Introduction
Dendritic cells (DCs) are potent antigen presenting
cells (APCs) that stimulate naïve T cell proliferation to initiate
the in vivo immune response (1). T cell negative selection may be induced
by the continuously unstable interaction between immature DCs and T
cells, due to a deficiency of mature DCs, which may further lead to
immune tolerance (2). DCs are
considered to be important cells for studying the initiation and
development of various inflammatory diseases (3).
Nasal polyps (NP) are one of the most common
diseases in otorhinolaryngology, as well as head and neck surgery
(4). The pathogenesis of NP remains
to be elucidates; however, it is thought a combination of factors,
including inflammation and allergies, cause this disease (5). Some scholars classify NP into
inflammatory NP and allergic NP, with inflammatory NP characterized
by the infiltration of lymphocytes and neutrophils, and allergic NP
characterized by the infiltration of lymphocytes, plasma cells,
eosinophils or neutrophils (5).
Eosinophil infiltration is the most characteristic pathological
change of NP and is caused by interactions between various
cytokines, of which interleukin (IL)-5 is the main cytokine that
causes eosinophil infiltration. During the initial stages of NP,
the main source of IL-5 is Th2 cells (6). Lymphocyte infiltration in NP tissues
may induce an imbalance of Th1/Th2, leading to the over expression
of Th2 compared with Th1 (7).
However, inflammatory cell infiltration and inflammatory mediators
in NP tissues are observed during the middle and high-stages of the
inflammatory response and the characteristics of early pathogenesis
of NP remain unclear (8).
A previous study by our group revealed that NP
tissues are characterized by the infiltration of a large number of
DCs, which are mainly distributed in the submucosa and are
concentrated in the epithelium (9).
The role of DCs in the pathogenesis of NP and the interaction
between DCs and T cells remain to be elucidated. The aim of the
present study was to investigate the expression of cluster of
differentiation (CD)1a and CD83, as well as the ratio of CD83+
DCs/CD1a+DCs, in NP tissues and the distribution of DCs in NP and
normal inferior turbinate mucosa (nITM) tissues.
Materials and methods
Patients
A total of 30 patients underwent endoscopic NP
resection (experiment group, E), 10 patients underwent simultaneous
nasal septal construction and inferior turbinate resection (control
group, C), and 1 patient (male, 53 years old) underwent esophageal
cancer resection (positive control group) (10) between October 2015 to September 2016
at Shantou Central Hospital (Shantou, China). Group E included 16
males and 14 females, aged 14–72 years old (mean, 36 years). Group
C included 7 males and 3 females, aged 16–60 years old (mean, 34
years). Exclusion criteria were as follows: History of allergic
rhinitis and application of systemic or local glucocorticoids,
immunosuppressive agents, or any kinds of nasal spray in the 2
weeks prior to the study. All specimens were confirmed as NP or
nITM tissues by HE staining.
All tissues were washed three times with PBS, fixed
with 4% paraformaldehyde at 4°C for >24 h, dehydrated with a
graded series of ethanol at room temperature, cleared with xylene
and embedded in paraffin. Sections were then cut (5 µm) and stained
at room temperature with haematoxylin (ZLI-9610; ZSGB-Bio Inc.,
Beijing, China) for 15 min and eosin solution for 10–30 sec at room
temperature (ZLI-9613; ZSJQ Biotechnology, Inc.). Each slide was
examined at a magnification of 40× using a light microscope
(Axioplan 2; Carl Zeiss AG; Oberkochen, Germany).
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Shantou Central Hospital (Shantou, China). Written
informed consent was obtained from all participants.
Immunohistochemistry
Tissues were fixed with 4% paraformaldehyde at 4°C
for >24 h, dehydrated with a graded series of ethanol at room
temperature, cleared in xylene and embedded in paraffin. Serial 5
µm sections were then cut and underwent conventional dewaxing and
rehydration, followed by incubation for 10 min at room temperature
with 3% hydrogen peroxide to block endogenous peroxidase activity.
Sections were rinsed and incubated with citrate buffer (pH 6.0) at
room temperature for 15 min. The solution was allowed to cool to
room temperature and sections were rinsed three times with PBS for
2 min. Sections were subsequently incubated overnight at 4°C with
primary antibodies against CD1a (1:50; cat. no. EP3622; Neomarkers,
Inc., Portsmouth, NH, USA) and CD83 (1:100; cat. no. LP0306577;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). Sections were
rinsed with PBS three times for 2 min followed by incubation with
mouse anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (1:100; cat. no. L111327; KPL, Inc., Gaithersburg, MD,
USA) at 37°C for 30 min. Sections were rinsed again with PBS three
times for 2 min and subjected to DAB staining for 5 min at room
temperature, hematoxylin counter-staining for 2 min at room
temperature, decolored in 1% HCl-alcohol, lithium carbonate
red-blue-staining for 15 sec at room temperature, conventional
dehydration and hyalinization, followed by sealing in natural
resin. Sections were digested in the 1 mM EDTA (pH 8.0) containing
CD1a in an autoclave for 7.5 min, followed by digestion in the
solution containing CD83 (cat. no. LP0306577; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and 0.1% trypsin-containing CaCl2
(Shanghai Huajing Biotech Corp., Shanghai, China) at 37°C for 10.3
min. PBS was used to replace the primary antibodies as the negative
control and one esophageal cancer sample was selected as the
positive control.
Result determination and observation
indexes
CD1a+ and CD83+DCs were identified according to the
staining and morphology of cells. For each section, the number of
DCs was counted in five high-magnification fields at a
magnification of ×40 with a light microscope (Axioplan 2; Carl
Zeiss AG) and the mean number of the positive cells in these fields
was calculated to determine the degree of DC infiltration in this
sample. The observation indices included the following: i) The
infiltration of CD1a+DCs; ii) the infiltration of CD83+DCs; and
iii) the ratio of CD83+/CD1a+DCs.
Double immunostaining
The 4-µm paraffin-embedded sections were subjected
to the following treatments: Gradual alcohol dewaxing, rinsing with
PBS, incubation in 3% H2O2 to block the
endogenous peroxidase activity, PBS rinsing, antigen repair in in
0.01 M citrate buffer and further PBS rinsing. Sections were then
incubated with primary antibodies against CD1a (cat. no. MA119314;
rat anti-human monoclonal antibody; LAbVision, AB, Värmdö, Sweden;
1:50) and CD40 (cat. no. PA137334; rabbit anti-human monoclonal
antibody Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:50) at
37.5°C for 2 h and washed with PBS. Subsequently, sections were
incubated with secondary anti-rat antibody IgG-fluorescein
isothiocyanate (FITC; Seracare, Milford, MA, USA; 1:100) and
secondary anti-rabbit IgG-phycoerithrin (PE; Santa Cruz
Biotechnology, Inc.; 1:100) at 37.5°C for 30 min and mounted.
Control tissues were treated as above with PBS in place of the
antibodies. In addition, the esophageal cancer sample was used as
the positive control.
Low-illumination fluorescence
microscopy analysis
An Axioplan 2 imaging multi-function automatic
fluorescence microscope, Axio Cam digital camera (magnification,
40×; resolution 3,900× 3,090 pixels) and a KS400 image analysis
system (version 3.0) were used in the present study (all Zeiss AG,
Oberkochen, Germany). The three-fluorescence-excitation light
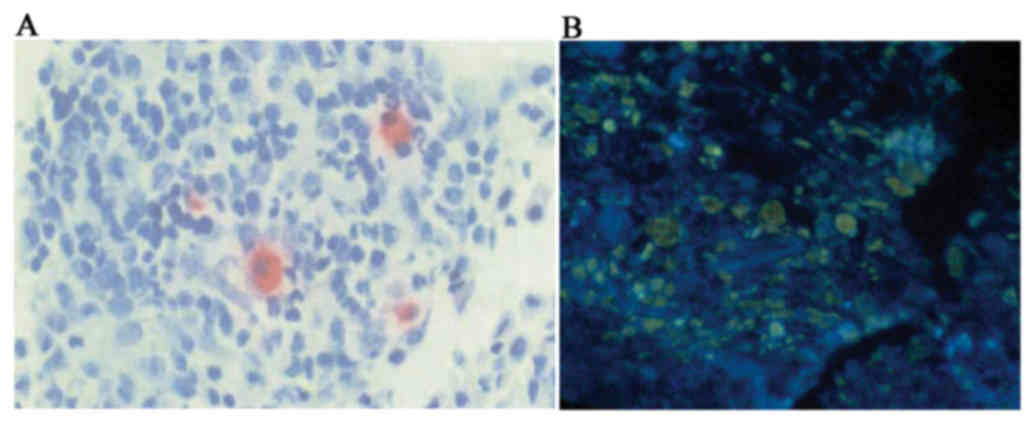
filter (Zeiss AG) was used to observe the cells. CD1a+cells
exhibited yellow-green fluorescence and CD40+ cells exhibited red
fluorescence. The area positive for double immunostaining per
section was measured using the KS400 image analysis system and
distribution density of double-positive cells was calculated.
Statistical analysis
SPSS10.0 (SPSS, Inc., Chicago, IL, USA) was used for
data analysis. All data are expressed as the mean ± standard
deviation and analyzed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Light microscopy
The DCs exhibited irregular shapes with dendrites of
varying lengths unevenly distributed in NP and nITM. Commonly
concentrated and formed foci were observed and DCs were mainly
distributed in T cell-concentrated areas. Many dendrites were in
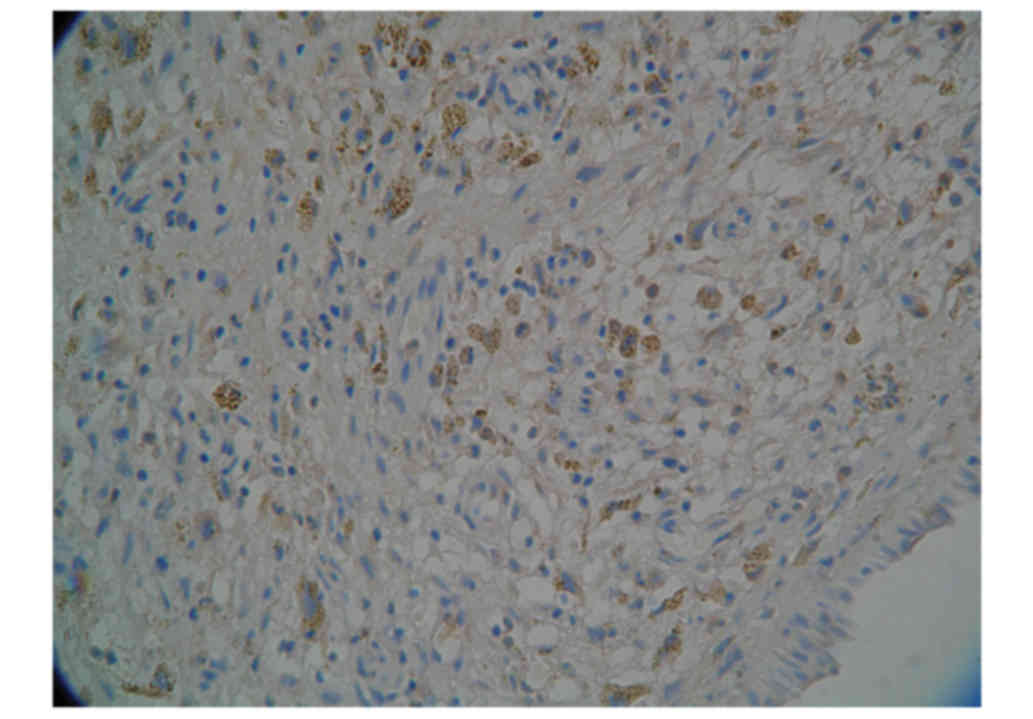
physical contact with the surrounding lymphocytes (Fig. 1).
Distribution density of CD1a+DCs and
CD83+DCs
As presented in Fig.
2 and Table I, the distribution
density [total number of CD1a+ cells in the unit area
(mm2)] of CD1a+DCs in NP tissues was significantly
higher compared with nITM tissues (39.8±3.5 and 11.1±4.9
cells/mm2, respectively). Similarly, the distribution
density of CD83+ DCs in NP tissues were also significantly higher
compared with nITM tissues (distribution density, 26.9±2.3 and
8.7±3.3 cells/mm2, respectively).
 | Table I.Comparison of distribution density and
ratio of CD1a+DCs and CD83+DCs between NP and nITM. |
Table I.
Comparison of distribution density and
ratio of CD1a+DCs and CD83+DCs between NP and nITM.
|
|
| Distribution density
of CD1a+ and CD83+DCs (cell/mm2) | Ratio of CD83+ and
CD1a+DCs |
|---|
|
|
|
|
|
|---|
| Group | n | CD1a+DCs | CD83+DCs | CD83+/CD1a+DCs |
|---|
| C | 10 | 11.1±4.9 | 8.7±3.3 | 0.78±0.67 |
| E | 30 | 39.8±3.5a | 26.9±2.3a |
0.68±0.66a |
Number of DCs
The results of the present study revealed that the
ratio of CD83+DCs/CD1a+DCs in Group C was significantly higher
compared with Group E (Fig. 3A,
Table I). The number of CD83+DCs was
significantly higher in nITM tissues compared with NP tissues.
Comparison of distribution area,
number and density of CD1a/CD40DC
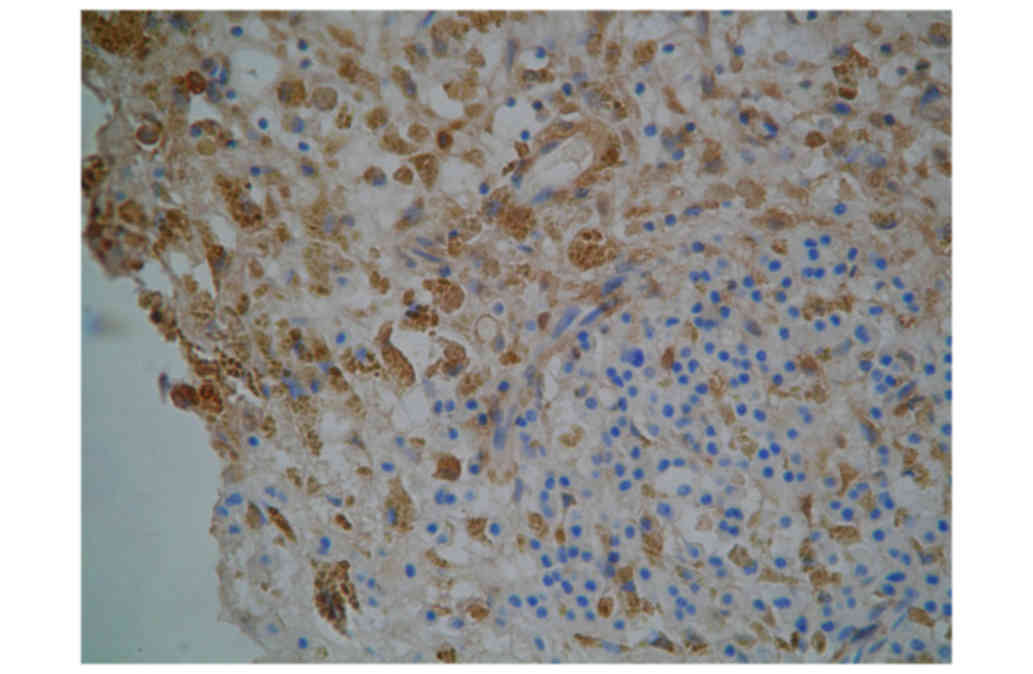
As presented in Fig.
3 and Table II, the total area
of CD1a/CD40 double stained DCs in nITM tissues (285.2±169.6
µm2) was significantly lower than compared with NP
tissues (3,417.3±755.1 µm2). Furthermore, the number and
density of CD1a/CD40 double stained DCs in nITM tissues (42.9±33.5
and 566±389 cells/cm2, respectively) were significantly
lower compared with NP tissues (692.3±247.1 and 7,327±2,429
cells/cm2, respectively).
 | Table II.Comparison of distribution area,
number and density of CD1a/CD40 DCs between NP and nITM. |
Table II.
Comparison of distribution area,
number and density of CD1a/CD40 DCs between NP and nITM.
| Group | n | Total area
(µm2) | Total number
(cells) | Density
(cells/cm2) |
|---|
| C | 10 |
285.2±169.6 | 42.9±33.5 | 566±389 |
| E | 30 | 3,417.3±755.1 | 692.3±247.1 | 7,327±2,429 |
| P-value |
| <0.01 | <0.01 | <0.01 |
Discussion
CD1a is a marker of DCs and the double phenotypes of
CD1a and CD40 used in the present study are specific DC markers.
Furthermore, CD40+ staining indicates that DCs are in the mature
phase and interacting with the T cells (11). The results of the present study
indicate that the number of DCs in nITM tissue is significantly
lower compared with NP tissues, which is consistent with previous
reports (12) and suggests that DCs
may serve an important role in the onset and development of NP.
CD40+ staining suggests that DCs may be associated with the
pathogenesis of NP, mainly via impacting the differentiation of T
cells (13). In addition, the
results of the present study revealed that CD83 was overexpressed
in the DCs of nITM tissues compared with NP, which was consistent
with previous studies (9,14). In addition, CD83 is a specific
surface molecule expressed in mature DCs, suggesting that DCs in NP
immature. Partially mature DCs express CD40, which then interacts
with T cells and mediates the immune responses (15).
In the present study it was demonstrated that DCs
are mainly distributed in the submucosal layer in NP tissues and
DCs near the epithelium are relatively dense. DCs most likely
infiltrate the lamina propria, explaining the gradual decrease from
the outside to the inside (14).
Such distribution characteristics of DCs may be due to the effect
of chemotactic adhesion factor; Yoshimi et al (16) observed that DCs are mainly located in
the squamous epithelium of NP and rarely distributed in the pseudo
stratified ciliated columnar epithelium. Yoshimi et al
considered that this may be due to the fact that keratinocytes are
mainly constructed by the squamous epithelium and are able to
secrete DC chemokines, including IL-1 and GM-CSF, to attract DCs
onto NP tissues via chemotaxis. The submucosal lamina propria is
the site in which DCs interact with Eosnophils (EOS) and T cells:
DCs are mainly distributed in the submucosal lamina propria and
EOS, T cells and other inflammatory cells mainly infiltrate the
submucosal lamina propria of NP (17). It has also been reported that (the
expression of Toll-like receptors (TRL-2/TRL-4) in NP are
significantly enhanced and mainly expressed in the submucosa
(18). DCs are able to upregulate
TRLs (19). The present study,
combined with these previous reports, suggests that DCs upregulate
TRLs in NP to identify pathogen associated molecular patterns
(PAMPs). Interactions of PAMPs with TLRs on DCs leads to the
maturation of DCs via the NF-κB pathway during an immune response,
which may result in the secretion of major histocompatibility
complex class II, over-expression of costimulatory molecules and
the secretion of proinflammatory cytokines (IL-1, TNF-α, IL-6, or
IL-12). T cells may then become activated by these costimulatory
molecules and cytokines, which further promotes the immune response
(20).
The results of the present study show that the DCs
in NP are significantly increased and are CD40-positive, suggesting
that DCs may activate the expression of B7-1 (CD80) and B7-2 (CD86)
by binding to T cells' CD40L; the B7 molecule expressed by DCs may
then activate the T cells via the CD28/CTLA-4 pathway (21). This antigen presentation process is a
two-way process, in which DC mediates the activation of T cells and
receives an activation signal feedback from T cell to enter the
so-called terminal mature stage, during which DCs highly express
CD80/CD86 on their surface and lose their adhesiveness and
phagocytosis (22). The interaction
between DCs and T cells may lead to T cell maturation and cytokine
secretion, which may induce a greater infiltration of DCs and T
cells into NP tissues, resulting in the increased number of DCs and
T cells observed in the present study, which further exacerbates
NP.
Increase numbers of DCs and T cells in NP tissues
produces several effects. The increase of DCs and T cells in NP is
crucial to maintain the Th1/Th2 ratio in NP (23), with predominant expression of the Th2
cytokine. This may be achieved via various mechanisms: i) Jahnsen
et al (24) demonstrated that
an increase in the number of DC2 in the mucosa induces Th2, thus
resulting in a significant increase in Th2 cells in NP and
upregulation of the Th2 cytokines; ii) DC-induced maturation and
differentiation of T cells may occur via connecting to surface
molecules and secreting cytokines. DC-expressed CD86 is the
costimulatory molecule that causes Th0 to differentiate into Th2.
CD80 mainly induces the differentiation of Th1 (25), and DC is able to cause an imbalance
of Th1/Th2 in NP by altering the CD80/CD86 ratio; iii) When T cells
interact with DCs, they are able to induce DCs to secrete IL-12 via
CD40/CD40L and IL-12 is the most important cytokine during Th0
differentiation into Th1 cells (26). However, IL-10 has been reported to be
highly expressed in NP (27). Reider
et al (28) also confirmed
that IL-10 acting on DCs may cause defective IL-12 secretion, thus
inhibiting the differentiation of T cells into Th1 cells while
inducing their polarization toward Th2; iv) DCM is able to induce
the production of IL-4 in DCs (29).
IL-4 is the most important cytokine during Th0 cell differentiation
into Th2 cells. IL-4 is increased in NP tissues, thus prompting the
Th0 cells to differentiate toward the Th2 cells. The Th2 cells can
also secrete IL-4 and this cycle can promote the dominance of Th2
in NP. In addition, IL-4 can promote the B cells to transform
toward the plasma cells and secrete IgE (30); IgE overexpression is known to be one
of the factors associated with NP onset and its increase is
positively correlated with EOS infiltration (31).
The increased number of DCs and T cells in NP is
also responsible for the overexpression of IL-5 in NP. It has been
reported that DCs are able to induce T cells to secrete IL-5
(32). IL-5 is one of the most
important cytokines and has been confirmed to be associated with
the onset and development of NP (33). During the early stages of NP, Th2
cells are the main source of IL-5; however, as the disease
progresses, EOS gradually replaces Th2 and becomes a direct source
of IL-5 in NP (34). It has been
reported that IL-5 is able to directly inhibit the differentiation
and maturation of DCs (35).
Therefore, DCs may promote the infiltration of EOS by inducing the
IL-5 secretion pathway in T cells in NP. The infiltration of EOS
may then inhibit the differentiation and maturation of DCs by
secreting IL-5, thus keeping the DCs in an immature stage to
promote the development of NP.
In summary, the present study demonstrated that the
submucosal layer of NP has a large number of DCs, whereas the ratio
of CD83+ DCs is relatively low in NP, indicating that more DCs are
in non-mature stages. Partially mature DCs interact with T cells
via the CD40/CD40L costimulatory factor, thus serving an important
role in the development and progression of NP. This process may be
regulated by the immune response signaling pathway. The results of
the present study indicate that the interaction between DCs and T
cells is associated with the pathogenesis of NP, which might
further contribute to Th1/Th2 imbalance and cytokine secretion.
However, there were a number of limitations in the present study,
including the small sample size, which needs to be addressed.
Future studies should investigate the signaling
pathways involved in the interaction between DC and T-cells in NP
at the cytological and molecular levels. Closer attention should be
paid to the interaction between DCs and T cells to develop targets
for the prevention and treatment of NP, thus helping to improve our
understanding of the pathogenesis of NP.
Acknowledgements
Not applicable.
Funding
The study was supported by the Medical Science
Research Foundation of Guangdong Province (A2015080).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL collected the samples; XZ performed
immunohistochemistry and immunostaining; CL performed statistical
analysis; XW planned the study and wrote the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Shantou Central Hospital (Shantou, China). Written
informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Collin M, McGovern N and Haniffa M: Human
dendritic cell subsets. Immunology. 140:22–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banchereau J, Briere F, Caux C, Davoust J,
Lebecque S, Liu YJ, Pulendran B and Palucka K: Immunobiology of
dendritic cells. Annu Rev Immunol. 18:767–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie ZX, Zhang HL, Wu XJ, Zhu J, Ma DH and
Jin T: Role of the immunogenic and tolerogenic subsets of dendritic
cells in multiple sclerosis. Mediators Inflamm. 2015:5132952015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanos V, Berry KE, Seikkula J, Raad Abi E,
Stavroulis A, Sleiman Z, Campo R and Gordts S: The management of
polyps in female reproductive organs. Int J Surg. 43:7–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hulse KE, Stevens WW, Tan BK and Schleimer
RP: Pathogenesis of nasal polyposis. Clin Exp Allergy. 45:328–346.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varricchi G, Bagnasco D, Borriello F,
Heffler E and Canonica GW: Interleukin-5 pathway inhibition in the
treatment of eosinophilic respiratory disorders: Evidence and unmet
needs. Curr Opin Allergy Clin Immunol. 16:186–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim DW, Eun KM, Jin HR, Cho SH and Kim DK:
Prolonged allergen exposure is associated with increased thymic
stromal lymphopoietin expression and Th2-skewing in mouse models of
chronic rhinosinusitis. Laryngoscope. 126:E265–E272. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng KJ, Zhou ML, Xu YY and Zhou SH: The
role of local allergy in the nasal inflammation. Eur Arch
Otorhinolaryngol. 274:3275–3281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin XS, Luo XY, Wang HG, Li CW, Lin X and
Yan C: Expression and distribution of dendritic cells in nasal
polyps. Exp Ther Med. 5:1476–1480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang WF and Wang SZ: Expression of CD1a,
CD80, CD86 in dendritic cell of tumor tissue and regional lymph
node in esophageal carcinoma. Ai Zheng. 23:189–192. 2004.(In
Chinese). PubMed/NCBI
|
|
11
|
Tay NQ, Lee DCP, Chua YL, Prabhu N,
Gascoigne NRJ and Kemeny DM: CD40L expression allows CD8+ T cells
to promote their own expansion and differentiation through
dendritic cells. Front Immunol. 8:14842017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Till SJ, Jacobson MR, O'Brien F, Durham
SR, KleinJan A, Fokkens WJ, Juliusson S and Löwhagen O: Recruitment
of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic
rhinitis and effects of topical corticosteroid therapy. Allergy.
56:126–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elizondo D, Andargie T, Kubhar D, Gugssa A
and Lipscomb M: CD40-CD40L cross-talk drives fascin expression in
dendritic cells for efficient antigen presentation to CD4+ T cells.
Int Immunol. 29:121–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang Z, Yang T, Xu W, Huang Y, Jiang L,
Yin Z and Qin G: The role of dendritic cells in immune regulation
of nasal polyps. Histol Histopathol. 32:87–97. 2017.PubMed/NCBI
|
|
15
|
Ju X, Silveira PA, Hsu WH, Elgundi Z,
Alingcastre R, Verma ND, Fromm PD, Hsu JL, Bryant C, Li Z, et al:
The analysis of CD83 expression on human immune cells identifies a
unique CD83+-activated t cell population. J Immunol. 197:4613–4625.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimi R, Takamura H, Takasaki K and
Kumagami H: Immunohistologic study of the nasal mucosa with
reference to Langerhans cells. Nihon Jibiinkoka Gakkai Kaiho.
96:1252–1257. 1993.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Rijt LS, Kuipers H, Vos N, Hijdra D,
Hoogsteden HC and Lambrecht BN: A rapid flow cytometric method for
determining the cellular composition of bronchoalveolar lavage
fluid cells in mouse models of asthma. J Immunol Methods.
288:111–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mulligan JK, White DR, Wang EW, Sansoni
SR, Moses H, Yawn RJ, Wagner C, Casey SE, Mulligan RM and Schlosser
RJ: Vitamin D3 deficiency increases sinus mucosa dendritic cells in
pediatric chronic rhinosinusitis with nasal polyps. Otolaryngol
Head Neck Surg. 147:773–781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berzi A, Varga N, Sattin S, Antonazzo P,
Biasin M, Cetin I, Trabattoni D, Bernardi A and Clerici M:
Pseudo-mannosylated DC-SIGN ligands as potential adjuvants for HIV
vaccines. Viruses. 6:391–403. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raymond CR and Wilkie BN: Toll-like
receptor, MHC II, B7 and cytokine expression by porcine monocytes
and monocyte-derived dendritic cells in response to microbial
pathogen-associated molecular patterns. Vet Immunol Immunopathol.
107:235–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grewal IS, Foellmer HG, Grewal KD, Xu J,
Hardardottir F, Baron JL, Janeway CA Jr and Flavell RA: Requirement
for CD40 ligand in costimulation induction, T cell activation, and
experimental allergic encephalomyelitis. Science. 273:1864–1867.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitajima T, Caceres-Dittmar G, Tapia FJ,
Jester J, Bergstresser PR and Takashima A: T cell-mediated terminal
maturation of dendritic cells: Loss of adhesive and phagocytotic
capacities. J Immunol. 157:2340–2347. 1996.PubMed/NCBI
|
|
23
|
Mulligan JK, Mulligan RM, Atkinson C and
Schlosser RJ: Human sinonasal epithelial cells direct dendritic
function and T-cell T helper 1/T helper 2 skewing following
Aspergillus exposure. Int Forum Allergy Rhinol. 1:268–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jahnsen FL, Lund-Johansen F, Dunne JF,
Farkas L, Haye R and Brandtzaeg P: Experimentally induced
recruitment of plasmacytoid (CD123high) dendritic cells in human
nasal allergy. J Immunol. 165:4062–4068. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuchroo VK, Das MP, Brown JA, Ranger AM,
Zamvil SS, Sobel RA, Weiner HL, Nabavi N and Glimcher LH: B7-1 and
B7-2 costimulatory molecules activate differentially the Th1/Th2
developmental pathways: Application to autoimmune disease therapy.
Cell. 80:707–718. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelsall BL, Stüber E, Neurath M and
Strober W: Interleukin-12 production by dendritic cells. The role
of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad
Sci. 795:116–126. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faith A, Singh N, Farooque S, Dimeloe S,
Richards DF, Lu H, Roberts D, Chevretton E, Lee TH, Corrigan CJ and
Hawrylowicz CM: T cells producing the anti-inflammatory cytokine
IL-10 regulate allergen-specific Th2 responses in human airways.
Allergy. 67:1007–1013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reider N, Reider D, Ebner S, Holzmann S,
Herold M, Fritsch P and Romani N: Dendritic cells contribute to the
development of atopy by an insufficiency in IL-12 production. J
Allergy Clin Immunol. 109:89–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Redecke V, Häcker H, Datta SK, Fermin A,
Pitha PM, Broide DH and Raz E: Cutting edge: Activation of
Toll-like receptor 2 induces a Th2 immune response and promotes
experimental asthma. J Immunol. 172:2739–2743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sánchez-Segura A, Brieva JA and Rodríguez
C: T lymphocytes that infiltrate nasal polyps have a specialized
phenotype and produce a mixed TH1/TH2 pattern of cytokines. J
Allergy Clin Immunol. 102:953–960. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sokolovska A, Hem SL and HogenEsch H:
Activation of dendritic cells and induction of CD4(+) T cell
differentiation by aluminum-containing adjuvants. Vaccine.
25:4575–4585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rissoan MC, Soumelis V, Kadowaki N,
Grouard G, Briere F, de Waal Malefyt R and Liu YJ: Reciprocal
control of T helper cell and dendritic cell differentiation.
Science. 283:1183–1186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun C, Ouyang H and Luo R: Distinct
characteristics of nasal polyps with and without eosinophilia. Braz
J Otorhinolaryngol. 83:66–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dhariwal J, Cameron A, Trujillo-Torralbo
MB, Del Rosario A, Bakhsoliani E, Paulsen M, Jackson DJ, Edwards
MR, Rana BMJ, Cousins DJ, et al: Mucosal type 2 innate Lymphoid
cells are a key component of the allergic response to
aeroallergens. Am J Respir Crit Care Med. 195:1586–1596. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yi H, Zhang L, Zhen Y, He X and Zhao Y:
Dendritic cells induced in the presence of GM-CSF and IL-5.
Cytokine. 37:35–43. 2007. View Article : Google Scholar : PubMed/NCBI
|