Introduction
Burn injury is a common traumatic injury with
considerable mortality and morbidity, requiring a long period of
hospitalization and rehabilitation (1). Severe injury may induce a series of
physiological changes, such as the change of metabolic rate, core
temperature and various substrate cycling (2). In addition, both complications and burn
wound of burn injury influence the physical and psychological
health (3).
In previous studies, the expression of various genes
was found to be altered after severe burns. Noronha et al
(4) found that several genes,
including C-X-C motif chemokine ligand 8, interleukin 6, tumor
necrosis factor and major histocompatibility complex, Class I, E
played a contributory role in wound infection induced by burns. In
addition, Padfield et al identified various genes that
encode chemokines, complement, oxidative-stress and immune
functions following severe burn trauma (5). Long non-coding RNAs (lncRNA) were
defined as non-protein coding transcripts longer than 200
nucleotides (6). Initially they were
regarded as ‘noise’ with no biological function in the
transcription of the genome. However, lncRNAs were confirmed to
regulate gene expression in epigenetics, transcription and at the
post-transcriptional level (7). In
addition, lncRNAs were closely associated with the occurrence,
development and prevention of disease by participating in chromatin
modification, transcriptional activation, transcriptional
interference and intranuclear transport (8). Haijun et al found a differential
expression of lncRNAs compared to controls in skeletal muscles of
rats with burn injuries (9).
However, lncRNA and related functions and pathways in human have
not been studied.
Identification of sub-pathways regulated by lncRNA
are essential for monitoring of the occurrence and development of
disease, and also help to research the functions of lncRNAs. In
this study, we suggested a new method for identification of
function regulated by lncRNA. The expression profiles of lncRNA and
mRNA were integrated. Moreover, the regulation role of miRNA-target
mRNA and topological analysis of pathways were processed to
identify sub-pathways regulated by lncRNA and predict the function
of lncRNAs in severe burn injury patients.
Materials and methods
Samples
The expression profiling of E-GEOD-37069 with 553
injury samples and 37 controls, were downloaded from ArrayExpress
Archive (http://www.ebi.ac.uk/arrayexpress/), which was based
on the platform of [HG-U133_Plus_2] Affymetrix Human Genome U133
Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA). These 553 injury
samples were gathered from 244 severe burns patients over time. The
patients had burns covering >20% of the total surface and were
admitted at 96 h. The raw profiles were downloaded for further
analysis.
Data preprocessing and genes
screening
Based on the database of hgu133plus2, the raw data
were preprocessed. Genes were obtained by combing various
corresponding probes, and the expression value of this gene was
assigned by the mean value of these probes.
Construction of candidate lncRNA-mRNA
interaction
miRNA-mRNA interactions and lncRNA-miRNA
interactions were downloaded from starBase v2.0 database. Moreover,
some mRNA-miRNA interactions were obtained from various databases
including TarBase, mirTarBase, mir2Disease, and miRecords (V4.0).
According to shared miRNA in the aforementioned interactions, the
relationships of lncRNA-miRNA-mRNA were constructed, and candidate
lncRNA-mRNA regulatory relationships were obtained. The lncRNA-mRNA
regulatory relationships were screened using the criteria: i)
Hypergeometric functions were used to assess the significance of
miRNA that have a regulatory relationship with both lncRNA and
mRNA. The Benjamini and Hochberg (BH) method was used to adjust the
P-value. The lncRNA-miRNA intersections with false discovery rate
(FDR) <0.05 were screened. ii) Top 20% lncRNA-mRNA intersections
with higher Jaccard coefficient were retained. The formula of
hypergeometric functions was shown as:
P=1-∑t=0x(Kt)(N-KM-t)(NM)
Construction of co-expression
lncGenePairs
The genes of expression profiling were intersected
with mRNA and lncRNA in a lncRNA-mRNA interaction, respectively.
Based on these matched expression profiles of lncRNA and mRNA, the
Pearson's correlation coefficient was used to assess candidate
lncRNA-mRNA intersections (10), and
Fisher Z transformation was processed to calculate the P-value of
lncRNA-mRNA-intersections (11).
Furthermore, the P-value was adjusted as per the FDR using the BH
method, and lncRNA-mRNA-intersections with FDR <0.05 were
screened with significance (12).
Sub-pathway analysis
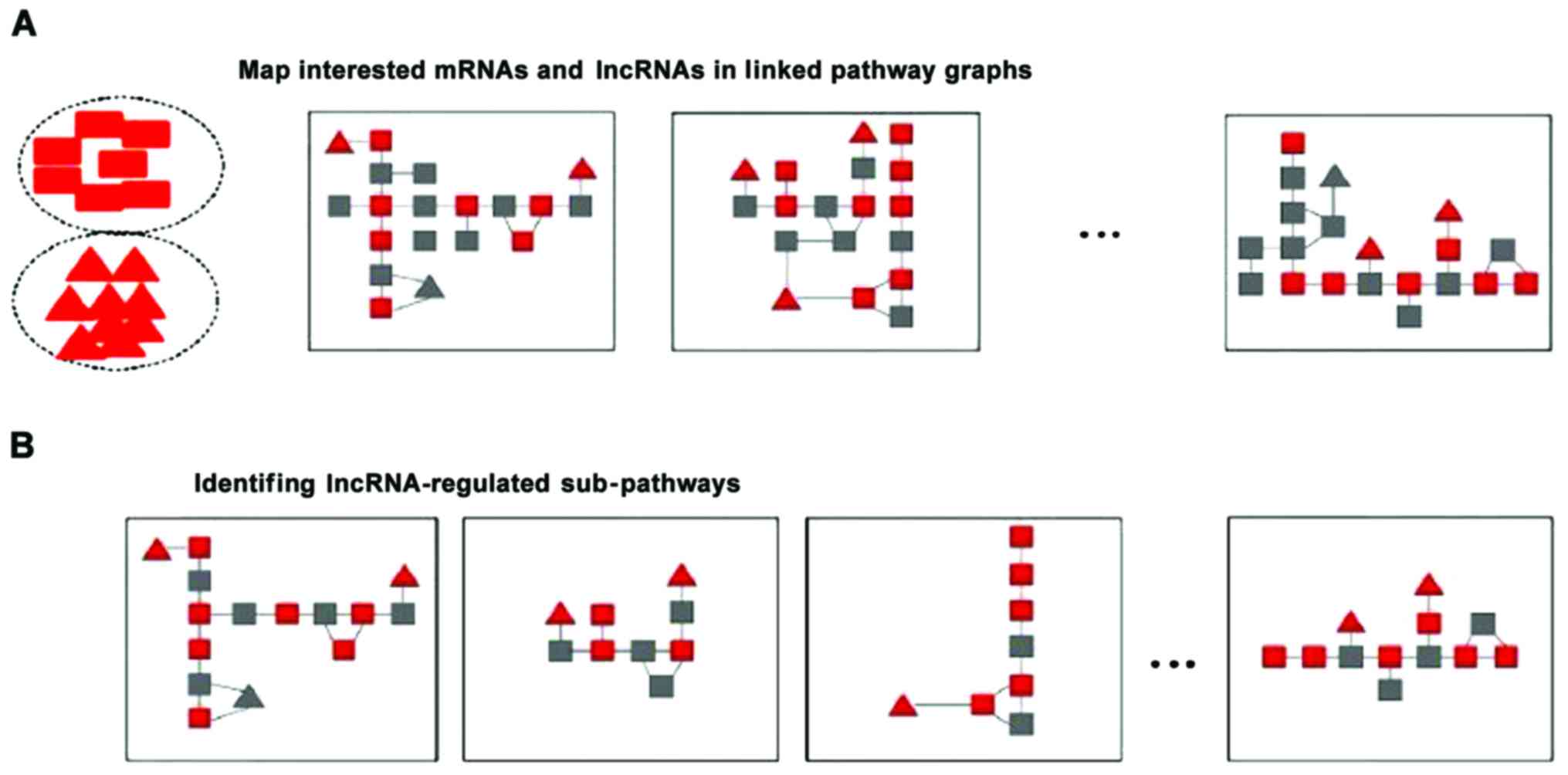
The lncRNA-regulated sub-pathway comprised two
steps: Mapping interested mRNAs and lncRNAs into linked pathways,
and identifying lncRNA-regulated sub-pathways (Fig. 1).
Pathway enrichment analysis of
screened mRNA
Based on the Kyoto Encyclopedia of Genes and Genomes
database, the screened mRNAs were enriched into various pathways.
Fisher's exact test was used to identify significant pathways.
Then, the P-value was adjusted into FDR by the BH method. The
pathways with FDR <0.01 were regarded as candidate different
pathways.
LncRNAs embed into enriched
pathways
The screened lncRNAs were embedded into candidate
pathways according to the results of lncGenePairs. Then, these
pathways enriched lncRNA and related genes were obtained.
Identification of sub-pathways
regulated by lncRNA
The screened lncRNA and related mRNA were regarded
as flag nodes. Based on the situation of lncRNA embedding, the
shortest route between two flag nodes was calculated. Once the
molecular number between two flag nodes ≥1, the two flag nodes were
combined to one node. In addition, the nodes in pathways were also
calculated, and pathways with ≥ 8 nodes were regarded as candidate
sub-pathways.
Assessment of sub-pathways and
identification of hub-lncRNA
The Wallenius approximation methods were used to
calculate the FDR value of each sub-pathway. In this process, the
pathway weight was calculated using the formula:
W=1+β(-log2(GLPG))
While the significance was evaluated using the
formula of P-value = F (x, m1, m2, n, weighti), where x,
represents the number of lncGenePairs that were enriched into this
hub-pathway; n, was the total number of mRNA; m1, represents the
number of mRNA enriched into this hub-pathway; m2, the number of
mRNA that could be annotated to lncGenePairs; w, was the weight of
this hub-subway; P and G were mRNA numbers in this sub-pathway; G
and L were mRNA numbers regulated by lncRNA; β=1. In addition, the
sub-pathway with FDR <0.01 was screened. Based on the results of
significant sub-pathways, the related lncRNA-mRNA network was
constructed. The lncRNAs with a degree more than the average degree
were regarded as hub-lncRNAs.
Results
Genes screening and obtaining of
lncRNA-mRNA interaction
After data preprocessing and combination, 18,081
genes were obtained. In addition, lncRNA-mRNA intersections
including 835 lncRNAs, 1,749 mRNAs and 7,693 interacting pairs were
constructed by several databases and sharing miRNAs.
Construction of co-expression
lncGenePairs
Taking intersection between screened genes and
lncRNA-mRNA, 1,641 mRNA expression profiles and 125 lncRNA
expression profiles were obtained. After Fisher Z transformation,
656 lncGenePairs including 101 lncRNAs, and 503 mRNAs were
constructed with P<0.05. The top 5 lncGenePairs were EMX2
opposite strand/antisense RNA-ACTN3 (P=4.80×1027),
TBX5-AS1-ADAMTS16 (P=3.79×1018), IQCH-AS1-ANKRD37
(P=0.02), MFI2-AS1-APLP1 (P=1.16×1026) and
HOXA-AS2-ARL16 (P=0.04) (Table
I).
 | Table I.The top 10 hub-lncRNA genes. |
Table I.
The top 10 hub-lncRNA genes.
| Lnc | Gene | corValue | P-value |
|---|
| EMX2OS | ACTN3 |
4.23×10−1 |
4.80×1027 |
| TBX5-AS1 |
ADAMTS16 |
3.47×10−1 |
3.79×1018 |
| IQCH-AS1 | ANKRD37 |
9.48×10−2 |
2.12×10−2 |
| MFI2-AS1 | APLP1 |
4.20×10−1 |
1.16×1026 |
| HOXA-AS2 | ARL16 |
8.5×10−2 |
3.8×10−2 |
| HOXA-AS2 | ASB9 |
9.97×10−2 |
1.53×10−2 |
| FOXN3-AS1 | ASPSCR1 |
3.31×10−1 |
1.22×1016 |
| LBX2-AS1 | ASTN1 |
1.77×10−1 |
1.43×1005 |
| LEF1-AS1 | BBIP1 |
2.97×10−2 |
1.71×1013 |
| TDRG1 | BNC1 |
2.55×10−2 |
6×1010 |
Sub-pathway analysis
The enriched mRNA were enriched into various
candidate pathways including ribosome biogenesis in eukaryotes (FDR
=8.19×105), neurotrophin signaling pathway (FDR
=8.07×105) and thyroid cancer (FDR =7.99×106)
(Table II).
 | Table II.The top 5 pathways enriched by
candidate mRNAs. |
Table II.
The top 5 pathways enriched by
candidate mRNAs.
| Pathway_id |
Pathway_P-value | P.adjust (FDR) |
|---|
| hsa03008: Ribosome
biogenesis in eukaryotes |
8.19×105 |
8.19×105 |
| hsa04722:
Neurotrophin signaling pathway |
8.07×105 |
8.07×105 |
| hsa05216: Thyroid
cancer |
7.99×106 |
7.99×106 |
| hsa05222: Small
cell lung cancer |
7.59×106 |
7.59×106 |
| hsa05145:
Toxoplasmosis |
7.15×105 |
7.15×105 |
After identification and assessment, several
sub-pathways were screened, including ribosome biogenesis in
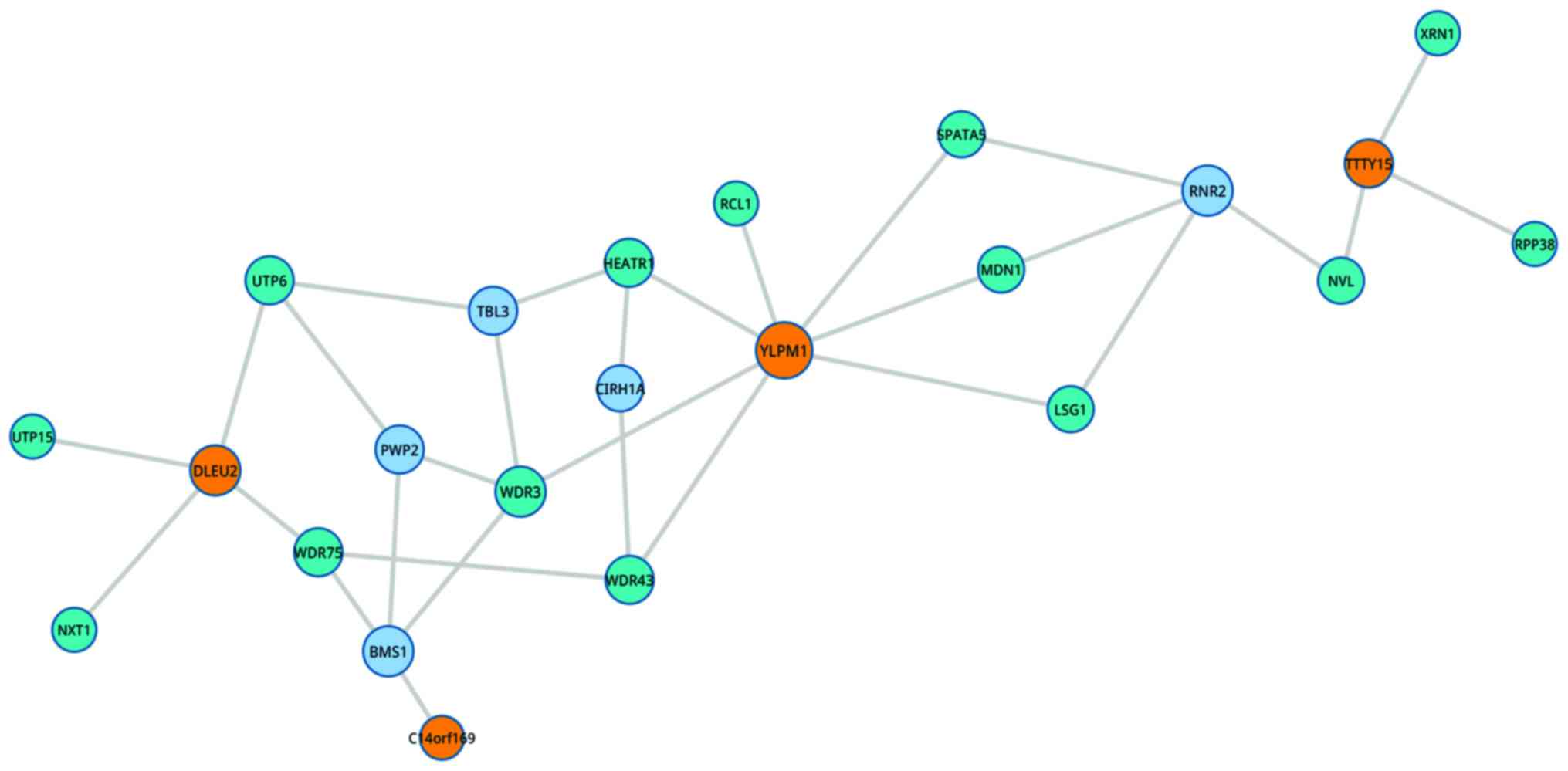
eukaryotes, and the MAPK and ErbB signaling pathways (Table III). In the top 3 pathways, various
lncRNA and related genes were involved. For example, YLP motif
containing 1 (YLPM1) in ribosome biogenesis pathway was closely
associated with heat repeat containing 1 (HEATR1), WD repeat domain
3 and spermatogenesis associated 5 (Fig.
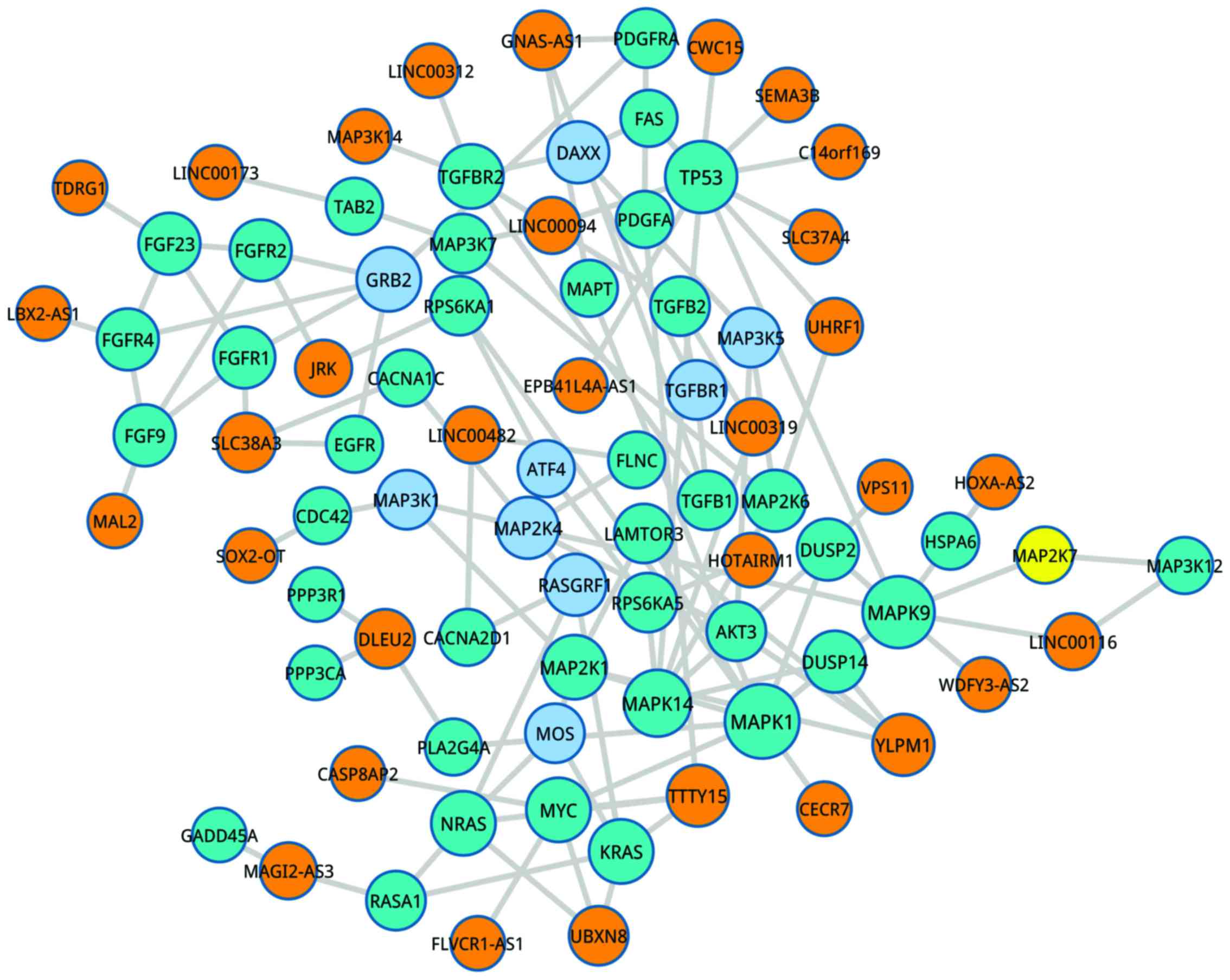
2). Besides, HOXA transcript antisense RNA, myeloid-specific 1
in MAPK signaling pathway was regulated by AKT serine/threonine
kinase 3 (AKT3), ribosomal protein S6 kinase A5 and
mitogen-activated protein kinase kinase 6 (Fig. 3). Furthermore, YLPMI in the ErbB
signaling pathway was associated with MAP2K1 and AKT3 (Fig. 4).
 | Table III.The top 5 hub-pathways. |
Table III.
The top 5 hub-pathways.
| Pathway_id | Pathway_name | Molecule ratio
(m2/x) | BgRatio (m1/n) | Weight | P-value | FDR |
|---|
| 03008_1 | Ribosome biogenesis
in eukaryotes | 15/489 | 19/26232 | 1.341037 | <0.001 | 0 |
| 04010_1 | MAPK signaling
pathway | 38/489 | 51/26232 | 1.387023 | <0.001 | 0 |
| 04012_1 | ErbB signaling
pathway | 18/489 | 25/26232 | 1.556393 | <0.001 | 0 |
| 04066_2 | HIF-1 signaling
pathway | 20/489 | 25/26232 | 1.395929 | <0.001 | 0 |
| 04110_1 | Cell cycle | 26/489 | 35/26232 | 1.428843 | <0.001 | 0 |
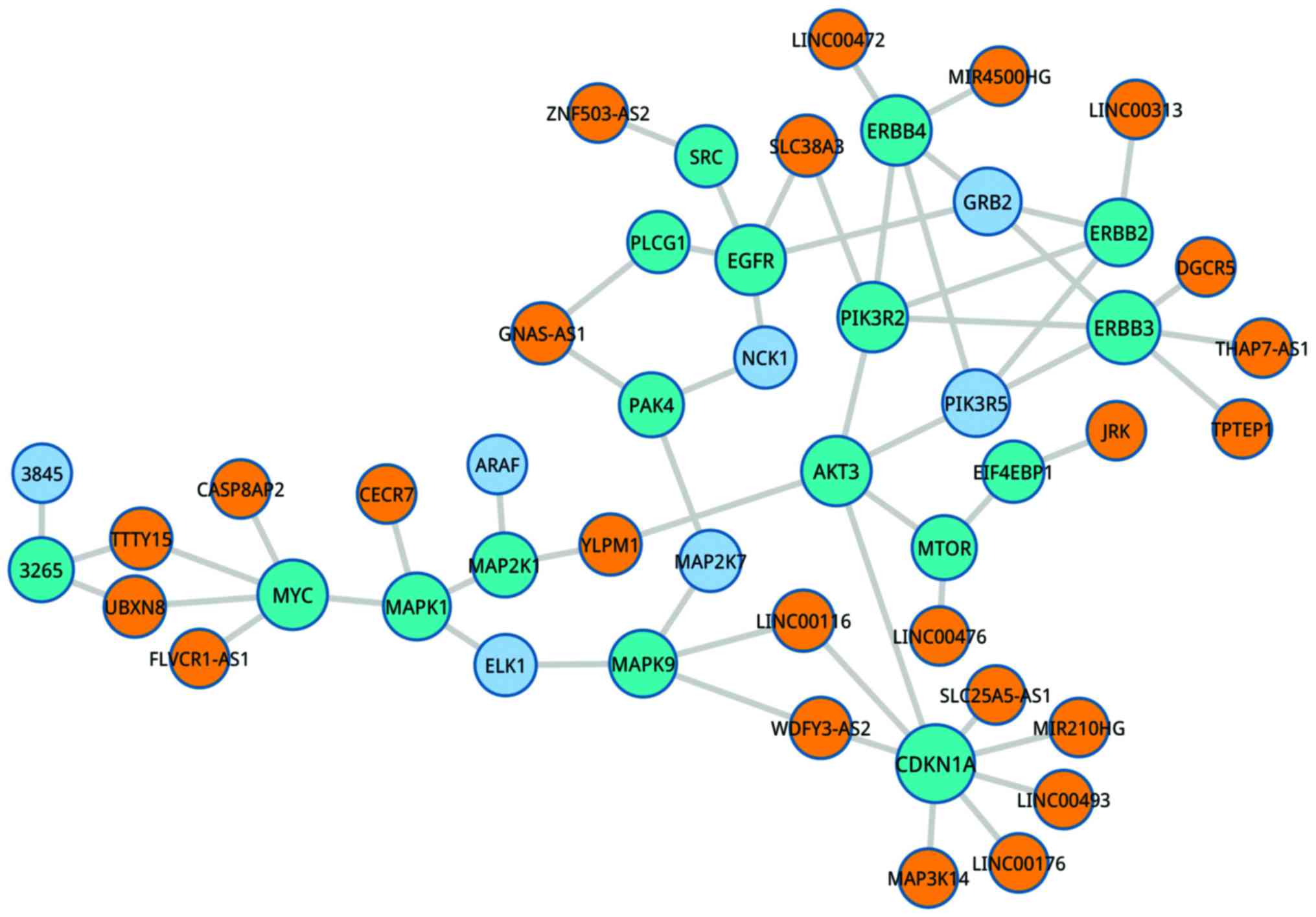
Identification of hub-lncRNA
Based on the above results, the network of
pathway-lncRNA-mRNA was constructed (Fig. 5). Through topological analyses of
this network, the hub-genes were identified, including chromosome
14 open reading frame 169 (C14orf169), (YLPM1), testis-specific
transcript, Y-linked 15 (TTTY15), PCBP1 antisense RNA 1 (PCBP1-AS1)
and deleted in lymphocytic leukemia 2. More importantly, several
hub-lncRNAs were obtained, including PRKAG2 antisense RNA 1, LEF1
antisense RNA 1, EMX2 opposite strand/antisense RNA (EMX2OS) and
BOLA3 antisense RNA 1.
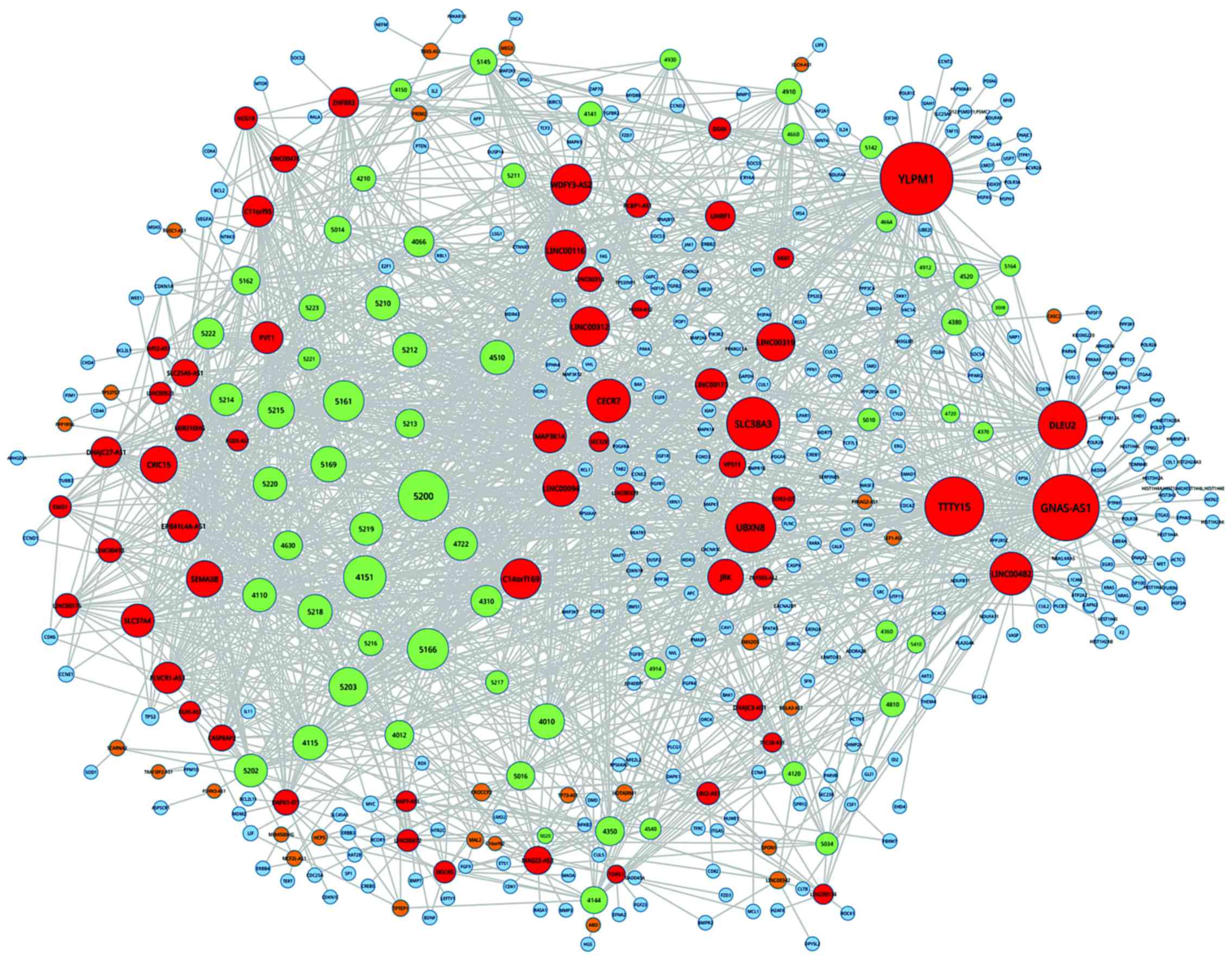 | Figure 5.The network of pathway-lncRNA-mRNA.
The red, yellow, light blue, dark blue and green nodes represent
hublnc, lnc, mRNA, mRNA and path, respectively. The size of node
represent degree of node. Edges represented regulated relationship
among hublnc, lnc, mRNA, mRNA& and path. |
Discussion
Increasing evidence has shown that lncRNAs were
important factors for regulating gene expression (13). However, the molecular mechanism in
burn injuries including lncRNA functions and regulatory genes
remain unknown. In the present study, an effective method for
identification of lncRNA-regulated functions was presented.
LncRNA-regulated subpathways were identified, including ribosome
biogenesis in eukaryotes, as well as MAPK and ErbB signaling
pathways. More importantly, several hub-lncRNAs were identified,
including C14orf169, YLPM1, TTTY15, and PCBP1-AS1. In addition, the
sub-pathways regulated by the lncRNAs were identified, and
functions of these lncRNAs were predicted.
C14orf169 was found to regulate various genes in
this study, including eukaryotic translation initiation factor 1
(EIF1) and PPP2R5C. Thus EIF1 was mainly present in nucleus and
cytosol. In a previous study, EIF1 was confirmed to be related to
the generation of stable 40S preinitiation complex (13). In addition, the eukaryotic
translation initiation factor contributed to muscle regeneration by
participating in the skeletal muscle stem cell differentiation
(14). Besides, PPP2R5C was found to
be overexpressed in myeloid leukemia cells, and closely related to
leukemic cell proliferation and differentiation (15). In the present study, PPP2R5C was
enriched into the PI3K-Akt and Wnt signaling pathways. Consistent
with the study of Shi et al (16), anti-fibrotic effects by activation of
the PIK3-Akt signaling pathway was beneficial for the reduction and
prevention of scar formation. Furthermore, alteration of the Wnt
signaling pathway could induce muscle fibrosis, and even lead to
failure in muscle generation and tissue disorganization (17). Thus, C14orf169 served as a potential
key lncRNA for the recovery of severe burn injury and
post-operative complications by regulating EIF1 and PPP2R5C.
Moreover, YLPM1 was screened as a hub-lncRNA in the
present study and resulted in the regulation of various genes, such
as RPL10L, PPT1, HEATR1 and RPL9. Both RPL10L
and RPL9 encoded ribosomal proteins, and were mainly present
in cytosol and nucleus. Kim et al (18) found that RPL9 interacted with Ftn-2,
and played an important role in the maintenance of iron
homeostasis. In addition, ribosomal protein phosphorylation was
confirmed to interact with myostatin, and further affect the mature
muscle (19). In mouce models,
knockout PPT1 induced neuronal ceroid lipofuscinosis (20). In several diseases, HEATR1 was
confirmed to regulate functional cytotoxic T lymphocytes and
enhance cell immunity (21,22). In the present study, HEATR1 was also
found to enrich into the hub-pathway of ribosome biogenesis in
eukaryotes. Based on this information, we inferred that YLPM1 was
an important lncRNA after severe burn injuries by regulating
RPL10L, PPT1, HEATR1 and RPL9.
TTTY15 has been widely researched and found to have
functions in the pathogenesis of prostate cancer, but it is less
known regarding other diseases. In the present study, the gene was
found to regulate the expression of different genes including
KCTD13, UFL1 and ZNF322. In zebrafish models,
KCTD13 was found to be closely associated with
neurodevelopmental phenotype (23).
In addition, KCTD13 interacted with Bacurd1, and further
influenced the dendritic maturation and long-term positioning of
cerebral neurons (24). It is well
known that UFL1 is mainly involved in ufmylation of
ubiquitin fold modifier 1 targets, which was necessary for
erythroid differentiation (25,26).
Besides, ZNF322 is a crucial gene that regulates
transcriptional activation in MAPK signaling pathways (27). Consequently, TTTY15 was
important for recovery of severe burn injury by regulating
KCTD13, UFL1 and ZNF322.
PCBP1-AS1, which was closer to the gene of
PCBP1, resulted in the regulation of the expression of
various genes including ZAP70, SOCS5 and SIRT1.
Moreover, these regulated genes were involved in the T-cell
receptor and Jak-STAT signaling pathways. In 2005, Wu et al
(21) found that ZAP70 activated the
generation of T-cell receptor microclusters and sustained T-cell
activation, and participated in the immune response. Furthermore,
SOCS5 was confirmed to be a specific inhibitor of IL-4 signaling,
which was important for eosinophil infiltration (28). Of note, Schug et al (29) found that SIRT1 had a beneficial role
in the treatment of inflammation and other associated diseases.
Thus, PCBP1-AS1 may be involved in the mechanism following severe
burn injury by regulating, the immune and inflammation process.
In conclusion, by integrating the expression
profiles of lncRNA and mRNA, several hub-lncRNAs including
C14orf169, YLPM1, TTTY15, and PCBP1-AS1 were screened. In addition,
the sub-pathways regulated by these lncRNAs were identified, and
functions of these lncRNAs were predicted.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brusselaers N, Monstrey S, Vogelaers D,
Hoste E and Blot S: Severe burn injury in Europe: A systematic
review of the incidence, etiology, morbidity, and mortality. Crit
Care. 14:R1882010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wolfe RR, Herndon DN, Jahoor F, Miyoshi H
and Wolfe M: Effect of severe burn injury on substrate cycling by
glucose and fatty acids. N Engl J Med. 317:403–408. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pavoni V, Gianesello L, Paparella L,
Buoninsegni LT and Barboni E: Outcome predictors and quality of
life of severe burn patients admitted to intensive care unit. Scand
J Trauma Resusc Emerg Med. 18:242010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Noronha SA, Noronha SM, Lanziani LE,
Ferreira LM and Gragnani A: Innate and adaptive immunity gene
expression of human keratinocytes cultured of severe burn injury.
Acta Cir Bras. 29:60–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Padfield KE, Zhang Q, Gopalan S, Tzika AA,
Mindrinos MN, Tompkins RG and Rahme LG: Local and distant burn
injury alter immuno-inflammatory gene expression in skeletal
muscle. J Trauma. 61:280–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pian C, Zhang G, Chen Z, Chen Y, Zhang J,
Yang T and Zhang L: LncRNApred: Classification of long non-coding
RNAs and protein-coding transcripts by the ensemble algorithm with
a new hybrid feature. PLoS One. 11:e01545672016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vadaie N and Morris KV: Long antisense
non-coding RNAs and the epigenetic regulation of gene expression.
Biomol Concepts. 4:411–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Liu X, Liu L, Deng H, Zhang J, Xu Q,
Cen B and Ji A: Regulation of lncRNA expression. Cell Mol Biol
Lett. 19:561–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haijun Z, Yonghui Y and Jiake C:
Expression signatures of lncRNAs in skeletal muscles at the early
flow phase revealed by microarray in burned rats. Ulus Travma Acil
Cerrahi Derg. 22:224–232. 2016.PubMed/NCBI
|
|
10
|
Pearson K and Galton F: Pearson
product-moment correlation coefficient. Covariance. 2014.
|
|
11
|
Bond CF Jr and Richardson K: Seeing the
Fisher Z -transformation. Psychometrika. 69:291–303. 2004.
View Article : Google Scholar
|
|
12
|
Thissen D, Steinberg L and Kuang D: Quick
and easy implementation of the Benjamini-Hochberg procedure for
controlling the false positive rate in multiple comparisons. J Educ
Behav Stat. 27:77–83. 2002. View Article : Google Scholar
|
|
13
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luchessi AD, Cambiaghi TD, Hirabara SM,
Lambertucci RH, Silveira LR, Baptista IL, Moriscot AS, Costa-Neto
CM and Curi R: Involvement of eukaryotic translation initiation
factor 5A (eIF5A) in skeletal muscle stem cell differentiation. J
Cell Physiol. 218:480–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng H, Chen Y, Chen S, Niu Y, Yang L, Li
B, Lu Y, Geng S, Du X and Li Y: Expression and distribution of
PPP2R5C gene in leukemia Open Access. J Hematol Oncol. 4:21–26.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi J, Li J, Guan H, Cai W, Bai X, Fang X,
Hu X, Wang Y, Wang H, Zheng Z, et al: Anti-fibrotic actions of
interleukin-10 against hypertrophic scarring by activation of
PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts.
PLoS One. 9:e982282014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cisternas P, Henriquez JP, Brandan E and
Inestrosa NC: Wnt signaling in skeletal muscle dynamics:
Myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol.
49:574–589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HS, Ahn CH, Park TS, Park HD, Koh KS,
Ryoo ZY, Park SC and Lee S: Gene expression profiling of a
cold-shocked earthworm Eisenia andrei. Cryo Letters. 33:1–11.
2012.PubMed/NCBI
|
|
19
|
Welle S, Burgess K and Mehta S:
Stimulation of skeletal muscle myofibrillar protein synthesis, p70
S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation
by inhibition of myostatin in mature mice. Am J Physiol Endocrinol
Metab. 296:E567–E572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta P, Soyombo AA, Atashband A,
Wisniewski KE, Shelton JM, Richardson JA, Hammer RE and Hofmann SL:
Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in
knockout mice. Proc Natl Acad Sci USA. 98:13566–13571. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu ZB, Qiu C, Zhang AL, Cai L, Lin SJ, Yao
Y, Tang QS, Xu M, Hua W, Chu YW, et al: Glioma-associated antigen
HEATR1 induces functional cytotoxic T lymphocytes in patients with
glioma. J Immunol Res. 2014:1314942014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Fang Y, Zhang H, Deng M, Gao B, Niu
N, Yu J, Lee S, Kim J, Qin B, et al: HEATR1 negatively regulates
Akt to help sensitize pancreatic cancer cells to chemotherapy.
Cancer Res. 76:572–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Golzio C1, Willer J, Talkowski ME, Oh EC,
Taniguchi Y, Jacquemont S, Reymond A, Sun M, Sawa A, Gusella JF, et
al: KCTD13 is a major driver of mirrored neuroanatomical phenotypes
associated with the 16p11.2 CNV. Nature. 485:363–367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gladwyn-Ng I, Huang L, Ngo L, Li SS, Qu Z,
Vanyai HK, Cullen HD, Davis JM and Heng JI: Bacurd1/Kctd13 and
Bacurd2/Tnfaip1 are interacting partners to Rnd proteins which
influence the long-term positioning and dendritic maturation of
cerebral cortical neurons. Neural Dev. 11:72016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Zhang M, Wu J, Lei G and Li H:
Transcriptional regulation of the Ufm1 conjugation system in
response to disturbance of the endoplasmic reticulum homeostasis
and inhibition of vesicle trafficking. PLoS One. 7:e485872012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tatsumi K, Yamamoto-Mukai H, Shimizu R,
Waguri S, Sou YS, Sakamoto A, Taya C, Shitara H, Hara T, Chung CH,
et al: The Ufm1-activating enzyme Uba5 is indispensable for
erythroid differentiation in mice. Nat Commun. 2:1812011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Wang Y, Zhang C, Yuan W, Wang J, Zhu
C, Chen L, Huang W, Zeng W, Wu X, et al: ZNF322, a novel human C2H2
Kruppel-like zinc-finger protein, regulates transcriptional
activation in MAPK signaling pathways. Biochem Biophys Res Commun.
325:1383–1392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ozaki A, Seki Y, Fukushima A and Kubo M:
The control of allergic conjunctivitis by suppressor of cytokine
signaling (SOCS)3 and SOCS5 in a murine model. J Immunol.
175:5489–5497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schug TT, Xu Q, Gao H, Peres-da-Silva A,
Draper DW, Fessler MB, Purushotham A and Li X: Myeloid deletion of
SIRT1 induces inflammatory signaling in response to environmental
stress. Mol Cell Biol. 30:4712–4721. 2010. View Article : Google Scholar : PubMed/NCBI
|