Introduction
Congenital hypothyroidism (CH) is defined as thyroid
hormone deficiency at birth and is one of the most frequent
preventable causes of mental retardation; however, the majority of
infants with CH do not exhibit obvious clinical manifestations at
birth (1). In hypothyroidism, type 2
deiodinase is adapted to increase the conversion of the prohormone
thyroxine to the biologically active triiodothyronine (2). Thyroid hormone serves a pivotal role in
the development of the mammalian brain and its dyssynthesis,
paracrisis or lack of biological effects may be caused by
hypothyroidism (3). CH results in
various developmental disorders (4).
The incidence in newborn infants ranges from 1 in 3,000 to 1 in
4,000 (5) and is markedly higher in
preterm compared with full term infants (6).
Previous studies have demonstrated the importance of
timely treatment for neurologic outcome, which was indicated by an
inverse correlation between intelligence quotient and the age of
diagnosis (7,8). Despite early diagnosis of CH,
neurologic maldevelopment may also occur when treatment is not
optimized in the first 2–3 years of life (9). It is therefore important for patients
with CH to receive early treatment and close follow up.
MicroRNAs (miRNAs or miRs) are short non-coding
RNAs, which regulate post-transcriptional gene silencing (10). Thousands of miRNAs have been
identified in a variety of species (11). Binding of miRNA to the 3′
untranslated region (UTR) may degrade target mRNA and block
translation (12). Previous studies
have suggested the role of miRNAs in nervous system diseases,
including autoimmune neuroinflammation (13), Alzheimer's disease (14) and Parkinson's disease (15). miR-124-3p may also attenuate matrix
metalloproteinase-induced neuronal injury via regulating signal
transducer and activator of transcription 3 expression in SH-SY5Y
cells (16). Huang et al
(17) suggested that miR-124-3p may
inhibit neuronal inflammation induced by traumatic brain injury and
contributes to neurite outgrowth. Furthermore, miR-124-3p serves a
neuroprotective role in the 6-hydroxydopamine-induced cell model of
Parkinson's disease via regulating ANAX5 (18). miR-124 was also demonstrated to
protect neurons from apoptosis in newborn rats with thyroid
hypofunction (19). However, the
expression and role of miR-124-3p in thyroid hypofunction remains
unclear. Therefore, the present study was performed to investigate
the role and precise molecular mechanism of miR-124-3p in CH.
Materials and methods
Animal model
A total of 12 pregnant Sprague Dawley rats (body
weight, 200±10 g; ~6 week old) were kept in Qingdao Women and
Children's Hospital (Shandong, China) in appropriate-sized cages at
room temperature and a humidity of 55%. Mice were subjected to a 12
h light/dark cycle and had ad libitum access to standard
pellet feed and water. Propylthiouracil (50 mg/day; Beyotime
Institute of Biotechnology, Haimen, China) was injected
intraperitoneally into pregnant rats at gestational day 15 each day
until parturition in order to establish the thyroid hypofunction
rat model as previously described (20). Following anesthetization with
intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg),
newborn rats (12 days old) were fixed on stereotaxic apparatus and
their skulls were opened at 1.0 mm from the former fontanel and 1.7
mm from the mid-line. Thereafter, a micro syringe was inserted
vertically 3.8 mm at 15 µm/sec and mice were injected with 5 µl 1
nmol/l miR-124-3p mimic solution or miR-124-3p negative control
(NC) solution (Shanghai GenePharma Co., Ltd., Shanghai, China) as
previously described (21). Newborn
rats were divided into the following four groups (each n=5): The
control group, the thyroid hypofunction group, the miR-124-3p mimic
group and the miR-124-3p NC group. The present study was approved
by the Animal Ethics Committee of Qingdao Women and Children's
Hospital (Qingdao, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
At day 15 following birth, newborn rats were
sacrificed and the hippocampus was harvested. Total RNA was
extracted from the hippocampus using a PrimeScript reverse
transcription reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China) in accordance with the manufacturers' protocols. cDNA was
synthesized by RT using the following primers: miR-124-3p,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCATT-3′; and U6,
5′-AAAATATGGAACGCTTCACGAATTTG-3′. PCR was conducted using the
following primers: miR-124-3p, forward, 5′-GCTAAGGCACGCGGTG-3′ and
reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6, forward
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse
5′-ACGCTTCACGAATTTGCGTGTC-3′; PDCD6, forward
5′-ATGGCCGCCTACTCTTACC3′ and reverse 5′TCCTGTCTTTATCGACCCTCTG3′;
GAPDH, forward 5′CTTTGGTATCGTGGAAGGACTC3′ and reverse
5′GTAGAGGCAGGGATGATGTTCT3′. The thermocycling conditions were as
follows: 5 min at 95°C and 40 cycles of 30 sec at 95°C, 30 sec at
60°C and 30 sec at 72°C. Gene expression was normalized to U6 with
using the 2−∆∆Cq method (22). This experiment was performed in
accordance with a previous study (19).
Cell culture
Neurons were isolated from the hippocampus of rats
in the four groups. The cerebral cortices of rats were dissected
under an inverted microscope (Olympus Corporation, Tokyo, Japan;
magnification: ×40). Neurons were seeded in poly-D lysine-coated
plates at a concentration of 1×106 cells/ml and cultured
in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) for 4–6 h at 37°C.
Subsequently, the cell culture medium was replenished with
neurobasal medium (Gibco; Thermo Fisher Scientific, Inc.), 2% B27,
100 U/ml penicillin, 100 g/ml streptomycin and 0.5 mM glutamine
(Gibco; Thermo Fisher Scientific, Inc.) and neurons were cultured
in a incubator at 37°C in an atmosphere containing 5%
CO2. The culture medium was changed every 3 days.
Transfection with miRNA mimics
Neurons were seeded in 6-well plates
(5×104 cells per well) and transfected with 50 nM
miR-124-3p mimics or 50 nM miR-124-3p negative control (NC; both
Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol, following the attainment of 60–70% confluence. After 24
h, neurons were collected for subsequent experiments.
Flow cytometry
Cell apoptosis and the cell cycle were analyzed in
the present study using a flow cytometer (FCM; BD Biosciences,
Franklin Lakes, NJ, USA). Neurons (1×105 cells/well)
were digested with 0.025% trypsin, washed with PBS and fixed in 70%
cold ethanol at 4°C overnight. Subsequently, propidium iodide (PI,
Sigma Aldrich; Merck KGaA, Darmstadt, Germany), RNaseA and 0.2%
Triton X-100 were added to cells and incubated at 4°C for 30 min in
the dark.
Neurons were adjusted to give a concentration of
1×106 cells/ml prior to the flow cytometry assay. The
cell apoptosis rate was detected at 1 h following the addition of 5
µl fluorescein isothiocyanate-labeled Annexin V and 5 µl PI (cat.
no. 6592; Cell Signaling Technology, Inc., Danvers, MA, USA). The
cell number at each phase was analyzed using FlowJo software 7.6.1
(Tree Star Inc., Ashland, OR, USA).
Western blot analysis
At 24 h post-transfection, neurons were digested
with lysis buffer (Cell Signaling Technology, Inc.). Proteins were
quantified using a BCA assay (Thermo Fisher Scientific, Inc.).
Total protein (25 µg per lane) was separated by 10% SDS-PAGE,
transferred to polyvinylidene fluoride membranes and blocked with
5% bovine serum at room temperature for 1.5 h. Membranes were
incubated with primary antibodies against caspase-3 (cat. no.
9665), B-cell lymphoma 2 (Bcl-2; cat no. 4223), Bcl-2-associated X
protein (Bax; cat. no. 5023), cleaved poly ADP-ribose polymerase
(PARP; cat. no. 5625) and PARP (cat. no. 9532) (all 1:1,000; all
Cell Signaling Technology, Inc.) at 4°C overnight. β-actin (cat.
no. 4970; 1:5,000; Cell Signaling Technology, Inc.) was used as an
internal control. Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. ab191866 and
ab218695; 1:1,000; Abcam, Cambridge, UK) at room temperature for 1
h. Bands were quantified using enhanced chemiluminescence (cat. no.
6883; Cell Signaling Technology, Inc.). Each protein level was
normalized to β-actin (cat. no. 4970). The band density was
quantified with Gel-Pro Analyzer densitometry software (Version
6.3, Media Cybernetics, Inc., Rockville, MD, USA).
Dual luciferase reporter assays
Bioinformatics software (http://www.microrna.org/microrna/home.do and
http://www.targetscan.org/vert_71/)
was used to predict the potential targets of miR-124-3p and
programmed cell death protein 6 (PDCD6) was identified as a
promising target of miR-124-3p. The vectors named PDCD6-3′UTR-wild
type (WT) and PDCD6-3′UTR-mutant (MUT) (GeneCopoeia, Inc.,
Rockville, MD, USA) with wild type and mutated 3′UTRs of PDCD6 mRNA
were used to investigate whether miR-124-3p directly targets the
3′UTR of PDCD6. Cells (5×104 cells per well) were seeded
in 24-well plates and co-transfected with PDCD6-3′UTR-WT (2 µg) or
PDCD6-3′UTR-MUT (2 µg) plasmid and miR-124-3p mimics (50 nM) or
miR-124-3p NC (50 nM) using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.). After 24 h, relative firefly and
Renilla luciferase activities were detected using a dual
luciferase reporter assay kit (Promega Corporation, Madison, WI,
USA) in accordance with the manufacturer's protocol. Firefly
luciferase activity was used as a control.
Statistical analysis
Statistical analyses were conducted using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Numerical data are expressed as the
mean ± standard deviation. Differences among multiple groups were
analyzed with one-way analysis of variance followed by a Student's
Newman Keul's-Q test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-124-3p was downregulated in rats
with CH
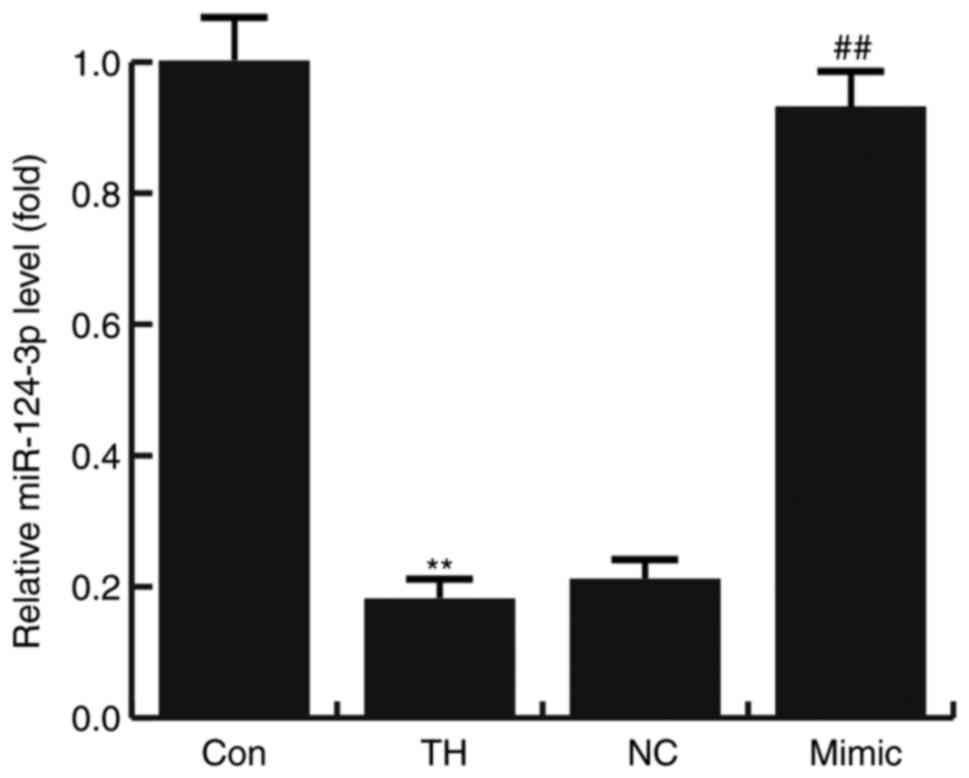
The results of RT-qPCR indicated that the expression
of miR-124-3p was significantly decreased in the hippocampus
tissues of rats with CH compared with the control group. Compared
with the TH model group, the level of miR-124-3p significantly
increased in the hippocampus tissues of CH rats treated with the
miR-124-3p mimic. However, no significant differences were
identified between the NC and TH groups (Fig. 1).
Bioinformatics analysis
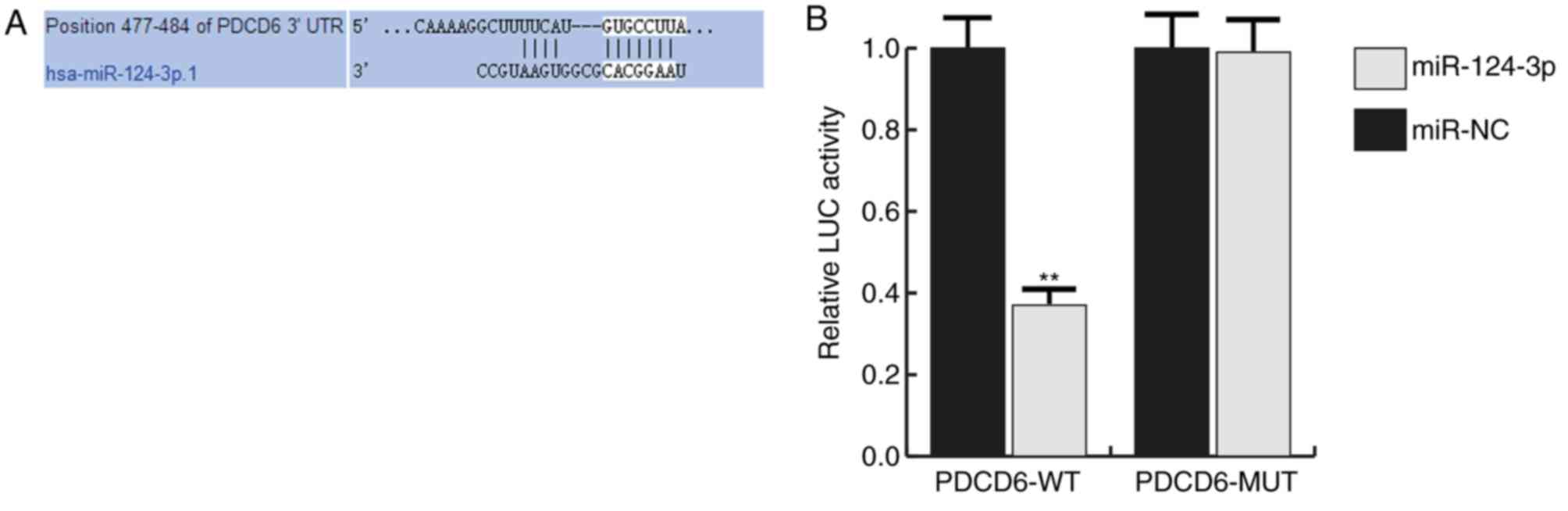
Many potential targets of miR-124-3p were
identified, however the PDCD6 3′UTR exhibited the seed sequence of
miR-124-3p (Fig. 2A). PDCD6 is a
calcium-binding modulator protein, which regulates cell
proliferation and apoptosis (23,24),
indicating a promising role in CH development. Therefore, PDCD6 was
used for subsequent experiments.
miR-124-3p targeted PDCD6 3′UTR
The interaction between miRNA-124-3p and PDCD6 was
verified using a dual-luciferase reporter assay system.
miRNA-124-3p significantly inhibited the luciferase activity in the
PDCD6-wt group but not in the PDCD6-mut group (Fig. 2B).
PDCD6 was upregulated in rats with
CH
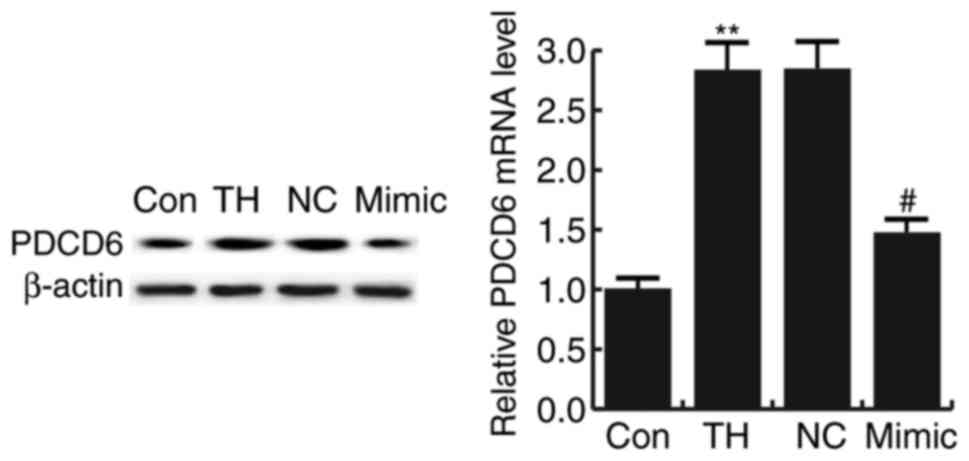
The results indicated that the mRNA and protein
levels of PDCD6 were significantly increased in the hippocampus
tissues of rats with CH compared with that in the control group.
Compared with the model group (TH), the level of PDCD6
significantly decreased in the hippocampus tissues of CH rats
treated with the miR-124-3p mimic. However, no significant
differences were identified between the NC and TH groups (Fig. 3).
miR-124-3p protected neurons from
apoptosis
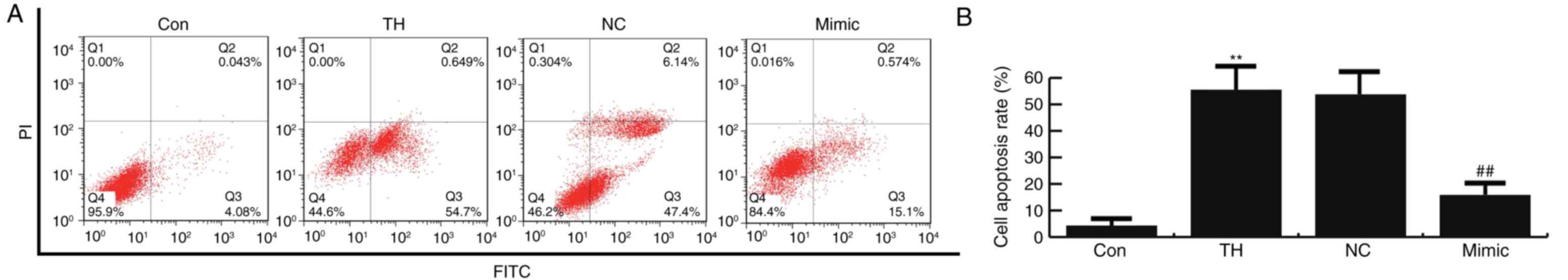
The results of flow cytometry demonstrated that
thyroid hypofunction induced cell apoptosis, which was
significantly inhibited by miR-124-3p mimics compared with the
control group (Fig. 4). No
significant difference in neuronal cell apoptosis was observed
between the thyroid hypofunction and miR-124-3p NC groups (Fig. 4). The results of the flow cytometric
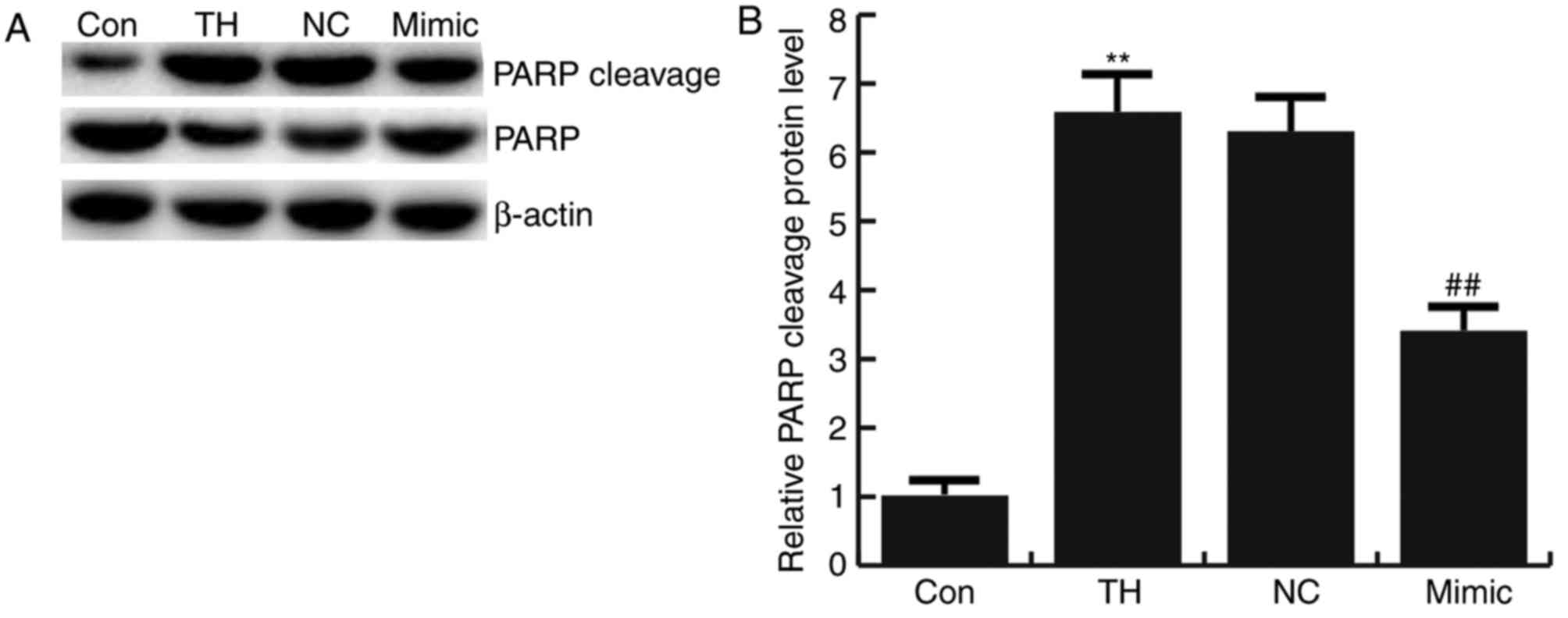
analysis were confirmed by analyzing PARP cleavage. Cleavage of
PARP was significantly increased in the thyroid hypofunction group
compared with the control group and this increase was significantly
reversed by transfection with miRNA-124-3p mimics. No significant
difference in the expression of PARP cleavage in neuronal cells was
observed between the thyroid hypofunction group and miR-124-3p NC
group (Fig. 5).
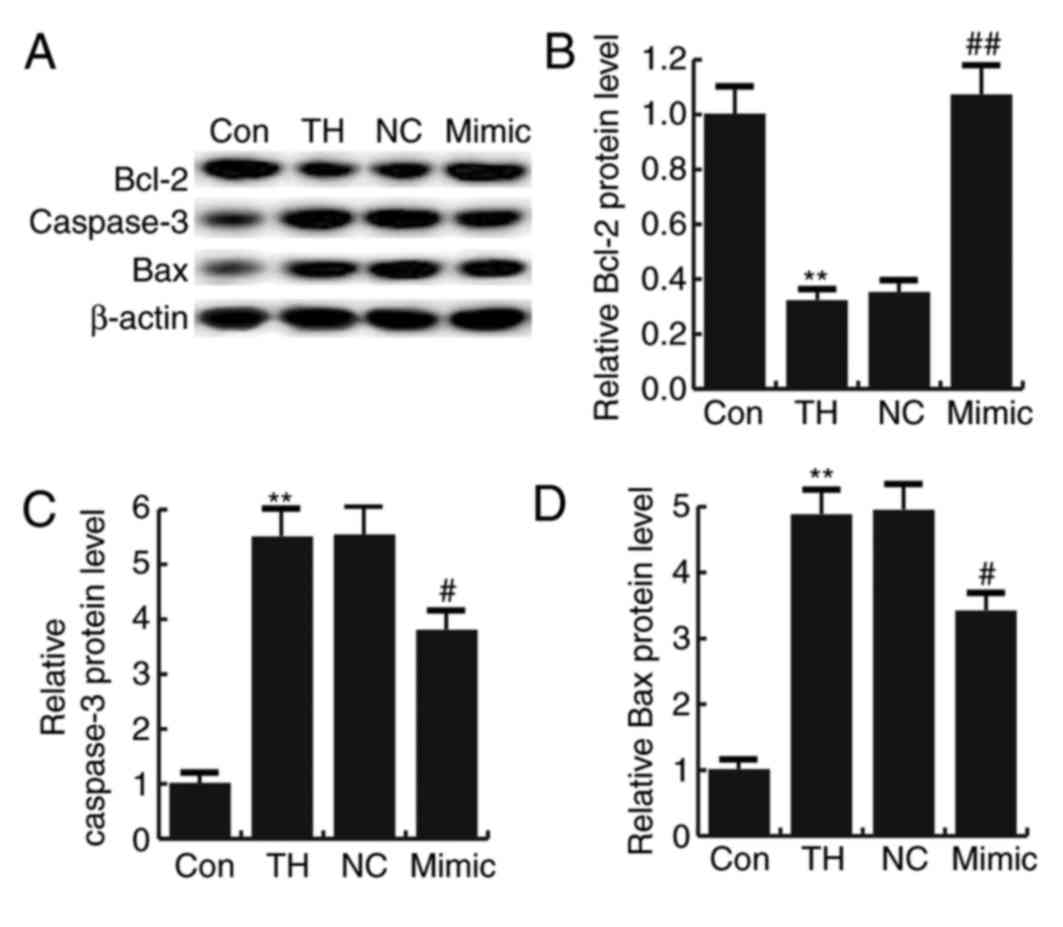
miR-124-3p reversed the thyroid
hypofunction-induced upregulation of caspase-3 and Bax and the
downregulation of Bcl-2
No significant difference was observed in the
expression of caspase-3, Bax and Bcl-2 in neuronal cells between
the thyroid hypofunction group and miR-124-3p NC group (Fig. 6). Thyroid hypofunction significantly
increased the expression of caspase-3 and Bax proteins, while the
expression of Bcl-2 protein was significantly decreased compared
with the control group; this effect was reversed by transfection
with miRNA-124-3p mimics (Fig.
6).
Discussion
The results of the present study indicate that the
expression of miR-124-3p was significantly decreased and the
expression of PDCD6 was significantly increased in the hippocampus
of rats with CH in comparison with the control group. miR-124-3p
protected neurons from thyroid hypofunction-induced apoptosis.
Thyroid hypofunction induced Caspase-3 and Bax protein expression
and reduced Bcl-2 protein expression. However, these changes were
eliminated by miR-124-3p via the targeting of PDCD6. In conclusion,
it was demonstrated that miRNA-124-3p serves a protective role in
CH.
CH is one of the most frequent preventable causes of
mental retardation and leads to multiple developmental disorders
(4). Despite early diagnosis of CH
in infants, neurologic maldevelopment may also occur if treatment
is not optimized in the first 2–3 years of life (9). Therefore, it is important for infants
to receive optimized treatment. miRNAs serve a role in nervous
system diseases, including autoimmune neuroinflammation (13), Alzheimer's disease (14) and Parkinson's diseases (15). A previous study indicated that the
expression of miR-124 was downregulated in newborn rats with
thyroid hypofunction (19). However,
the target genes of miR-124 remain unknown. Furthermore, as a
sub-member of miR-124, the level and role of miR-124-3p in CH
remains unclear. Therefore, the present study was performed to
investigate the role and precise molecular mechanism of miR-124-3p
in CH.
RT-qPCR was performed to investigate changes in the
expression of miR-124-3p in CH. The results indicated that
miR-124-3p was significantly decreased in the hippocampus of rats
with CH compared with the control group, which is consistent with a
previous study (19). Bioinformatics
analysis was used to predict the possible target genes of
miR-124-3p. The PDCD6 3′UTR exhibited the seed sequence of
miR-124-3p. The interaction between miRNA-124-3p and PDCD6 was then
verified using a dual Luciferase reporter assay system. PDCD6 is a
calcium-binding modulator protein that regulates cell proliferation
and death (23,24). Aberrant expression of PDCD6 has been
observed in various types of human cancer and may act as either an
oncogene or a tumor suppressor. For instance, PDCD6 was recently
reported to be a tumor suppressor and may be a potential
therapeutic target in glioblastoma (25); reintroduction of PDCD6 significantly
reverses the effects of miR-183 in enhanced cell
cycle/proliferation and inhibits the apoptosis of leukemia cell
lines (26); and PDCD6 was highly
expressed in metastatic ovarian cancer and induced cell
migration/invasion (27). In the
present study, RT-qPCR was used to detect the differential
expression of PDCD6 in CH and the results indicated that PDCD6 was
significantly increased in the hippocampus of rats with CH compared
with the control group. These results were consistent with those of
a previous study (27). The results
also indicated a negative correlation between miR-124-3p and
PDCD6.
It has previously been demonstrated that miR-124
protects neurons against apoptosis in cerebral ischemic stroke
(28). The results of the present
study demonstrated that the thyroid hypofunction group had the
highest rate of apoptosis, whereas transfection with miR-124-3p
mimics significantly decreased this effect, consistent with
previous studies (19,28). Apoptosis-associated proteins were
further detected in the present study using western blot
analysis.
Caspase-3 is a member of the cysteine-aspartic acid
protease family, which is encoded by the CASP3 gene
(29). Caspase-3 is activated in
apoptotic cells by the extrinsic and intrinsic pathways (30). In addition, Bax was the first
identified pro-apoptotic member of the Bcl-2 protein family
(31) and Bcl-2 itself is an
important anti-apoptotic protein (32). In the present study, the protein
levels of Caspase-3 and Bax were significantly increased, whereas
the expression of Bcl-2 was significantly decreased in the thyroid
hypofunction group. These effects were reversed by transfection
with miRNA-124-3p mimics.
In conclusion, the results of the present study
demonstrate that miRNA-124-3p serves a protective role in CH via
targeting PDCD6. miR-124-3p may therefore be a potential
therapeutic target for the treatment of infants with CH. However,
as miR-124-3p has various target genes, the role and underlying
mechanism of miR-124-3p in CH progression remain largely unknown.
Thus, further study is required to support the conclusions drawn
from the present study.
References
|
1
|
Kollati Y, Ambati RR, Reddy PN, Kumar NSS,
Patel RK and Dirisala VR: Congenital hypothyroidism: Facts, Facets
& Therapy. Curr Pharm Des. 23:2308–2313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Oña Ruiz C, Obregón MJ, del Rey Escobar
F and de Escobar Morreale G: Developmental changes in rat brain
5′-deiodinase and thyroid hormones during the fetal period: The
effects of fetal hypothyroidism and maternal thyroid hormones.
Pediatr Res. 24:588–594. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai X: Effects of thyroid hormone
replacement therapy on thyroid hormone levels and electrocardiogram
changes in geriatric patients with hypothyroidism. Pak J Pharm Sci
(5 Suppl). 30:S1939–S1942. 2017.
|
|
4
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
5
|
Fisher DA: Second International conference
on neonatal thyroid screening: Progress report. J Pediatr.
102:653–654. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris KB and Pass KA: Increase in
congenital hypothyroidism in New York State and in the United
States. Mol Genet Metab. 91:268–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alm J, Hagenfeldt L, Larsson A and
Lundberg K: Incidence of congenital hypothyroidism: Retrospective
study of neonatal laboratory screening versus clinical symptoms as
indicators leading to diagnosis. Br Med J (Clin Res Ed).
289:1171–1175. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
LaFranchi SH and Austin J: How should we
be treating children with congenital hypothyroidism? J Pediatr
Endocrinol Metab. 20:559–578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bongers-Schokking JJ and de Muinck
Keizer-Schrama SM: Influence of timing and dose of thyroid hormone
replacement on mental psychomotor, and behavioral development in
children with congenital hypothyroidism. J Pediatr. 147:768–774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kozomara A and Griffiths-Jones S: miRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39:(Database Issue). D152–D157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghorbani S, Talebi F, Chan WF, Masoumi F,
Vojgani M, Power C and Noorbakhsh F: MicroRNA-181 variants regulate
T cell phenotype in the context of autoimmune neuroinflammation.
Front Immunol. 8:7582017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J and Kim YK: Identification of the
Role of miR-142-5p in Alzheimer's Disease by Comparative
Bioinformatics and Cellular Analysis. Front Mol Neurosci.
10:2272017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grasso M, Piscopo P, Confaloni A and Denti
MA: Circulating miRNAs as biomarkers for neurodegenerative
disorders. Molecules. 19:6891–6910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geng L, Liu W and Chen Y: miR-124-3p
attenuates MPP+-induced neuronal injury by targeting STAT3 in
SH-SY5Y cells. Exp Biol Med (Maywood). 242:1757–1764. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y,
Chen F, Wang H, Zhang J and Lei P: Increased miR-124-3p in
microglial exosomes following traumatic brain injury inhibits
neuronal inflammation and contributes to neurite outgrowth via
their transfer into neurons. FASEB J: fj.201700673R. 2017.
|
|
18
|
Dong RF, Zhang B, Tai LW, Liu HM, Shi FK
and Liu NN: The Neuroprotective role of MiR-124-3p in a
6-Hydroxydopamine-induced cell model of Parkinson's disease via the
regulation of ANAX5. J Cell Biochem. 116:269–277. 2017.
|
|
19
|
Shao Q, Jiang W and Jin Y: MiR-124 effect
in neuron apoptosis in newborn rat with thyroid hypofunction. Int J
Clin Exp Pathol. 8:14465–14471. 2015.PubMed/NCBI
|
|
20
|
Fabian ID, Rosner M, Fabian I,
Vishnevskia-Dai V, Zloto O, Maman Shinderman E, Cohen K, Ellis M,
Lin HY, Hercbergs A, et al: Low thyroid hormone levels improve
survival in murine model for ocular melanoma. Oncotarget.
6:11038–11046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu LJ, Ouyang YB, Xiong X, Stary CM and
Giffard RG: Post-stroke treatment with miR-181 antagomir reduces
injury and improves long-termbehavioral recovery in mice after
focal cerebral ischemia. Exp Neurol. 264:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashemi M, Yousefi J, Hashemi SM, Amininia
S, Ebrahimi M, Taheri M and Ghavami S: Association between
programmed cell death 6 interacting protein insertion/deletion
polymorphism and the risk of breast cancer in a sample of Iranian
Population. Dis Markers. 2015:8546212015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou B, Bai P, Xue H, Zhang Z, Shi S,
Zhang K, Wang Y, Wang K, Quan Y, Song Y and Zhang L: Single
nucleotide polymorphisms in PDCD6 gene are associated with the
development of cervical squamous cell carcinoma. Fam Cancer.
14:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D, Wang F, Pang Y, Zhao E, Zhu S,
Chen F and Cui H: ALG2 regulates glioblastoma cell proliferation,
migration and tumorigenicity. Biochem Biophys Res Commun.
486:300–306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Zuo D, Yuan Y, Yang X, Hong Z and
Zhang R: MicroRNA-183 promotes cell proliferation via regulating
programmed cell death 6 in pediatric acute myeloid leukemia. J
Cancer Res Clin Oncol. 143:169–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su D, Xu H, Feng J, Gao Y, Gu L, Ying L,
Katsaros D, Yu H, Xu S and Qi M: PDCD6 is an independent predictor
of progression free survival in epithelial ovarian cancer. J Transl
Med. 10:312012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan
JL and Liu X: MicroRNA-124 protects neurons against apoptosis in
cerebral ischemic stroke. CNS Neuroscience Ther. 19:813–819.
2013.
|
|
29
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for bcl-2 and a hybrid
bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|