added to incubate for 120 min at 37°C, followed by rinsing
3 times (5 min/time); and the PVDF membrane was placed into the
cassette with X-ray film for exposure for 10 min, followed by
conventional development and fixation. The relative expression
level of AMPKα protein was expressed by the gray level ratio of
AMPKα mRNA to β-actin protein.
Detection of other indexes
At 1, 4, 6 and 8 weeks after drug intervention,
blood (4 ml) was collected from the abdominal aorta of rats, placed
in ethylene diamine tetraacetic acid (EDTA) anticoagulant tube and
centrifuged at 1,400 × g for 10 min. Subsequently, the supernatant
was removed and stored at −20°C, and 24 h urine was collected to be
tested. The levels of hs-CRP, FcγR, BUN and 24 h U-ALB in rats in
each group were detected. The levels of hs-CRP and FcγR in rats
were detected via ELISA according to the instructions of the kit. A
microplate reader was used to read the OD value at the wavelength
of 450 nm to calculate the concentrations of hs-CRP and FcγR. The
serum BUN level was detected by continuous monitoring assay with
urease-glutamate dehydrogenase and 24 h U-ALB was detected via
immunological transmission turbidimetry.
Statistical analysis
Data were processed using SPSS 19.0 (SPSS Inc.,
Chicago, IL, USA) software. Measurement data were presented as mean
± standard deviation (SD) and Students' t-test was used for
intergroup comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological changes in renal
tissues
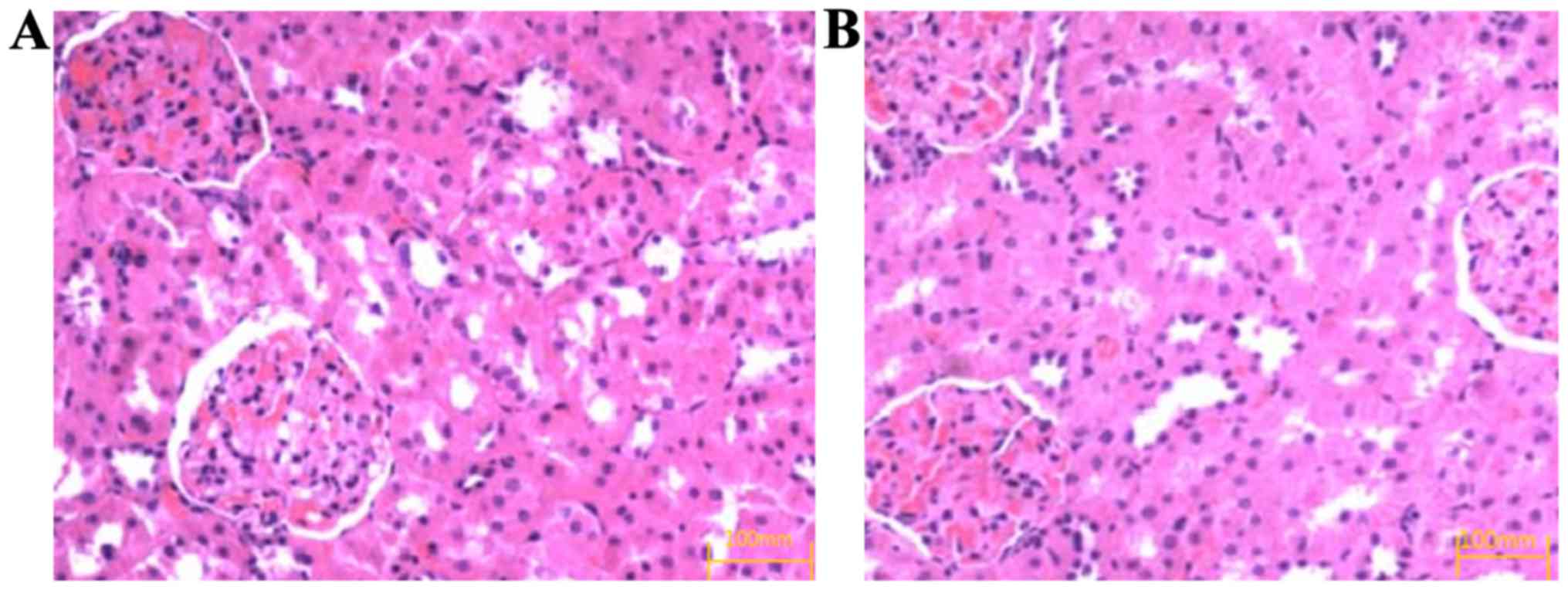
H&E staining showed that after 8 weeks of drug
intervention, the glomerular sclerosis, mesangial cell and
mesangial matrix hyperplasia occurred in the control group
(Fig. 1A) and the degree of renal
pathological change in the observation group was significantly
relieved compared with that in the control group (Fig. 1B).
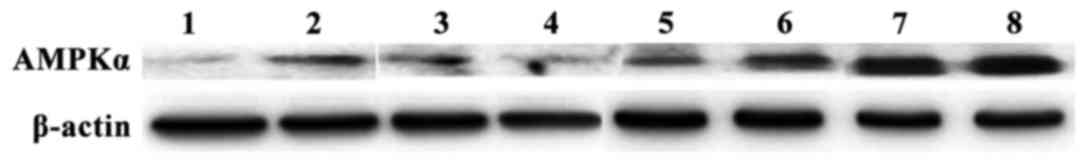
After 8 weeks of drug intervention, the relative
expression levels of AMPKα mRNA and protein in the observation
group were higher than those in the control group (p<0.05)
(Table II). The results of gel
electrophoresis of RT-PCR products and western blot analysis are
shown in Figs. 2 and 3.
 | Table II.Comparison of AMPKα expression in rats
between the two groups. |
Table II.
Comparison of AMPKα expression in rats
between the two groups.
| Group | Relative expression
level of AMPKα mRNA | Relative expression
level of AMPKα protein |
|---|
| Observation | 0.493±0.074 | 0.978±0.124 |
| group |
|
|
| Control | 0.278±0.062 | 0.456±0.083 |
| group |
|
|
| t-test | 12.198 | 19.161 |
| P-value | <0.001 | <0.001 |
Comparison of serum hs-CRP level in
rats between the two groups after drug intervention
The hs-CRP levels in the observation group at 1, 4,
6 and 8 weeks after intervention were significantly lower than
those in the control group (p<0.05) (Table III).
 | Table III.Comparison of hs-CRP level in rats
between the two groups after drug intervention (mg/l). |
Table III.
Comparison of hs-CRP level in rats
between the two groups after drug intervention (mg/l).
| Group | Case | 1 week after
intervention | 4 weeks after
intervention | 6 weeks after
intervention | 8 weeks after
intervention |
|---|
| Observation
group | 30 | 7.52±2.73 | 5.75±2.26 | 3.47±1.35 | 1.83±0.56 |
| Control group | 30 | 12.36±3.42 | 13.43±3.63 | 14.22±3.15 | 15.45±3.53 |
| t-test |
| 6.058 | 9.837 | 17.181 | 20.872 |
| P-value |
| <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of serum FcγR level in rats
between the two groups after drug intervention
FcγR levels in the observation group at 1, 4, 6 and
8 weeks after intervention were significantly higher than those in
the control group (p<0.05) (Table
IV).
 | Table IV.Comparison of FcγR level in rats
between the two groups after drug intervention (ng/ml). |
Table IV.
Comparison of FcγR level in rats
between the two groups after drug intervention (ng/ml).
| Group | Case | 1 week after
intervention | 4 weeks after
intervention | 6 weeks after
intervention | 8 weeks after
intervention |
|---|
| Observation
group | 30 | 22.57±3.74 | 25.68±3.16 | 26.67±3.38 | 27.63±3.58 |
| Control group | 30 | 17.46±3.47 | 18.53±3.62 | 18.42±3.16 | 19.15±3.23 |
| t-test |
| 5.486 | 8.150 | 9.766 | 9.633 |
| P-value |
| <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of serum BUN level in rats
between the two groups after drug intervention
BUN levels in the observation group at 1, 4, 6 and 8
weeks after intervention were significantly lower than those in the
control group (p<0.05) (Table
V).
 | Table V.Comparison of BUN level in rats
between the two groups after drug intervention (mmol/l). |
Table V.
Comparison of BUN level in rats
between the two groups after drug intervention (mmol/l).
| Group | Case | 1 week after
intervention | 4 weeks after
intervention | 6 weeks after
intervention | 8 weeks after
intervention |
|---|
| Observation
group | 30 | 9.08±2.53 | 8.23±2.16 | 6.87±1.75 | 4.43±1.35 |
| Control group | 30 | 18.35±2.46 | 18.73±2.13 | 19.82±2.14 | 24.45±2.43 |
| t-test |
| 14.388 | 18.958 | 25.658 | 39.446 |
| P-value |
| <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of 24 h U-ALB level in rats
between the two groups after drug intervention
U-ALB levels in the observation group at 1, 4, 6 and
8 weeks after intervention were significantly lower than those in
the control group (p<0.05) (Table
VI).
 | Table VI.Comparison of 24 h U-ALB level in
rats between the two groups after drug intervention (mg). |
Table VI.
Comparison of 24 h U-ALB level in
rats between the two groups after drug intervention (mg).
| Group | Case | 1 week after
intervention | 4 weeks after
intervention | 6 weeks after
intervention | 8 weeks after
intervention |
|---|
| Observation
group | 30 | 85.53±7.35 | 78.75±5.16 | 59.48±4.38 | 47.83±3.57 |
| Control group | 30 | 164.39±9.47 | 193.43±9.65 | 203.22±9.36 | 209.45±9.43 |
| t-test |
| 36.032 | 57.410 | 76.184 | 87.793 |
| P-value |
| <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
DN is one of the major complications of DM, and DM
is the main cause of end-stage renal disease (9). The main cause of DN is the damage to
glomerular microvascular structure and function caused by the
long-term high-glucose environment, which is generally regarded as
the result of environmental and genetic factors. Its pathogenesis
is very complex, including the mutual influences of insulin
resistance, hemodynamic changes, cytokines, oxidative stress,
glucose metabolism disorders and genetic background (9). DN has the characteristics of nodular or
diffuse glomerular sclerosis; thus, it is also known as diabetic
glomerulopathy with non-specific manifestations, whose early
manifestations include renal tubular hypertrophy and hyperplasia,
renal tubular fibrosis and thickening of basilar membrane (10). Additionally, high glucose increases
the glucose filtration rate of glomeruli and directly stimulates
the basilar side of renal tubules, resulting in the increased
glucose load in renal tubules and damage to the renal tubular
epithelial cells (11). High glucose
also induces platelet aggregation, forms microthrombus, promotes
glomerular sclerosis, and increases glomerular permeability.
Consequently, proteinuria is increased, thus causing
tubulointerstitial damage, forming a vicious cycle and
deteriorating the effects of the disease (12).
AMPK is a heterotrimer comprising three subunits: α,
β and γ, with α playing a catalytic role and the other two subunits
playing roles of maintaining stability (13). AMPK widely exists in a number of
systems, including liver, skeletal muscle, adipose tissue, kidney
and pancreas. AMPK is a cellular energy metabolic regulator that
realizes the complex activity regulation via sensitization of
changes in cellular energy state to maintain the energy
supply-demand balance in various links of cellular material
metabolism (14). AMPK blocks
glucogenesis-related enzymes, leading to reduction of glucogenesis,
which plays a key role in fatty acid and sugar metabolism and is
closely related to insulin resistance. After activation of AMPK,
the blood lipids and blood sugar can be decreased, thereby
alleviating the symptoms of DM (15). At the same time, AMPK can promote the
glucose uptake and utilization for peripheral tissues, which is
realized in two ways. Firstly, AMPK can induce the transfer of
glucose transporter 4 to serosa, thereby increasing the rate of
glucose transfer; secondly, AMPK can promote the activity of
phosphofructokinase, thereby regulating and enhancing the
glycolysis to enhance the glucose uptake capacity of peripheral
tissues and ensure normal sugar metabolism. The results of the
present study showed that the expression of AMPKα mRNA and protein
in the observation group at 8 weeks after drug intervention was
higher than those in the control group (p<0.05), suggesting that
AMPKα expression is upregulated after drug intervention and AMPK
activation can decrease the fat and cholesterol synthesis, enhance
the mechanization of fatty acids, and regulate lipid and glucose
metabolism, thereby alleviating the symptoms of DN.
Hs-CRP is an acute-phase index of micro-inflammatory
response that can activate the complement system in the body and
enhance the leukocyte phagocytosis by binding to the chromatin and
can play a regulatory role by stimulating cell activation (16). Hs-CRP can cause inflammatory response
of the body, which is an important pathological process of DN
(17). Previous findings have shown
that hs-CRP is an independent risk factor of obesity and type 2
diabetes and hs-CRP is closely related to DN (18). An increasing number of studies have
shown that the immune inflammatory mechanism is important in the
occurrence and development of DN. FcγR belongs to the Ig
superfamily, which is widely expressed in the hematopoietic system
and can regulate the inflammatory and immune response and ensure
the dynamic balance of DN (19).
Clinical treatment of DN usually includes correcting
lipid metabolism disorders, controlling blood pressure and blood
sugar, reducing proteinuria and protecting renal function (20). A large number of studies have shown
that traditional Chinese medicine has a unique effect on DN
prevention and treatment). Astragalus injection is the medicine
refined by Astragaloside extracted from Astragalus, which can
invigorate qi strengthening superficies, arrest sweat and
detoxify, promote granulation, eliminate the swelling and promote
urination (21). The results of the
present study showed that after the intervention with Astragalus
injection at different time-points, the hc-CRP level in the control
group was significantly higher than that in the observation group,
whereas the FcγR level was significantly lower than that in the
observation group (p<0.05), which may be because Astragalus has
an anti-inflammatory effect and can downregulate the expression of
chemokines and adhesion molecules and inhibit the release of
inflammatory factors, thereby reducing the infiltration of
inflammatory cells. At the same time, Astragalus can adjust the
immune dysfunction and activate FcγR, thereby delaying the
progression of DN.
BUN and U-ALB are the main indexes of evaluating the
renal function and the long-term hyperglycemia state may cause
damage to the glomerular filtration membrane, filtering out U-ALB,
can reflect the degree of glomerular injury (22). Findings have confirmed that one of
the key factors of forming proteinuria in DN is the podocyte injury
(23). The results of the present
study showed that the concentrations of BUN and U-ALB in
observation group at different time-points after drug intervention
were significantly lower than those in control group (p<0.05).
H&E staining showed that the degree of renal pathological
changes in the observation group was significantly relieved
compared with that in the control group, which may be because the
anti-oxidative stress effect of baicalein may downregulate the
expression of podocyte integrin-linked protease, thus delaying the
progression of disease. Baicalein effectively inhibited the
accumulation of tubulointerstitial extracellular matrix, eliminated
the free radicals, improved the microcirculation, increased the
renal blood flow and reduced the urinary protein, thus protecting
the kidney.
In conclusion, AMPKα, hs-CRP and FcγR play important
roles in the development and progression of DN. The interference in
AMPKα, hs-CRP and FcγR expression via baicalein can delay the
progression of DN, thus increasing the survival time and life
quality of patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
d'Emden MC, Shaw JE, Jones GR and Cheung
NW: Guidance concerning the use of glycated haemoglobin (HbA1c) for
the diagnosis of diabetes mellitus. Med J Aust. 203:89–90. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mellitus D and Glucose O: American
Diabetes Association: Diagnosis and classification of diabetes
mellitus. Diabetes Care. 36 Suppl 1:S67–S74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai SF, Su CW, Wu MJ, Chen CH, Fu CP, Liu
CS and Hsieh M: Urinary Cyclophilin A as a new marker for diabetic
nephropathy: A cross-sectional analysis of diabetes mellitus.
Medicine (Baltimore). 94:e18022015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper ME, Gilbert RE and Epstein M:
Pathophysiology of diabetic nephropathy. Metabolism. 47 Suppl
1:3–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maezawa Y, Takemoto M and Yokote K: Cell
biology of diabetic nephropathy: Roles of endothelial cells,
tubulointerstitial cells and podocytes. J Diabetes Investig.
6:3–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pineda CT, Ramanathan S, Tacer Fon K, Weon
JL, Potts MB, Ou YH, White MA and Potts PR: Degradation of AMPK by
a cancer-specific ubiquitin ligase. Cell. 160:715–728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liao L, Lei MX, Chen HL, Wu J and Guo LJ:
High-sensitive C-reactive protein and Type 2 diabetic nephropathy.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 29:627–630. 2004.(In Chinese).
PubMed/NCBI
|
|
8
|
Nimmerjahn F, Gordan S and Lux A: FcγR
dependent mechanisms of cytotoxic, agonistic, and neutralizing
antibody activities. Trends Immunol. 36:325–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmad J: Management of diabetic
nephropathy: Recent progress and future perspective. Diabetes Metab
Syndr. 9:343–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bangstad HJ, Osterby R, Rudberg S,
Hartmann A, Brabrand K and Hanssen KF: Kidney function and
glomerulopathy over 8 years in young patients with Type I
(insulin-dependent) diabetesmellitus and microalbuminuria.
Diabetologia. 45:253–261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rigalleau V, Lasseur C, Raffaitin C,
Perlemoine C, Barthe N, Chauveau P, Combe C and Gin H: Glucose
control influencesglomerular filtration rate and its prediction in
diabetic subjects. Diabetes Care. 29:1491–1495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haneda M, Utsunomiya K, Koya D, Babazono
T, Moriya T, Makino H, Kimura K, Suzuki Y, Wada T, Ogawa S, et al:
A new classification of Diabetic Nephropathy 2014: A report from
Joint Committee on Diabetic Nephropathy. Clin Exp Nephrol. 19:1–5.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent EE, Coelho PP, Blagih J, Griss T,
Viollet B and Jones RG: Differential effects of AMPK agonists on
cell growth and metabolism. Oncogene. 34:3627–3639. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blagih J, Coulombe F, Vincent EE, Dupuy F,
Galicia-Vázquez G, Yurchenko E, Raissi TC, van der Windt GJ,
Viollet B, Pearce EL, et al: The energy sensor AMPK regulates T
cell metabolic adaptation and effector responses in vivo. Immunity.
42:41–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ford RJ, Fullerton MD, Pinkosky SL, Day
EA, Scott JW, Oakhill JS, Bujak AL, Smith BK, Crane JD, Blümer RM,
et al: Metformin and salicylate synergistically activate liver
AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem
J. 468:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimoda M, Kaneto H, Yoshioka H, Okauchi
S, Hirukawa H, Kimura T, Kanda-Kimura Y, Kohara K, Kamei S,
Kawasaki F, et al: Influence of atherosclerosis-related risk
factors on serum high-sensitivity C-reactive protein levels in
patients with type 2 diabetes: Comparison of their influence
between in obese and non-obese subjects. J Diabetes Investig.
7:197–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varma V, Varma M, Varma A, Kumar R,
Bharosay A and Vyas S: Serum total sialic acid and highly sensitive
C-reactive protein: Prognostic markers for the diabetic
nephropathy. J Lab Physicians. 8:25–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alam F, Fatima F, Orakzai S, Iqbal N and
Fatima SS: Elevated levels of ferritin and hs-CRP in type 2
diabetes. J Pak Med Assoc. 64:1389–1391. 2014.PubMed/NCBI
|
|
19
|
Bosques CJ and Manning AM: Fc-gamma
receptors: Attractive targets for autoimmune drug discovery
searching for intelligent therapeutic designs. Autoimmun Rev.
15:1081–1088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bakris GL, Agarwal R, Chan JC, Cooper ME,
Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack
C, et al: Mineralocorticoid Receptor Antagonist Tolerability Study
- Diabetic Nephropathy (ARTS-DN) Study Group: Effect of finerenone
on albuminuria in patients with diabetic nephropathy: A randomized
clinical trial. JAMA. 314:884–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu ZQ, Li QZ and Qin GJ: Effect of
Astragalus injection onplatelet function and plasma endothelin in
patients with earlystage diabetic nephropathy. Zhongguo Zhong Xi Yi
Jie He ZaZhi. 21:274–276. 2001.(In Chinese).
|
|
22
|
Ford ES: Urinary albumin-creatinine ratio,
estimated glomerular filtration rate, and all-cause mortality among
US adults with obstructive lung function. Chest. 147:56–67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meneses MJ, Silva BM, Sousa M, Sá R,
Oliveira PF and Alves MG: Antidiabetic drugs: Mechanisms of action
and potential outcomes on cellular metabolism. Curr Pharm Des.
21:3606–3620. 2015. View Article : Google Scholar : PubMed/NCBI
|