Introduction
Bisphosphonates are analogues of pyrophosphate and
have been used in the treatment of various clinical conditions (not
only osteolytic cancers and bone metastases but also conditions
involving osteoclast-mediated bone loss) since the 1960s. As
important anti-resorptive agents, bisphosphonates have an important
role in metabolic bone diseases and bone metastases (1). Their anti-osteoclastic actions make
them important candidates for the adjuvant treatment of giant cell
tumors (2). Relative to other
bisphosphonates, zoledronic acid has the highest mineral binding
affinity, indicating high potency and a long duration of action
(3).
As the most potent bisphosphonate, zoledronic acid
is widely used in the treatment of patients with osteoporosis,
Paget's disease, hypercalcemia, bone metastases and multiple
myeloma (3–5). Large doses of bisphosphonates may
result in high bioavailability and long, intermittent treatment
periods may improve the compliance and persistence of
bisphosphonate treatment, particularly for those patients with an
overall frail constitution (6).
However, the adverse effects of zoledronic acid are not to be
underestimated, particularly the renal side effects, which may
occur with large dosages. When the dosage of zoledronic acid was
increased from 4 to 8 mg, the risk of kidney hypofunction and even
kidney failure is significantly increased.
The side effects of zoledronic acid may be grouped
into four major categories: Acute-phase reactions, renal side
effects, gastrointestinal effects and osteonecrosis of the jaw
(7–11). Due to the imposing risks, it is
important to know the minimal effective concentration of zoledronic
acid. However, this number has rarely been determined and the
underlying mechanisms of the anti-osteoclastic actions of
zoledronic acid remain elusive (12). The aim of the present study was to
analyze the efficacy of zoledronic acid to inhibit osteoclast
formation in vitro. Specifically, the
concentration-dependent influence of zoledronic acid was evaluated
in a primary murine osteoclast cell line. The results indicated
that zoledronic acid impacted osteoclastogenesis, as well as the
adhesive, migratory and bone resorption abilities of the cells. The
present study provided a basis for future optimization of
zoledronic acid use in the clinic and sheds light on certain
previously unreported effects of the drug on osteoclasts.
Materials and methods
Reagents
Zoledronic acid was obtained from Novartis
Pharmaceuticals Ltd. (Basel, Switzerland). Macrophage colony
stimulating factor (M-CSF) and receptor activator for nuclear
factor-κB ligand (RANKL) were obtained from Abcam (Cambridge, UK).
Fetal bovine serum (FBS) and tartrate-resistant acid phosphatase
(TRAP) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Stock solutions of zoledronic acid were prepared in PBS.
Next, 0.1 mol/l NaOH was slowly added dropwise to adjusted the pH
to 7.4, and the stock was sterilized by filtration (Millipore
syringe-fitted filter, 0.45 µm).
Establishment of primary cell
culture
All experiments were approved by the Animal Care and
Use Committee of Hebei Medical University (Shijiazhuang, China) and
performed by experienced personnel. C57 female mice (age 6 weeks;
n=20; weight, 18–20 g) were obtained from the Animal House of Hebei
Medical University. Bone marrow cells were obtained from the long
limb bone of C57 mice as previously described with minor
modifications (13). In brief, mouse
bone marrow mononuclear cells were collected by centrifugation (3 ×
g, 5 min, 37°C), cultured in α-modified minimal essential medium
(α-MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and incubated at 37°C in a humidified atmosphere with 5%
CO2 for 72 h. Next, the cells were incubated with a
α-MEM supplemented with 3×10−5 g/l M-CSF,
2×10−5 g/l murine recombinant RANKL and 10% FBS
(13).
After 72 h, the cells were incubated at a density of
10,000 cells/ml with 5 ml α-MEM. Cells were passaged following
confluence (80%) and they were separated into six groups.
Zoledronic acid was added at a concentration of 1×10−4,
1×10−5, 1×10−6, 1×10−7 or
1×10−8 mol/l, while equal volumes of PBS (1 ml) were
added to control wells. Zolendronic acid was added during
osteoclastogenesis. This process usually takes 48 h, therefore the
effect of zolendronic acid was to inhibit osteoclastogenesis. After
24 h, the cell culture medium was replaced with α-MEM containing
4×10−5 g/l M-CSF, 6×10−5 g/l murine
recombinant RANKL and 10% FBS, followed by incubation for another
120 h (13).
TRAP staining
After 120 h, the cells were fixed with 10%
formaldehyde in PBS. Adherent cells were treated with
ethanol-acetone at a ratio of 1:1. TRAP staining was subsequently
performed as previously described (13). To identify osteoclasts at the end of
the culture period, the cells were observed with an inverted
microscope (Nikon TE2000S inverted microscope; Nikon Corp., Tokyo,
Japan). A scientific research camera and QCapture Pro 7 image and
analysis software (QImaging Ltd., Surrey, BC, Canada) were used to
capture images and process them. Image-Pro Plus 6.0 software (Media
Cybernetic, Rockville, MD, USA) was applied to score and quantify
the multinuclear giant cells with positive TRAP staining. Cells
with >3 nuclei were regarded as mature osteoclasts).
In vitro adhesion assay of
osteoclasts
Bone marrow mononuclear cells were incubated with
α-MEM containing 4×10−5 g/l M-CSF, 6×10−5 g/l
murine recombinant RANKL and 10% FBS. After 120 h, bone marrow
mononuclear cells gradually differentiated into osteoclasts. Then
the single-cell suspension of osteoclast cells were prepared. These
cells were cultured in 96-well plates coated with 4×10−5
g/l M-CSF and 6×10−5 g/l murine recombinant RANKL
(5×104 cells/well; Thermo Fisher Scientific, Inc.) and
incubated with 1×10−6 mol/l zoledronic acid or PBS for 1
h. Following the incubation, PBS (pH 7.4) washed the cells three
times, removing non-adherent cells, the attached cells then
subjected to TRAP staining, and TRAP-positive cells were fixed,
observed and counted as previously described (13).
In vitro osteoclast migration
assay
First, bone marrow cells were suspended and
incubated for 30–40 min. It is known that cells adhere to the wall
of the cell culture dish, and that the bone marrow mononuclear
cells gradually grow and differentiate into osteoclasts. At the
beginning, other cells, including bone marrow stromal cells, were
present; however, as the culture time progressed and the media was
changed several times, the osteoclasts adhered to the wall of the
well more firmly. Following the washing out of other impurities,
the majority of the osteoclasts remained as they adhere to the
walls firmly. A Transwell system (24-pore plate; 8-µm pore size;
Corning-Costar, Corning, NY, USA) was used to analyze and measure
osteoclast migration as previously described (13). Osteoclasts (1×104
cells/chamber) induced by 3×10−5 g/l M-CSF or
1×10−6 mol/l zoledronic acid were added to the Transwell
system to the experimental chambers, and 200 µl PBS and
3×10−5 g/l M-CSF were added to the control chambers. All
these cells were incubated for 4 h. Then, the cells that had
transgressed through the Transwell membrane were observed and
quantified.
In vitro resorption of osteoclasts on
dentine slice assay
Bone resorption is the characteristic function of
osteoclasts (they are also known as bone-resorbing cells). The bone
marrow mononuclear cells were incubated in 96-well plates at
5×104 cells/well, bone marrow mononuclear cells
gradually differentiated into osteoclasts as specified above.
Single-cell suspensions of purified osteoclasts (1×104
cells) were obtained and seeded on pre-wetted dentine slices
(American Laboratory Products Co., Windham, NH, USA) in the
presence of 4×10−5 g/l M-CSF and 6×10−5 g/l
murine recombinant RANKL, followed culture at 37°C for 16 h.
Zoledronic acid (1×10−6 mol/l) was then added to the
experimental wells, while equal volumes of PBS were added to the
control wells. After toluidine blue staining, bone lacuna was
observed and measured in vitro. Reflective light microscopy
was used to observe each bone slice under low-power magnification,
and the bone lacuna area was calculated with Image-Pro Plus 6.0
software (Media Cybernetics).
Statistical analysis
Values are expressed as the mean ± standard
deviation. SPSS software for Windows (version 19.0; IBM Corp.,
Armonk, NY, USA) was used for all statistical analyses. The results
of the osteoclast formation assay were compared between groups
using analysis of variance according to post hoc testing by the
Tukey-Kramer method. This method was used to determine the
association between various concentrations of zoledronic acid. The
results of the osteoclast adhesion assay, migration assay and bone
resorption assay were statistically analyzed using the Student's
t-test. P<0.05 indicated that the difference between groups was
statistically significant.
Results
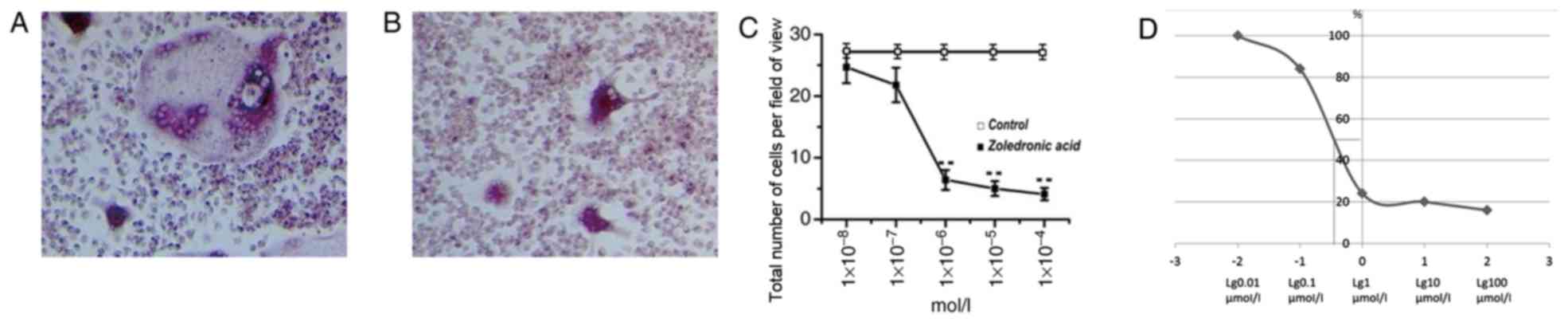
Osteoclast formation declines at
1×10−6 mol/l of zoledronic acid
Zoledronic acid was effective across all
experimental concentrations according to the quantitative
evaluation of cells stained with TRAP. Compared with the control
group, zoledronic acid directly suppressed osteoclast formation at
all concentrations. However, the effectiveness was not obvious at
the concentrations of 1×10−8 and 1×10−7
mol/l. At the concentration of 1×10−6 mol/l, the total
area of mature mouse osteoclasts was significantly decreased
(P<0.01). This inhibitory effect was further enhanced at the
concentration of 1×10−5 mol/l. Furthermore, at the
concentration of 1×10−4 mol/l, the suppressive effect
was slightly greater compared with zoledronic acid at
1×10−5 mol/l. However, no significant suppressive effect
was detectable at the concentration of 1×10−4 mol/l.
Importantly, a simple and fitted S-shaped curve was generated and
the 50% effective concentration of zoledronic acid was
0.6×10−6 mol/l (Fig.
1).
Zoledronic acid at 1×10−6
mol/l exerts a suppressive effect on osteoclast adhesion
Based on the observation of inhibition of osteoclast
formation by 1×10−6 mol/l zoledronic acid, the possible
inhibitory effect of this concentration of zoledronic acid on the
adhesion ability of osteoclasts was next examined. Attached
TRAP-positive cells were fixed and quantitatively evaluated. The
number of attached, TRAP-stained cells was 139.7±16.8 cells per
high-power field (HPF) in the control group and 72.6±13.1 cells/HPF
in the experimental group. Thus, compared with the control group,
1×10−6 mol/l zoledronic acid (equivalent to
pharmacologically administered dose, 2–4 mg) was sufficient to
significantly inhibit the adhesion ability of osteoclasts
(P<0.01; Fig. 2).
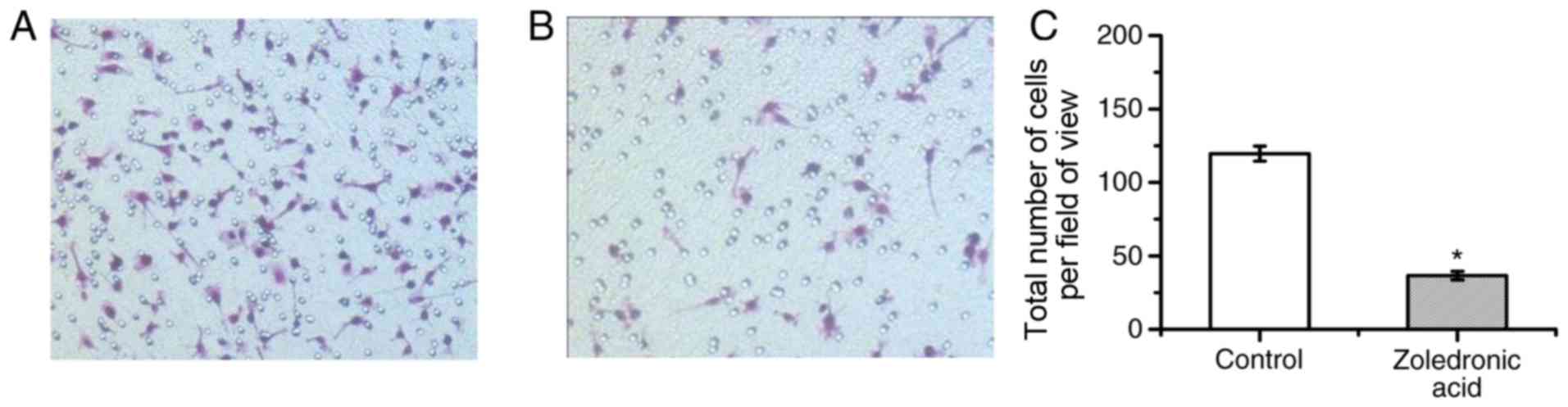
Zoledronic acid at 1×10−6
mol/l markedly suppresses the migration of osteoclasts
One pre-condition for bone resorption is the
migration of osteoclasts. Therefore, the impact of
1×10−6 mol/l zoledronic acid on the migration ability of
osteoclasts was examined. The number of osteoclasts transgressing
through a Transwell membrane was determined. The results indicated
that in control group 119.6±16.2 cells/HPF were migratory as
compared with 36.6±6.1 cells/HPF in the experimental group. This
result demonstrated that 1×10−6 mol/l zoledronic acid
was sufficient to significantly decrease the number of migratory
cells compared with that in the control group (P<0.01; Fig. 3).
Zoledronic acid (1×10−6
mol/l) appreciably suppresses bone resorption of osteoclasts
Osteolytic destruction and bone resorption are
universally regarded as basic cellular functions of osteoclasts.
Bone resorption of osteoclasts may be observed and measured by the
size of Howship's lacuna in vitro. In the present study, the
demarcation of Howship's lacuna was examined and quantified in the
two groups treated with 1×10−6 mol/l zoledronic acid or
vehicle in vitro. Microscopically, it was observed that
Howship's lacuna in the control group was obviously longer and
deeper than that in the experimental group. Specifically, the
volume of the bone lacuna was 30.9±6.5 cells/HPF in the control
group and 5.1±1.5 cells/HPF in the experimental group. Expressed as
percentages, the bone lacuna area was 20.8±3.65% in the control
group and 2.12±0.44% in the experimental group. A statistically
significant decline in the size of Howship's lacuna was observed
after zoledronic acid administration in vitro (P<0.01;
Fig. 4).
Discussion
Bisphosphonates are non-toxic analogues of
pyrophosphate. They share a similar core structure, with one key
binding of two molecules (P-C-P), and two side chains or groups, R1
and R2, attached to the central carbon atom. Small changes to the
structure of the R2 side chain may alter the anti-resorptive
potency by affecting the ability of bisphosphonates to inhibit
farnesyl diphosphate synthase (14).
The differences in the physicochemical and biological properties of
bisphosphonates are due to the differences in the R2 group
(14–18). For instance, the presence of nitrogen
and its orientation within the R2 side chain may influence the
overall potency of various bisphosphonates, and small modifications
of the structure of the R2 side chain may afford substantial
changes in the anti-resorptive properties of these compounds
(19,20).
Due to their anti-osteoclastogenic actions,
bisphosphonates have demonstrated efficacy not only in the
treatment of osteolytic cancers and bone metastases, but also in
other clinical conditions involving osteoclast mediated bone loss
(21–23). At present, >10 bisphosphonates
have been approved for various clinical applications in various
countries, bisphosphonates exhibit differences in the dosages,
routes of administration, therapeutic effects and adverse
reactions; these differences are meaningful to the patients and
clinicians (24–28). The bisphosphonate family is large and
a strong structure-activity association of their anti-resorptive
potency prevails, likely owing to the fact that each derivation has
its own specific mode of action (29).
There are marked differences in the pharmacokinetics
of bisphosphonates. Zoledronic acid has the strongest ability to
inhibit osteoclastogenesis, followed by alendronate, ibandronate,
risedronate and etidronate (14).
Alendronate can be taken orally. High mineral binding affinity and
intermediate enzyme inhibitory potency are characteristic features
of alendronate (29). Therefore, the
bone turnover rate is reduced the most with alendronate treatment
and its duration of action is the longest (14,29–31). In
contrast, due to its moderate mineral binding affinity, risedronate
can distribute more widely in the bone (14). The relatively fast onset of action of
risedronate is due to its high enzyme potency, although it is lower
compared with zoledronic acid (14).
Conversely, the enzyme inhibitory potency of ibandronate is higher
compared with alendronate (14).
Compared with alendronate and risedronate, ibandronate has medium
mineral binding affinity (32).
As a highly potent, third-generation
nitrogen-containing bisphosphonate, zoledronic acid is the
strongest inhibitor of farnesyl pyrophosphate synthase, compared
with alendronate, ibandronate, risedronate and etidronate (14). The mineral binding affinity of
zoledronic acid is the highest, therefore it has the longest
duration of action and the highest potency (33). Zoledronic acid can serve an important
role in the loss of osteoclast activity and induction of apoptosis
by effectively inhibiting enzymes in the mevalonate signalling
pathway, including key regulatory proteins, such as mitochondrial
ADP/ATP translocase (34). Moreover,
the activity of zoledronic acid on the reduction of osteoclasts and
induction of apoptosis is through inhibiting protein prenylation
(34). Zoledronic acid is a
nitrogen-containing bisphosphonate (35). Clinically, zoledronic acid is
approved for the treatment of osteoporosis and cancer patients with
osteolysis as it exhibits a high efficacy. In addition, zoledronic
acid has a direct effect on cancer cells (9). At the low concentration of
1×10−6 mol/l, zoledronic acid can inhibit the invasion
of cells (36). In the current
study, zoledronic acid was demonstrated to inhibit osteoclast
formation, adhesion and migration, and bone resorption at the
minimal effective concentration. A study revealed that zoledronic
acid can inhibit osteoclast maturation, differentiation and
migration, to prevent inflammatory lesion osteolysis (37). Clodronate is a first generation
non-nitrogen-containing bisphosphonate and pamidronate is a second
generation bisphosphonate nitrogen-containing bisphosphonate
(38). Compared with clodronate and
pamidronate, zoledronic acid is the most effective drug for
inducing apoptosis and inhibiting reabsorption (38). The aforementioned results have
important clinical significance.
Zoledronic acid is the most potent bisphosphonate
currently known. It is effective in the prevention and treatment of
bone-associated conditions. When other bisphosphonates are
ineffective, zoledronic acid still relieves cancerous bone pain.
The characteristics of bisphosphonates are dose-dependent. However,
it was identified that high doses of zoledronic acid did not
achieve the desired effect in the treatment of carcinomatous pain
(39). Furthermore, the adverse
reactions to zoledronic acid were more prominent at high doses.
Furthermore, the renal excretion of zoledronic acid is longer than
that of bisphosphonates of the same generation (e.g., ibandronic
acid). The toxicity to the kidney is relatively high. It remains
elusive why high doses of zoledronic acid do not achieve the
desired effect. Furthermore, the detailed mechanisms via which
zoledronic acid inhibits osteoclasts have remained to be
determined. Therefore, the present in vitro study examined
the effect zoledronic acid on the differentiation of bone marrow
cells into osteoclasts, the primary biological characteristics of
osteoclasts and the minimal inhibitory concentration. The current
study also analyzed and compared the effects of different
concentrations of zoledronic acid on the activity of osteoclasts,
in order to provide a scientific theoretical basis for clinical
treatment. Certain modes of inhibitory action of zoledronic acid on
osteoclasts were identified and the lowest effective concentration
of zoledronic acid was determined.
In the present study, the influence of zoledronic
acid on osteoclastogenesis, adhesion, migration and bone resorption
of bone marrow cells was evaluated in vitro. Osteoclast
formation has been previously reported to be suppressed by high
concentrations of zoledronic acid (39,40).
Furthermore, a subtoxic concentration (10−5 mol/l) of
zoledronic acid can prolong the osteoblastic stage span of primary
human osteoblasts (40). On the
contrary, zoledronic acid at the lowest concentrations,
10−6-10−11 mol/l, had no effect on the
proliferation of osteoblasts (40).
In the present study, the higher concentrations had greater
effects, the most pronounced effect of zoledronic acid occurred at
a concentration of 1×10−4 mol/l. However,
1×10−6 mol/l was the minimum inhibitory concentration.
The number of mature mouse osteoclasts at this dose was
significantly decreased. As other bisphosphates, zoledronic acid
suppressed the formation of osteoclasts in a
concentration-dependent manner (13,32).
This inhibitory effect was further enhanced at the concentration of
1×10−5 mol/l. However, the dose-response was not linear,
given that the suppressive effectiveness at the concentration of
1×10−4 mol/l was not significantly higher than that at
the precedent concentration. This phenomenon is supported by
previous observations describing that the clinical administration
of high-dose zoledronic acid did not achieve the desired
therapeutic results, particularly in the treatment of osseous
metastases of malignant tumors (39). As demonstrated in the current study,
the inhibitory effect of zoledronic acid on osteoclasts in culture
was not reduced, however no further increases were observed with
increasing doses and the dose-response curve was not linear. This
characteristic inhibitory effect of zoledronic acid on osteoclasts
is supported by clinical studies (40–44).
Furthermore, a recent study indicated that in patients with bone
metastases due to breast cancer, prostate cancer or multiple
myeloma, the use of 4 mg zoledronic acid every 12 weeks (which is a
greater administration interval, but required a smaller dose
compared with the regime of the current study) did not result in an
increased risk of skeletal events compared with the standard dosing
interval of every 4 weeks over 2 years (45). In addition, an increased dosage of
zoledronic acid did not achieve the desired therapeutic results in
another study (42). This is
significant particularly due to the known side effects of
bisphosphonates, which may be grouped into three major categories:
Acute-phase reactions, gastrointestinal effects and renal side
effects (41). While certain studies
indicate that the anti-angiogenetic effect of bisphosphonates may
influence the wound healing process after injury, the side effects
of high doses may actually pose more risks than benefits (43–46).
This may explain why the clinical administration of high doses of
zoledronic acid does not typically achieve the desired
efficacy.
The present in vitro study provided an
appropriate and efficacious concentration of zoledronic acid, and
explored its effects on the osteoclastogenesis of bone marrow
mononuclear cells and their biological behaviour. As zoledronic
acid has been approved for clinical use, the effective and
appropriate doses should be established. Several calculations were
performed and it was found that 1×10−6 mol/l zoledronic
acid is equivalent to the pharmacologically administered dose of
2–4 mg. In particular, the administration dose (2–4 mg) is required
to achieve plasma levels of 1×10−6 mol/l in an adult
person (47). However, the
concentration of zoledronic acid in the bone tissue will be higher
due to preferential concentration and enrichment of zoledronic acid
to bone tissue (47). Through a new
experimental model, the appropriate concentration of zoledronic
acid in the bone tissue of the patients was calculated to be
between 0.4×10−6 and 4.6×10−6 mol/l (47). We determined with the effective dose
2–4 mg of zoledronic acid that should be available. When the
administration dose comes to 6–8 mg, it demands careful
consideration. The effective dose of zoledronic acid was 2–4 mg,
which is the appropriate dose in clinical practice; larger doses of
6–8 mg are not recommended. In a future study, an appropriate
concentration should be translated into the corresponding dose for
clinical administration, to validate its therapeutic effectiveness
in vivo. The present study also supports the proposition
that there is no requirement for large doses of zoledronic acid,
due to side effects associated with a high concentration and long
durations of treatment. Therefore, the honing in on the appropriate
dosage and duration of zoledronic acid treatment is important for
reducing the incidence of adverse events in humans. Toward this
aim, future studies should be performed to assess the
pharmacological effects, at a cytological and pathological basis,
of zoledronic acid at a dose comparable to the minimum effective
concentration determined in the present study. This in turn will
ensure the optimal use of zoledronic acid and similar
bisphosphonates for the treatment of various diseases.
The inhibition of osteoclasts formation and bone
resorption by zoledronic acid may have already been performed
during the early stages of the development of zoledronic acid as a
drug to treat bone diseases (14).
In contrast to other studies, the present study aimed to determine
the minimally effective concentration of zoledronic acid to
suppress osteoclast formation in vitro, as an unconventional
approach to identify means of administering zoledronic acid in an
optimized way without any side effects. Therefore, the present
study is pushing back the frontiers and opening doors to reveal why
high doses of zoledronic acid did not achieve the desired effect
and how zoledronic acid inhibits osteoclasts.
One limitation of the present study is that the
dose-dependent effects of zoledronic acid on cell adhesion,
migration and bone resorption were not determined. Another
limitation is that the in vitro experiments cannot imitate
the complex drug metabolism and progression of the bone diseases
associated with osteoclastogenesis in vivo. The present
in vitro study only explains certain details and features of
the pharmacological mechanisms of osteoclast suppression. The
effect of zoledronic acid in vitro is different from that
in vivo; therefore, further studies are required to verify
the optimal administration schedule and dosing of zoledronic acid
in vivo. To assess this, clinical trials will be
performed.
Acknowledgements
The authors would like to thank Professor Depei Li
(Anderson Cancer Center, University of Texas, Houston, TX, USA) for
scientific suggestions and discussion.
Funding
This study was funded by the Medical Foundation of
Hebei Province (grant no. 20130030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and XJ designed the study. YS and HL performed
the experiments. LW and CN collected and analyzed the data. PL
performed the interpretation of the data and was a major
contributor in writing the manuscript. All authors reviewed the
initial manuscript and revised it critically for important
intellectual content. The final version of the manuscript has been
read and approved by all authors, and each author believes that the
manuscript represents honest work.
Ethical approval and consent to
participate
The design of the present study was in line with
scientific and ethical principles. All animal experimental
protocols were reviewed and approved by the Animal Care and Use
Committee of Hebei Medical University (Shijiazhuang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Insalaco L, Di Gaudio F, Terrasi M, Amodeo
V, Caruso S, Corsini LR, Fanale D, Margarese N, Santini D, Bazan V
and Russo A: Analysis of molecular mechanisms and anti-tumoural
effects of zoledronic acid in breast cancer cells. J Cell Mol Med.
16:2186–2195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soki FN, Li X, Berry J, Koh A, Sinder BP,
Qian X, Kozloff KM, Taichman RS and McCauley LK: The effects of
zoledronic acid in the bone and vasculature support of
hematopoietic stem cell niches. J Cell Biochem. 114:67–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphy CM, Schindeler A, Gleeson JP, Yu
NY, Cantrill LC, Mikulec K, Peacock L, O'Brien FJ and Little DG: A
collagen-hydroxyapatite scaffold allows for binding and co-delivery
of recombinant bone morphogenetic proteins and bisphosphonates.
Acta Biomater. 10:2250–2258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brennan MA, Gleeson JP, O'Brien FJ and
McNamara LM: Effects of ageing, prolonged estrogen deficiency and
zoledronate on bone tissue mineral distribution. J Mech Behav
Biomed Mater. 29:161–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voskaridou E, Christoulas D, Plata E,
Bratengeier C, Anastasilakis AD, Komninaka V, Kaliontzi D,
Gkotzamanidou M, Polyzos SA, Dimopoulou M and Terpos E: High
circulating sclerostin is present in patients with
thalassemia-associated osteoporosis and correlates with bone
mineral density. Horm Metab Res. 44:909–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das S, Edwards PA, Crockett JC and Rogers
MJ: Upregulation of endogenous farnesyl diphosphate synthase
overcomes the inhibitory effect of bisphosphonate on protein
prenylation in Hela cells. Biochim Biophys Acta. 1841:569–573.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dedes PG, Kanakis I, Gialeli Ch,
Theocharis AD, Tsegenidis T, Kletsas D, Tzanakakis GN and Karamanos
NK: Preclinical evaluation of zoledronate using an in vitro mimetic
cellular model for breast cancer metastatic bone disease. Biochim
Biophys Acta. 1830:3625–3634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evdokiou A, Labrinidis A, Bouralexis S,
Hay S and Findlay DM: Induction of cell death of human osteogenic
sarcoma cells by zoledronic acid resembles anoikis. Bone.
33:216–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Majithia N, Atherton PJ, Lafky JM,
Wagner-Johnston N, Olson J, Dakhil SR, Perez EA, Loprinzi CL and
Hines SL: Zoledronic acid for treatment of osteopenia and
osteoporosis in women with primary breast cancer undergoing
adjuvant aromatase inhibitor therapy: A 5-year follow-up. Support
Care Cancer. 24:1219–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tada Y, Hiroshima K, Shimada H, Shingyoji
M, Suzuki T, Umezawa H, Sekine I, Takiguchi Y, Tatsumi K and Tagawa
M: An intrapleural administration of zoledronic acid for inoperable
malignant mesothelioma patients: A phase I clinical study protocol.
Springerplus. 5:1952016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kroep JR, Charehbili A, Coleman RE, Aft
RL, Hasegawa Y, Winter MC, Weilbaecher K, Akazawa K, Hinsley S,
Putter H, et al: Effects of neoadjuvant chemotherapy with or
without zoledronic acid on pathological response: A meta-analysis
of randomized trials. Eur J Cancer. 54:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
James ND, Sydes MR, Clarke NW, Mason MD,
Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard
G, et al: Addition of docetaxel, zoledronic acid, or both to
first-line long-term hormone therapy in prostate cancer (stampede):
Survival results from an adaptive, multiarm, multistage, platform
randomised controlled trial. Lancet. 387:1163–1177. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kellinsalmi M, Mönkkönen H, Mönkkönen J,
Leskelä HV, Parikka V, Hämäläinen M and Lehenkari P: In vitro
comparison of clodronate, pamidronate and zoledronic acid effects
on rat osteoclasts and human stem cell-derived osteoblasts. Basic
Clin Pharmacol Toxicol. 97:382–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russell RG, Xia Z, Dunford JE, Oppermann
U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps
RJ, et al: Bisphosphonates: An update on mechanisms of action and
how these relate to clinical efficacy. Ann N Y Acad Sci.
1117:209–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Little DG, Peacock L, Mikulec K, Kneissel
M, Kramer I, Cheng TL, Schindeler A and Munns C: Combination
sclerostin antibody and zoledronic acid treatment outperforms
either treatment alone in a mouse model of osteogenesis imperfecta.
Bone. 101:96–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raje N, Terpos E, Willenbacher W, Shimizu
K, García-Sanz R, Durie B, Legieć W, Krejčí M, Laribi K, Zhu L, et
al: Denosumab versus zoledronic acid in bone disease treatment of
newly diagnosed multiple myeloma: An international, double-blind,
double-dummy, randomised, controlled, phase 3 study. Lancet Oncol.
19:370–381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Byun JH, Jang S, Lee S, Park S, Yoon HK,
Yoon BH and Ha YC: The efficacy of bisphosphonates for prevention
of osteoporotic fracture: An update meta-analysis. J Bone Metab.
24:37–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Bari JA, Nead JA and Schurman SJ:
Zoledronic acid for neonatal subcutaneous fat necrosis. Clin Case
Rep. 5:567–569. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayilvaganan S, Sarathi Vijaya HA and
Shivaprasad C: Preoperative zoledronic acid therapy prevent hungry
bone syndrome in patients with primary hyperparathyroidism. Indian
J Endocrinol Metab. 21:76–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayer PS, Deane AK, Agrawal A, Maheshwari
R and Juyal A: Effect of zoledronic acid on fracture healing in
osteoporotic patients with intertrochanteric fractures. Int J Appl
Basic Med Res. 7:48–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anastasilakis AD, Polyzos SA, Gkiomisi A,
Saridakis ZG, Digkas D, Bisbinas I, Sakellariou GT, Papatheodorou
A, Kokkoris P and Makras P: Denosumab versus zoledronic acid in
patients previously treated with zoledronic acid. Osteoporos Int.
26:2521–2527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hattori Y, Yamashita J, Sakaida C, Kawano
K and Yonemochi E: Evaluation of antitumor effect of zoledronic
acid entrapped in folate-linked liposome for targeting to
tumor-associated macrophages. J Liposome Res. 25:131–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lézot F, Chesneau J, Navet B, Gobin B,
Amiaud J, Choi Y, Yagita H, Castaneda B, Berdal A, Mueller CG, et
al: Skeletal consequences of RANKL-blocking antibody (IK22-5)
injections during growth: Mouse strain disparities and synergic
effect with zoledronic acid. Bone. 73:51–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishizuna K, Ota D, Fukuuchi A, Teraoka M,
Fujii A, Mori M and Nishi T: A case of femoral diaphyseal fracture
after long-term treatment with zoledronic acid. Breast Cancer.
22:90–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen KH, Wu PK, Chen CF and Chen WM:
Zoledronic acid-loaded bone cement as a local adjuvant therapy for
giant cell tumor of the sacrum after intralesional curettage. Eur
Spine J. 24:2182–2188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hattori Y, Shibuya K, Kojima K, Miatmoko
A, Kawano K, Ozaki K and Yonemochi E: Zoledronic acid enhances
antitumor efficacy of liposomal doxorubicin. Int J Oncol.
47:211–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tisdale JE, Allen MR, Overholser BR,
Jaynes HA and Kovacs RJ: Influence of zoledronic acid on atrial
electrophysiological parameters and electrocardiographic
measurements. J Cardiovasc Electrophysiol. 26:671–677. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo KW, Ko CH, Yue GG, Gao S, Lee JK, Li
G, Fung KP, Leung PC, Evdokiou A and Lau CB: The combined use of
Camellia sinensis and metronomic zoledronic acid in a breast
cancer-induced osteolysis mouse model. J Cancer Res Clin Oncol.
141:1025–1036. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin CK, Dirksen WP, Carlton MM, Lanigan
LG, Pillai SP, Werbeck JL, Simmons JK, Hildreth BE III, London CA,
Toribio RE and Rosol TJ: Combined zoledronic acid and meloxicam
reduced bone loss and tumour growth in an orthotopic mouse model of
bone-invasive oral squamous cell carcinoma. Vet Comp Oncol.
13:203–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Günaldi M, Afsar CU, Duman BB, Kara IO,
Tatli U and Sahin B: Effect of the cumulative dose of zoledronic
acid on the pathogenesis of osteonecrosis of the jaws. Oncol Lett.
10:439–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hiroshima Y, Maawy AA, Katz MH, Fleming
JB, Bouvet M, Endo I and Hoffman RM: Selective efficacy of
zoledronic acid on metastasis in a patient-derived orthotopic
xenograph (PDOX) nude-mouse model of human pancreatic cancer. J
Surg Oncol. 111:311–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luedders DW, Steinhoff J, Thill M, Rody A
and Bohlmann MK: Lack of difference in acute nephrotoxicity of
intravenous bisphosphonates zoledronic acid and ibandronate in
women with breast cancer and bone metastases. Anticancer Res.
35:1797–1802. 2015.PubMed/NCBI
|
|
33
|
Wu D, Ma J, Bao S and Guan H: Continuous
effect with long-term safety in zoledronic acid therapy for
polyostotic fibrous dysplasia with severe bone destruction.
Rheumatol Int. 35:767–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mönkkönen H, Ottewell PD, Kuokkanen J,
Mönkkönen J, Auriola S and Holen I: Zoledronic acid-induced
IPP/ApppI production in vivo. Life Sci. 81:1066–1070. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka Y, Nagai Y, Dohdoh M, Oizumi T,
Ohki A, Kuroishi T, Sugawara S and Endo Y: In vitro cytotoxicity of
zoledronate (nitrogen-containing bisphosphonate: NBP) and/or
etidronate (non-NBP) in tumour cells and periodontal cells. Arch
Oral Biol. 58:628–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kimachi K, Kajiya H, Nakayama S, Ikebe T
and Okabe K: Zoledronic acid inhibits RANK expression and migration
of osteoclast precursors during osteoclastogenesis. Naunyn
Schmiedebergs Arch Pharmacol. 383:297–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Denoyelle C, Hong L, Vannier JP, Soria J
and Soria C: New insights into the actions of bisphosphonate
zoledronic acid in breast cancer cells by dual RhoA-dependent and
-independent effects. Br J Cancer. 88:1631–1640. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Z, Guan H, Duan X and Kleinerman ES:
Zoledronic acid inhibits primary bone tumor growth in Ewing
sarcoma. Cancer. 104:1713–1720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen T, Berenson J, Vescio R, Swift R,
Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, et al:
Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer
patients with bone metastases. J Clin Pharmacol. 42:1228–1236.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zara S, De Colli M, di Giacomo V, Zizzari
VL, Di Nisio C, Di Tore U, Salini V, Gallorini M, Tetè S and
Cataldi A: Zoledronic acid at subtoxic dose extends osteoblastic
stage span of primary human osteoblasts. Clin Oral Investig.
19:601–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gnant M, Mlineritsch B, Stoeger H,
Luschin-Ebengreuth G, Knauer M, Moik M, Jakesz R, Seifert M,
Taucher S, Bjelic-Radisic V, et al: Zoledronic acid combined with
adjuvant endocrine therapy of tamoxifen versus anastrozol plus
ovarian function suppression in premenopausal early breast cancer:
Final analysis of the Austrian Breast and Colorectal Cancer Study
Group Trial 12. Ann Oncol. 26:313–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chien MC, Mascarenhas L, Hammoudeh JA and
Venkatramani R: Zoledronic acid for the treatment of children with
refractory central giant cell granuloma. J Pediatr Hematol Oncol.
37:e399–e401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Luo X, Yan F, Jiang Z, Li Y, Fang
C and Shen J: Effect of zoledronic acid on vertebral marrow
adiposity in postmenopausal osteoporosis assessed by MR
spectroscopy. Skeletal Radiol. 44:1499–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Honda Y, Takahashi S, Zhang Y, Ono A,
Murakami E, Shi N, Kawaoka T, Miki D, Tsuge M, Hiraga N, et al:
Effects of bisphosphonate zoledronic acid in hepatocellular
carcinoma, depending on mevalonate pathway. J Gastroenterol
Hepatol. 30:619–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kalder M, Kyvernitakis I, Albert US,
Baier-Ebert M and Hadji P: Effects of zoledronic acid versus
placebo on bone mineral density and bone texture analysis assessed
by the trabecular bone score in premenopausal women with breast
cancer treatment-induced bone loss: Results of the ProBONE II
substudy. Osteoporos Int. 26:353–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Himelstein AL, Foster JC, Khatcheressian
JL, Roberts JD, Seisler DK, Novotny PJ, Qin R, Go RS, Grubbs SS,
O'Connor T, et al: Effect of longer-interval vs standard dosing of
zoledronic acid on skeletal events in patients with bone
metastases: A randomized clinical trial. JAMA. 317:48–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scheper MA, Badros A, Salama AR, Warburton
G, Cullen KJ, Weikel DS and Meiller TF: A novel bioassay model to
determine clinically significant bisphosphonate levels. Support
Care Cancer. 17:1553–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|