Introduction
Intracerebral hemorrhage (ICH) accounts for 10–15%
of all strokes and is associated with high rates of mortality and
morbidity (1,2). Despite decades of research, the
mortality rates of patients that have experienced ICH have scarcely
improved (3). Primary brain injury
caused by hematoma formation following ICH occurs, which causes
mechanical damage to adjacent tissues. As hematoma is the most
important factor for ICH outcome, early removal of the hematoma and
prevention of hematoma expansion are potentially therapeutic
(4,5). However, the Surgical Trial in
Intracerebral Hemorrhage (STICH) has failed to provide convincing
evidence to support the efficacy of early surgical removal of
hematoma (6). An increasing number
of studies have focused on the mechanisms underlying ICH-induced
secondary injury in order to identify novel therapeutic targets for
ICH (4,5). Several mechanisms contributing to
secondary brain injury following ICH involve cytotoxic,
excitotoxic, oxidative and inflammatory pathways (5,7). It has
been demonstrated that the inflammatory reaction that occurs
following ICH serves a vital role in ICH-induced secondary brain
injury.
Innate immunity and inflammatory responses are
activated following ICH, potentially contributing towards the
pathogenesis of secondary injury (8). Toll-like receptors (TLRs), which
recognize distinct pathogen- and damage-associated molecular
patterns, serve an important role in innate immunity and
inflammatory responses (9,10). It has been demonstrated that TLR4 is
involved in the inflammatory response that occurs following ICH,
which subsequently activates nuclear factor (NF)-κB via the
downstream myeloid differentiation primary response 88
(MyD88)/TIR-domain-containing adapter-inducing interferon-β (TRIF)
signaling pathway (11). Some
natural compounds, including Curcumin (12) and Vitamin D (13), attenuated TLR4-induced inflammation
injury in atherosclerosis and hyperoxia-induced lung injury. These
results indicated that inhibiting the TLR4 signaling pathway may
alleviate inflammation injury.
Previous studies have demonstrated that Sparstolonin
B (SsnB), a natural compound extracted from the Chinese herb
Scirpus yagara, may block the recruitment of MyD88 by TLR4
(14,15). MyD88 is critical for the TLR
signaling pathway; inhibition of MyD88 results in the inhibition of
the TLR signaling pathway and subsequently decreases inflammation
(14). SsnB exhibits high
liposolubility and has a low molecular weight, meaning that it can
easily cross the blood-brain barrier (16). Therefore, SsnB may induce a
therapeutic effect on the ICH-induced inflammatory response.
Therefore, the aim of the current study was to
assess whether SsnB attenuates the inflammatory response following
ICH. Autologous blood-induced mice ICH models were used to explore
the efficacy of SsnB on ICH neurological outcomes in the present
study. The Morris water maze (MWM) was used to observe the efficacy
of SsnB on short memory following ICH. The neurological deficit
scores (NDS), brain water content and the expression of
inflammation-associated proteins were measured following the MWM
testing.
Materials and methods
Animals
A total of 90 male C57BL/6 mice (8–10 weeks old,
weighing 20–23 g) were obtained from the Animal Center of the Third
Military Medical University (Chongqing, China). Animals were housed
in individual cages in a specific pathogen-free facility (25°C,
50–60% humidity and a 12–12 h light-dark cycle) and had ad
libitum access to sterile acidified water and radiation-treated
food. The present study was approved by the Animal Ethics Committee
of Ba-Nan People's Hospital (Chongqing, China) and were also
conducted in accordance with the ARRIVE guidelines (17). Mice were randomly divided into the
SsnB-treated group (45 mice) and the vehicle-treated group (45
mice) in the next experiment. No mice succumbed during the
experiments.
Morris water maze (MWM)
The MWM test was performed to assess spatial memory,
as previously described (18,19). The
test requires the mice to swim to find a hidden transparent
platform (10 cm in diameter; submerged 1 cm below the surface of
the water) and to use distal spatial cues and their configuration
to remember its location. The mice could not see the submerged
platform as the water was made opaque using non-toxic paint
(Primalex; PPG Deco, Břasy, Czech Republic). The MWM pool, had a
diameter of 160 cm and was filled up with water up to 40 cm. The
pool was placed in a room with an abundance of visual landmarks and
water temperature was maintained at 20°C.
In the training phase, mice underwent 4 trials/day
over 5 consecutive days. Each mouse was given 60 sec to find the
platform. If the mice found the platform in <60 sec, the mice
were allowed to rest on the platform for 30 sec. If the mice could
not find the platform in the 60 sec time period, it was gently
guided towards it by the experimenter and subsequently allowed to
rest for 30 sec to emphasize the platform.
In the testing phase, a computerized video tracking
system (Supermaze Morris; Shanghai Xinruan Information Technology
Co., Ltd., Shanghai, China) recorded the path latency from entering
the water until the platform was found and calculated the swimming
time. The first 5 days were the training phase prior to the
establishment of ICH models on day 5. The actual trials were
conducted over 3 successive days on day 2 following the
establishment of the ICH model. Mice were released in the quadrant
diagonally opposite to the location of the previous platform and
allowed to swim for 60 sec. If mice found the platform in <60
sec, the time spent in the water and path latency were recorded as
the actual time and path latency. If the mice could not find the
platform after 60 sec time, the time spent was recorded as 60 sec,
and the actual path latency was recorded for 60 sec.
ICH model
The ICH model was established in all mice following
a previously reported protocol (20). All animals were anesthetized with 4%
chloral hydrate at a dose of 400 mg/kg by intrapenitoneal injection
and immobilized in a stereotaxic frame (Stoelting Co., Wood Dale,
IL, USA). Autologous whole blood (25 µl, without anticoagulant,
drawn from the tip of the tail) was injected into the striatum (0.2
mm anterior and 2.3 mm lateral to the bregma, 3.5 mm deep from the
skull) of each mouse using a syringe pump (Lagato100, KD
Scientific, Inc., Holliston, MA, USA). Each mouse initially
received a 5 µl injection of whole blood and after 7 min, received
a second injection of blood (20 µl) at 2 µl/min.
SsnB treatment
SsnB was extracted and prepared from the rhizome of
the Chinese herb Scirpus yagara (Kangmei Century Chinese
Medicine Co. Ltd., Bozhou, China) as described previously (14,15).
Scirpus yagara was widely used in traditional Chinese
medicine (14). A previous study
revealed that SsnB reduced lipopolysaccharide (LPS)-induced nuclear
factor (NF)-κB activity in a dose-dependent manner and indicated
that the half-maximal inhibitory concentration of SsnB is ~5
mg/kg/day (14). Therefore, 5 mg/kg
SsnB, dissolved in polyethylene glycol 400 (cat. no. 1546445; Sigma
Aldrich, Merck KGaA, Darmstadt, Germany), was injected
intraperitoneally into 45 mice 2 h after the establishment of the
ICH model and subsequently once a day at the same time everyday
over 3 consecutive days. The mice in the control group (45 mice)
were injected intraperitoneally with the same volume (0.1 ml) of
vehicle (polyethylene glycol 400) 2 h after the establishment of
the ICH model and subsequently once a day at the same time everyday
over 3 consecutive days.
A total of 40 mice (20 mice/group) were monitored by
two blinded observers during the MWM and neurological deficit score
(NDS) tests. Following these tests the 40 mice were sacrificed. A
total of 20 were sacrificed 1 day after the ICH model was
established (10 mice/group) and 20 were sacrificed 3 days after the
ICH model was established (10 mice/group).
The remaining 50 mice (25 mice/group) were
sacrificed 1 day (a total of 20 mice, 10 mice/group) and 3 days (a
total of 30 mice, 15 mice/group) after ICH was established. These
mice were sacrificed for brain water content, ELISA or western blot
analysis.
NDS
Different behavioral tests, including postural
flexing, forelimb placing, circling and foot fault tests, were used
to assess neurological deficits, as reported previously (21,22).
Each test was scored from 0–4, with a maximum possible deficit
score of 16. Two trained investigators who were blinded to animal
grouping scored the tests and the mean score of the subscales was
the final score for each mouse.
Determination of brain water
content
Brain water content was measured following a
previously described protocol (20,23).
Brains were removed and 3-mm coronal slices were obtained. Each
brain slice was divided into two hemispheres along the midline and
for each hemisphere, the cortex and basal ganglia were dissected.
Wet and dry weights were recorded for each section prior to and
following incubation of the sections at 95–100°C for 24 h,
respectively. The brain water content was calculated using the
following formula: (Wet weight-dry weight)/wet weight ×100%.
Enzyme-linked immunosorbent assay
(ELISA)
Perihematomal brain tissue samples from 10 mice
(n=5/group) were centrifuged (4°C at 12,000 × g for 15 min) and the
concentrations of tumor necrosis factor (TNF)-α (mouse TNF-α ELISA
kit; cat. no., DKW12-2720-096), interleukin (IL)-1β (mouse IL-1β
ELISA kit; cat. no., DKW12-1012-096) and IL-6 (mouse IL-6 kit; cat.
no., DKW12-1060-096) in the supernatant were measured using ELISA
kits following the manufacturer's protocol (Dakewe, Shenzhen,
China).
Western blotting
A total of 10 mice (n=5/group) were perfused with
0.01 mol/l PBS 3 days after ICH, and the cerebral tissues from the
perihematomal region were isolated (n=5). The protein was extracted
and the protein concentration was determined using the nuclear and
cytoplasmic protein extraction kit (cat. no. P0027) and enhanced
BCA protein assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology, Haimen, China), respectively, according to the
manufacturer's protocol. A total of 50 µg protein was loaded per
lane by SDS-PAGE in a 8% separation gel and 5% spacer gel. The
proteins were then transferred onto polyvinylidene fluoride
membranes (GE Healthcare, Chicago, IL, USA). Membranes were blocked
using 0.5% goat serum (cat. no. C0265; Beyotime Institute of
Biotechnology) at 25°C for 2 h and then incubated with rabbit
anti-mouse TLR4 (1:200; cat. no. ab13556), rabbit anti-mouse TRIF
(1:400; cat. no. ab13810), rabbit anti-mouse MyD88 (1:400; cat. no.
ab135693; all Abcam, Cambridge, UK) or rabbit anti-mouse NF-κB
(1:400; cat. no. AN365; Beyotime Institute of Biotechnology)
antibodies at 4°C overnight. Rabbit monoclonal anti-mouse GAPDH
antibodies (1:1,000; cat. no. AF1186; Beyotime Institute of
Biotechnology) were used as a loading control. Subsequently,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibodies (1:3,000; cat. no. sc2004,
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 25°C for 1.5 h.
Immunoreactive bands were visualized using a chemiluminescence
detection system (BeyoECL Star; Beyotime Institute of
Biotechnology) in the chemiluminescence imager
(FluorQuant™ AC600; Acuronbio Technology LLC, Houston,
TX, USA). Densitometric analysis was performed using Image-Pro Plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analyses were performed using the
statistical software SPSS version 13.0 (SPSS, Inc., Chicago, IL,
USA). Levene's homogeneity of variances test was used to test the
variance homogeneity. Student t test was used to compare
differences between two groups and P<0.05 was considered to
indicate a statistically significant difference.
Results
SsnB improves neurobehavioral
performance following ICH
The coronal level (and volume) of mice following ICH
is presented in Fig. 1. The hematoma
was located at the caudate nucleus of the mice. At the end of the
training period, there were no differences in average swimming path
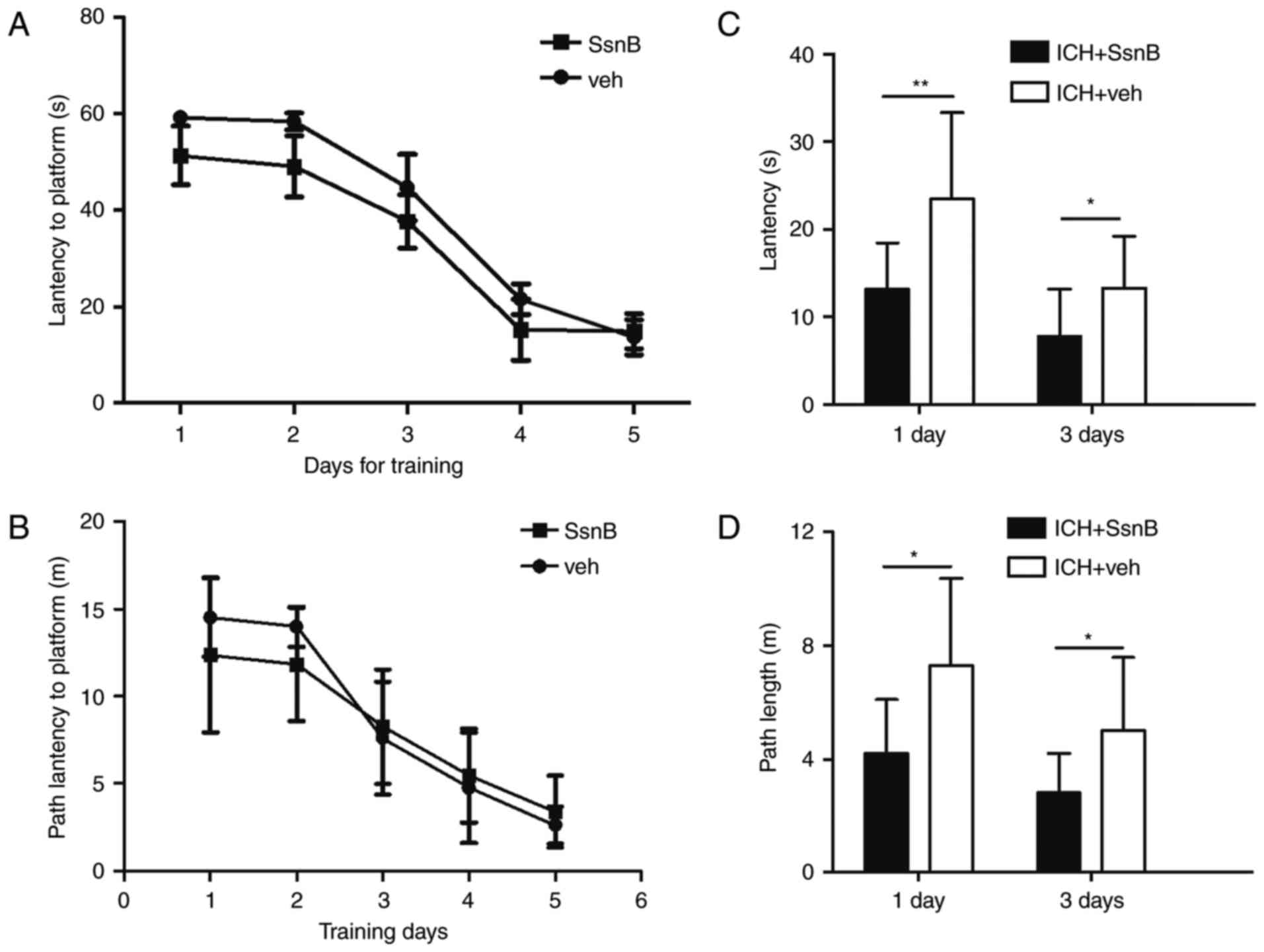
latency and time latency between the two groups (Fig. 2A and B). To determine the effect of
SsnB on spatial memory; time and path latency were measured 1 and 3
days following the establishment of ICH models. Mice in the ICH +
SsnB group exhibited significantly lower latency compared with the
ICH + vehicle group at each time point (13.1±5.3 vs. 23.4±9.9,
P=0.01; 7.8±5.4 vs. 13.3±5.9, P=0.04, respectively; Fig. 2C). Path latency in the ICH + SsnB
group was also significantly lower than that of the ICH + vehicle
group 1 and 3 days following ICH establishment (4.2±1.9 vs.
7.3±3.1, P=0.01; 2.8±1.4 vs. 5.0±2.5, P=0.03 respectively, Fig. 2D).
To evaluate neurobehavioral performance between the
two groups, NDS were measured. The NDS of the ICH + SsnB group were
significantly lower than those of the ICH + vehicle group 1 and 3
days following the establishment of ICH models (8.5±2.1 vs.
11.2±1.7, P=0.003; 6.6±1.8 vs. 9.1±1.6, P=0.004, respectively,
Fig. 3A).
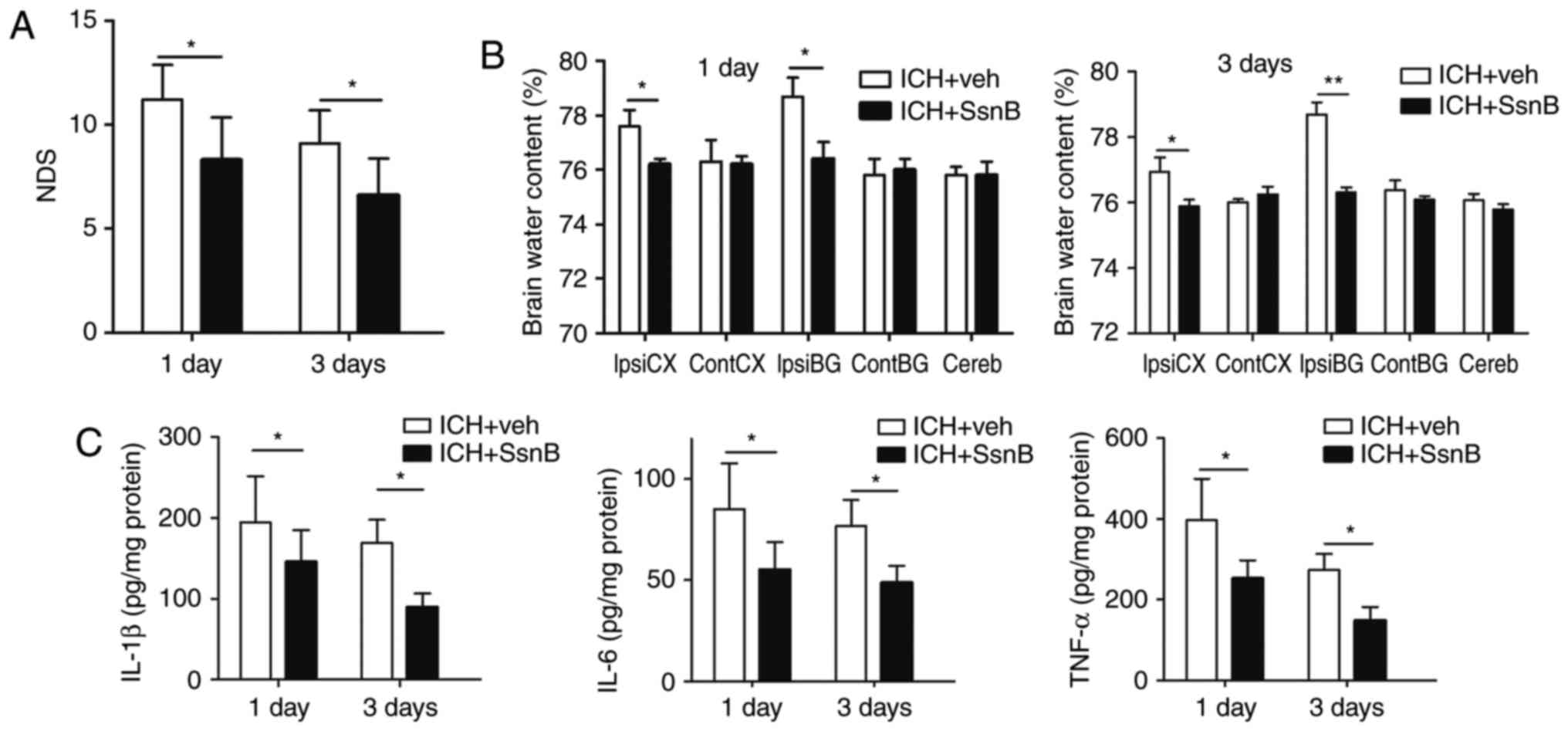 | Figure 3.SsnB reduces the inflammatory response
in autologous blood-induced ICH mouse models. (A) Intravenous
injection of SsnB reduced NDS in autologous-induced ICH mice
(n=10). (B) Intravenous injection of SsnB reduced brain water
content following ICH (n=5). (C) The concentrations of TNF-α,
IL-1β, and IL-6 in perihematomal tissues in ICH mice (n=5). All
data are presented as the mean ± standard deviation. Data were
obtained for samples pooled from 5 mice, and the experiments were
repeated 3 times. *P<0.05 and **P<0.01. ContCX, contralateral
cortex; ContBG, contralateral basal ganglia; IpsiCX, ipsilateral
cortex; IpsiBG, ipsilateral basal ganglia; Cereb, cerebellum; ICH,
intracerebral hemorrhage; SsnB, Sparstolonin B; IL, interleukin;
TNF, tumor necrosis factor; NDS, neurological deficit scores. |
SsnB reduces brain edema and the
expression of inflammatory factors in mice following ICH
Brain water content and the expression of
inflammatory factors were subsequently evaluated following
euthanasia. The dry-wet weight method was used to determine the
effect of SsnB on brain edema following ICH. The results indicated
that SsnB significantly decreased brain water content in the
ipsilateral cortex and basal ganglia of mice compared with the ICH
+ vehicle group 1 and 3 days following the induction of ICH
(P<0.05, Fig. 3B). These results
suggest that SsnB significantly reduces brain edema in mice with
ICH.
Inflammatory factors are critical indices for
inflammatory reaction following ICH. ELISA was used to determine
levels of pro-inflammatory factors, including TNF-α, IL-1β, and
IL-6, in the perihematomal tissue of mice following ICH. Levels of
TNF-α, IL-1β, and IL-6 were all significantly decreased in the ICH
+ SsnB group compared with the ICH + vehicle group at 1 and 3 days
following the establishment of ICH (P<0.05; Fig. 3C). These results indicate that SsnB
reduces the production of inflammatory factors in perihematoma
tissue in a mouse model of ICH.
SsnB affects the expression of
signaling molecules associated with TLR4
Previous studies indicated that SsnB
suppressedTLR4-mediated inflammatory reaction in an LPS-induced
sepsis mouse model (14,15). To further evaluate the underlying
neuroprotective mechanism of SsnB in ICH, western blotting was
performed to examine the expression of signaling molecules
downstream of TLR4.
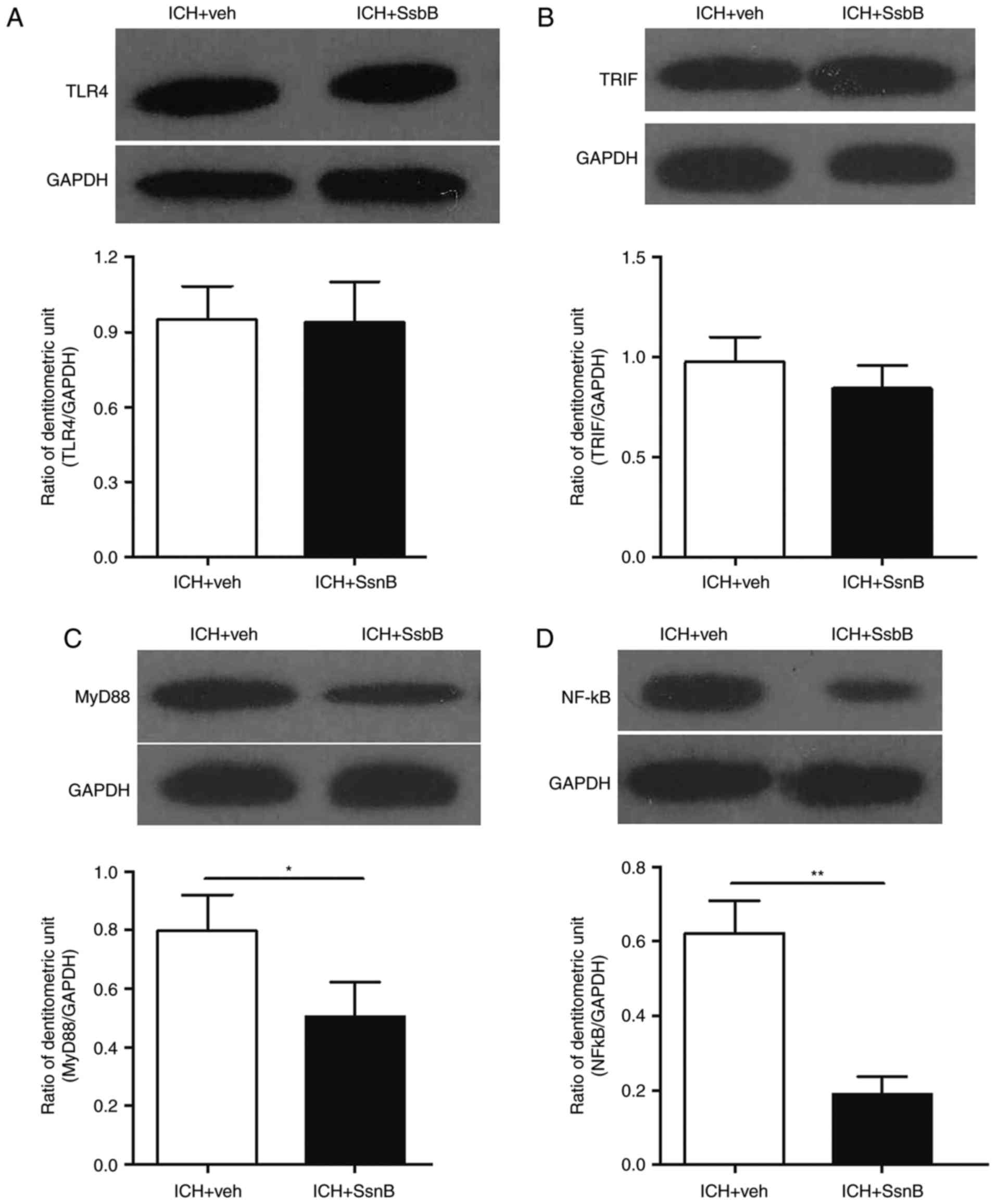
The expression of TLR4, TRIF, MyD88 and NF-κBp65 in
perihematomal tissues 3 days following ICH were measured. The
results indicated that there were no differences in the expression
of TLR4 and TRIF between mice in the ICH + vehicle group compared
with those in the ICH + SsnB group 3 days following ICH (Fig. 4A and B). However, the expression of
MyD88 and NF-κB were significantly decreased in the perihematomal
tissues of mice in the ICH + SsnB group compared with the ICH +
vehicle group 3 days following ICH (P<0.05 and P<0.01,
respectively; Fig. 4C and D).
Discussion
Inflammatory processes serve a critical role in the
secondary damage following ICH; indeed, several studies have
indicated that the inflammatory reaction is one of the main
mechanisms that induces the secondary damage associated with ICH
(24,25). Secondary brain injury following ICH
includes hematoma toxicity, high metabolic injury, excitotoxicity,
oxidative stress and inflammatory injury (1,9–11). Previous studies into injury following
ICH have focused downstream of inflammation and have not identified
reagents that induce sufficient therapeutic effects in patients
with ICH (5,8).
Toll-like receptors (TLRs) serve a key role in
innate immunity and inflammatory responses (9,10). TLR4
signaling in inflammatory responses induces secondary injury
following ICH, which subsequently activates NF-κB via the
downstream MyD88/TRIF signaling pathway (11). Furthermore, it has been demonstrated
that TLR4 contributes to poor outcomes following intracerebral
hemorrhage. Therefore, the aim of the current study was to suppress
secondary injury following the initial inflammation reaction, thus
alleviating ICH-induced injury and reducing associated neurological
deficits.
SsnB is a natural compound extracted from the
Chinese herb, Scirpus yagara, which is a perennial, aquatic
plant whose tubers have long been used in traditional Chinese
medicine to treat several inflammatory diseases (14). It has been demonstrated that high
concentrations of SsnB have no toxic effects on macrophages in rats
with sepsis (15). Therefore, it was
speculated that it may induce no side effect in the ICH model. The
current study did not measure the side effects of SsnB; therefore,
it remains unknown whether SsnB induces side effects in a mouse
model of ICH and further studies are required. SsnB has a low
molecular weight and high lipid solubility, which facilitates its
ability to cross the blood brain barrier and target an ICH-induced
inflammatory response (15,16). Therefore SsnB may be developed as a
potential method of treating patients with ICH.
It has been reported that SsnB inhibits the
production of multiple cytokines and protects mice against
endotoxin shock (26). Liu et
al (27) demonstrated that SsnB
attenuates hypoxia-reoxygenation-induced cardiomyocyte inflammation
by inhibiting the TLR2/4-mediated inflammatory response. The
results of the present study may provide valuable insights into the
mechanisms and potential treatments of other bleeding diseases,
including gastrointestinal bleeding and Crush syndrome.
The results of the current study demonstrated that
SsnB suppresses the expression of MyD88 and NF-κB p65, which
ultimately induces the downregulation of pro-inflammatory factors,
including IL-1β, IL-6 and TNF-α. SsnB did not affect the expression
of TLR4 and TRIF. This indicates that SsnB does not affect TLR4
expression or TLR4-TRIF signaling, which induces the expression of
interferon regulatory factor 3 (IRF-3). Consecutively, activation
of the IRF-3 signaling pathway induces the expression of
interferon, which is an antiviral, antitumor and immune regulatory
factor (28). The results of the
current study indicate that the underlying neuroprotective
mechanism of SsnB may be associated with MyD88 and are consistent
with the results of a study by Zhong et al (16), which indicated that SsnB may inhibit
TLR2/TLR4 heterodimer formation on cultured bone marrow-derived
dendritic cells and that this mechanism was associated with MyD88
Arg196. The current study did not examine SsnB concentrations in
perihematomal tissues following ICH. Therefore, further studies are
required to determine the plasma concentration and pharmacokinetics
of SsnB in mice following ICH.
There were no differences between the time and path
latencies in the two groups on days 4 and 5 of the MWM training.
This indicates that swimming path and latency tend to be stable.
Hence, ICH models were established on day 5 following the training
experiment.
In the mouse models of ICH, the time and path
latencies of mice in the SsnB treated group were significantly
lower compared with the vehicle group. These results indicate that
SsnB may improve short-term spatial memory following ICH; however,
the effect of SsnB on long-term spatial memory was not tested in
the current study. It remains unknown whether SsnB may improve
short-term memory in other neurological diseases.
In conclusion, SsnB significantly improved the MWM
path and time latency. The neurological deficit scores were
decreased in SsnB-treated mice. Brain water content, the levels of
inflammatory cytokines and the expression of
inflammation-associated proteins were also significantly reduced in
the SsnB-treated group. These results indicate that SsnB treatment
stimulates short-term neurobehavioral recovery and reduces
neurological deficits. SsnB may attenuate the ICH-induced
inflammatory response and may be developed as a natural therapeutic
candidate for ICH treatment.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81400974).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW conceived of and coordinated the study, and
designed, performed and analyzed the experiments. SJ and JX
performed the data analysis and collected the data. YW wrote the
paper. QL and MT designed and revised the paper. All authors
reviewed the results and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Ba-Nan People's Hospital (Chongqing, China)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
Glossary
Abbreviations
Abbreviations:
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ICH
|
intracerebral hemorrhage
|
|
MWM
|
morris water maze
|
|
NDS
|
neurological deficit scores
|
|
SsnB
|
sparstolonin B
|
|
TLRs
|
toll-like receptors
|
References
|
1
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Asch CJ, Luitse MJ, Rinkel GJ, van der
Tweel I, Algra A and Klijn CJ: Incidence, case fatality, and
functional outcome of intracerebral haemorrhage over time,
according to age, sex, and ethnic origin: A systematic review and
meta-analysis. Lancet Neurol. 9:167–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zahuranec DB, Lisabeth LD, Sánchez BN,
Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF,
Adelman EE and Morgenstern LB: Intracerebral hemorrhage mortality
is not changing despite declining incidence. Neurology.
82:2180–2186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xi G, Strahle J, Hua Y and Keep RF:
Progress in translational research on intracerebral hemorrhage: Is
there an end in sight? Prog Neurobiol. 115:45–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Y, Wang Y, Wang J, Stetler Anne R and
Yang QW: Inflammation in intracerebral hemorrhage: From mechanisms
to clinical translation. Prog Neurobiol. 115:25–44. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendelow AD, Gregson BA, Fernandes HM,
Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD and Barer DH:
STICH investigators: Early surgery versus initial conservative
treatment in patients with spontaneous supratentorial intracerebral
haematomas in the International Surgical Trial in Intracerebral
Haemorrhage (STICH): A randomised trial. Lancet. 365:387–397. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aronowski J and Zhao X: Molecular
pathophysiology of cerebral hemorrhage: Secondary brain injury.
Stroke. 42:1781–1786. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J: Preclinical and clinical research
on inflammation after intracerebral hemorrhage. Prog Neurobiol.
92:463–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong Y and Le Y: Toll-like receptors in
inflammation of the central nervous system. Int Immunopharmacol.
11:1407–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YC, Zhou Y, Fang H, Lin S, Wang PF,
Xiong RP, Chen J, Xiong XY, Lv FL, Liang QL and Yang QW: Toll-like
receptor 2/4 heterodimer mediates inflammatory injury in
intracerebral hemorrhage. Ann Neurol. 75:876–889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Zou J, Li P, Zheng X and Feng D:
Curcumin protects against atherosclerosis in apolipoprotein
E-knockout mice by inhibiting toll-like receptor 4. expression. J
Agr Food Chem. 66:449–456. 2018. View Article : Google Scholar
|
|
13
|
Yao L, Shi Y, Zhao X, Hou A, Xing Y, Fu J
and Xue X: Vitamin D attenuates hyperoxia-induced lung injury
through downregulation of Toll-like receptor 4. Int J Mol Med.
39:1403–1408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang Q, Wu Q, Jiang J, Duan J, Wang C,
Smith MD, Lu H, Wang Q, Nagarkatti P and Fan D: Characterization of
sparstolonin B, a Chinese herb-derived compound, as a selective
Toll-like receptor antagonist with potent anti-inflammatory
properties. J Biol Chem. 286:26470–26479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang QL, Lei LL, Cui X, Zou NS and Duan
JA: Bioactive cis-stilbenoids from the tubers of Scirpus yagara.
Fitoterapia. 84:170–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong Q, Zhou K, Liang QL, Lin S, Wang YC,
Xiong XY, Meng ZY, Zhao T, Zhu WY, Yang YR, et al: Interleukin-23
secreted by activated macrophages drives γδT cell production of
interleukin-17 to aggravate secondary injury after intracerebral
hemorrhage. J Am Heart Assoc. 5:e0043402016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vorhees CV and Williams MT: Value of water
mazes for assessing spatial and egocentric learning and memory in
rodent basic research and regulatory studies. Neurotoxicol Teratol.
45:75–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terry AV Jr: Spatial navigation (Water
Maze) tasksMethods of behavior analysis in neuroscience. 2nd
edition. Buccafusco JJ: Boca Raton (FL): 2009
|
|
20
|
Wang YC, Wang PF, Fang H, Chen J, Xiong XY
and Yang QW: Toll-like receptor 4 antagonist attenuates
intracerebral hemorrhage-induced brain injury. Stroke.
44:2545–2552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao X, Zhang Y, Strong R, Grotta JC and
Aronowski J: 15d-Prostaglandin J2 activates peroxisome
proliferator-activated receptor-gamma, promotes expression of
catalase, and reduces inflammation, behavioral dysfunction, and
neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood
Flow Metab. 26:811–820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su BY and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflamm. 9:462012. View Article : Google Scholar
|
|
23
|
Chang CF, Chen SF, Lee TS, Lee HF and
Shyue SK: Caveolin-1 deletion reduces early brain injury after
experimental intracerebral hemorrhage. Am J Pathol. 178:1749–1761.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belur PK, Chang JJ, He S, Emanuel BA and
Mack WJ: Emerging experimental therapies for intracerebral
hemorrhage: Targeting mechanisms of secondary brain injury.
Neurosurg Focus. 34:E92013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ziai WC: Hematology and inflammatory
signaling of intracerebral hemorrhage. Stroke. 44 6 Suppl
1:S74–S78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Q, Dong S, Lei L, Liu J, Zhang J, Li
J, Duan J and Fan D: Protective effects of Sparstolonin B, a
selective TLR2 and TLR4 antagonist, on mouse endotoxin shock.
Cytokine. 75:302–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q, Wang J, Liang Q, Wang D, Luo Y, Li
J, Janicki JS and Fan D: Sparstolonin B attenuates
hypoxia-reoxygenation-induced cardiomyocyte inflammation. Exp Biol
Med (Maywood). 239:376–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao Y, Guan K, He X, Wei CW, Zheng ZR,
Zhang YH, Ma SL, Zhong H and Shi W: Yersinia YopJ negatively
regulates IRF3-mediated antibacterial response through disruption
of STING-mediated cytosolic DNA signaling. Biochim Biophys Acta.
1863:3148–3159. 2016. View Article : Google Scholar : PubMed/NCBI
|