Introduction
Lung cancer is one of the most prevalent types of
human cancer and is the second highest cause of cancer-associated
mortality worldwide (1). Previous
studies have indicated that lung cancer is typically diagnosed when
the disease has reached an advanced stage (2,3). There
are different types of lung cancer, including large cell carcinoma,
squamous cell carcinoma and adenocarcinoma, and the incidence rates
of all types are increasing (4,5). The
ability of physicians to diagnose lung cancer using imaging
techniques has a substantial impact on the therapeutic decisions
that are made and the survival time of patients (6). A missed diagnosis of lung cancer based
on imaging tests may deny patients the opportunity to receive
effective cancer therapy and result in a diminished survival time
(7–9). Therefore, more sensitive methods of
cancer diagnosis are required to improve therapeutic options and
increase the 5-year survival rate (10).
At present, ultrasound, fludeoxyglucose-positron
emission tomography (PET), computed tomography (CT) and magnetic
resonance imaging (MRI) are widely used to diagnose and determine
the disease stage of human lung cancer (11,12). In
a clinical setting, MRI represents the most sensitive and accurate
method of diagnosis for lung cancer and metastases (13). A previous study has indicated that
the efficacy of contrast-enhanced (CE)MRI in diagnosing brain
metastasis in lung cancer is increased compared with regular MRI
scanning (14). Additionally, a
recent comparison between PET/CT and MRI for diagnosis, staging and
follow-up of patients with lung cancer revealed that MRI is useful
for distinguishing benign and malignant pulmonary nodules, has a
higher sensitivity and specificity for nodal staging and is more
beneficial in evaluating an early response to systemic chemotherapy
(9). Freedman et al (15) have previously demonstrated that
nanodelivery of MRI contrast agent (scL-gad-d nanocomplex) enhances
the sensitivity of MRI to detect lung cancer metastases.
Liposomal-iodinated contrast agent may facilitate the early
detection and diagnosis of pulmonary lesions, and have implications
on treatment response and monitoring (16). Additionally, although a number of
reports have introduced various diagnoses of early-stage lung
cancer by CEMRI using a contrast agent, nano-particle sized
contrast agents present additional benefits than other contrast
agents for the diagnosis of lung cancer, including high sensitivity
and specificity (17–19). Therefore, in the present study the
auxiliary role of
chistosan/Fe3O4-encapsulated bispecific
antibodies (BsAbCENS) in CEMRI-diagnosed lung cancer was
investigated.
At present, tumor markers carcinoembryonic antigen
(CEA) and neuron-specific enolase (NSE) are sensitive methods used
for the diagnosis and treatment of lung cancer (20). Additionally, the combined detection
of hematoporphyrin, CEA, NSE and CYFRA21-1 have been reported to
significantly improve the sensitivity and specificity of lung
cancer diagnosis, and may be useful for pathological typing
(21). In the present study, the
efficacy of CEMRI-BsAbCENS in the diagnosis of patients with lung
cancer was investigated. It was revealed that CEMRI-BsAbCENS
improves the signal intensity at the lung cancer location and also
enhances the accuracy and sensitivity of MRI in the diagnosis of
clinical patients with suspected lung cancer.
Materials and methods
Targeted contrast agent
Nano-sized
chistosan/Fe3O4-enclosed bispecific
antibodies (BsAbCENS, obtained from the department of
bio-pharmaceuticals, Shandong University, Jinan, China) were
produced using the covalent bond method as previously described
(22). The bsAbCENS was taken using
an atomizer (NE-J01; Contec Medical Systems Co., Ltd., Qinhuangdao,
China). It was possible to visualize the nano-particles contrast
agent via an MRI system. The BsAbCENS contrast agent was
administered via atomizer 30, 60 and 90 min prior to MRI.
Patients
The inclusion and exclusion criteria of the present
study were the same as previously reported (23). A total of 182 patients (104 males and
78 females; mean age, 48.4 years) with suspected early stage lung
cancer (NSE ≥15 µg/ml; CEA >10 µg/ml) were enrolled in the
present study from Shandong Medical Imaging Research Institute
(Jinan, China) between May 2011 and July 2016. Patients were
diagnosed according to the European Society for Medical Oncology
clinical practice guidelines for the diagnosis of lung cancer
(24).
A total of 182 healthy volunteers (105 males, 77
females; mean age, 48.6 years) were recruited from Shandong Medical
Imaging Research institute (Jinan, China). All patients and healthy
volunteers underwent a CEMRI scan and CEMRI-BsAbCENS for the
detection of early-stage lung cancer. The characteristics of all
participants within the present study are summarized in Table I.
 | Table I.Characteristics of study
patients. |
Table I.
Characteristics of study
patients.
| Characteristic | Patients | Healthy
volunteers |
|---|
| Male (n) | 104 | 105 |
| Female (n) | 78 | 87 |
| Age range
(years) | 28.8–64.6 | 28.6–64.8 |
| Medical history of
cancer (n) | 8 | 0 |
| Serum CEA
(µg/l) | 29.4±13.3 | 2.6±1.8 |
| Serum NSE
(µg/ml) | 27.8±15.0 | 7.8±4.1 |
The experiments in the present study were performed
in accordance with the recommendations of the Guide for the Care
and Use of Clinical Study of China (approval no. BUCMT20070612M25).
The present study was approved by ethics committee of Shandong
Medical Imaging Research Institute (Jinan, China) and all patients
provided written informed consent prior to their inclusion within
the present study.
Cells and reagents
Lung cancer cell line A549 and normal lung cell line
MRC-5 were purchased from the American Type Culture Collection
(Manassas, VA, USA). A549 cells were cultured in Dulbecco's
modified Eagle medium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.). MRC-5 cells were cultured in 1640
medium (Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS. All
cells were cultured at 37°C in a 5% CO2 humidified
atmosphere.
ELISA
The serum concentration levels of CEA (cat. no.
DY4128), fibroblast growth factor receptor (cat. no. 661FR) and NSE
(cat. no. DY5169-05; all R&D Systems, Inc., Minneapolis, MN,
USA) were analyzed by commercialized ELISA kits. The operational
procedures were performed according to the manufacturer's protocol.
The results were analyzed using an ELISA reader system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
MRI scanning
An MRI diagnosis system was used to diagnose
patients with suspected lung cancer using preprogrammed settings.
The settings were optimized to provide the optimal image formation.
The whole lungs of all patients in the present study underwent MRI
scanning according to the manufacturer's protocol (Ingenia 1.5T CX;
Philips Medical Systems, Inc., Bothell, WA, USA). The details of
the principles and settings used for MRI scanning were described in
a previous study (25).
Image analysis
The outcomes generated by MRI scanning were analyzed
using integral software in the MRI machine. Lung cancer location
was diagnosed by three physicians using an image produced by the
MRI. Patients with lung cancer were analyzed using an MRI scan and
the signal enhancement of MRI induced by BsAbCENS was also measured
via a pre-prepared program (T1-weighted, T2-weighted and
fluid-attenuated inversion recovery sequences) in the MRI
machine.
Treatments of patients with lung
cancer diagnosed by BsAbCENS-MRI
All Patients with suspected early-stage lung cancer
diagnosed via CEMRI-BsAbCENS received different treatments,
including chemoradiotherapy, Traditional Chinese Medicine,
biological therapy and comprehensive therapy after surgery. The
clinical treatment methods for patients with lung cancer are listed
in Table II. The median overall
survival rate was analyzed as previously described (26).
 | Table II.Treatment of patients with lung
cancer. |
Table II.
Treatment of patients with lung
cancer.
| Treatment | Male (n=78) | Female (n=52) |
|---|
|
Chemoradiotherapy | 40 | 27 |
| Chinese
medicine | 10 | 13 |
| Biological
therapy | 14 | 5 |
| Comprehensive
therapy | 14 | 7 |
| following
surgery |
|
|
Immunofluorescence and histological
staining
BsAbCENS (2 mg/ml) was labeled with fluorescein
isothiocyanate (FITC; 1 mg/ml in dimethylsulphoxide) as described
in a previous study (27). Prior to
immunofluorescence staining, A549 and MRC-5 cells were cultured to
85% confluence. All cells were fixed with 10% paraformaldehyde for
30 min at 37°C and subsequently incubated with rabbit anti-human
FITC-labeled antibodies targeting CEA (1:1,000; cat. no. ab133633,
Abcam, Cambridge, UK) or NSE (1:1,000; cat. no. ab53025; Abcam) for
1 h at 25°C. The cells were washed three times with PBS and
observed using a fluorescence microscope (CKX53; Olympus
Corporation, Tokyo, Japan). For histological staining 4-µm-thick
tumor sections were fixed with 10% formaldehyde for 10 min at 37°C
and then stained with hematoxylin and eosin for 1 h at 37°C as
previously described (28).
Statistical analysis
All data are presented as the mean ± standard
deviation of 3 experiments. Unpaired data was analyzed using
Student's t-test. Kaplan-Meier analysis was used to estimate the
survival rate every 5-month call visits during 60 months
observation (to observe overall survival rate and tumor
recurrence). P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of patients
A total of 182 patients with suspected lung cancer
and 182 age-matched healthy volunteers were enrolled in the present
study. The age range was 28.8–64.6 and 28.6–64.8 years in the
patients and healthy volunteers, respectively. The numbers of male
and female participants were similar in the patient and healthy
volunteer groups. The characteristics of all patients included in
the present study are summarized in Table I.
Analysis of the serum expression of
CEA and NSE in lung cancer tissues and normal cells
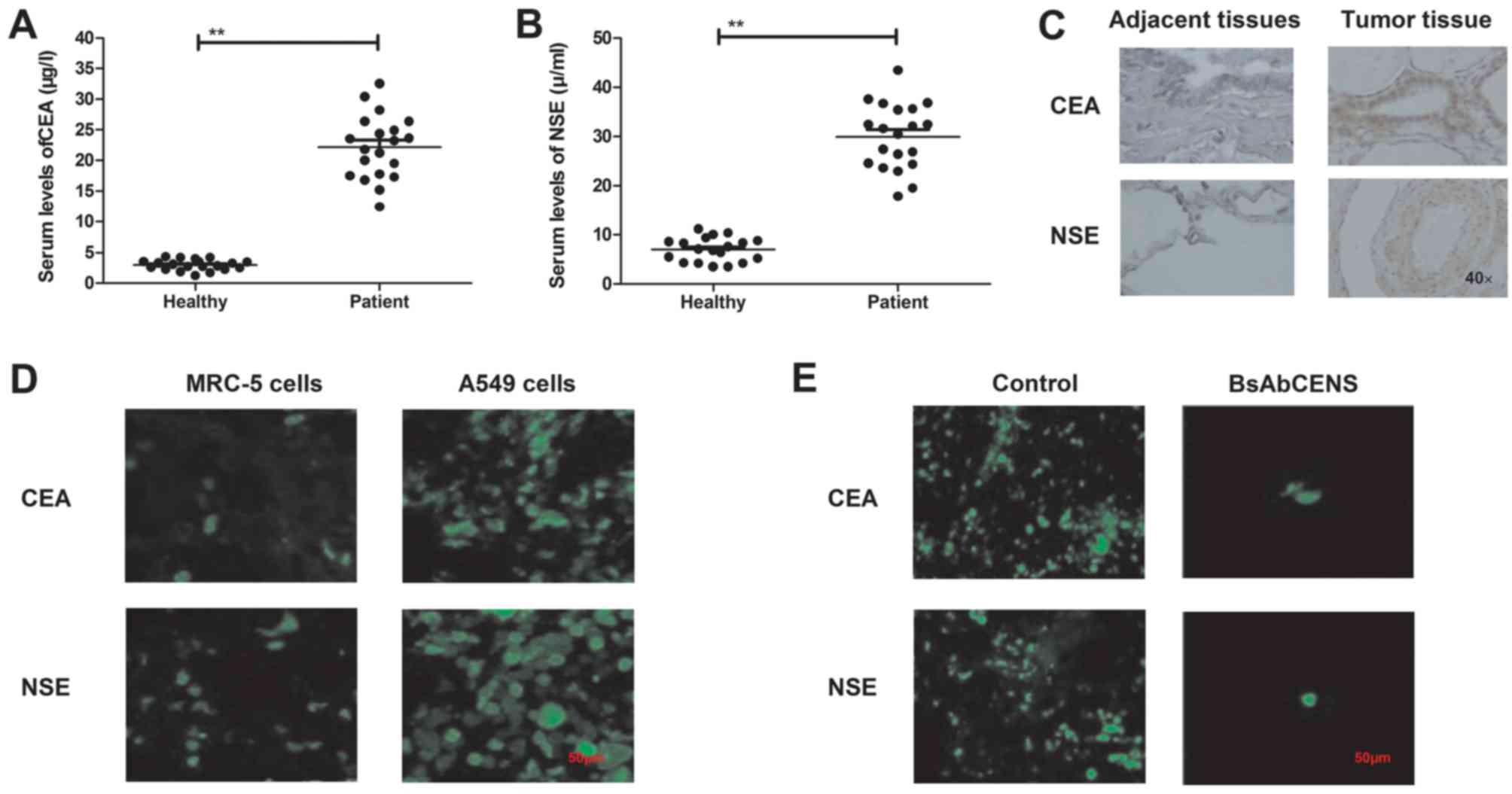
The serum levels of CEA and NSE were analyzed in
patients with lung cancer and healthy volunteers. It was observed
that the serum levels of CEA and NSE were significantly higher in
patients with lung cancer compared with healthy individuals
(Fig. 1A and B). It was demonstrated
that CEA and NSE were overexpressed in lung cancer tissues compared
with the adjacent normal tissues (Fig.
1C). Immunofluorescence analysis revealed that the expression
levels of CEA and NSE were upregulated in the A549 lung cancer cell
line compared with the MRC-5 normal lung cell line (Fig. 1D). It was also demonstrated that
BsAbCENS notably decreased the CEA and NSE expression levels in
lung cancer cells (Fig. 1E). These
results indicate that patients with lung cancer exhibited higher
plasma CEA and NSE levels than healthy volunteers.
Efficacy of CEMRI-BsAbCENS in early
clinical diagnosis for patients with suspected lung cancer
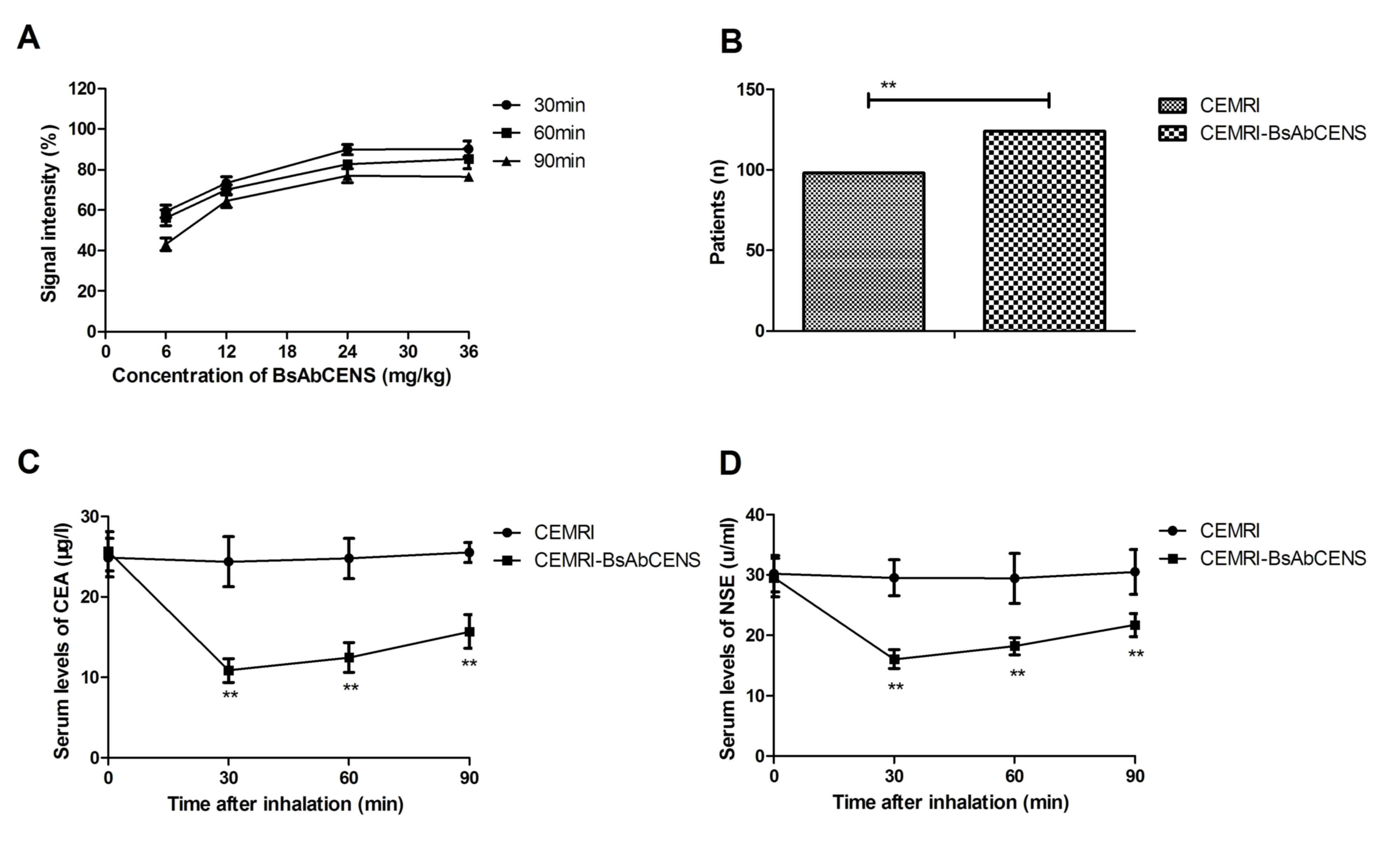
The diagnostic efficacy of CEMRI-BsAbCENS was
investigated in patients with suspected lung cancer. The dose of
CEMRI-BsAbCENS that provided the optimal signal intensity for CEMIR
detection was 24 mg/kg for 30 min (Fig.
2A). CEMRI-BsAbCENS diagnosed 122 (68.13%; 76 male and 46
female) patients with lung cancer, whereas CEMRI diagnosed 98
(53.85%; 62 male and 36 female) patients with lung cancer. This
demonstrated that CEMRI-BsAbCENS diagnosed a significantly higher
number of patients with lung cancer compared with CEMRI (Fig 2B). Following 60 min inhalation of
BsAbCENS it was also observed that there were significantly
decreased serum concentrations of CEA and NSE in patients with lung
cancer compared with those who had not inhaled BsAbCENS (Fig. 2C and D). These results suggest that
CEMRI-BsAbCENS is more effective than CEMRI due to binding with CEA
and NSE for the diagnosis of lung cancer than CEMRI.
Histopathology confirms the diagnosis
of CEMRI-BsAbCENS for lung cancer cases
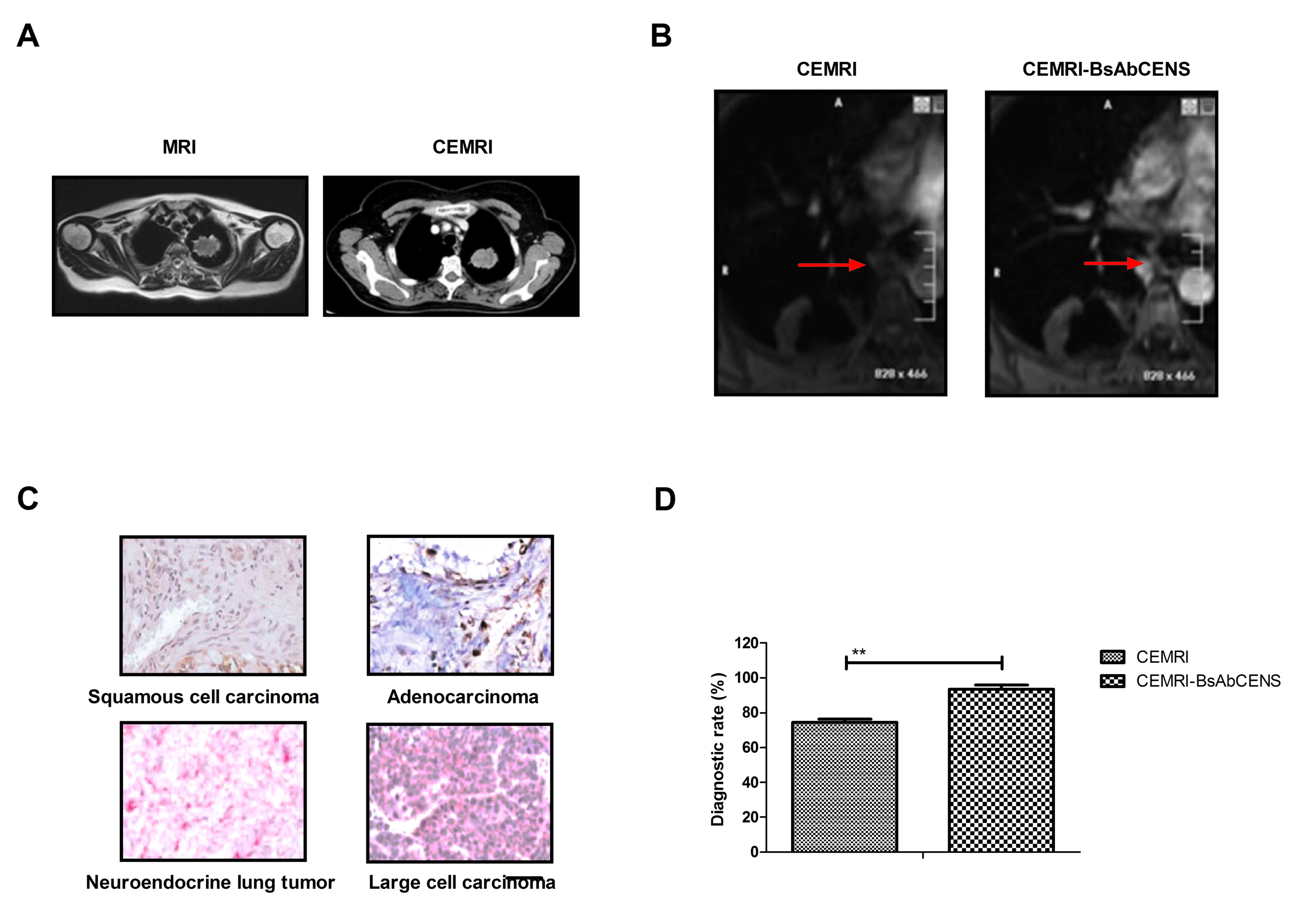
Histopathology images were used to further confirm
the results of the CEMRI-BsAbCENS and CEMRIs. It was demonstrated
that the BsAbCENS contrast agent improved image quality generated
by MRI (Fig. 3A). The representative
images demonstrate that CEMRI-BsAbCENS defined the image of the
tumor more clearly than the CEMRI (Fig.
3B). The CEMRI-BsAbCENS diagnosis of patients with lung cancer
was confirmed by histological analysis of lung tissues (Fig. 3C). Histopathology confirmed that 130
(78 male patients and 52 female patients) patients had lung cancer
and CEMRI-BsAbCENS diagnosed 124 of these patients. The diagnostic
rate was significantly higher for CEMRI-BsAbCENS compared with
CEMRI for patients with suspected lung cancer (Fig. 3D). These results suggest that
CEMRI-BsAbCENS is accurate in diagnosing patients with lung
cancer.
Pharmacodynamics of BsAbCENS in the
serum of patients with lung cancer
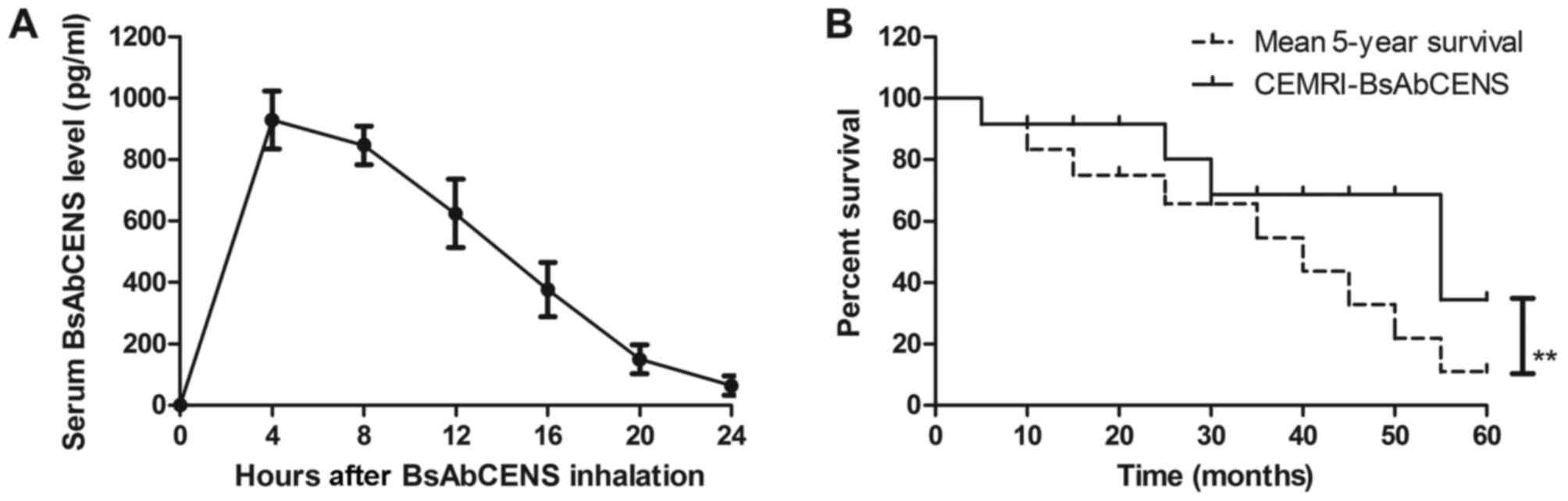
The signal intensity of CEMRI-BsAbCENS following the
administration of BsAbCENS was investigated in patients with lung
cancer. The results revealed that BsAbCENS was almost fully
metabolized from the blood at 24 h following inhalation (Fig. 4A). It was observed that patients
diagnosed with lung cancer by CEMRI-BsAbCENS had a significantly
improved survival rate compared with the mean 5-year survival rate
(Fig. 4B). These results indicate
that BsAbCENS increased the signal intensity for CEMRIs in patients
with lung cancer.
Discussion
MRI is a type of imaging technique that is currently
available for the noninvasive diagnosis of human carcinomas
(29,30). CEMRI has been demonstrated to be more
effective than MRI in differentiating between melanoma and lung
cancer brain metastases (31). In
the present study, the diagnostic efficacy of CEMRI-BsAbCENS was
further analyzed in a total of 182 patients with suspected lung
cancer who had high serum levels of CEA and NSE. The results
revealed that contrast agent BsAbCENS was able to bind with CEA and
NSE on lung tumor cells and present a higher signal intensity than
MRI in the same patients. CEMRI-BsAbCENS markedly improved
sensitivity and significantly improved the diagnostic accuracy for
patients with suspected lung cancer compared with MRI.
Early diagnosis of lung cancer allows for the
implementation of cancer treatments that may improve the overall
survival rate of patients (32–34). A
previous study has demonstrated that CEMRI was able to identify
patients who would benefit from bevacizumab and erlotinib treatment
compared with CT based on molecular imaging for an earlier
diagnosis (35). The present study
reported that CEMRI-BsAbCENS diagnosed 124/182 patients with lung
cancer and significantly improved the sensitivity and specificity
of diagnosis. Nensa et al (36) have previously suggested that dynamic
CEMRI parameters may act as biomarkers for the therapeutic effects
of vatalanib in patients with non-small-cell lung cancer. The
present study has revealed that BsAbCENS binds with the lung cancer
biomarkers CEA and NSE, which increased the signal intensity
produced by MRI. Lung cancer patients had a higher survival rate
following diagnosis by CEMRI-BsAbCENS compared with the mean 5-year
survival rate, suggesting that CEMRI-BsAbCENS has a potential
application for the early diagnosis of lung cancer. However,
patients received different anti-cancer treatments, which may have
affected survival rate, thus making it a limitation of this
study.
Serum CEA is higher in patients with small cell lung
cancer and non-small-cell lung cancer and has been used to improve
the diagnostic sensitivity for human lung cancer (20,37). The
clinical use of NSE has significantly improved the sensitivity and
specificity of lung cancer diagnosis and may be useful for
pathological typing (21). The
combination of CEA and NSE may be an effective clinical
confirmation and exclusive diagnostic indictor of meningeal
carcinomatosis in lung cancer (38).
In the present study, it was observed that BsAbCENS targeted CEA
and NSE on lung cancer cells. A previous study has indicated that
MRI contrast agent was able to detect an early stage glioma
(39). In addition,
fibronectin-targeting contrast agent in combination with MRI may
detect breast cancer micrometastases (40). The present study revealed that
BsAbCENS, when used with MRI, significantly improved the diagnostic
accuracy and sensitivity for patients with lung cancer.
Furthermore, contrast-agent MRI targeting of CA1 may produce
high-resolution MR molecular imaging of human lung adenocarcinoma
A549 cells (41). The results of the
present study revealed that CEMRI-BsAbCENS was more effective than
MRI for the diagnosis of patients with lung cancer. However,
stratification for NSE ≥15 µ/ml was not conducted for patients with
suspected lung cancer, which indicates a potential bias in the
analysis.
In conclusion, the present study has described a
novel approach for improved diagnostic accuracy of patients with
lung cancer within a clinical setting, using MRI images with a
targeting contrast agent. The effect of this approach on the
overall survival of patients with lung cancer has also been
discussed. These results suggest that CEMRI-BsAbCENS may present a
significant clinical advantage to clinicians diagnosing patients
with suspected lung cancer. However, further research should be
conducted with a larger cohort to confirm these findings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG, LL, Xl and RG performed the experiments and BZ
designed and analyzed the data of the present study.
Ethics approval and consent to
participate
The experiments in the present study were performed
in accordance with the recommendations of the Guide for the Care
and Use of Clinical Study of China (approval no. BUCMT20070612M25).
The present study was approved by ethics committee of Shandong
Medical Imaging Research Institute (Jinan, China) and all patients
provided written informed consent prior to their inclusion within
the present study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou C and Yao LD: Strategies to improve
outcomes of patients with EGRF-mutant non-small cell lung cancer:
Review of the literature. J Thorac Oncol. 11:174–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H, Gao L, Zhang B, Zhang L and Wang
C: Prognostic value of platelet to lymphocyte ratio in non-small
cell lung cancer: A systematic review and meta-analysis. Sci Rep.
6:226182016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao S, Jiang T, Zhang L, Yang H, Liu X,
Jia Y and Zhou C: Clinicopathological and prognostic significance
of regulatory T cells in patients with non-small cell lung cancer:
A systematic review with meta-analysis. Oncotarget. 7:36065–36073.
2016.PubMed/NCBI
|
|
4
|
Tomos I, Vlami A, Karakatsani A, Korbila
I, Manali ED and Papiris SA: Diffuse idiopathic skeletal
hyperostosis (DISH) and non small cell lung cancer: Case
presentation and review of the literature. Pneumonol Alergol Pol.
84:116–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Wekken AJ, Saber A, Hiltermann TJ,
Kok K, van den Berg A and Groen HJ: Resistance mechanisms after
tyrosine kinase inhibitors afatinib and crizotinib in non-small
cell lung cancer, a review of the literature. Crit Rev Oncol
Hematol. 100:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folch E, Costa DB, Wright J and VanderLaan
PA: Lung cancer diagnosis and staging in the minimally invasive age
with increasing demands for tissue analysis. Transl Lung Cancer
Res. 4:392–403. 2015.PubMed/NCBI
|
|
7
|
Ju F, Lee HK, Osarogiagbon RU, Yu X, Faris
N and Li J: Computer modeling of lung cancer diagnosis-to-treatment
process. Transl Lung Cancer Res. 4:404–414. 2015.PubMed/NCBI
|
|
8
|
Khan M, Wasim A, Mirrakhimov AE, McMahon
BA, Judge DP, Chu LC, Banavali A and Zeidan AM: Case report of a
patient with left ventricular assistance device undergoing
chemotherapy for a new diagnosis of lung cancer. Case Rep Oncol
Med. 2015:1637272015.PubMed/NCBI
|
|
9
|
Kim HS, Lee KS, Ohno Y, van Beek EJ and
Biederer J: PET/CT versus MRI for diagnosis, staging, and follow-up
of lung cancer. J Magn Reson Imaging. 42:247–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blumenthal GM, Karuri SW, Zhang H, Zhang
L, Khozin S, Kazandjian D, Tang S, Sridhara R, Keegan P and Pazdur
R: Overall response rate, progression-free survival, and overall
survival with targeted and standard therapies in advanced
non-small-cell lung cancer: US food and drug administration
trial-level and patient-level analyses. J Clin Oncol. 33:1008–1014.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jimenez-Bonilla JF, Quirce R,
Martinez-Rodriguez I, De Arcocha-Torres M, Carril JM and Banzo I:
The role of PET/CT molecular imaging in the diagnosis of recurrence
and surveillance of patients treated for non-small cell lung
cancer. Diagnostics (Basel). 6:E362016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baco E, Rud E, Ukimura O, Vlatkovic L,
Svindland A, Matsugasumi T, Bernhard JC, Rewcastle JC and Eggesbø
HB: Effect of targeted biopsy guided by elastic image fusion of MRI
with 3D-TRUS on diagnosis of anterior prostate cancer. Urol Oncol.
32:1300–1307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krüger S, Mottaghy FM, Buck AK, Maschke S,
Kley H, Frechen D, Wibmer T, Reske SN and Pauls S: Brain metastasis
in lung cancer. Comparison of cerebral MRI and 18F-FDG-PET/CT for
diagnosis in the initial staging. Nuklearmedizin. 50:101–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li ZL, Chen X, Xia CC, Sun JY, Li CX, Tang
HH and Song B: Comparison of the efficacy of gadobutrol and
multihance in contrast-enhanced MRI for diagnosis of brain
metastasis in lung cancer. Sichuan Da Xue Xue Bao Yi Xue Ban.
43:601–604. 2012.(In Chinese). PubMed/NCBI
|
|
15
|
Freedman M, Chang EH, Zhou Q and Pirollo
KF: Nanodelivery of MRI contrast agent enhances sensitivity of
detection of lung cancer metastases. Acad Radiol. 16:627–637. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Badea CT, Athreya KK, Espinosa G, Clark D,
Ghafoori AP, Li Y, Kirsch DG, Johnson GA, Annapragada A and
Ghaghada KB: Computed tomography imaging of primary lung cancer in
mice using a liposomal-iodinated contrast agent. PloS One.
7:e344962012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pauls S, Mottaghy FM, Schmidt SA, Krüger
S, Möller P, Brambs HJ and Wunderlich A: Evaluation of lung tumor
perfusion by dynamic contrast-enhanced MRI. Magn Reson Imaging.
26:1334–1341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mistry NN, Pollaro J, Song J, De Lin M and
Johnson GA: Pulmonary perfusion imaging in the rodent lung using
dynamic contrast-enhanced MRI. Magn Reson Med. 59:289–297. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eichinger M, Puderbach M, Fink C, Gahr J,
Ley S, Plathow C, Tuengerthal S, Zuna I, Müller FM and Kauczor HU:
Contrast-enhanced 3D MRI of lung perfusion in children with cystic
fibrosis-initial results. Eur Radiol. 16:2147–2152. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lazarev SM, Massard Zh, Reshetov AV,
Nikolaev GV, Volgin GN, Osipov EV, Lomteva EIu, Nokhrin AV and
Kakysheva OE: Role of biological tumor markers CEA, Cyfra-21, NSE,
TU M2-PK in diagnosis and treatment of lung cancer. Vestn Khir Im I
I Grek. 169:39–43. 2010.(In Russian). PubMed/NCBI
|
|
21
|
Wang B, He YJ, Tian YX, Yang RN, Zhu YR
and Qiu H: Clinical utility of haptoglobin in combination with CEA,
NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac J Cancer
Prev. 15:9611–9614. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
23
|
Preskorn SH, Macaluso M and Trivedi M: How
commonly used inclusion and exclusion criteria in antidepressant
registration trials affect study enrollment. J Psychiatr Pract.
21:267–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crinὸ L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group, : Early stage and locally
advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol 21 Suppl. 5:v103–v115. 2010. View Article : Google Scholar
|
|
25
|
Sahibzada I, Batura D and Hellawell G:
Validating multiparametric MRI for diagnosis and monitoring of
prostate cancer in patients for active surveillance. Int Urol
Nephrol. 48:529–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boehm K, Schiffmann J, Tian Z, Lesmana H,
Larcher A, Mandel P, Karakiewicz PI, Graefen M, Schwarz R, Krüll A
and Tilki D: Five-year biochemical recurrence-free and overall
survival following high-dose-rate brachytherapy with additional
external beam or radical prostatectomy in patients with clinically
localized prostate cancer. Urol Oncol. 34:119.e11–e18. 2016.
View Article : Google Scholar
|
|
27
|
Zhao JJ, Chen J, Wang ZP, Pan J and Huang
YH: Double labeling and comparison of fluorescence intensity and
photostability between quantum dots and FITC in oral tumors. Mol
Med Rep. 4:425–429. 2011.PubMed/NCBI
|
|
28
|
Kargahi N, Razavi SM, Deyhimi P and
Homayouni S: Comparative evaluation of eosinophils in normal
mucosa, dysplastic mucosa and oral squamous cell carcinoma with
hematoxylin-eosin, Congo red, and EMR1 immunohistochemical staining
techniques. Electron Physician. 7:1019–1026. 2015.PubMed/NCBI
|
|
29
|
Ocak I, Bernardo M, Metzger G, Barrett T,
Pinto P, Albert PS and Choyke PL: Dynamic contrast-enhanced MRI of
prostate cancer at 3 T: A study of pharmacokinetic parameters. AJR
Am J Roentgenol. 189:8492007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rappeport ED, Loft A, Berthelsen AK, von
der Recke P, Larsen PN, Mogensen AM, Wettergren A, Rasmussen A,
Hillingsoe J, Kirkegaard P and Thomsen C: Contrast-enhanced
FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients
with liver metastases from colorectal cancer: A prospective study
with intraoperative confirmation. Acta Radiol. 48:369–378. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hatzoglou V, Tisnado J, Mehta A, Peck KK,
Daras M, Omuro AM, Beal K and Holodny AI: Dynamic contrast-enhanced
MRI perfusion for differentiating between melanoma and lung cancer
brain metastases. Cancer Med. 6:761–767. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ye ZJ, Qiu HZ, Li PF, Liang MZ, Zhu YF,
Zeng Z, Hu GY, Wang SN and Quan XM: Predicting changes in quality
of life and emotional distress in chinese patients with lung,
gastric, and colon-rectal cancer diagnoses: The role of
psychological resilience. Psychooncology. 26:829–835. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaseda K, Watanabe K, Asakura K, Kazama A
and Ozawa Y: Identification of false-negative and false-positive
diagnoses of lymph node metastases in non-small cell lung cancer
patients staged by integrated (18F-)fluorodeoxyglucose-positron
emission tomography/computed tomography: A retrospective cohort
study. Thorac Cancer. 7:473–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abraha I, Giovannini G, Serraino D, Fusco
M and Montedori A: Validity of breast, lung and colorectal cancer
diagnoses in administrative databases: A systematic review
protocol. BMJ Open. 6:e0104092016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Langen AJ, van den Boogaart V,
Lubberink M, Backes WH, Marcus JT, van Tinteren H, Pruim J, Brans
B, Leffers P, Dingemans AM, et al: Monitoring response to
antiangiogenic therapy in non-small cell lung cancer using imaging
markers derived from PET and dynamic contrast-enhanced MRI. J Nucl
Med. 52:48–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nensa F, Stattaus J, Morgan B, Horsfield
MA, Soria JC, Besse B, Gounant V, Khalil A, Seng K, Fischer B, et
al: Dynamic contrast-enhanced MRI parameters as biomarkers for the
effect of vatalanib in patients with non-small-cell lung cancer.
Future Oncol. 10:823–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao W, Yu H, Han Z, Gao N, Xue J and Wang
Y: Clinical significance of joint detection of serum CEA, SCCA, and
bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol.
8:9506–9511. 2015.PubMed/NCBI
|
|
38
|
Wang P, Piao Y, Zhang X, Li W and Hao X:
The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal
fluid can be useful indicators for diagnosis of meningeal
carcinomatosis of lung cancer. Cancer Biomark. 13:123–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu X, Madhankumar AB, Miller PA, Duck KA,
Hafenstein S, Rizk E, Slagle-Webb B, Sheehan JM, Connor JR and Yang
QX: MRI contrast agent for targeting glioma: Interleukin-13 labeled
liposome encapsulating gadolinium-DTPA. Neuro Oncol. 18:691–699.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y,
Wilson DL and Lu ZR: MRI detection of breast cancer micrometastases
with a fibronectin-targeting contrast agent. Nat Commun.
6:79842015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Zhou J and Yu K: Design,
synthesis, and in vitro evaluation of a binary targeting MRI
contrast agent for imaging tumor cells. Amino Acids. 46:449–457.
2014. View Article : Google Scholar : PubMed/NCBI
|