Introduction
Acute myeloid leukemia (AML), is the most common
type of leukemia in adults, is a disease that leads to impaired
hematopoiesis and bone marrow failure induced by clonal expansion
of undifferentiated myeloid precursors (1). Epidemiological studies demonstrate that
in 2017 there were 19,950 cases of AML in the USA, (11,130 males
and 8,820 females) and 10,430 individuals succumbed (2). In China, the incidence and mortality
rates of all types of leukemia have markedly increased between 2011
and 2015 (3). There were ~18,000 new
cases in 2011 and ~75,300 new cases in 2015, while 24,526 people
succumbed to the disease in 2011 compared with 53,400 in 2015
(3). As the population of China is
ageing rapidly, the incidence of AML is expected to increase by
~50% by 2030 (4).
The etiology of most cases of AML is unknown.
Environmental and genetic factors are considered to be the most
possible risks for AML (5,6). It is likely that many different
mutations, epigenetic aberrations and downstream abnormalities are
involved in development of AML (7).
The results of whole-genome sequencing have indicated that AML is a
complex, dynamic disease (8,9). Despite the improvement of chemotherapy
for AML, refractory disease is common and relapse is the primary
cause of treatment failure (10,11). The
exact etiology of AML remains unknown and current treatments of AML
are not as effective as they could be; therefore, it is important
to determine the molecular biological mechanisms of AML and
identify novel potential treatment targets for AML in order to
develop novel potential treatments for AML.
The natural tetrapeptide acetyl-N-Ser-Asp-Lys-Pro
(ACSDKP) is generated from the N-terminus of thymosin-β4 via
enzymatic cleavage by prolyl oligopeptidase (POP) (12). It has been demonstrated that ACSDKP
is associated with endothelial cell proliferation (13), the promotion of angiogenesis
(14) and the inhibition of
myofibroblast differentiation (15).
ACSDKP is also considered to be abnormally expressed in some human
malignant tumors, including tumors of the thyroid gland (16), breast, colon, head and neck, kidney,
lung, skin, ovary and prostate (17,18). It
has been demonstrated that ACSDKP expression varies during
chemotherapy to treat patients with AML, in certain patients the
ACSDKP level increased sharply during treatment, whereas in others
it did not change or decreased (19); however, few studies investigating
ACSDKP have been conducted since. It has been demonstrated that
ADSCKP is upregulated in human leukemia cells and may enhance the
proliferation of U87-MG glioblastoma cells via the
phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α
(PI3KCA)/protein kinase B (Akt) signaling pathway (17). Furthermore, it was indicated that
prolyl oligopeptidase inhibitor (POPi) may inhibit the stimulation
of U87-MG glioblastoma cell proliferation by ADSCKP (20). Therefore, the present study focused
on the expression of ADSCKP and its effects on AML.
In present study, the serum level of ADSCKP in
patients with AML was determined and the effect of POPi on ADSCKP
expression and the proliferation of bone marrow stromal cells in
patients with AML was assessed. The aim of the present study may
identify a novel target for the treatment of patients with AML.
Materials and methods
Patients
Serum and bone marrow stromal cell samples were
collected from 33 patients with AML admitted to the Wuxi Second
People's Hospital, Nanjing Medical University (Wuxi, China) between
September 2011 and August 2016. All patients were diagnosed with
AML according to the World Health Organization criteria and no
patients received chemotherapy prior to the study (21). The mean age of patients was
57.33±9.32 (range, 18–77) years, and patients consisted of 23 males
and 10 females. Patients were classified according to the French,
American and British (FAB) classification system for AML (22). Sera samples were obtained from 25
healthy individuals who underwent a physical examination at the
Wuxi Second People's Hospital over the same time period. The
control patients had a mean age of 53.48±12.50 (range, 18–75) years
and consisted of 17 males and 8 females. A total of 5 ml blood was
obtained from the ulnar vein of each participant. To obtain the
sera the blood samples were centrifuged for 10 min at room
temperature at 2,795 × g and stored at −20°C. Written informed
consent was obtained from all participants and the present study
was approved by the Research Committee of Wuxi Second People's
Hospital (Wuxi, China). Baseline clinical data from the patients
and healthy volunteers is presented in Table I.
 | Table I.Basic clinical data of all
participants. |
Table I.
Basic clinical data of all
participants.
| Characteristic | Patients (n=33) | Healthy controls
(n=25) | P-value |
|---|
| Mean age ± SD,
years | 57.30±15.1 | 55.36±12.57 | 0.198 |
| Age range, years | (18–77) | (18–73) |
|
| Sex
(male:female) | 23:10 | 18:7 | 0.720 |
| FAB group |
|
|
|
| 0 | 1 | N/A | N/A |
| 1 | 8 | N/A | N/A |
| 2 | 6 | N/A | N/A |
| 3 | 4 | N/A | N/A |
| 4 | 4 | N/A | N/A |
| 5 | 3 | N/A | N/A |
| 6 | 2 | N/A | N/A |
| 7 | 1 | N/A | N/A |
| Unc | 4 | N/A | N/A |
ACSDKP measurement
ACSDKP concentration was measured using a highly
specific ACSDKP ELISA kit (cat. no. 69-99161; MSKBio, Ltd.; Merck
KGaA, Darmstadt, Germany) with acetylcholinoesterase conjugate as a
tracer (Bertin Pharma, Montigney le Brettoneux, France), as
described previously (19,20). ACSDKF levels were measured using
ELISA on the serum fraction following extraction by methanol,
evaporation and reconstitution in saline buffer. The extracts were
then added to 0.5 ml buffer and used to determine ACSDKP
concentration, according to the manufacturer's protocol.
Bone marrow stromal cell culture and
treatment
Bone marrow stromal cell samples were collected from
all patients with AML. Briefly, 3–5 ml marrow fluid was extracted
using a sterile injector with heparin sodium (20 IU/M1)
anticoagulant (Sigma Aldrich; Merck KGaA). Bone marrow fluid was
diluted with RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and centrifuged for 10 min at room
temperature at 2,795 × g and the cell supernatant was extracted.
Subsequently, the cell fluid was cultured with RPMI 1620 medium in
a humidified incubator with 5% CO2 at 37°C. Following 72
h culture, all supernatant and suspension cells were discarded and
the complete culture medium of the aforementioned system was added.
When the adherent cells were spread across >90% of the bottom of
the culture flask, cells were collected and stored at −20°C prior
to further investigation.
The cells were subsequently treated with 10 µM/ml
synthetic ACSDKP (Genepep, Saint-Jean de Védas, France) or
different concentrations of POPi S17092 (25, 50 and 100 µg/ml;
Institut de Recherche Servier, Croissy, France) and cultured for 72
h. Untreated cells were used as control.
MTT assay
An MTT assay was then conducted to measure the
proliferation of bone marrow stromal cells, following a previously
described protocol in which the formazan crystals were dissolved in
0.2 ml dimethyl sulfoxide for 30 min at 37°C (23). The optical density (OD) of each well
was measured at 590 nm using a BioTek microplate reader (BioTek
Instruments, Winooski, VT, USA). Values are expressed as the
percentage of the OD of the control cells.
Statistical analysis
All data are expressed as the mean ± standard
deviation, apart from the particular ADSCKP values of each patient.
All analyses were conducted using SPSS 18.0 (SPSS, Inc., Chicago,
IL, USA). A χ2 test was performed to analyze
characteristics, such as the gender ratio. A Student's t-test was
used to compare differences between continuous variables, including
the ages of patients and controls. A Kruskal-Wallis test followed
by Turkey's post hoc test was used to compare levels of ADSCKP in
patients with different FAB stages of AML. The Mann-Whitney U test
was used in the in vitro study to compare differences
between the groups. P<0.05 was considered to indicate a
significant difference.
Results
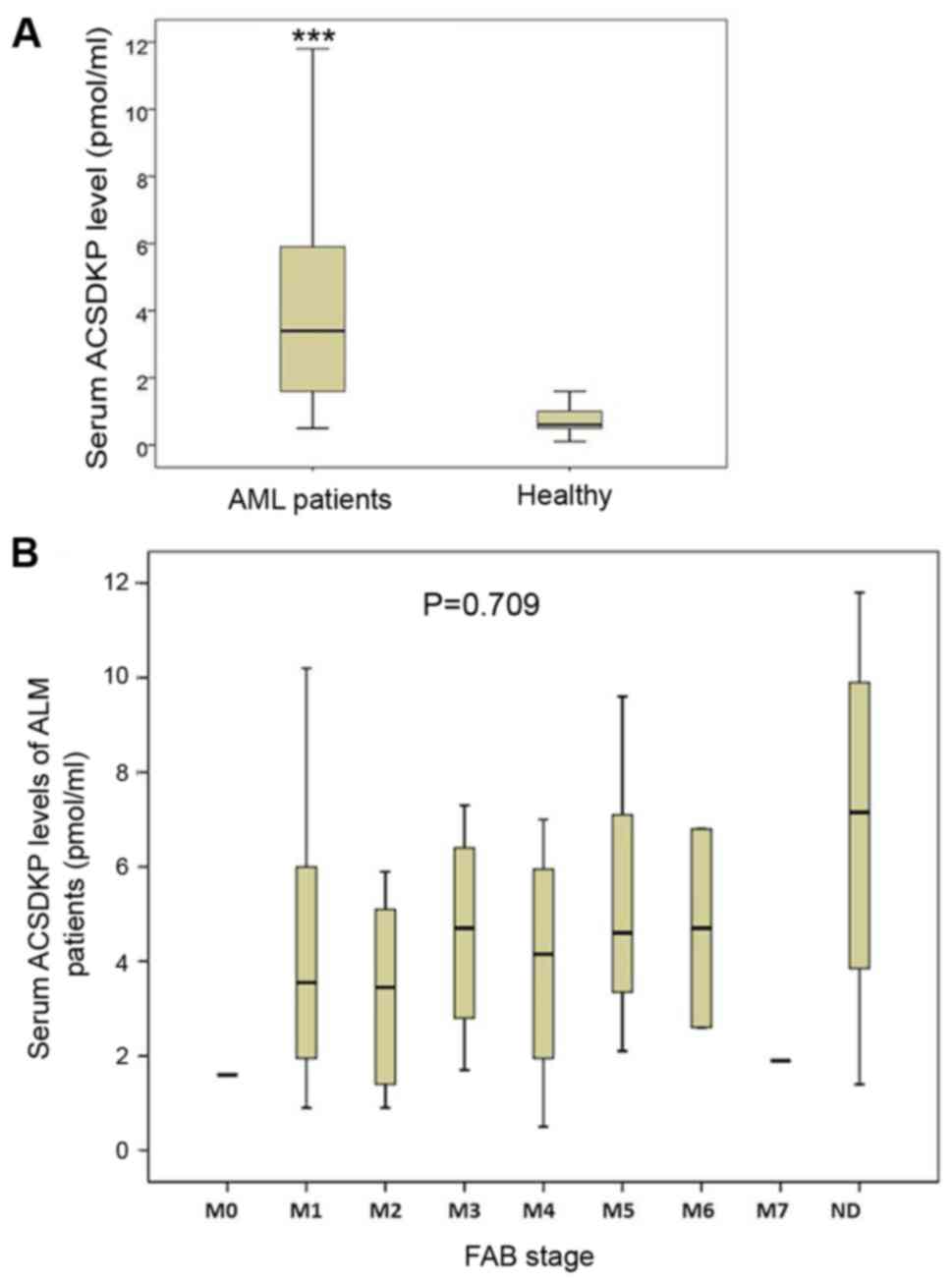
Serum levels of ACSDKP are upregulated
in patients with AML
The baseline clinical characteristics of all
participants are presented in Table
I. Serum levels of ACSDKP in patients with AML and healthy
controls were measured and compared. The results demonstrated that
serum levels of ACSDKP in patients with AML were significantly
higher than those of control group (P<0.05; Fig. 1A). This indicates that serum ACSDKP
levels are upregulated in patients with AML. Subsequently, serum
levels of ACSDKP were analyzed in patients with different FAB
stages of AML; however, no significant differences were observed
among these groups (Fig. 1B).
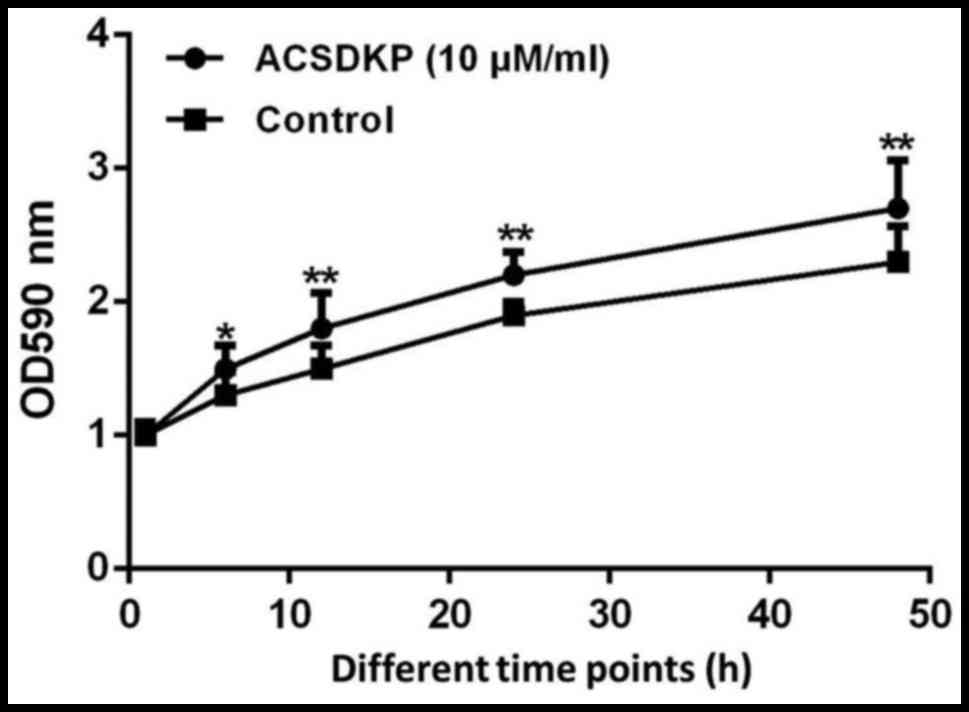
ACSDKP promotes the proliferation of
bone marrow stromal cells of patients with AML
To determine the effect of ACSDKP on bone marrow
stromal cell proliferation in patients with AML, 10 µM/ml ACSDKP
was used to treat cells and proliferation was measured using MTT.
The results demonstrated that following treatment with ACSDKP,
proliferation was significantly promoted compared with untreated
cells (P<0.05; Fig. 2),
indicating that ACSDKP stimulates the progression of AML.
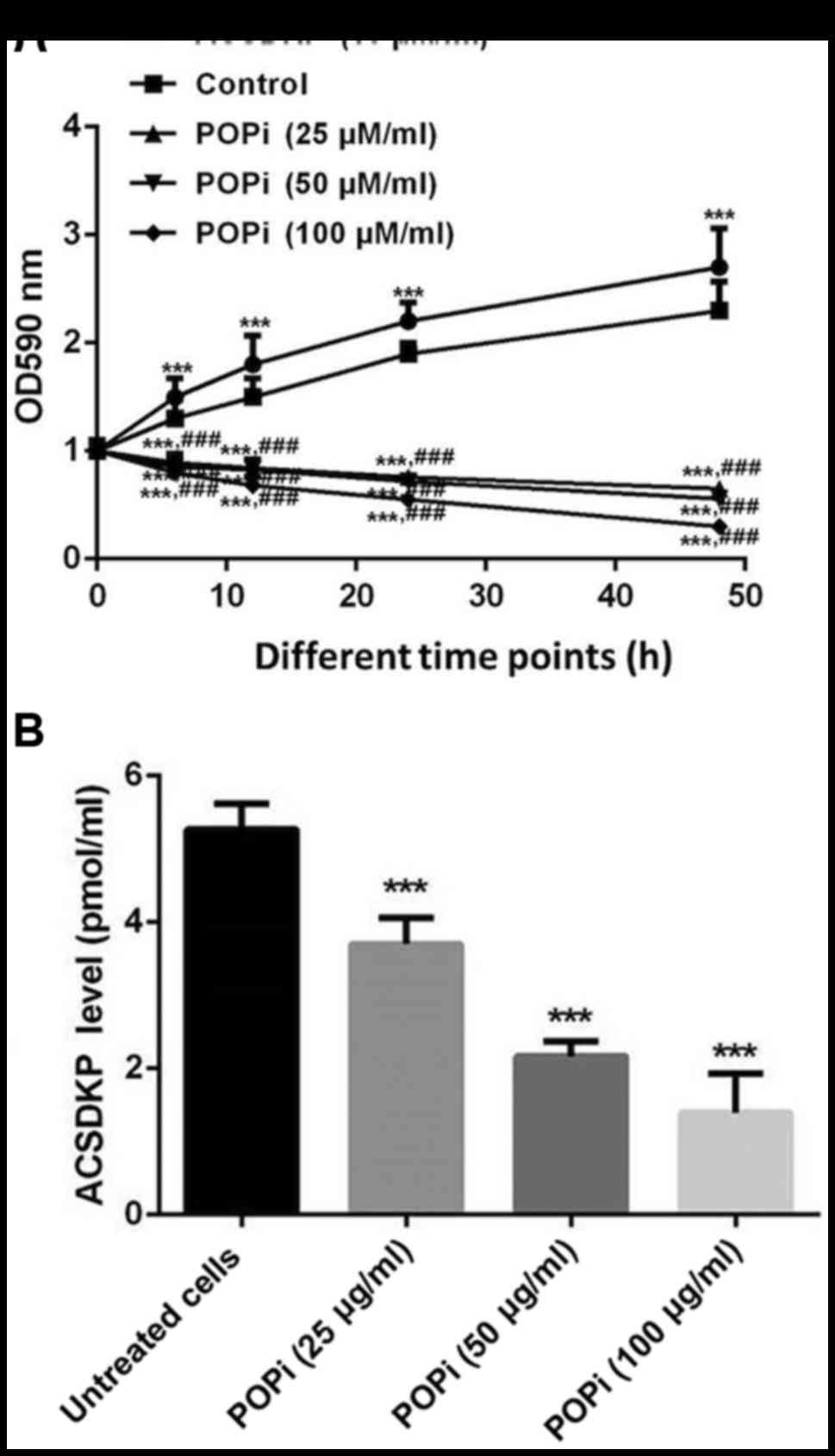
POPi reverses the effects of ACSDKP on
bone marrow stromal cells taken from patients with AML
Finally the effects of POPi on ACSDKP expression and
the proliferation of bone marrow stromal cells of patients with AML
were determined. The results indicated that cell proliferation was
significantly inhibited following POPi treatment (P<0.05;
Fig. 3A). Furthermore, treatment of
bone marrow stromal cells with different concentrations of POPi
(25, 50 and 100 µg/ml) decreased the expression of ACSDKP in a
dose-dependent manner (P<0.05; Fig.
3B).
Discussion
High-risk AML is characterized primarily by
cytogenetic features of the blast population and less often by
immunophenotypic abnormalities (24). Patients with AML usually respond to
induction therapy but may also experience secondary disease
manifestation following myelodysplastic syndrome or cytotoxic
treatment for another malignant disease (25). Numerous studies have focused on the
pathogenesis of AML; however, the mechanisms by which AML develops
remain unknown.
The function of ACSDKP in angiogenesis and its
association with leukemia has been characterized (19,26).
However the precise role that ACSDKP serves in leukemia has not yet
been elucidated. It was identified that ACSDKP expression is
upregulated in human leukemia (17)
and it was demonstrated that ACSDKP could enhance the proliferation
of U87-MG glioblastoma cells via the PI3KCA/Akt signaling pathway
(20). Therefore, the current study
aimed to determine the role of ACSDKP in AML.
In the present study, the serum level of ACSDKP in
patients with AML and healthy controls were assessed and compared
and it was demonstrated that ACSDKP expression was significantly
increased in patients with AML. Subsequently, the proliferation of
bone marrow stromal cells of patients with AML was determined and
the results indicated that ACSDKP promoted bone marrow stromal cell
proliferation. Finally it was determined that POPi inhibits ACSDKP
expression and the proliferation of bone marrow stromal cells from
patients with AML in a dose-dependent manner.
Hu et al (20)
identified that ACSDKP expression was significantly upregulated in
U87-MG glioblastoma cells; furthermore, the expression of ACSDKP in
K562 human leukemia cells was not particularly high. Other studies
obtained different results; for example, Liozon et al
(19) indicated that serum levels of
ACSDKP varied between 0.6 and 13.0 pmol/ml in patients with AML.
Liu et al (17) demonstrated
that serum levels of ACSDKP were significantly increased in a mouse
model of leukemia and in patients with leukemia. In the present
study, it was demonstrated that ACSDKP levels were enhanced in the
serum and bone marrow stromal cells of patients with AML.
There were certain limitations of the current study.
The molecular mechanisms underlying the ACSDKP-mediated increase in
bone marrow stromal cell proliferation remain unclear. Furthermore,
the mechanism by which POPi suppresses the ADSCKP-induced increase
in bone marrow stromal cell proliferation remains unknown. Further
studies are required to elucidate these mechanisms of action.
In conclusion, the current study measured the serum
levels of ADSCKP in patients with AML and determined the effect of
POPi on ADSCKP expression and the proliferation of bone marrow
stromal cells of patients with AML. The results indicated that
ACSDKP levels were enhanced in the serum and bone marrow stromal
cells of patients with AML and indicated that ACSDKP may promote
the proliferation of bone marrow stromal cells. Treatment with POPi
reversed this increase in bone marrow stromal cell proliferation.
Therefore, the current study indicated that POPi treatment may
present a novel therapeutic strategy for the treatment of AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HC wrote the manuscript and conducted the
experiments. XZ and SL collected and analyzed the data. ZW designed
the study and approved the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants and the present study was approved by the Research
Committee of Wuxi Second People's Hospital (Wuxi, China).
Consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stelljes M, Beelen DW, Braess J, Sauerland
MC, Heinecke A, Berning B, Kolb HJ, Holler E, Schwerdtfeger R,
Arnold R, et al: Allogeneic transplantation as post-remission
therapy for cytogenetically high-risk acute myeloid leukemia:
Landmark analysis from a single prospective multicenter trial.
Haematologica. 96:972–979. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medeiros BC, Othus M, Estey EH, Fang M and
Appelbaum FR: Impact of body-mass index on the outcome of adult
patients with acute myeloid leukemia. Haematologica. 97:1401–1404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai RJ, Luckhaupt SE, Schumacher P, Cress
RD, Deapen DM and Calvert GM: Acute myeloid leukemia risk by
industry and occupation. Leuk Lymphoma. 55:2584–2591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ML and Bailey NG: Acute myeloid
leukemia genetics: Risk stratification and implications. Arch
Pathol Lab Med. 139:1215–1223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papaemmanuil E, Gerstung M, Bullinger L,
Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F,
Bolli N, et al: Genomic classification and prognosis in acute
myeloid leukemia. N Engl J Med. 374:2209–2221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shlush LI, Zandi S, Mitchell A, Chen WC,
Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW,
et al: Identification of pre-leukaemic haematopoietic stem cells in
acute leukaemia. Nature. 506:328–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ritchie DS, Neeson PJ, Khot A, Peinert S,
Tai T, Tainton K, Chen K, Shin M, Wall DM, Hönemann D, et al:
Persistence and efficacy of second generation CAR T cell against
the LeY antigen in acute myeloid leukemia. Mol Ther. 21:2122–2129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding G, Zhang Z, Chopp M, Li L, Zhang L,
Li Q, Wei M and Jiang Q: MRI evaluation of BBB disruption after
adjuvant AcSDKP treatment of stroke with tPA in rat. Neuroscience.
271:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang WQ, Wang BH and Wang QR: Thymosin
beta4 and AcSDKP inhibit the proliferation of HL-60 cells and
induce their differentiation and apoptosis. Cell Biol Int.
30:514–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JM, Lawrence F, Kovacevic M, Bignon J,
Papadimitriou E, Lallemand JY, Katsoris P, Potier P, Fromes Y and
Wdzieczak-Bakala J: The tetrapeptide AcSDKP, an inhibitor of
primitive hematopoietic cell proliferation, induces angiogenesis in
vitro and in vivo. Blood. 101:3014–3020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Yang F, Sun Y, Yuan Y, Cheng H, Wei
Z, Li S, Cheng T, Brann D and Wang R: A new antifibrotic target of
Ac-SDKP: Inhibition of myofibroblast differentiation in rat lung
with silicosis. PloS One. 7:e403012012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kusinski M, Wdzieczak-Bakala J, Liu JM,
Bignon J and Kuzdak K: Acsdkp: A new potential marker of malignancy
of the thyroid gland. Langenbecks Arch Surg. 391:9–12. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu JM, Garcia-Alvarez MC, Bignon J,
Kusinski M, Kuzdak K, Riches A and Wdzieczak-Bakala J:
Overexpression of the natural tetrapeptide acetyl-N-ser-asp-lys-pro
derived from thymosin beta4 in neoplastic diseases. Ann N Y Acad
Sci. 1194:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu JM, Kusinski M, Ilic V, Bignon J,
Hajem N, Komorowski J, Kuzdak K, Stepien H and Wdzieczak-Bakala J:
Overexpression of the angiogenic tetrapeptide acsdkp in human
malignant tumors. Anticancer Res. 28:2813–2817. 2008.PubMed/NCBI
|
|
19
|
Liozon E, Volkov L, Comte L, Trimoreau F,
Pradelles P, Bordessoule D, Frindel E and Praloran V: Acsdkp serum
concentrations vary during chemotherapy in patients with acute
myeloid leukaemia. Br J Haematol. 89:917–920. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu P, Li B, Zhang W, Li Y, Li G, Jiang X,
Wdzieczak-Bakala J and Liu J: AcSDKP regulates cell proliferation
through the PI3KCA/Akt signaling pathway. PloS One. 8:e793212013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vardiman JW, Harris NL and Brunning RD:
The World Health Organization (WHO) classification of the myeloid
neoplasms. Blood. 100:2292–2302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Drexler HG: Classification of acute
myeloid leukemias-a comparison of FAB and immunophenotyping.
Leukemia. 1:697–705. 1987.PubMed/NCBI
|
|
23
|
Lv H, Che T, Tang X, Liu L and Cheng J:
Puerarin enhances proliferation and osteoblastic differentiation of
human bone marrow stromal cells via a nitric oxide/cyclic guanosine
monophosphate signaling pathway. Mol Med Rep. 12:2283–2290. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Juopperi TA, Bienzle D, Bernreuter DC,
Vernau W, Thrall MA and McManus PM: Prognostic markers for myeloid
neoplasms: A comparative review of the literature and goals for
future investigation. Vet Pathol. 48:182–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cîrstea M, Coliță A, Ionescu B, Ghiaur A,
Vasilescu D, Dobrea C, Jardan C, Dragomir M, Gheorghe A, Várady Z
and Lupu AR: Therapy-related myelodysplastic syndrome after
successful treatment of acute promyelocytic leukemia: Case report
and literature review. Revista Romana De Medicina De Laborator.
25:165–179. 2017. View Article : Google Scholar
|
|
26
|
Frindel E, Masse A, Pradelles P, Volkov L
and Rigaud M: Correlation of endogenous acetyl-ser-asp-lys-pro
plasma levels in mice and the kinetics of pluripotent hemopoietic
stem cells entry into the cycle after cytosine arabinoside
treatment: Fundamental and clinical aspects. Leukemia. 6:599–601.
1992.PubMed/NCBI
|