Introduction
Acute lung injury (ALI) and its more severe form,
acute respiratory distress syndrome (ARDS), are generally diagnosed
in critically sick patients characterized by widespread
inflammation of the lung. The mortality rate for ARDS is as high as
36–44% (1). ALI and ARDS are caused
by pneumonia, sepsis, severe trauma with shock, transfusion, drug
toxicity, or aspiration of gastric contents. Lung inflammation,
impaired gas exchange, destruction of the epithelium-capillary
interface, and refractory hypoxemia are characteristic features of
ARDS. There is currently no effective pharmacotherapy to improve
survival of patients with ARDS (2,3).
Mesenchymal stem cells (MSCs) are multipotent
progenitor cells that are isolated from various mesenchymal
tissues, including umbilical cord, bone marrow (BM), placenta and
adipose tissue (4). MSCs are
attractive therapeutic candidates for the treatment of ARDS. The
paracrine effects of MSCs modulate inflammation, endothelial
injury, alveolar fluid clearance and apoptosis in ARDS. MSCs
display anti-inflammatory, anti-apoptotic, neoangiogenic and
immunomodulatory effects in various immune cells (5–9).
microRNAs (miRNAs or miRs) are short non-coding
single-stranded RNA species approximately 19–25 nucleotides long.
miRNAs modulate gene expression by translational inhibition, and
are associated with diverse biological pathways, as diagnostic
biomarkers and potential therapeutic targets (10–11).
Altered miRNA expression levels have been associated with disease
processes or therapeutic effects of different therapies (12). Previous studies have suggested that
specific miRNAs are upregulated and others are downregulated in ALI
and ARDS (13–16). Altered expression of miRNAs in the
regulation of the inflammatory pathway and tissue repair in ALI and
ARDS are correlated with inflammatory mediators and recruitment of
B cells, T cells, and other immune cells in the lung (17,18).
In the present study, miRNA expression was profiled
following treatment with BM-MSCs. Microarray analysis was used to
investigate dysregulated miRNAs associated with the effects of
BM-MSCs in a rat model of lipopolysaccharide (LPS)-induced ALI. To
the best of our knowledge, the present study is the first attempt
to estimate the miRNA expression profile in rat ALI following
BM-MSC treatment.
Materials and methods
Induction of ALI with LPS and
administration of BM-MSCs
A total of 15 male Sprague-Dawley rats (age, 8–9
weeks; weight, 200–250 g) provided by Samtako Bio Korea (Osan,
Korea) were used. All experimental procedures were approved by the
Institutional Animal Care and Use Committee in Daejeon St. Mary's
Hospital, Catholic University of Korea (Daejeon, Korea). All rats
were housed under a 12 h light/dark cycle, a 50–60% humidity, and
an ambient temperature of 22–24°C. In addition, rats received ad
libitum access to food and water. All procedures were conducted
by the same individual to minimize variation. In order to induce
ALI, LPS extracted from Escherichia coli 055:B5
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted in saline
was used (20 mg/kg). ALI rats were injected intraperitoneally with
LPS (5 mg/kg). In the control group, sham intervention was
performed using the same amount of saline. Human MSCs were provided
by The Catholic Institute of Cell Therapy (Seoul, Korea). The cells
were preserved with Dulbecco's modified Eagle's medium containing
1,000 mg/l glucose, sodium bicarbonate, and pyridoxine (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere of 5% CO2 at 37°C. The cells at
passages 3–4 were isolated for in vivo experiments. All 15
rats were assigned randomly to one out of the following three
groups (n=5/group): Saline-treated controls, LPS-induced ALI with
saline (ALI) and LPS-induced ALI with BM-MSC (LPS+BM-MSC). At 30
min following administration with LPS, BM-MSCs (2×106;
100 µl) or saline (100 µl) were injected slowly into the tail vein
over 20 min.
Laboratory tests and histopathological
examination
Rats were sacrificed at 6 h following administration
of saline or BM-MSCs. Blood was harvested via cardiac puncture and
plasma was centrifuged for 10 min at 3,000 × g at 37°C. Plasma
samples were frozen at −70°C prior to analyze alanine
aminotransferase (ALT), aspartate aminotransferase (AST), lactate,
blood urea nitrogen (BUN), and creatinine (CREA) using an IDEXX
VetTest® Chemistry Analyzer (IDEXX Laboratories, Inc.,
Westbrook, ME, USA).
The trachea was incised and bronchoalveolar lavage
(BAL) fluid was obtained from the right lung. Total cells were
counted using the LUNA automated cell counter (Logos Biosystems,
Annandale, VA, USA) following the manufacturer's instructions. An
aliquot of 200 µl of the diluted 500 µl pellet was cytospinned at a
speed of 180 × g at 4°C, transferred to a slide, and stained with
Wright-Giemsa stain at 24°C for 6 min. The 100-cell differential
count was performed for estimating the percentage of neutrophils
under a light microscope (magnification, ×400; Olympus Corporation,
Tokyo, Japan) in 4 ideal slide zones. Rat left upper lobe lung
tissue was fixed with 10% formalin overnight at 24°C, embedded in
paraffin, and stained with hematoxylin and eosin at 24°C for 1 min.
Each lung section was assessed independently by two clinical
pathologists using microscopy (magnification, ×100) to evaluate the
severity of lung injury. The lung injury score (LIS) comprises four
components (hemorrhage, alveolar capillary congestion, inflammatory
cells infiltrating the interstitium or airspace, and the alveolar
wall thickness), with each component scored on a 5 point scale (0 =
minimal damage, 1 = mild damage, 2 = moderate damage, 3 = severe
damage, 4 = maximal damage). The LSI is the sum of all four
component scores (9). The left lower
lobe was frozen at −70°C prior to analysis of miRNA expression.
Microarray analysis and functional
annotation
Total RNA was isolated from the rat lung tissue
using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH,
USA) following the manufacturer's protocols. The total RNA pellet
was dissolved in nuclease-free water and its quantity and yield was
estimated using a 2100 Bioanalyzer (Agilent Technologies, Inc.,
Santa Clara, CA, USA). Rat miRNA expression profiling was performed
using miRCURY LNA miRNA PCR Assays (Exiqon; Qiagen GmbH, Hilden,
Germany). The seventh generation array included with this assay
contains ~3,100 capture probes, covering all human, mouse and rat
miRNAs annotated in miRBase (www.mirbase.org), as well as all viral miRNAs related
to these species. Processed microarray slides were scanned using a
G2565CA microarray scanner system (Agilent Technologies, Inc.) and
imported using Feature Extraction software ver. 10.7.3.1 (Agilent
Technologies, Inc.). The fluorescence intensities of each slide
were quantified according to the Exiqon protocol. The results of
miRNA expression were calculated as the mean ± standard error of
the mean. Target prediction for functional estimation of the
differentially expressed miRNAs was conducted using miRanda
(34.236.212.39/microrna/home.do) and Targetscan ver. 7.0
(http://www.targetscan.org). The target
lists of dysregulated miRNAs were submitted separately to the
functional annotation tool provided by the Database for Annotation,
Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov), ver. 6.7. The predicted
targets were annotated according to the Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway analysis (19,20).
Visualization and analysis of
dysregulated miRNAs
To visualize the predicted target genes associated
with dysregulated miRNAs, the Network Analyzer plug-in (apps.cytoscape.org/apps/with_tag/networkanalysis) of
Cytoscape 3.6 was used (21).
Statistical analysis
The Kruskal-Wallis test and a one-way analysis of
variance for non-normally distributed data was used to test the
median difference between each variable in the three groups. A post
hoc Tamhane's T2 test was performed for pairwise comparison of
subgroups. The box-and-whisker plot was used to present the data
distribution in each figure. A line is drawn inside the box at the
median and the box portion of the plot is defined by two lines at
the 25th percentile and 75th percentile. The distance between the
lower (25th percentile) and upper (75th percentile) lines of the
box is defined as the inter-quartile range. The two whisker
boundaries indicate the 10th (lower) and 90th (upper) percentiles.
MedCalc Statistical Software Version 17.6 (MedCalc Software bvba,
Ostend, Belgium) was used for statistical investigation. P<0.05
was considered to indicate a statistically significant
difference.
Results
BM-MSCs reduce LPS-induced ALI
The presence of moderate pulmonary injuries
including hemorrhage, congestive alveolar capillaries, inflammatory
cell infiltration, and alveolar wall thickening in the LPS group
were revealed via histopathological examination, compared with the
LPS+BM-MSC group. The LIS was used to estimate the influence of
BM-MSCs on lung injury. Similar to the histopathological
examination, LIS (median, 10) in LPS rats was significantly higher
than in controls (P<0.05). LIS (6) in BM-MSC rats was significantly lower
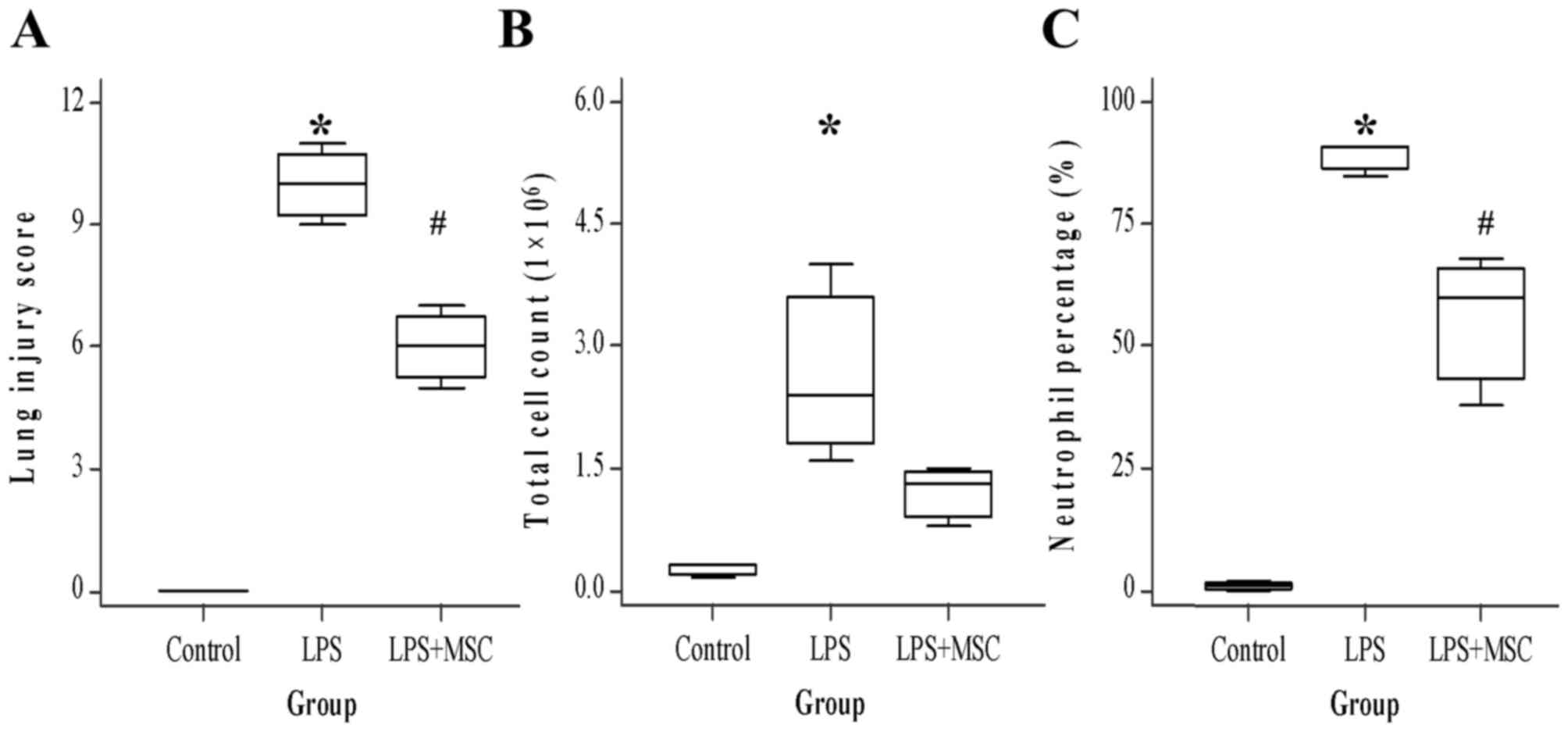
than in LPS rats (P<0.05). The total cell count and neutrophil
percentage in BAL fluid were counted to evaluate the protective
role of BM-MSCs in LPS-induced ALI. As a result, BM-MSCs markedly
reduced the number of total cell count (control, 0.3; LPS, 2.4;
LPS+BM-MSC, 1.3), and significantly reduced the neutrophil
percentage (Control, 1; LPS, 91; LPS+BM-MSC, 60; P<0.05) in the
BAL fluid compared with the LPS group (Fig. 1).
BM-MSC treatment improves multi-organ
damage induced by LPS
Organ damage was estimated by measuring serum
biochemical indicators 6 h following administration with LPS. The
levels of four analytes (excluding ALT) were significantly elevated
by LPS (Fig. 2). The levels of liver
enzymes ALT and AST released into circulation upon injury were
lower in ALI treated with BM-MSCs than in ALI only (ALT, P=0.223;
AST, P<0.05). In particular, AST was significantly decreased
(control, 32; LPS, 300; LPS+BM-MSC, 70). The concentration of
lactate, typically used as an indicator of tissue hypoperfusion,
was lower (control, 1.3; LPS, 5.2; LPS+BM-MSC, 2.7) in ALI with
BM-MSCs compared with ALI alone (P<0.05). In kidney injury,
blood urea nitrogen (BUN; control, 15; LPS, 49; LPS+BM-MSC, 18) and
creatinine (CREA; control, 0.2; LPS, 0.5; LPS+BM-MSC, 0.2) levels
were also significantly lower in ALI with BM-MSCs, compared with
ALI alone (BUN, P<0.05; CREA, P<0.05).
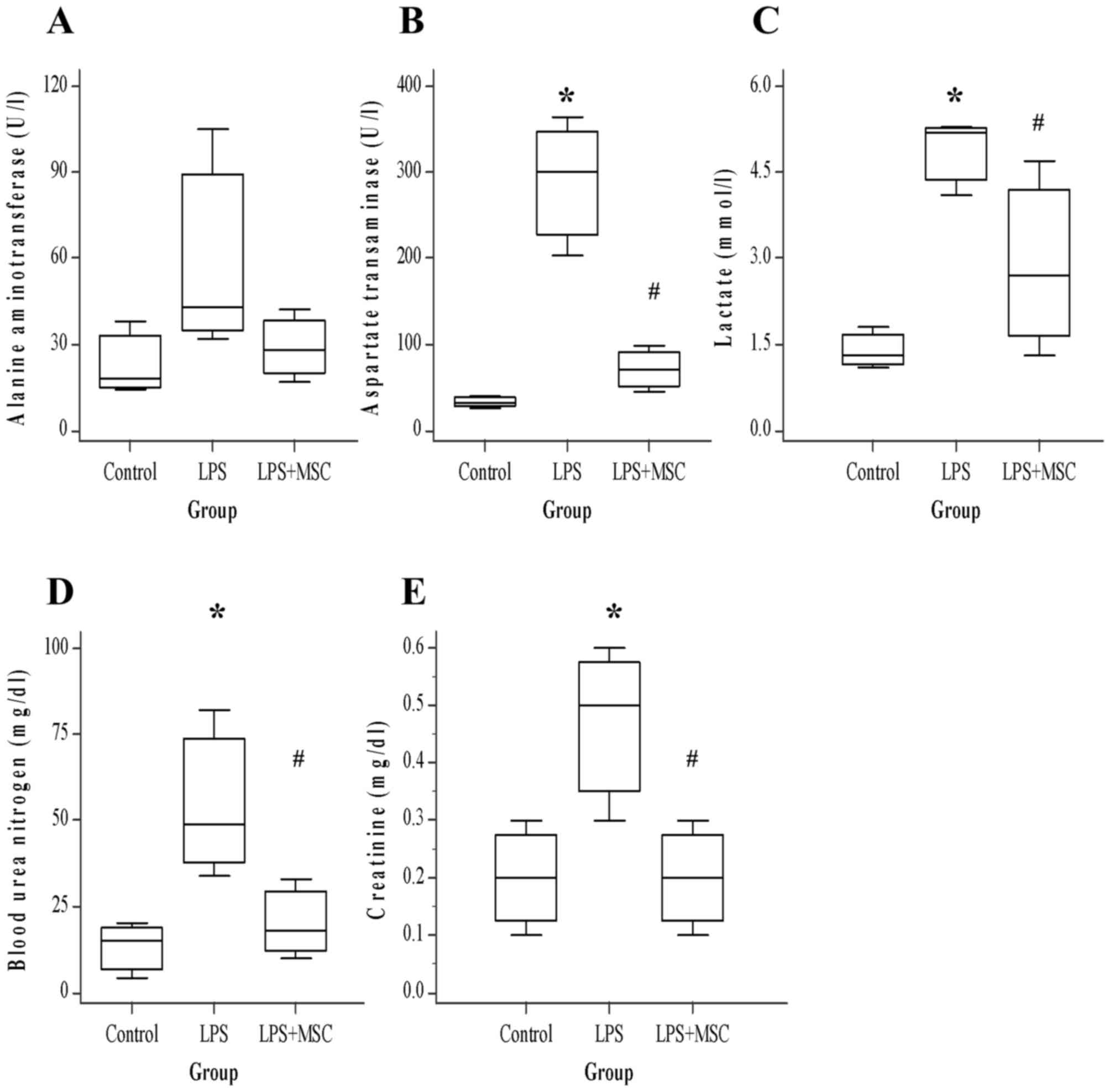 | Figure 2.Blood chemistry levels (n=5 for each
group). BM-MSC ameliorates LPS-induced aggravation in multi-organ
damage. Evaluation of (A) ALT, (B) AST, (C) lactate, (D) BUN and
(E) CREA. Compared with the control rats, the LPS group exhibited
significantly increased AST, lactate, BUN and CREA. In contrast,
the BM-MSC group exhibited significantly decreased AST, lactate,
BUN and CREA (all P<0.05) compared with the LPS group.
*P<0.05 vs. control; #P<0.05 vs. LPS. BM, bone
marrow-derived; MSC, mesenchymal stem cell; LPS,
lipopolysaccharide; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; BUN, blood urea nitrogen; CREA, creatinine. |
miRNA expression profiles in ALI
miRNA expression profiling was performed to identify
the alteration in miRNAs in the lungs of rats with LPS-induced ALI.
A total of 128/690 rat miRNAs were expressed differently between
the ALI and control groups. They included 68 upregulated and 60
downregulated miRNAs, respectively. Furthermore, 15 miRNAs were
significantly upregulated or downregulated (fold-change ≥2) in the
ALI group, compared with the control group (P<0.05). Five of
these miRNAs (miR-760-3p, miR-223-3p, miR-449c-3p, miR-503-3p and
miR-142-3p) were upregulated and 10 (miR-100-5p, miR-199a-5p,
miR-99a-5p, miR-199a-3p, miR-181a-5p, miR-497-5p, miR-191a-5p,
miR-28-5p, miR-3065-5p and miR-196b-3p) were downregulated
following LPS treatment (Table
I).
 | Table I.miRNAs implicated in rats with
ALI. |
Table I.
miRNAs implicated in rats with
ALI.
| miRNA name |
Fold-Regulation | change
direction | P-value |
|---|
| rno-miR-760-3p | 3.7 | Up | 0.030 |
| rno-miR-223-3p | 2.9 | Up | 0.048 |
|
rno-miR-449c-3p | 2.1 | Up | 0.040 |
| rno-miR-503-3p | 2.1 | Up | 0.045 |
| rno-miR-142-3p | 2.0 | Up | 0.047 |
| rno-miR-100-5p | 2.4 | Down | 0.020 |
|
rno-miR-199a-5p | 2.3 | Down | 0.030 |
| rno-miR-99a-5p | 2.3 | Down | 0.010 |
|
rno-miR-199a-3p | 2.2 | Down | 0.030 |
|
rno-miR-181a-5p | 2.1 | Down | 0.040 |
| rno-miR-497-5p | 2.1 | Down | 0.030 |
|
rno-miR-191a-5p | 2.1 | Down | 0.010 |
| rno-miR-28-5p | 2.0 | Down | 0.030 |
|
rno-miR-3065-5p | 2.0 | Down | 0.040 |
|
rno-miR-196b-3p | 2.0 | Down | 0.046 |
miRNA expression profiles in ALI
following treatment with BM-MSCs
Among 690 rat miRNAs, 95 were differentially
expressed between ALI in the BM-MSCs group and the control group.
They included 66 upregulated and 29 downregulated miRNAs.
Furthermore, nine miRNAs were significantly upregulated or
downregulated in the ALI group, compared with the control group
(fold-change ≥2; P<0.05). Among the nine miRNAs, five
(miR-1843-3p, miR-323-3p, miR-183-5p, miR-182 and miR-196b-3p) were
upregulated and four (miR-547-3p, miR-301b-5p, miR-503-3p and
miR-142-3p) were downregulated following treatment with BM-MSCs
(Table II). To investigate the
effects of BM-MSCs in ALI, the inversely expressed miRNAs in ALI
with BM-MSCs compared with ALI were selected. Three miRNAs were
inversely expressed in the two groups. The expression of two of
these miRNAs (miR-503-3p and miR-142-3p) was increased in the LPS
group, and decreased in the BM-MSC group. The miR-196b-3p was
downregulated in the LPS group, but upregulated in the BM-MSC
group.
 | Table II.Altered miRNAs in rats with ALI
following BM-MSC treatment. |
Table II.
Altered miRNAs in rats with ALI
following BM-MSC treatment.
| miRNA name |
Fold-Regulation | change
direction | P-value |
|---|
|
rno-miR-1843-3p | 4.5 | Up | 0.013 |
| rno-miR-323-3p | 3.8 | Up | 0.015 |
| rno-miR-183-5p | 3.7 | Up | 0.048 |
| rno-miR-182 | 2.9 | Up | 0.015 |
|
rno-miR-196b-3p | 2.5 | Up | 0.043 |
| rno-miR-547-3p | 2.0 | Down | 0.010 |
|
rno-miR-301b-5p | 2.1 | Down | 0.045 |
| rno-miR-503-3p | 2.0 | Down | 0.048 |
| rno-miR-142-3p | 2.0 | Down | 0.049 |
Pathway analysis of altered miRNAs in
ALI following treatment with BM-MSCs
Gene ontology and KEGG pathway annotation analyses
via DAVID ver. 6.7 revealed annotated KEGG pathways for the altered
expressed miRNAs (Tables III and
IV). The miRNA pathways were
associated with inflammation, the immune response and cellular
apoptosis.
 | Table III.Functional annotation of the altered
microRNAs in rats with acute lung injury. |
Table III.
Functional annotation of the altered
microRNAs in rats with acute lung injury.
| Term | Count | P-value |
|---|
| hsa05200:Pathways
in cancer | 35 | 0.004 |
| hsa04010:MAPK
signaling pathway | 31 | 0.002 |
| hsa04810:Regulation
of actin cytoskeleton | 24 | 0.013 |
|
hsa04722:Neurotrophin signaling
pathway | 22 | <0.001 |
| hsa04630:JAK-STAT
signaling pathway | 21 | 0.002 |
| hsa04510:Focal
adhesion | 21 | 0.040 |
| hsa04310:Wnt
signaling pathway | 20 | 0.004 |
| hsa04360:Axon
guidance | 19 | 0.001 |
| hsa04910:Insulin
signaling pathway | 18 | 0.006 |
| hsa04530:Tight
junction | 17 | 0.014 |
| hsa05211:Renal cell
carcinoma | 14 | <0.001 |
| hsa05210:Colorectal
cancer | 14 | 0.003 |
| hsa05322:Systemic
lupus erythematosus | 14 | 0.012 |
|
hsa04916:Melanogenesis | 14 | 0.012 |
| hsa04012:ErbB
signaling pathway | 13 | 0.010 |
| hsa05215:Prostate
cancer | 13 | 0.013 |
| hsa04520:Adherens
junction | 12 | 0.011 |
| hsa05217:Basal cell
carcinoma | 10 | 0.008 |
| hsa05212:Pancreatic
cancer | 10 | 0.045 |
 | Table IV.Functional annotation of the altered
microRNAs in rats with acute lung injury following bone
marrow-derived mesenchymal stem cell treatment. |
Table IV.
Functional annotation of the altered
microRNAs in rats with acute lung injury following bone
marrow-derived mesenchymal stem cell treatment.
| Term | Count | P-value |
|---|
| hsa04810:Regulation
of actin cytoskeleton | 23 | <0.001 |
| hsa04010:MAPK
signaling pathway | 22 | 0.005 |
| hsa04360:Axon
guidance | 15 | 0.001 |
| hsa04310:Wnt
signaling pathway | 14 | 0.013 |
|
hsa04722:Neurotrophin signaling
pathway | 12 | 0.018 |
| hsa04540:Gap
junction | 10 | 0.014 |
| hsa04666:Fc gamma
R-mediated phagocytosis | 10 | 0.021 |
| hsa04912:GnRH
signaling pathway | 10 | 0.025 |
Pathway analysis of altered miRNAs in
ALI following treatment with BM-MSCs
It was observed that miR-503-3p and miR-142-3p were
associated with myeloid/lymphoid or mixed-lineage translocated to,
1; cyclin T2; and granzyme B gene is a serine protease with a
notable role in the rapid induction of target cell apoptosis
(22). It was also predicted that
miR-503-3p and miR-196-3p were correlated with muscleblind-like
protein 1 and DNA damage regulated autophagy modulator 1 (DRAM1)
genes, whereas miR-142-3p and miR-196b-3p were associated with
activator of heat shock protein ATPase 2, tyrosine-protein kinase
ABL2 (ABL2), homeobox protein Nkx2-3 (Nkx2-3), Ras-responsive
element-binding protein 1 (RREB1) and Musashi RNA binding protein
2.
Discussion
In the present study, the therapeutic effects of
BM-MSCs were observed in an LPS-induced ALI rat model. The number
of total inflammatory cells and neutrophil percentage in the BAL
fluid were reduced in the ALI group treated with BM-MSCs, compared
with the ALI group. AST/ALT (hepatic damage), BUN/CREA (renal
damage) and lactate (tissue hypoperfusion) levels were measured to
determine organ damage. BM-MSCs attenuated liver and kidney injury
and improved tissue perfusion. Histological examination indicated
that lung injury in the ALI group treated with BM-MSCs was less
prominent than in the ALI group.
Immunomodulatory or immunosuppressive properties of
BM-MSCs have been studied for many years (23–27).
BM-MSCs have been considered as potential candidates for treatment
of ALI and ARDS. Gupta et al (28) previously reported that intrabronchial
infusion of BM-MSCs increased survival and reduced pulmonary edema.
Improvement in lung histopathology was associated with decreased
expression of pro-inflammatory cytokines including macrophage
inflammatory protein-2, tumor necrosis factor-α (TNF-α) and
elevation of anti-inflammatory cytokines, such as interleukin
(IL)-1ra, IL-10, and IL-13 (28).
Administration of BM-MSCs reduces not only systemic and pulmonary
inflammation, but also organ damage, in mouse sepsis models
(29). BM-MSCs mediate
anti-inflammatory effects via concurrent downregulation of
inflammation-related genes (IL-6 and IL-10) (29). Overall mortality in septic mice
receiving MSCs was significantly decreased, likely due to decreased
inflammation as evidenced by a reduction in protein and gene
expression levels of pro-inflammatory cytokines, such as IL-6
(29).
miRNAs are non-coding small (~22 nucleotides)
regulatory RNAs that affect the translation or stability of target
mRNAs. The significance of miRNAs in various biological processes
has been described previously (30).
As miRNA regulation serves a crucial role in the immunomodulatory
effects of MSCs, it may be associated with different miRNA
expression patterns. MSCs suppress T cell proliferation via
indoleamine 2,3-dioxygenase (IDO) (31) and prostaglandin E2, and act along
with T-cells in inflammation (32).
miR-181 is associated with T and B cell development (33), and enhances IL-6 and IDO expression
when its expression is increased in MSCs (34). The expression of aberrant miRNAs
associated with immune regulation was evaluated in ALI rats with or
without BM-MSC treatment. It was demonstrated that 128 of the total
of 690 miRNAs were expressed differently in ALI rats. This included
68 upregulated and 60 downregulated miRNAs. Significantly
upregulated miRNAs included miR-760-3p, miR-223-3p, miR-449c-3p,
miR-503-3p and miR-142-3p. Significantly downregulated miRNAs
included miR-100-5p, miR-199a-5p, miR-99a-5p, miR-199a-3p,
miR-181a-5p, miR-497-5p, miR-191a-5p, miR-28-5p, miR-3065-5p and
miR-196b-3p.
The anti-inflammatory effects of miR-181a may be
mediated via targeting of importin α3, and miR-181b may inhibit
nuclear factor-κ-gene binding (NF-κB)-mediated inflammatory
responses (35). miR-223 is
hematopoietic-specific miRNA and is a key modulator of
hematopoietic lineage differentiation. It is deregulated in various
inflammatory disorders (36).
miR-223 is also upregulated in autoimmune diseases such as
inflammatory bowel disease and rheumatoid arthritis (37). Serum levels of miR-146a and miR-223
are significantly decreased in sepsis, compared with systemic
inflammatory response syndrome (SIRS) and healthy populations.
However, there were no significant differences in levels of miR-223
in SIRS, compared with the controls (38). Another study profiling serum miRNAs
from 214 patients with sepsis (117 survivors and 97 non-survivors)
reported that miR-223 levels were significantly decreased in
patients with non-surviving sepsis compared with surviving sepsis
(39). Various miRNAs modulated the
expression of pro-inflammatory cytokines TNF-α and IL-6. The
expression of miR-181 and miR-191 was associated with TNF-α,
whereas miR-142, miR-223, miR-181 and miR-199 were associated with
IL-6 (40).
In the present study, 95 out of 690 miRNAs were
differentially expressed following the treatment of BM-MSCs in ALI
rats. Of these 95 miRNAs, 66 were upregulated and 29 were
downregulated. Among them, 9 miRNAs (upregulated 5 miRNAs:
miR-1843-3p, miR-323-3p, miR-183-5p, miR-182 and miR-196b-3p;
downregulated 4 miRNAs: miR-547-3p, miR-301b-5p, miR-503-3p and
miR-142-3p) were significantly upregulated or downregulated.
Differently expressed miRNAs in ALI rats were
associated with mitogen-activated protein kinase (MAPK), janus
kinase-signal transducer and activator of transcription, Wnt and
ErbB signaling pathways, which are controlled by altered levels of
miRNAs in ALI rats. Altered miRNAs in these rats following
treatment with BM-MSCs were likely associated with the MAPK and Wnt
signaling pathways. miR323-3p has been implicated in the Wnt
signaling pathway and the cadherin signaling pathway (41).
In particular, three miRNAs were significantly
inversely expressed in ALI with BM-MSCs compared with ALI: The
expression of two miRNAs (miR-503-3p and miR-142-3p) was increased
in the LPS group and decreased in the BM-MSC group. miR-196b-3p was
downregulated in the LPS group and upregulated in the BM-MSC group.
miR-196b controls granulocytic colony numbers and suppresses
granulocyte-colony stimulating factor-stimulated granulopoiesis
(42). Therefore, miR-196b is a
negative regulator of granulocytic differentiation (42). miR-196b significantly enhanced cell
proliferation and partially inhibited the differentiation of mouse
normal bone marrow precursors (43,44).
miR-142 is expressed in hematopoietic or dendritic cells, and
regulates immune response. It serves a critical role in LPS-induced
endogeneous expression of IL-6, which is a significant component of
LPS-induced endotoxemia (45). It
was predicted that miR-142 mediated the regulation of apoptosis,
which is a major metabolic process activated in the lungs of
patients with ALI/ARDS. miR-503 inhibits cell proliferation, and
induces cellular apoptosis and G0/G1 arrest
by directly targeting E2F3, as an important transcription factor in
proliferation and cell cycle distribution (46). These miRNAs are associated with cell
proliferation, immune response, inflammation and apoptosis, and
associated with the therapeutic effects of BM-MSCs in ALI.
Among the predicted target genes associated with
dysregulated miRNAs, DRAM1 mediates autophagic defense against a
broader range of intracellular pathogens, because the common
bacterial endotoxin lipopolysaccharide induces DRAM1 expression
(47). ABL2 suppresses fms-like
tyrosine kinase 3 (FLT3)-internal tandem duplication-induced cell
proliferation as negative regulator of signaling downstream of FLT3
by partially blocking FLT3-induced protein kinase B phosphorylation
(48). Increased expression of
Nkx2-3 at both RNA and protein level was demonstrated in intestinal
specimens of Crohn's disease (49).
RREB1 is activated by the MAPK pathway and negatively represses the
miR-143/145 promoter through interaction with two Ras responsive
elements and establishes complex network of regulation through
which the miR-143/145 cluster is able to modulate KRAS signaling in
colorectal cancer (50).
There are a few study limitations. First, the small
sample size may render the result less powerful. Second, the
temporal variation in the miRNA expression following LPS injection
was not analyzed, which may be associated with discrepancies in
miRNA expression levels in previous studies associated with
ALI/ARDS. Third, dysregulated miRNAs following LPS injections or
BM-MSC infusions were not quantified using reverse
transcription-quantitative polymerase chain reaction because of
small sample volumes.
In spite of these limitations, the present study
identified the miRNA expression profiles in ALI rats following
BM-MSC treatment, and revealed that BM-MSCs improved multiorgan
damage and attenuated lung injury. Furthermore, BM-MSC treatment of
ALI rats dysregulated miRNA profiles. Dysregulated miRNAs mediated
the immunomodulation of BM-MSCs in ALI. Further studies are
required to elucidate the putative targets of dysregulated
miRNAs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Catholic
Medical Center Research Foundation made in the program year of 2016
(grant no. 52015B000100173).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP was involved in revising the manuscript and was
responsible for the interpretation of general data and the
statistical analysis. SJ contributed to the conception of the
study. KP and KY made substantial contributions to the acquisition,
analysis, and interpretation of the experimental data. SS collected
the fund for this study, made substantial contributions to the
conception and design of the study, revised it critically for
important intellectual content, and gave final approval of the
version to be published. All authors approved the final manuscript
and agree to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Institutional Animal Care and Use Committee of Daejeon St. Mary's
Hospital, Catholic University of Korea (Seoul, Korea).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Phua J, Badia JR, Adhikari NK, Friedrich
JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et
al: Has mortality from acute respiratory distress syndrome
decreased over time?: A systematic review. Am J Respir Crit Care
Med. 179:220–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crisostomo PR, Markel TA, Wang Y and
Meldrum DR: Surgically relevant aspects of stem cell paracrine
effects. Surgery. 143:577–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nemeth K, Leelahavanichkul A, Yuen PS,
Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller
BH, Brown JM, et al: Bone marrow stromal cells attenuate sepsis via
prostaglandin E(2)-dependent reprogramming of host macrophages to
increase their interleukin-10 production. Nat Med. 15:42–49. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mazhari R and Hare JM: Mechanisms of
action of mesenchymal stem cells in cardiac repair: potential
influences on the cardiac stem cell niche. Nat Clin Pract
Cardiovasc Med. 4 Suppl 1:S21–S26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staszel T, Zapala B, Polus A,
Sadakierska-Chudy A, Kiec-Wilk B, Stepien E, Wybranska I, Chojnacka
M and Dembinska-Kiec A: Role of microRNAs in endothelial cell
pathophysiology. Pol Arch Med Wewn. 121:361–366. 2011.PubMed/NCBI
|
|
11
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luck ME, Muljo SA and Collins CB:
Prospects for therapeutic targeting of micrornas in human
immunological diseases. J Immunol. 194:5047–5052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie T, Liang J, Liu N, Wang Q, Li Y, Noble
PW and Jiang D: Microrna-127 inhibits lung inflammation by
targeting igg fcgamma receptor i. J Immunol. 188:2437–2444. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai ZG, Zhang SM, Zhang Y, Zhou YY, Wu HB
and Xu XP: MicroRNAs are dynamically regulated and play an
important role in LPS-induced lung injury. Can J Physiol Pharmacol.
90:37–43. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaporidi K, Vergadi E, Kaniaris E,
Hatziapostolou M, Lagoudaki E, Georgopoulos D, Zapol WM, Bloch KD
and Iliopoulos D: Pulmonary microRNA profiling in a mouse model of
ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol.
303:L199–L207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tili E, Michaille JJ, Cimino A, Costinean
S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA and
Croce CM: Modulation of miR-155 and miR-125b levels following
lipopolysaccharide/TNF-alpha stimulation and their possible roles
in regulating the response to endotoxin shock. J Immunol.
179:5082–5089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhargava M and Wendt CH: Biomarkers in
acute lung injury. Transl Res. 159:205–217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao Y, Lyu YI, Tang J and Li Y: MicroRNAs:
Novel regulatory molecules in acute lung injury/acute respiratory
distress syndrome. Biomed Rep. 4:523–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
da Huang W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su G, Morris JH, Demchak B and Bader GD:
Biological network exploration with Cytoscape 3. Curr Protoc
Bioinformatics. 47:8.13.11. –24. 2014.PubMed/NCBI
|
|
22
|
Lieberman J: Granzyme A activates another
way to die. Immunol Rev. 235:93–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horwitz EM, Gordon PL, Koo WK, Marx JC,
Neel MD, McNall RY, Muul L and Hofmann T: Isolated allogeneic bone
marrow-derived mesenchymal cells engraft and stimulate growth in
children with osteogenesis imperfecta: Implications for cell
therapy of bone. Proc Natl Acad Sci USA. 99:8932–8937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koc ON, Day J, Nieder M, Gerson SL,
Lazarus HM and Krivit W: Allogeneic mesenchymal stem cell infusion
for treatment of metachromatic leukodystrophy (MLD) and Hurler
syndrome (MPS-IH). Bone Marrow Transplant. 30:215–222. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krampera M, Cosmi L, Angeli R, Pasini A,
Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G,
Vinante F, et al: Role for interferon-gamma in the immunomodulatory
activity of human bone marrow mesenchymal stem cells. Stem Cells.
24:386–398. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Le Blanc K: Immunomodulatory effects of
fetal and adult mesenchymal stem cells. Cytotherapy. 5:485–489.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mei SH, Haitsma JJ, Dos Santos CC, Deng Y,
Lai PF, Slutsky AS, Liles WC and Stewart DJ: Mesenchymal stem cells
reduce inflammation while enhancing bacterial clearance and
improving survival in sepsis. Am J Respir Crit Care Med.
182:1047–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meisel R, Zibert A, Laryea M, Gobel U,
Daubener W and Dilloo D: Human bone marrow stromal cells inhibit
allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated
tryptophan degradation. Blood. 103:4619–4621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kroesen BJ, Teteloshvili N,
Smigielska-Czepiel K, Brouwer E, Boots AM, van den Berg A and
Kluiver J: Immuno-miRs: Critical regulators of T-cell development,
function and ageing. Immunology. 144:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ebert PJ, Jiang S, Xie J, Li QJ and Davis
MM: An endogenous positively selecting peptide enhances mature T
cell responses and becomes an autoantigen in the absence of
microRNA miR-181a. Nat Immunol. 10:1162–1169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Wang Y, Fan H, Zhao X, Liu D, Hu Y,
Kidd AR III, Bao J and Hou Y: MicroRNA-181a regulates local immune
balance by inhibiting proliferation and immunosuppressive
properties of mesenchymal stem cells. Stem Cells. 30:1756–1770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X, He S, Wara AKM, Icli B, Shvartz E,
Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, et al:
Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB
activation, vascular inflammation, and atherosclerosis in
apolipoprotein E-deficient mice. Circ Res. 114:32–40. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haneklaus M, Gerlic M, O'Neill LA and
Masters SL: miR-223: infection, inflammation and cancer. J Intern
Med. 274:215–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li YT, Chen SY, Wang CR, Liu MF, Lin CC,
Jou IM, Shiau AL and Wu CL: Brief report: amelioration of
collagen-induced arthritis in mice by lentivirus-mediated silencing
of microRNA-223. Arthritis Rheum. 64:3240–3245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang JF, Yu ML, Yu G, Bian JJ, Deng XM,
Wan XJ and Zhu KM: Serum miR-146a and miR-223 as potential new
biomarkers for sepsis. Biochem Biophys Res Commun. 394:184–188.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Zhang P, Chen W, Feng D, Jia Y and
Xie L: Serum microRNA signatures identified by Solexa sequencing
predict sepsis patients' mortality: A prospective observational
study. PLoS One. 7:e388852012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Benz F, Roy S, Trautwein C, Roderburg C
and Luedde T: Circulating micrornas as biomarkers for sepsis. Int J
Mol Sci. 17:pii: E78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pandis I, Ospelt C, Karagianni N, Denis
MC, Reczko M, Camps C, Hatzigeorgiou AG, Ragoussis J, Gay S and
Kollias G: Identification of microRNA-221/222 and microRNA-323-3p
association with rheumatoid arthritis via predictions using the
human tumour necrosis factor transgenic mouse model. Ann Rheum Dis.
71:1716–1723. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Velu CS, Baktula AM and Grimes HL: Gfi1
regulates miR-21 and miR-196b to control myelopoiesis. Blood.
113:4720–4728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Georgantas RW III, Hildreth R, Morisot S,
Alder J, Liu CG, Heimfeld S, Calin GA, Croce CM and Civin CI: CD34+
hematopoietic stem-progenitor cell microRNA expression and
function: A circuit diagram of differentiation control. Proc Natl
Acad Sci USA. 104:2750–2755. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Y, Varambally S, Maher CA, Cao Q,
Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, et al:
Targeting of microRNA-142-3p in dendritic cells regulates
endotoxin-induced mortality. Blood. 117:6172–6183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang SW, Yue J, Wang BC and Zhang XL:
miR-503 inhibits cell proliferation and induces apoptosis in
colorectal cancer cells by targeting E2F3. Int J Clin Exp Pathol.
8:12853–12860. 2015.PubMed/NCBI
|
|
47
|
van der Vaart M, Korbee CJ, Lamers GE,
Tengeler AC, Hosseini R, Haks MC, Ottenhoff TH, Spaink HP and
Meijer AH: The DNA damage-regulated autophagy modulator DRAM1 links
mycobacterial recognition via TLR-MYD88 to autophagic defense
[corrected]. Cell Host Microbe. 15:753–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kazi JU, Rupar K, Marhall A, Moharram SA,
Khanum F, Shah K, Gazi M, Nagaraj SR, Sun J, Chougule RA and
Rönnstrand L: ABL2 suppresses FLT3-ITD-induced cell proliferation
through negative regulation of AKT signaling. Oncotarget.
8:12194–12202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu W, Lin Z, Kelly AA, Hegarty JP, Poritz
LS, Wang Y, Li T, Schreiber S and Koltun WA: Association of a
Nkx2-3 polymorphism with Crohn's disease and expression of Nkx2-3
is up-regulated in B cell lines and intestinal tissues with Crohn's
disease. J Crohns Colitis. 3:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kent OA, Fox-Talbot K and Halushka MK:
RREB1 repressed miR-143/145 modulates KRAS signaling through
downregulation of multiple targets. Oncogene. 32:2576–2585. 2013.
View Article : Google Scholar : PubMed/NCBI
|