Introduction
Gastric carcinoma is one of the most prevalent types
of human cancer and remains the second leading cause of
cancer-associated mortality worldwide (1). Gastric signet ring cell carcinoma is
typically associated with diffuse, infiltrating myositis that
increases the difficulty of clinical diagnosis and treatments for
patients with suspected gastric cancer (2). Furthermore, gastric cancer has a higher
morbidity and mortality rate than other carcinomas concerning the
digestive system (3,4). In addition, previous studies have
demonstrated that apoptotic resistance of gastric cancer is
inevitable in cancer progression (5–7).
Furthermore, previous findings have suggested that target therapies
for advanced gastric cancer are more efficient compared with
alternative treatments for patients with gastric cancer (8,9).
Therefore, the investigation into efficient target molecules has
attracted more interest in researchers and clinicians in the field
of cancer research and clinical therapy.
Acidosis is a key physiological and pathological
feature that is commonly associated with gastric cancer (10). Previous studies have demonstrated
that acidosis induces a hypoxia-inducible factor (HIF)-1α signaling
pathway chain reaction in the gastric cancer cells (11,12). In
addition, previous studies have also indicated that acidosis
induces the activation of transforming growth factor (TGF)-β and
epithelial-mesenchymal transition (EMT) signaling pathways, which
contribute to the growth, invasion and apoptotic resistance of
gastric carcinoma cells (13,14).
Furthermore, a previous study indicated that the association of low
PH, tumor-associated macrophages, microvessel density, vascular
endothelial growth factor and matrix metalloproteinases (MMPs) in
human gastric cancer affect survival (15). These reports suggest that the
TGF-β/EMT signaling pathway is crucial for growth, migration and
invasion of gastric carcinoma cells.
Bone morphogenetic protein and activin
membrane-bound inhibitor (BAMBI) is a pseudo-receptor of mothers
against decapentaplegic homolog (SMAD)7 and is homologous to TGF-β
receptor type 1 (TGFβR1), but lacks the functional domain of active
kinase (16,17). BAMBI is similar to TGF-βRI and
participates in the regulation of the TGF-β-mediated signaling
pathway in various cancer cells (18). Although loss of BAMBI has been
identified in a large number of cancer tissues, including non-small
cell lung cancer, bladder cancer and colorectal cancer, a limited
number of studies have explored its function in gastric cancer
(18–20). In addition, Pils et al
(21) previously reported BAMBI is
overexpressed in ovarian cancer and co-translocates with SMADs into
the nucleus upon TGF-β treatment. Νotably, BAMBI overexpression is
beneficial for suppressing metastasis of gastric cancer cells by
inhibiting β-catenin and TGF-β (22). These findings suggest that BAMBI may
be associated with the progression of gastric cancer.
In the present study, it was speculated that BAMBI
expression levels may be associated with the growth and
aggressiveness of gastric carcinoma cells by regulating the
TGF-β/EMT signaling pathway. Although, previous studies have
indicated the role of BAMBI in bladder cancer, non-small-cell lung
cancer, ovarian cancer and gastric cancer (16,18–23), to
the best of our knowledge the present study is the first to have
comprehensively investigated BAMBI-mediated TGF-β/EMT processes in
gastric carcinoma cells in vitro and in vivo and
suggest BAMBI may be a promising therapeutic target for the
treatment of gastric cancer.
Materials and methods
Ethics statement
The present study was implemented legitimately
according to the Guide for the Care and Use of Laboratory Animals
of the Affiliated Tumor Hospital of Guangxi Medical University
(Nanning, China) (24). The present
study was performed in accordance with the Ethics of Animal
Experiments Defense Research (25)
and approved by the Ethics Committee of the Affiliated Tumor
Hospital of Guangxi Medical University.
Cell culture and reagents
Gastric tumor cell lines HGC-27 and BGC-823, and
human gastric mucosa epithelial cells GES-1 were purchased from
Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China). All tumor cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). GES-1 cells were
cultured in Eagle's minimal essential medium (Sigma-Aldrich; Merck
KGaA) supplemented with 10% fetal calf serum. All cells were
cultured in a humidified atmosphere containing 5% CO2 at
37°C. Intracellular pH was analyzed as previously described
(26).
Apoptosis assay
Apoptosis of gastric tumor cells was assessed using
flow cytometry. BGC-823 cells (1×106/well) were cultured
in 6-well plates with BAMBI (2.0 mg/ml) for 24 h at 37°C.
Subsequently, cells were harvested via trypsinization, washed in
cold PBS and adjusted to 1×106 cells/ml with PBS.
Following double staining with fluorescein isothiocyanate
(FITC)-Annexin V and propidium iodide using the FITC Annexin V
Apoptosis Detection kit I (BestBio, Shanghai, China) for 2 h at
37°C according to manufacturer's protocol, cells were analyzed
using a FACScan flow cytometer equipped with Cell Quest software
1.2 (BD Biosciences, San Jose, CA, USA) according to the
manufacturer's protocol in order to detect the apoptotic rate of
BGC-823 cells. All experiments were performed in triplicate.
siRNA transfection
Knockdown of BAMBI was performed via transfection of
specific small interfering (si)RNA designed by siDirect2.0 (version
2.0; sidirect2.rnai.jp/). All siRNAs were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.), including siRNA-BAMBI (Si-BAMBI;
gene accession no. WO 2005116204-A/725618; sense,
5′-GCAAGCAGAGCUCAGUAAUTT-3′ and antisense,
5′-AUUACUGAGCUCUGCUUGCTT-3′) or siRNA-mimic (control; sense,
5′-GCAAGCAGAGCUCAGUAAUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′). BGC-823 cells (1×107) were
transfected with 100 pmol Si-BAMBI or Si-vector using a Cell Line
Nucleofector kit L (Lonza, Slough, UK) according to the
manufacturer's protocol (27). Cells
were used further analysis after 72 h transfection. BAMBI knockdown
BGC-823 cells were treated with 2 mg/ml TGFβ (Si-BAMBI-TGFβ) for 12
h at 37°C for further analysis.
Endogenous BAMBI overexpression
BAMBI gene was cloned into PMD-18-T vector (Takara
Biotechnology Co., Ltd., Dalian, China) and sequenced to identify
its sequence according to previous report (28). BAMBI gene was subsequently cloned
into eukaryotic expression vector pCMVp-NEO-BAN (pBAMBI; Takara
Biotechnology Co., Ltd.) to generate BAMBI-overexpressed BGC-823
cells. Subsequently, pBAMBI (1.0 µg) or an empty vector (pvector;
1.0 µg) was transfected into cultured BGC-823 cells
(5×106) using Lipofectamine® 2000
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Stable BAMBI-overexpression BGC-823 cells were selected
using a G418 screening system (29).
Cells were incubated with TGF-β (2 mg/ml) for 12 h at 37°C and used
for further analysis after 72 h transfection.
Cell invasion and migration
assays
BGC-823 cells (1×106) were transfected
with Si-vector, Si-BAMBI, pvector (control) or pBAMBI to analyze
the efficacy of BAMBI. For the migration assay, transfected BGC-823
cells were incubated for 96 h at 37°C using a Transwell insert (BD
Biosciences, San Jose, CA, USA) instead of a Matrigel Invasion
Chamber. For the invasion assay, Si-vector, Si-BAMBI, control or
pBAMBI-treated cells were suspended at a density of
1×105 in 500 µl serum-free DMEM in the upper chamber and
500 µl serum-free DMEM with 5% FBS in the lower chamber of BD
BioCoat Matrigel Invasion Chambers (BD Biosciences) according to
the manufacturer's protocol. Cells were then stained with 0.1%
crystal violet dye (Sigma-Aldrich; Merck KGaA) for 20 min at 37°C.
Tumor cell invasion and migration were counted in at least three
random fields of view using a light microscope (BX51; Olympus
Corporation, Tokyo, Japan).
DNA methylation analysis
Methylation analysis of HOPE®-fixed,
paraffin-embedded (HOPE® Fixative system I;
Polysciences, Inc., Warrington, PA, USA) BAMBI-overexpressed or
pvector-overexpressed BGC-823 cells was performed according to the
manufacturer's protocol as previously described (30). DNA methylation was measured using the
EZ DNA Methylation kit (ZymoResearch, Irvine, CA, USA) and the
Infinium HumanMethylation450k BeadChip (HM450KBC; Illumina Inc.,
San Diego, CA, USA) according to the manufacturer's protocol.
Bisulfite pyrosequencing of the selected loci was performed as
indicated in a previous study to determine the DNA methylation
state (31).
Western blot analysis
Total protein from BGC-823 or BAMBI-overexpressed
BGC-823 cells (1×107) was extracted using
radioimmunoprecipitation assay lysis buffer containing phenylme
thanesulfonylfluoride (Sigma-Aldrich; Merck KGaA). Protein
concentration was measured with a bicinchoninic acid protein assay
kit (Thermo Fisher Scientific, Inc.). A total of 30 µg protein
lysates were subjected to 12% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes as previously described
(32). The following antibodies were
blocked in 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA)
for 1 h at 37°C and subsequently used to incubate protein samples
for 1 h at room temperature: Monoclonal goat anti-mouse BAMBI
(1:500; cat. no. AF2387; R&D Systems, Inc., Minneapolis, MN,
USA), TGF-β, (1:1,000; cat. no. AF532; R&D Systems, Inc.) SNAI1
(1:500; cat. no. PAB29288; Abnova Corporation, Taipei, Taiwan),
α-actin-2 (ACTA2; 1:200; cat. no. MA515806; Invitrogen; Thermo
Fisher Scientific, Inc.), vimentin (VIM; 1:500; cat. no. PAB24865;
Abnova Corporation), twist-related protein 1 (TWIST1; 1:500; cat.
no. DR1088100UG; EMD Millipore), MMP9 (1:500; cat. no. PAB19095;
Abnova Corporation), SRY-box 4 (SOX4; 1:500; cat. no. PAB14092;
Abnova Corporation), N-cadherin (1:200; cat. no. NBP238856; Novus
Biologicals), collagen-I (CT-I, 1:200; cat. no. NB6004080.01MG;
Novus Biologicals, Littleton, CO, USA) IPO-38 (cat. no. ab1045),
Ki68 (cat. no. ab15580), Smad6 (cat. no. ab214009), Smad7 (cat. no.
ab216428), pMAPK (cat. no. ab32047), pSmad2 (cat. no. ab53100),
β-actin (cat. no. ab8227) (all 1:1,000; Abcam, Cambridge, MA, USA)
and fibronectin (FIB; 1:500; cat. no. P1H11; R&D Systems, Inc.)
for 12 h at 4°C. Samples were subsequently incubated with
HRP-conjugated polyclonal anti-rabbit IgG antibody (1:10,000; cat.
no. PV-6001; R&D Systems, Inc.) for 1 h at room temperature.
Signals were visualized by using an enhanced chemiluminescence
western blot analysis detection reagent (EMD Millipore). The
density of the bands was analyzed using Quantity One software
(version 4.6; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Animal study
A total of 20 female C57BL/6 nude mice
(specific-pathogen-free; body weight, 30–35 g; age, 6 weeks) were
purchased from Slack Experimental Animals Co., Ltd. (Shanghai,
China). All mice were housed in a 12-h light/dark cycle,
temperature-controlled facility at 23±1°C with a relative humidity
of 50±5%. All mice were free access to food and water. Mice were
divided into two groups (n=10/group). A total volume of 200 µl
BGC-823 cells (5×105; control group) or Si-BAMBI-BGC-823
cells (5×105; experimental group) was administered by
subcutaneous injection into C57BL/6 nude mice. Tumor diameters were
recorded every 2 days and tumor volume was calculated using the
formula: 0.52× smallest diameter2 × largest diameter.
The experimental mice were sacrificed when tumors reached 10 mm. On
day 25, all mice in each group were sacrificed for further
analysis.
Histological immunostaining
Tumors tissues were isolated from gastric carcinoma
xenograft mice and were fixed using 10% formaldehyde for 2 h at
37°C and embedded in paraffin. Tumor samples were cut into sections
(4-µm-thick) and antigen retrieval was performed as described
previously (33). Tumor sections
were blocked in 5% BSA for 1 h at 37°C and incubated with the
following primary antibodies: Rabbit anti-mouse ki67 (1:1,000; cat.
no. 652401), HIF (1:500; cat. both no. 580809; Biolegend, Inc., San
Diego, CA, USA), TWIST1 (1:500; cat. no. 573208; Biolegend, Inc.),
MMP9 (1:500; cat. no. PAB19095), SOX4 (1:500; cat. no. PAB14092;
both Abnova Corporation), N-cadherin (1:200; cat. no. NBP238856),
CT-I (1:200; cat. no. NB6004080.01MG; both Novus Biologicals) and
FIB (1:500; cat. no. P1H11; R&D Systems, Inc.) for 12 h at 4°C.
Subsequently, tumor sections were incubated with HRP-conjugated
anti-IgG (1:10,000; cat. no. ab6721, Abcam) for 24 h at 4°C. The
results were visualized using a LumiGLO chemiluminescence system
(Cell Signaling Technology, Inc., Danvers, MA, USA). Images were
obtained using a fluorescent microscope (BZ-9000; Keyence
Corporation, Osaka, Japan) at magnification, ×40.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
For apoptotic analysis of tumor cells, the DeadEnd
Colorimetric TUNEL System from Promega Corporation (Madison, WI,
USA) was used to determine the number of apoptotic cells in tumor
sections. Tumor tissues from xenogeneic mice were fixed with 4%
paraformaldehyde solution for 12 h at 4°C. Serial 5-µm thick
sections were prepared and stained with TUNEL (5%) for 1 h at 37°C.
Biotinylated nucleotides were used to incorporate at the 3-OH DNA
ends using the enzyme terminal deoxynucleotidyl transferase for 2 h
at 37°C. Horseradish peroxidase (HRP)-conjugated streptavidin
(1:2,000; cat. no. PV-6001; OriGene Technologies, Inc., Beijing,
China) was then bound to these biotinylated nucleotides for 2 h at
37°C. Peroxidase activity was calculated using the liquid
3,3′-diaminobenzidine substrate chromogen system (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA). The percentage of
TUNEL-positive tumor cells in xylene was analyzed by counting
1×103 cells from six random selected fields of view in
this assay.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed using GraphPad Prism software 19.0
(GraphPad Software, Inc., La Jolla, CA, USA). Analysis on two
experimental groups was performed using a two-tailed Student's
t-test. Multiple comparisons (in more than two groups) were
analyzed using one-way analysis of variance followed by
Kruskal-Wallis test or Dunn's post-test. Bisulfite pyrosequencing
and HM450KBC analysis was used to analyze similar methylation
patterns of loci located in the BAMBI gene region using a Pearson
correlation coefficient. P<0.05, was considered to indicate a
statistically significant difference.
Results
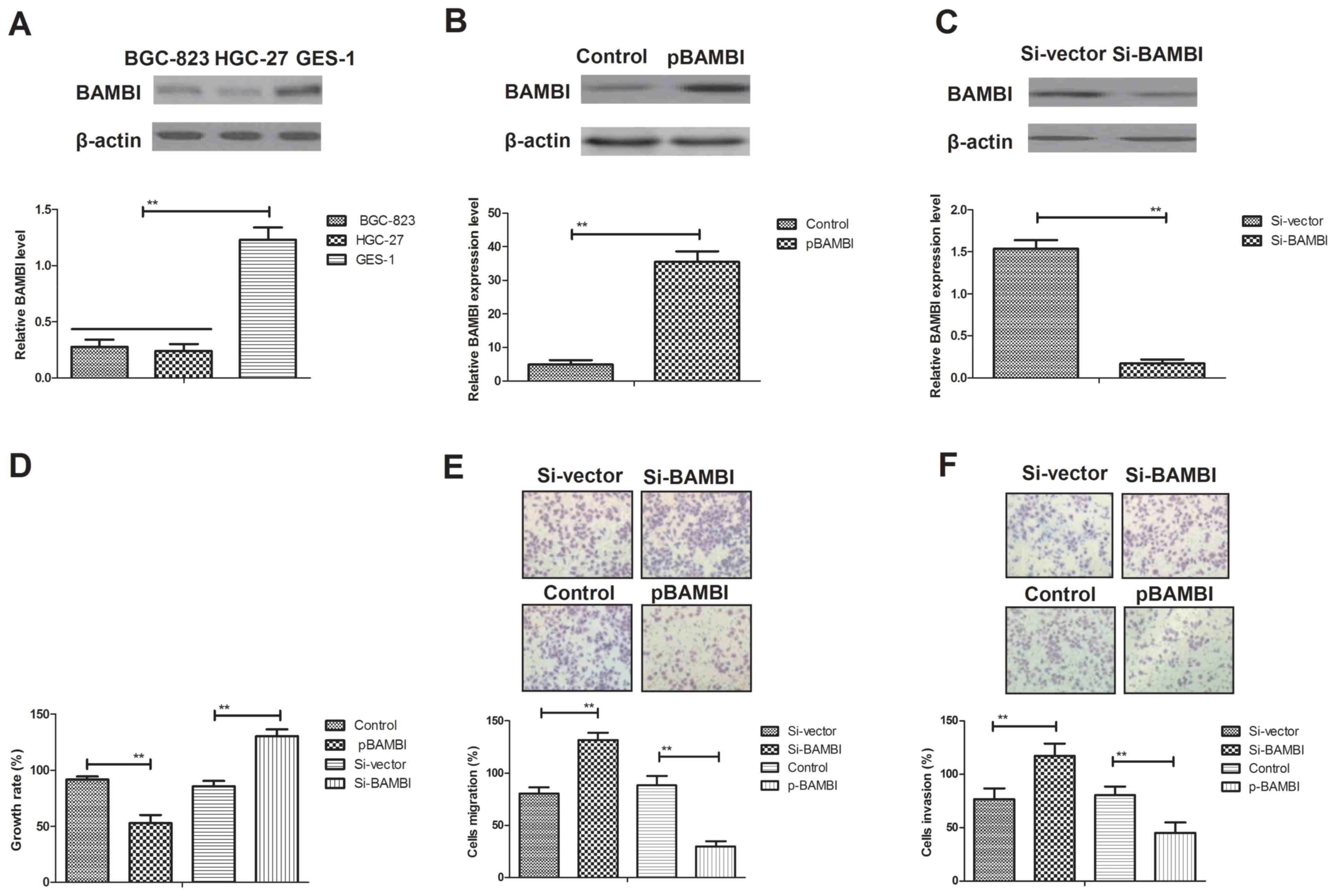
Analysis of BAMBI expression levels
its function in gastric cancer cells
Previous studies have indicated that BAMBI is
downregulated in gastric cancer cells (22,23). In
order to analyze the function of BAMBI in gastric cancer cells,
BAMBI expression levels in BGC-823 and HGC-27 gastric cancer cells
were compared with normal GES-1 gastric cells (control). BAMBI
expression levels were significantly downregulated in BGC-823 cells
compared with GES-1 cells (P<0.01; Fig. 1A). The effects of BAMBI on the growth
and aggressiveness on gastric cancer cells were also assessed.
BAMBI overexpression resulted in a 7.4-fold increase in expression
level whereas BAMBI inhibition resulted in a 9.1-fold decrease in
BAMBI expression level in BGC-823 cells compared with the control
(P<0.01; Fig. 1B and C). As
indicated in Fig. 1D, endogenous
BAMBI overexpression significantly inhibited BGC-823 cell growth,
whereas endogenous inhibition of BAMBI expression significantly
promoted the growth of BGC-823 cells compared with the control and
Si-vector, respectively (P<0.01). In addition, the migratory
ability of BGC-823 cells was significantly inhibited by the
treatment of pBAMBI, whereas BAMBI inhibition significantly
increased cell migration compared with the control and Si-vector,
respectively (P<0.01; Fig. 1E).
Furthermore, the invasive ability of BGC-823 cells was also
significantly suppressed following endogenous overexpression of
BAMBI, whereas the invasive characteristics were significantly
enhanced following knockdown of BAMBI compared with the control and
Si-vector, respectively (P<0.01; Fig.
1F). These results suggest that BAMBI expression may be
associated with the growth, migration and invasion of gastric
cancer cells.
BAMBI overexpression downregulates EMT
marker expression levels mediated by TGF-β in gastric cancer
The signaling pathway mediated by BAMBI was further
analyzed in gastric carcinoma cells. TGF-β expression and
differential expression levels of EMT markers in gastric cancer
cells following overexpression and knockdown of BAMBI were examined
in the present study. As indicated in Fig. 2A, BAMBI overexpression markedly
inhibited proliferation marker IPO-38 and Ki67 expression levels in
BGC-823 cells compared with the control. Notably, BAMBI
overexpression significantly increased the intracellular pH in
BGC-823 cells compared with the control (P<0.01; Fig. 2B). As indicated in Fig. 2C, the protein expression levels of
EMT markers TWIST1, MMP9 and SOX4 were also markedly downregulated
in BAMBI-overexpressed BGC-823 cells compared with the control. In
addition, EMT transcription factors SNAI1, ACTA2 and VIM exhibited
reduced levels of protein expression in BGC-823 cells following
BAMBI overexpression compared with the control (Fig. 2D). Furthermore, it was observed that
the protein expression levels of TGF-β and its regulatory molecules
(N-cadherin, CT-I and FIB) were inhibited in BAMBI-overexpressed
BGC-823 cells compared with the control (Fig. 2E). Notably, it was also demonstrated
that the protein expression levels of TGF-β, N-cadherin, CT-I and
FIB were increased following BAMBI knockdown in BGC-823 cells
compared with the control (Fig. 2F).
However, TGF-β treatment reversed the inhibitory effects of BAMBI
overexpression on EMT signaling pathway markers TWIST1, SOX4, MMP9,
SNAI1, ACTA2 and VIM (Fig. 2G).
Notably, BAMBI silencing promoted these expression levels in
BGC-823 cells compared with the control (Fig. 2H). These results suggest that BAMBI
overexpression downregulates EMT marker protein expression via
TGF-β in gastric cancer.
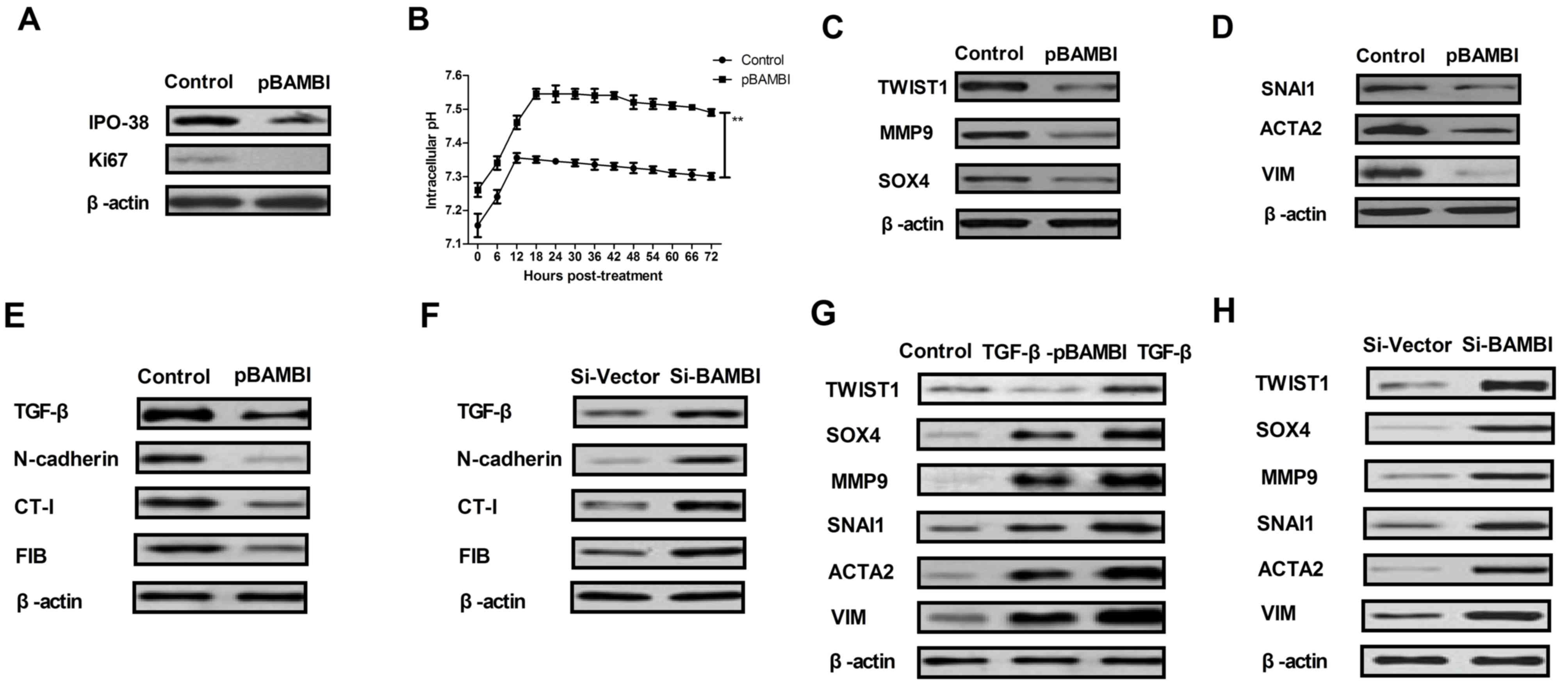 | Figure 2.BAMBI regulated the growth and
aggressiveness of gastric cancer cells through the TGF-β/EMT
signaling pathway. (A) Proliferation marker IPO-38 and Ki67 protein
expression levels in BGC-823 cells following BAMBI overexpression
were assessed. (B) Intracellular pH changes in BAMBI-overexpressed
BGC-823 cells were analyzed. (C) EMT marker TWIST1, MMP9 and SOX4
protein expression levels in BGC-823 cells following transfection
with BAMBI were indicated. (D) EMT transcription factor SNAI1,
ACTA2 and VIM protein expression levels in BGC-823 cells following
transfection with BAMBI were determined. Effects of BAMBI (E)
overexpression or (F) knockdown on TGF-β and its regulatory
molecules expression levels of N-cadherin, CT-I and FIB in BGC-823
cells. Effects of BAMBI (G) overexpression or (H) knockdown on
TGF-β-induced EMT signal pathway in BGC-823 cells. One-way analysis
of variance or two-tailed Student's t-test were performed.
**P<0.01 as indicated. Control, pvector. BAMBI, bone
morphogenetic protein and activin membrane-bound inhibitor; TGF-β,
transforming growth factor-β; EMT, epithelial-mesenchymal
transition; Si, small interfering RNA; pBAMBI, overexpressed BAMBI;
TWIST1, twist-related protein 1; MMP9, matrix metallopeptidase;
SOX4, SRY-box 4; ACTA2, α-actin-2; VIM, vimentin; FIB, fibronectin;
CT-I; collagen-I. |
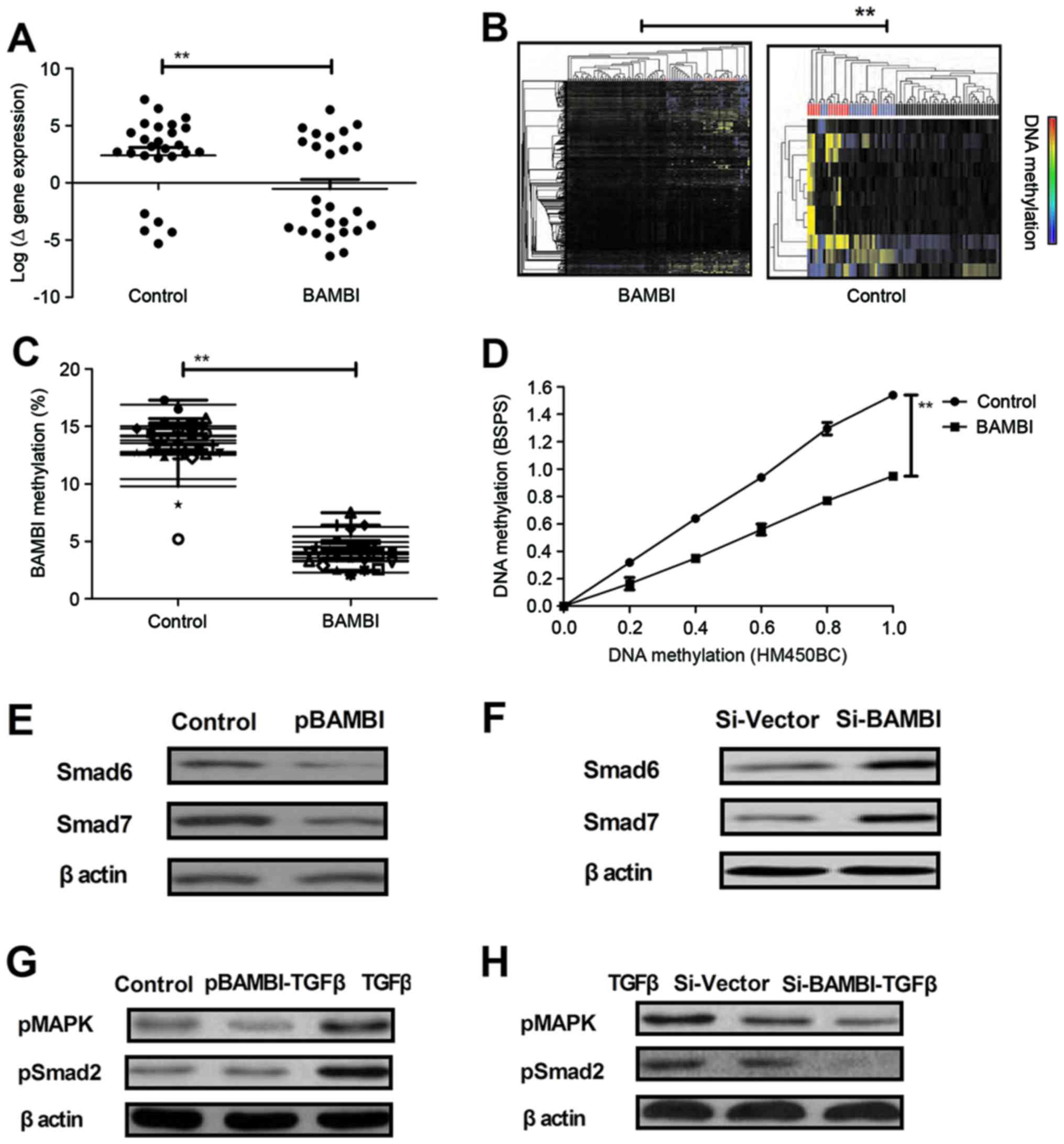
BAMBI overexpression regulates
methylation patterns of TGF-β signaling pathway members in gastric
cancer cells
A previous study has indicated that changes in
methylated modifications are observed during carcinogenesis and
growth, migration and invasion in gastric tumors (34). To determine the influence of BAMBI on
the methylation patterns of TGF-β signaling pathway members and
mediators in gastric cancer, gastric cells were analyzed using
Ilumina's BeadChip technology. Data indicated that BAMBI
overexpression significantly decreased the DNA methylation of genes
for molecules associated with TGF-β-signal transduction in BGC-823
cells compared with the control (P<0.01; Fig. 3A). Furthermore, BAMBI overexpression
decreased BAMBI methylation based on epigenetic modifications of
the CpG loci located in the BAMBI gene region compared with the
control (Fig. 3B and C). Bisulfite
pyrosequencing and HumanMethylation450K BeadChip analysis indicated
a similar methylation patterns of loci located in the BAMBI gene
region with a Pearson correlation coefficient of r=0.926 following
BAMBI overexpression (Fig. 3D). In
addition, it was demonstrated that BAMBI overexpression decreased
the BAMBI-induced upregulation of TGF-β target genes Smad6 and
Smad7, whereas knockdown of TGF-β induced the upregulation of TGF-β
target genes (Fig. 3E and F). It was
also indicated that BAMBI overexpression abolished TGF-β-induced
phosphorylation of mitogen-activated protein kinase (MAPK) and
Smad2, whereas knockdown of BAMBI (Si-BAMBI-TGFβ) decreased the
protein expression levels of pMAPK and pSmad2 in BGC-823 cells
(Fig. 3G and H). These findings
suggest that the BAMBI overexpression decreased the signal
molecular expression levels following TGF-β-stimulation.
BAMBI overexpression inhibits tumor
growth in BGC-823-bearing mice
In order to investigate the efficacy of BAMBI on the
progression of gastric carcinoma, BGC-823-bearing C57BL/6 nude mice
were established in the present study. BAMBI-overexpressed BGC-823
cells significantly suppressed tumor formation compared with PBS in
experimental mice (P<0.01; Fig.
4A). Intracellular pH was significantly increased in the tumors
induced with BAMBI-overexpressed BGC-823 cells compared with PBS in
mice (P<0.01; Fig. 4B). In
addition, the expression levels of key regulatory factors in the
TGF-β/EMT signaling pathway were analyzed. As indicated in Fig. 4C, ki67 and HIF expression levels were
markedly downregulated in tumors induced with BAMBI-overexpressed
BGC-823 cells compared with PBS. In addition, EMT signaling pathway
mediators TWIST1, MMP9 and SOX4 were markedly downregulated in
tumors induced with overexpressed BAMBI compared with PBS (Fig. 4D). Furthermore, it was also observed
that the expression levels of TGF-β signaling pathway mediators
N-cadherin, CT-I and FIB were markedly downregulated in tumors
treated with overexpressed BAMBI cells compared with PBS (Fig. 4E). Additionally, the apoptotic rate
in tumor cells was significantly upregulated following BAMBI
overexpression compared with PBS in tissues from the in vivo
model (P<0.01; Fig. 4F). These
results suggest that BAMBI overexpression may inhibit the formation
of gastric tumors by regulating the activation of the TGF-β/EMT
signaling pathway in vivo, which contributes to inhibition
of gastric tumor growth.
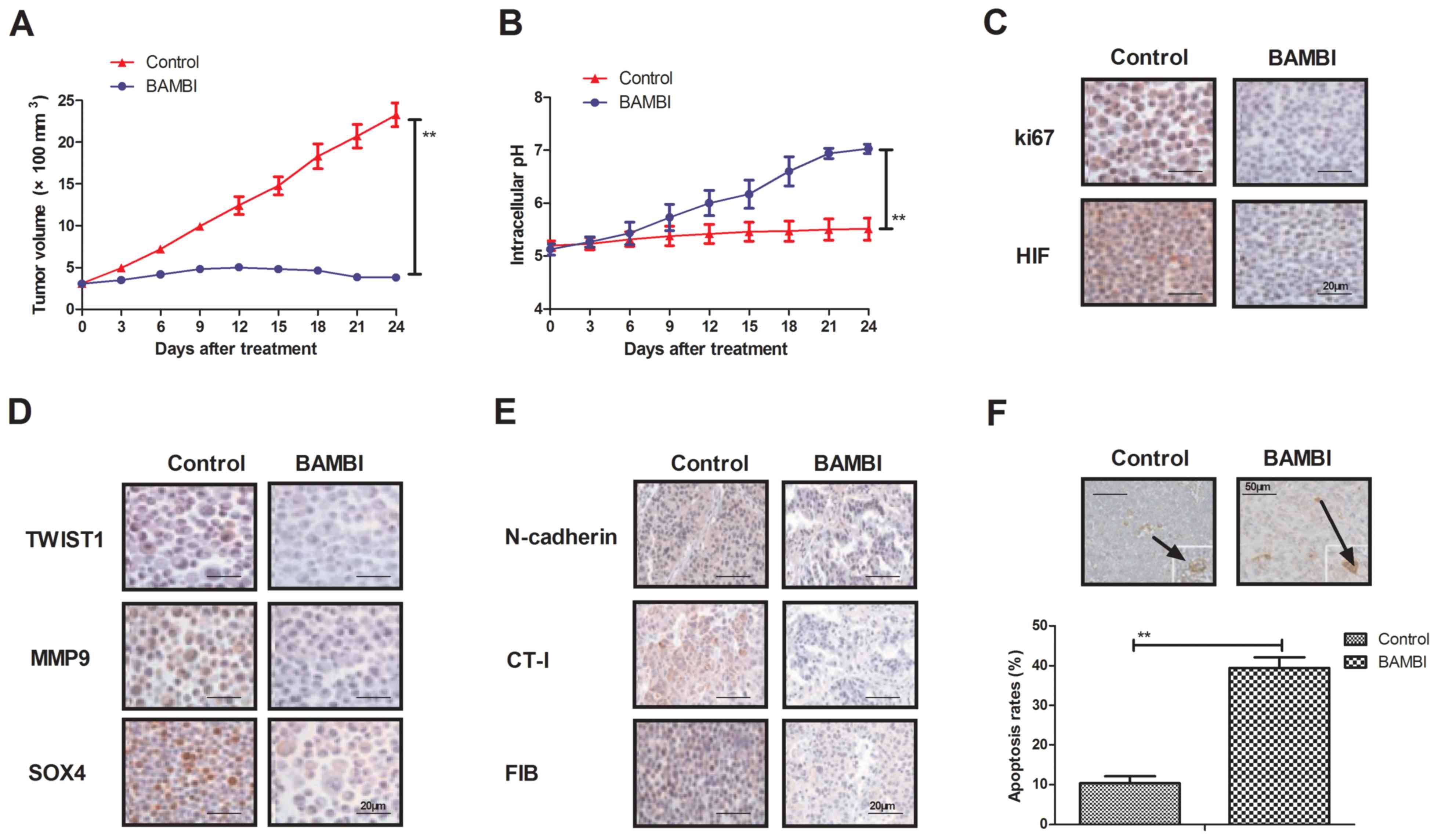 | Figure 4.In vivo effects of BAMBI
overexpression on gastric tumor progression. (A) Overexpression of
BAMBI inhibited gastric cancer tissues growth. (B) Endogenic BAMBI
overexpression improved intracellular pH in gastric tumors from
xenograft mice. (C) Protein expression levels of ki67 and HIF in
BAMBI overexpressed tumors were assessed. (D) TWIST1, MMP9 and SOX4
protein expression levels involved in the EMT signaling pathway in
tumors were overexpressed BAMBI. (E) Protein expression levels of
N-cadherin, CT-I and FIB were also assessed in BAMBI-overexpressed
gastric tumors. (F) Apoptosis rate in gastric tumors transfected
with BAMBI in vivo were indicated. White arrows indicate the
apoptotic cells. One-way analysis of variance or two-tailed
Student's t-test revealed a significant effect. **P<0.01 as
indicated. BAMBI, bone morphogenetic protein and activin
membrane-bound inhibitor; TGF-β, transforming growth factor-β; Si,
small interfering; pBAMBI, overexpressed BAMBI; TWIST1,
twist-related protein 1; MMP9, matrix metallopeptidase; SOX4,
SRY-box 4; ACTA2, α-actin-2; VIM, vimentin; FIB, fibronectin; CT-I;
collagen-I; HIF, hypoxia-inducible factor. |
Discussion
Gastric cancer is an important healthcare problem
that is difficult to treat due to apoptotic resistance, as gastric
tumor cells typically exist in the hydrochloric acid gastric juice,
which is a more acidic environment compared with other human cancer
types and further inhibits the efficacy of anticancer drugs
(35,36). Previous reports have demonstrated
that TGF-β and EMT signaling pathways are correlated with the
malignancy of gastric carcinoma and are responsible for its growth,
migration and metastasis (13,37–39). The
aim of the present study was to investigate the expression and
function of BAMBI in gastric carcinoma and explore the association
between BAMBI and gastric tumor formation. In addition, the
mechanism associated with the BAMBI-mediated signaling pathway in
gastric cancer cells was investigated in vitro and in
vivo. It was speculated that BAMBI overexpression relieved the
intracellular acidity by regulating the TGF-β signaling pathway in
gastric cancer cells. The present data indicated that BAMBI serves
a critical role in pH regulation and the growth of gastric
carcinoma cells. Furthermore, the present results demonstrated that
BAMBI downregulation drives the invasiveness of gastric cancer via
the TGF-β/EMT signaling pathway.
Previous studies have suggested that TGF-β is a
pleiotropic cytokine during the inflammatory response and tissue
homeostasis, which regulates differentiation, proliferation,
survival and apoptosis in tumor cells (40,41). Fu
et al (42) indicated that
TGF-β promotes migration and metastasis of gastric cancer cells by
regulating ERK and JNK signaling pathways. Notably, TGF-β serves a
dual function in the process of carcinogenesis by suppressing
apoptosis and regulating proliferation via the EMT signaling
pathway (43,44). The EMT signaling pathway is a vital
process in tumor cell progression and metastasis that is stimulated
by the TGF-β signaling pathway, which ultimately leads to complex
biochemical reaction processes in tumor cells (45,46).
TGF-β regulates the EMT signaling pathway by binding to ligands
TGFβR1 and TGF-β2 (47). A previous
study revealed the molecular mechanisms of the EMT signaling
pathway are associated with Ras-induced signaling, which regulates
the EMT process in human tumor cells (48). In the present analysis, it was
indicated that TGF-β regulated BAMBI-mediated growth and invasion
of gastric carcinoma through the regulation of EMT signaling.
The EMT signaling pathway is associated with the
progression of gastric cancer (13).
The present data indicated that the TGF-β signaling pathway may be
targeted via TGF-β in BAMBI-overexpressed gastric cells. Distinct
upregulation in the DNA methylation of the gene regions encoding
the TGF-β signaling pathway components was observed in
BAMBI-overexpressed gastric cells and resulted in a marked
reduction of TGF-β-induced growth, migration and invasion. However,
DNA methylation was decreased in the present study. In addition,
BAMBI overexpression abolished TGF-β-induced phosphorylation of
MAPK and Smad2 and knockdown of BAMBI decreased the protein
expression levels of pMAPK and pSmad2 in BGC-823 cells.
Furthermore, endogenous BAMBI overexpression significantly promoted
apoptosis in gastric cancer tissues and markedly inhibited the
growth of gastric tumors in murine xenografts. Therefore, these
results suggest that BAMBI overexpression in gastric tumor
regression may be dependent on the inhibition of TGF-β-induced
signaling at receptor level.
Although the association between progression and
TGF-β signaling has been investigated in gastric cells, a limited
number of studies have evaluated the association of BAMBI and TGF-β
in cancer cells (49). It was
observed that lower expression of BAMBI was induced by lower PH,
sensitized gastric cancer cells to TGF-β-induced aggression and
promoted EMT-dependent malignant processes. A previous study has
identified that BAMBI may inhibit TGF-β signaling in colorectal
tumor cells (50). Similarly, the
present results supported this hypothesis in xenograft mice and
demonstrated that overexpression of BAMBI in gastric cancer cells
resulted in the significant inhibition of gastric tumor growth.
In conclusion, the present findings suggest that
gastric cancer epigenetic overexpression of BAMBI results in
reduced TGF-β/EMT signaling, which contributes to the inhibition of
tumor growth and EMT-mediated signaling (51). Notably, epigenetic activating of
BAMBI results in inactivation of the TGF-β/EMT signaling pathway
in vitro and in vivo, which may enhance the
therapeutic effects of BAMBI in the treatment of gastric cancer.
These findings suggest that BAMBI overexpression may suppress the
growth and invasiveness of gastric tumors through regulation of the
TGF-β/EMT signaling pathway, suggesting that BAMBI may be a
potential target for the treatment of patients with gastric
carcinoma. However, future studies are required to investigate
other gastric carcinoma cell lines in order to fully elucidate the
therapeutic effects of BAMBI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CLY performed data analysis and wrote the
manuscript. YL designed the study and revised the manuscript. RL,
ZHL and YQL performed experiments and collected data. XLL and JZY
performed data analysis.
Ethics approval and consent to
participate
The present study was implemented legitimately
according to the Guide for the Care and Use of Laboratory Animals
of the Affiliated Tumor Hospital of Guangxi Medical University
(Nanning, China). The present study was performed in accordance
with the Ethics of Animal Experiments Defense Research and approved
by the Ethics Committee of the Affiliated Tumor Hospital of Guangxi
Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Areia M, Carvalho R, Cadime AT, Goncalves
Rocha F and Dinis-Ribeiro M: Screening for gastric cancer and
surveillance of premalignant lesions: A systematic review of
cost-effectiveness studies. Helicobacter. 18:325–337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Futtrup TB, Hasselby JP and Baeksgaard L:
Gastric signet ring cell carcinoma presenting as diffuse,
infiltrating myositis-a case report and review of the literature. J
Gastrointest Cancer. 45 Suppl 1:S62–S65. 2014. View Article : Google Scholar
|
|
3
|
Pimenta-Melo AR, Monteiro-Soares M,
Libanio D and Dinis-Ribeiro M: Missing rate for gastric cancer
during upper gastrointestinal endoscopy: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1041–1049. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veisani Y and Delpisheh A: Survival rate
of gastric cancer in Iran; a systematic review and meta-analysis.
Gastroenterol Hepatol Bed Bench. 9:78–86. 2016.PubMed/NCBI
|
|
5
|
Jung SA, Park YM, Hong SW, Moon JH, Shin
JS, Lee HR, Ha SH, Lee DH, Kim JH, Kim SM, et al: Cellular
inhibitor of apoptosis protein 1 (cIAP1) stability contributes to
YM155 resistance in human gastric cancer cells. J Biol Chem.
290:9974–9985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Izawa M, Mori T, Satoh T, Teramachi K and
Sairenji T: Identification of an alternative form of caspase-9 in
human gastric cancer cell lines: A role of a caspase-9 variant in
apoptosis resistance. Apoptosis. 4:321–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oberstein PE, Kenney B, Krishnamoorthy SK,
Woo Y and Saif MW: Metastatic gastric large cell neuroendocrine
carcinoma: A case report and review of literature. Clin Colorectal
Cancer. 11:218–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K and Li J: Current molecular targeted
therapy in advanced gastric cancer: A comprehensive review of
therapeutic mechanism, clinical trials, and practical application.
Gastroenterol Res Pract. 2016:41056152016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruzzo A, Catalano V, Canestrari E,
Giacomini E, Santini D, Tonini G, Vincenzi B, Fiorentini G, Magnani
M and Graziano F: Genetic modulation of the interleukin 6 (IL-6)
system in patients with advanced gastric cancer: A background for
an alternative target therapy. BMC Cancer. 14:3572014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thews O, Riemann A, Nowak M and Gekle M:
Impact of hypoxia-related tumor acidosis on cytotoxicity of
different chemotherapeutic drugs in vitro and in vivo. Adv Exp Med
Biol. 812:51–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujikuni N, Yamamoto H, Tanabe K, Naito Y,
Sakamoto N, Tanaka Y, Yanagihara K, Oue N, Yasui W and Ohdan H:
Hypoxia-mediated CD24 expression is correlated with gastric cancer
aggressiveness by promoting cell migration and invasion. Cancer
Sci. 105:1411–1420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao ZF, Wang ZN, Zhao TT, Xu YY, Gao J,
Miao F and Xu HM: Peritoneal milky spots serve as a hypoxic niche
and favor gastric cancer stem/progenitor cell peritoneal
dissemination through hypoxia-inducible factor 1α. Stem Cells.
32:3062–3074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, et
al: Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun XP, Dong X, Lin L, Jiang X, Wei Z,
Zhai B, Sun B, Zhang Q, Wang X, Jiang H, et al: Up-regulation of
survivin by AKT and hypoxia-inducible factor 1α contributes to
cisplatin resistance in gastric cancer. FEBS J. 281:115–128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osinsky S, Bubnovskaya L, Ganusevich I,
Kovelskaya A, Gumenyuk L, Olijnichenko G and Merentsev S: Hypoxia,
tumour-associated macrophages, microvessel density, VEGF and matrix
metalloproteinases in human gastric cancer: Interaction and impact
on survival. Clin Transl Oncol. 13:133–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marwitz S, Depner S, Dvornikov D, Merkle
R, Szczygieł M, Müller-Decker K, Lucarelli P, Wäsch M, Mairbäurl H,
Rabe KF, et al: Downregulation of the TGFβ pseudoreceptor BAMBI in
non-small cell lung cancer enhances TGFβ signaling and invasion.
Cancer Res. 76:3785–3801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Legg K: Autoimmunity: A controlled
performance by BAMBI. Nat Rev Rheumatol. 12:722016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao S, Zhao L, Gao J, Wang H and Cui Z:
Distribution and mRNA expression of BAMBI in Non-small-cell lung
cancer. Zhongguo Fei Ai Za Zhi. 12:203–207. 2009.(In Chinese).
PubMed/NCBI
|
|
19
|
Fritzmann J, Morkel M, Besser D, Budczies
J, Kosel F, Brembeck FH, Stein U, Fichtner I, Schlag PM and
Birchmeier W: A colorectal cancer expression profile that includes
transforming growth factor beta inhibitor BAMBI predicts metastatic
potential. Gastroenterology. 137:165–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khin SS, Kitazawa R, Win N, Aye TT, Mori
K, Kondo T and Kitazawa S: BAMBI gene is epigenetically silenced in
subset of high-grade bladder cancer. Int J Cancer. 125:328–338.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pils D, Wittinger M, Petz M, Gugerell A,
Gregor W, Alfanz A, Horvat R, Braicu EI, Sehouli J, Zeillinger R,
et al: BAMBI is overexpressed in ovarian cancer and co-translocates
with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol.
117:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu K, Song X, Ma H, Liu L, Wen X, Yu J,
Wang L and Hu S: Knockdown of BAMBI inhibits β-catenin and
transforming growth factor β to suppress metastasis of gastric
cancer cells. Mol Med Rep. 10:874–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Yu Z, Xiao Q, Sun X, Zhu Z, Zhang
J, Xu H, Wei M and Sun M: Expression of BAMBI and its combination
with Smad7 correlates with tumor invasion and poor prognosis in
gastric cancer. Tumour Biol. 35:7047–7056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foley PL: Current options for providing
sustained analgesia to laboratory animals. Lab Anim (NY).
43:364–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng L, Liang W, Pan J, Cao Y, Liu J, Wang
Q, Wang L, Zou Y, Wang K, Kong L, et al: Do Chinese researchers
conduct ethical research and use ethics committee review in
clinical trials of Anti-dementia drugs? An analysis of biomedical
publications originating from China. J Alzheimers Dis. 52:813–823.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo L, Zhong H, Liu S, Deng L, Luo Y,
Zhang Q, Zhu Y, Tian Y, Sun Y and Tian X: Intracellular ‘activated’
two-photon photodynamic therapy by fluorescent conveyor and
photosensitizer co-encapsulating pH-responsive micelles against
breast cancer. Int J Nanomedicine. 12:5189–5201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scherer O, Maeß MB, Lindner S, Garscha U,
Weinigel C, Rummler S, Werz O and Lorkowski S: A procedure for
efficient non-viral siRNA transfection of primary human monocytes
using nucleofection. J Immunol Methods. 422:118–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Babiker HA, Saito T, Nakatsu Y, Takasuga
S, Morita M, Sugimoto Y, Ueda J and Watanabe T: Molecular cloning,
polymorphism, and functional activity of the bovine and water
buffalo Mx2 gene promoter region. Springerplus. 5:21092016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Renshaw A and Elsheikh TM: A validation
study of the Focalpoint GS imaging system for gynecologic cytology
screening. Cancer Cytopathol. 121:737–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joubert BR, Felix JF, Yousefi P, Bakulski
KM, Just AC, Breton C, Reese SE, Markunas CA, Richmond RC, Xu CJ,
et al: DNA methylation in newborns and maternal smoking in
pregnancy: Genome-wide consortium Meta-analysis. Am J Hum Genet.
98:680–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iijima K and Kiyokawa N: Analysis of gene
expression and DNA methylation patterns in childhood acute
lymphoblastic leukemia. Rinsho Ketsueki. 57:425–429. 2016.(In
Japanese). PubMed/NCBI
|
|
32
|
Yaghoubi A, Aryan E, Zare H, Alami SH,
Teimourpour R and Meshkat Z: Design and construction of a cloning
vector containing the hspX gene of mycobacterium tuberculosis. Rep
Biochem Mol Biol. 5:46–50. 2016.PubMed/NCBI
|
|
33
|
Koole K, Clausen MJ, van Es RJ, van Kempen
PM, Melchers LJ, Koole R, Langendijk JA, van Diest PJ, Roodenburg
JL, Schuuring E and Willems SM: Fgfr family members protein
expression as prognostic markers in oral cavity and oropharyngeal
squamous cell carcinoma. Mol Diagn Ther. 20:363–374. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakamoto A, Akiyama Y, Shimada S, Zhu WG,
Yuasa Y and Tanaka S: DNA methylation in the Exon 1 region and
complex regulation of Twist1 expression in gastric cancer cells.
PLoS One. 10:e01456302015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi HS, Ha SY, Kim HM, Ahn SM, Kang MS,
Kim KM, Choi MG, Lee JH, Sohn TS, Bae JM, et al: The prognostic
effects of tumor infiltrating regulatory T cells and myeloid
derived suppressor cells assessed by multicolor flow cytometry in
gastric cancer patients. Oncotarget. 7:7940–7951. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mori F, Canu V, Lorenzon L, Garofalo A,
Blandino G and Strano S: Cancer gastric chemoprevention: Isolation
of gastric Tumor-initiating cells. Methods Mol Biol. 1379:129–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park D II, Son HJ, Song SY, Choe WH, Lim
YJ, Park SJ, Kim JJ, Kim YH, Rhee PL, Paik SW, et al: Role of
TGF-beta 1 and TGF-beta type II receptor in gastric cancer. Korean
J Intern Med. 17:160–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morita K, Fujimori T, Ono Y, Hiraishi H,
Yoshiura K, Shimada T, Sugaya H and Terano A: Identification of the
prelinitis condition in gastric cancer and analysis of TGF-beta,
TGF-beta RII and pS2 expression. Pathobiology. 69:321–328. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muhrer KH, Schwemmle K, Stambolis C and
Kahle M: KCl-tumor extracts in the electrophoretic mobility test
[EMT]. -Clinical relevance to diagnosis of gastric cancer (author's
transl). Z Gastroenterol. 20:376–383. 1982.(In German). PubMed/NCBI
|
|
40
|
Donatelli SS, Zhou JM, Gilvary DL,
Eksioglu EA, Chen X, Cress WD, Haura EB, Schabath MB, Coppola D,
Wei S and Djeu JY: TGF-β-inducible microRNA-183 silences
tumor-associated natural killer cells. Proc Natl Acad Sci USA.
111:4203–4208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohnuki H, Jiang K, Wang D, Salvucci O,
Kwak H, Sánchez-Martín D, Maric D and Tosato G: Tumor-infiltrating
myeloid cells activate Dll4/Notch/TGF-β signaling to drive
malignant progression. Cancer Res. 74:2038–2049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin (Shanghai). 41:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guan Z, Song B, Liu F, Sun D, Wang K and
Qu H: TGF-β induces HLA-G expression through inhibiting miR-152 in
gastric cancer cells. J Biomed Sci. 22:1072015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gu J, Qian H, Shen L, Zhang X, Zhu W,
Huang L, Yan Y, Mao F, Zhao C, Shi Y and Xu W: Gastric cancer
exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-β/Smad pathway. PLoS One. 7:e524652012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma Y, Liu H, Zhang H and Shao RG: The
TGF-β signaling pathway induced EMT in breast cancer. Yao Xue Xue
Bao. 50:385–392. 2015.(In Chinese). PubMed/NCBI
|
|
46
|
Elsum IA, Martin C and Humbert PO:
Scribble regulates an EMT polarity pathway through modulation of
MAPK-ERK signaling to mediate junction formation. J Cell Sci.
126:3990–3999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Ma J, Qian X, Wu Q, Xia J, Miele L,
Sarkar FH and Wang Z: Regulation of EMT by Notch signaling pathway
in tumor progression. Curr Cancer Drug Targets. 13:957–962. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pak KH, Kim DH, Kim H, Lee DH and Cheong
JH: Erratum to: Differences in TGF-β1 signaling and
clinicopathologic characteristics of histologic subtypes of gastric
cancer. BMC Cancer. 16:992016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sekiya T, Adachi S, Kohu K, Yamada T,
Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S,
et al: Identification of BMP and activin membrane-bound inhibitor
(BAMBI), an inhibitor of transforming growth factor-beta signaling,
as a target of the beta-catenin pathway in colorectal tumor cells.
J Biol Chem. 279:6840–6846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kitazawa S, Kitazawa R, Obayashi C and
Yamamoto T: Desmoid tumor with ossification in chest wall: Possible
involvement of BAMBI promoter hypermethylation in metaplastic bone
formation. J Bone Miner Res. 20:1472–1477. 2005. View Article : Google Scholar : PubMed/NCBI
|