Introduction
Explosives are major lethal weapons in modern
warfare, and blast-related traumatic brain injury (bTBI), which is
known as the ‘signature damage’ (1),
is of considerable interest to researchers. bTBI is a complex
injury, which includes primary (injury caused by blast waves),
secondary (injury caused by high-speed shrapnel and fragments),
ternary (injuries caused by the throwing, rotation and distortion
of shock waves or by squeezing occurring due to the collision of
objects) and quaternary (damage caused by other non-high-speed
injury factors, including harmful gases, heat and light) (1).
Injury to parts of the body other than the head may
also affect the evolution of brain injury, which further
complicates the study of brain injury (2–11).
Currently, there is no mature and stable animal model of brain
blast injury (12,13), and the current major types of injury
used in modelling include hydraulic, shock tube and weight drop
injuries (7–21). Previously, a simple brain blast
injury model using real blasts with protection of the trunk was
designed by the present research group (22), and this model had aroused the
attention and discussion of experts (23,24),
thereby laying a reliable foundation for the present study.
Diffuse axonal injury (DAI) is a common cause of
severe disabilities, vegetative states and fatality in patients
with traumatic brain injury, and is typically associated with
severe conditions, poor prognoses, treatment difficulties and high
mortality rates (25,26). Currently, the mechanisms of DAI are
considered to involve linear or angular acceleration initially
generated through external forces, followed by a shearing force
generated within the brain tissue, which results in axonal nerve
damage or fracture and capillary damage (27,28).
Previous studies have suggested a variety of animal models for the
study of DAI. These include the instantaneous rotation injury model
in which Gennarelli et al (14) designed a shearing stress-induced
axonal injury model through instantaneous head rotation, which
caused angular acceleration and generated shearing stress in the
brain. Secondly, a hydraulic shock injury model has been created;
the traumatic brain injury model was first established by Dixon
et al (15) in 1987, who
induced axonal injury through drilling in the middle of the animal
skull, causing injury to the midline structure with the impact.
Meythaler et al (26) applied
this model in the study of DAI. A third model is Marmarou's weight
drop model. Marmarou et al (16) improved the weight drop injury model,
which is typically used to study local craniocerebral trauma, by
placing rats on foam pads, gluing a steel helmet with dental
acrylic onto the skull vertex of the rat, and causing DAI by
dropping a weight onto the helmet. Fourthly, a complex injury model
of rotation and pounding has been proposed. Wang et al
(17) generated a complex injury
model through the combination of the instantaneous rotation and
weight drop injury models, in which DAI was created with
instantaneous linear and angular acceleration to induce compound
injury. Fifthly, an acceleration or deceleration injury model has
also been established (18). In this
model, whole brain tissue was subjected to an inertia load through
accelerated or decelerated motion to generate stress in the brain
tissue, which resulted in neuronal and fibre injury (18). A sixth model is the stretch injury
model. Gennarelli et al (14)
exerted traction force directly on the nerve fibres in vitro
to generate injuries similar to those observed in rotation,
acceleration or deceleration. Seventhly, a local blast injury model
was described by Garman et al (19), who conducted blast exposure in rats
with body shielding. Finally, a whole-body injury model has been
established, in which Säljö et al (20) conducted shock tube injury without any
shielding. However, these models generally exhibit poor stability
and do not fully represent the DAI observed in bTBI.
Based on the previous bTBI model (22), the present study investigated a means
of creating DAI through real blast injury using a novel approach
involving instantaneous high-speed swinging of the rat head,
thereby establishing a stable animal model of blast DAI.
Materials and methods
Animals and equipment
The Experimental Animal Center of Sichuan University
(Chengdu, China) provided 32 adult (1:1 ratio of male:female)
Sprague-Dawley rats [animal certification number: SCXK (Chuan)
2009-09], weighing 212.2±16.2 g and aged 42.2±1.7 days. They were
housed in polycarbonate cages with hard wood chips at a temperature
of 23±2°C and a humidity of 55±5% with a 12 light/dark cycle. Food
and drinking water were available ad libitum. After a 1-week
acclimation period, the animals were subjected to the treatments.
The present study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and the present study was approved by the
Institutional Animal Care and Use Committee (IACUC) of Sichuan
University.
Rats were randomly divided into experimental (n=16),
control (n=10) and sham control (n=6) groups. The frontal, parietal
and occipital cortices of rats in the experimental group were
exposed to the blast; rats in the control group were not exposed to
the blast, and rats in the sham control group were anesthetized as
described below and attached to the craniocerebral blast device but
not subjected to blasting. A self-designed craniocerebral blast
injury modelling apparatus was used (Fig. 1) to fix the rats in position. The
device was assembled using 19 aluminium alloy bars and 4 aluminium
alloy plates, with a size of 1,000×460×350 mm and a weight of 9.0
kg. Electric detonators (Chongqing Shun'an Civil Explosive
Equipment Co., Ltd., Chongqing, China) and sensors were used to
deliver a blast equivalent to 400 mg trinitrotoluene (containing
100 mg di-N-nonyl phthalate and 250 mg
cyclotrimethylenetrinitramine; density, 1.816 g/cm3;
detonation velocity, 4,000 m/sec; detonation pressure, 280 kPa).
Following a blast injury, the head was scanned a Bruker Biospec
70/30 7.0T magnetic resonance imaging (MRI) scanner (Bruker
Corporation, Billerica, MA, USA) at the Research Center of
Molecular Imaging, West China Hospital of Sichuan University.
Wavebook/516A Stress Test System (IOtech, Inc.; National
Instruments Corporation, Austin, TX, USA) and piezoelectric
pressure sensors (113A31; Piezotronics, Inc., Depew, NY, USA) were
used to the collection and analysis of shock wave parameters. A
Redlake HG-LE high-speed camera device from the Red River Computer
Co., Inc. (Claremont, NH, USA) at Daping Hospital, Third Military
Medical University was used for the collection and analysis of head
swelling parameters. A Leica RM2135 microtome (Leica Microsystems
GmbH; Wetzlar, Germany) and BP3100s electronic scales (Sartorius,
Tokyo, Japan) were used in the generation of paraffin sections for
pathological examination. For evaluation the load-stroke curves of
the spring, an electronic universal mechanical testing machine
(AG-IC 20 kN; Shimadzu Corporation, Tokyo, Japan) was used at the
Shimadzu Department of Material Engineering, Sichuan
University.
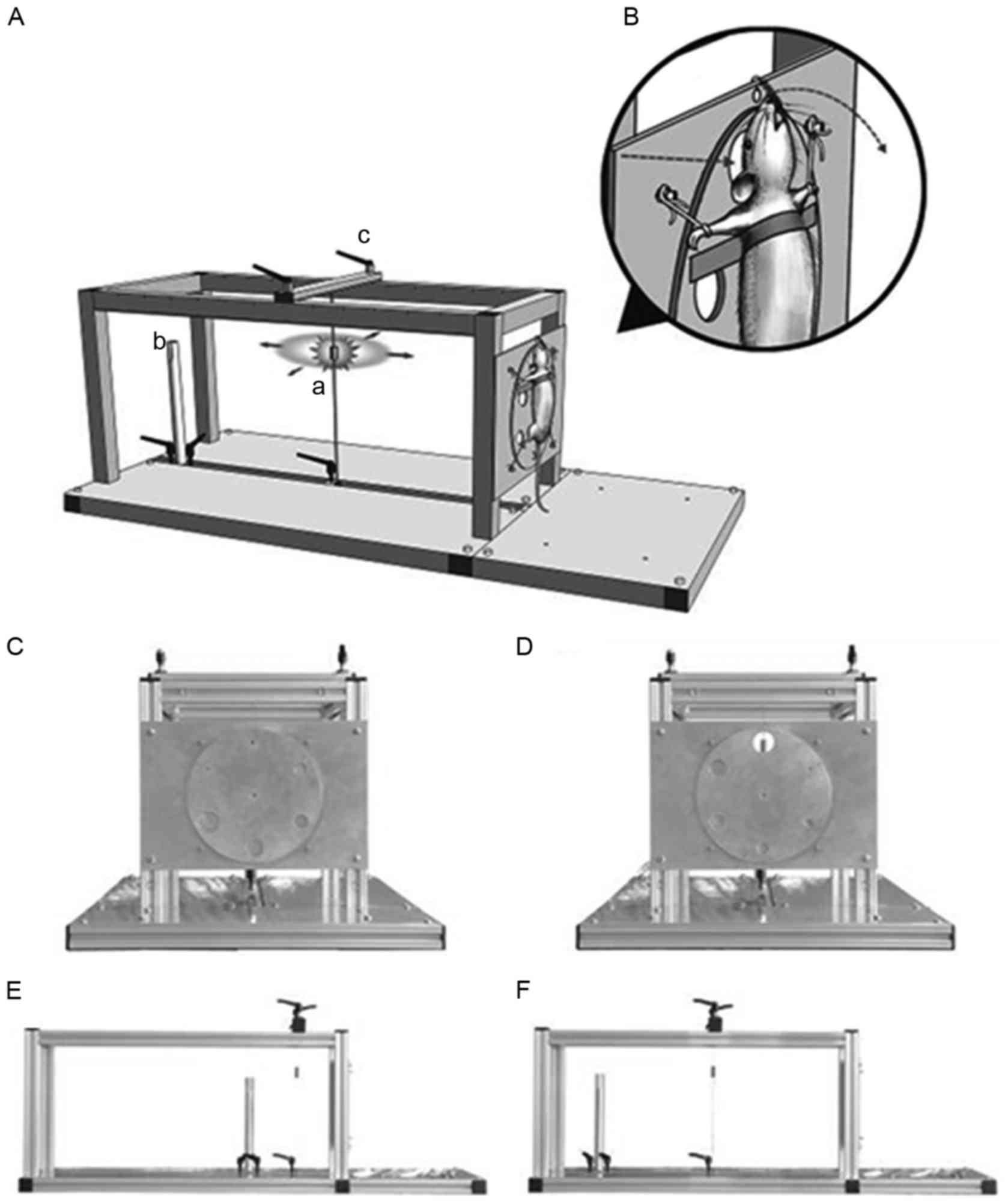 | Figure 1.(A) Craniocerebral blast device. The
device was assembled using 19 aluminium alloy bars and 4 aluminium
alloy plates, with a size of 1,000×460×350 mm, and a weight of 9.0
kg. a, blast source (electric detonators); b, piezoelectric
pressure detector fixtures; and c, locking screws for the securing
of the sliding blocks. (B) Diagram of the local model. The straight
arrow indicates that the explosive shock wave caused head injury
through the blast window; the curved arrow indicates the direction
of head swing at the time of explosion. (C and D) Six round holes
with a diameter of 5–30 mm (pitch, 5 mm) were distributed evenly
around the disc (diameter, 240 mm). The disc could be flipped to
adjust the aperture size and change the exposure range. The blast
source, piezoelectric pressure sensors and the centre of blast
window were aligned. (E and F) The distance between the blast
source and the blast window was adjustable to change the shock wave
overpressure. The distance between piezoelectric pressure sensors
and the detonator was approximately midway between the detonators
and the blast window, and the overpressure of the explosion shock
wave was indirectly reflected using the values measured. |
Model preparation
Rats were anesthetized through the intraperitoneal
injection of 50 mg/kg sodium pentobarbital and then fixed on a
protective plate in an erect position; the head was fixed in an
upward position using a carbon spring steel wire GB4357 65 Mn tooth
hook (Shenzhen Nai Li Da Hardware Products Co., Ltd., Shenzhen,
China; Fig. 2), and the position of
the rat and the round blast window were adjusted to place the
frontal, parietal and occipital parts of rats from the experimental
group in the blast window, while the body below the foramen magnum,
and the mouth and face above the inner canthus of the eyes were
fully protected. The blast window was closed to rats in the control
group, so that they were not directly exposed to the blast.
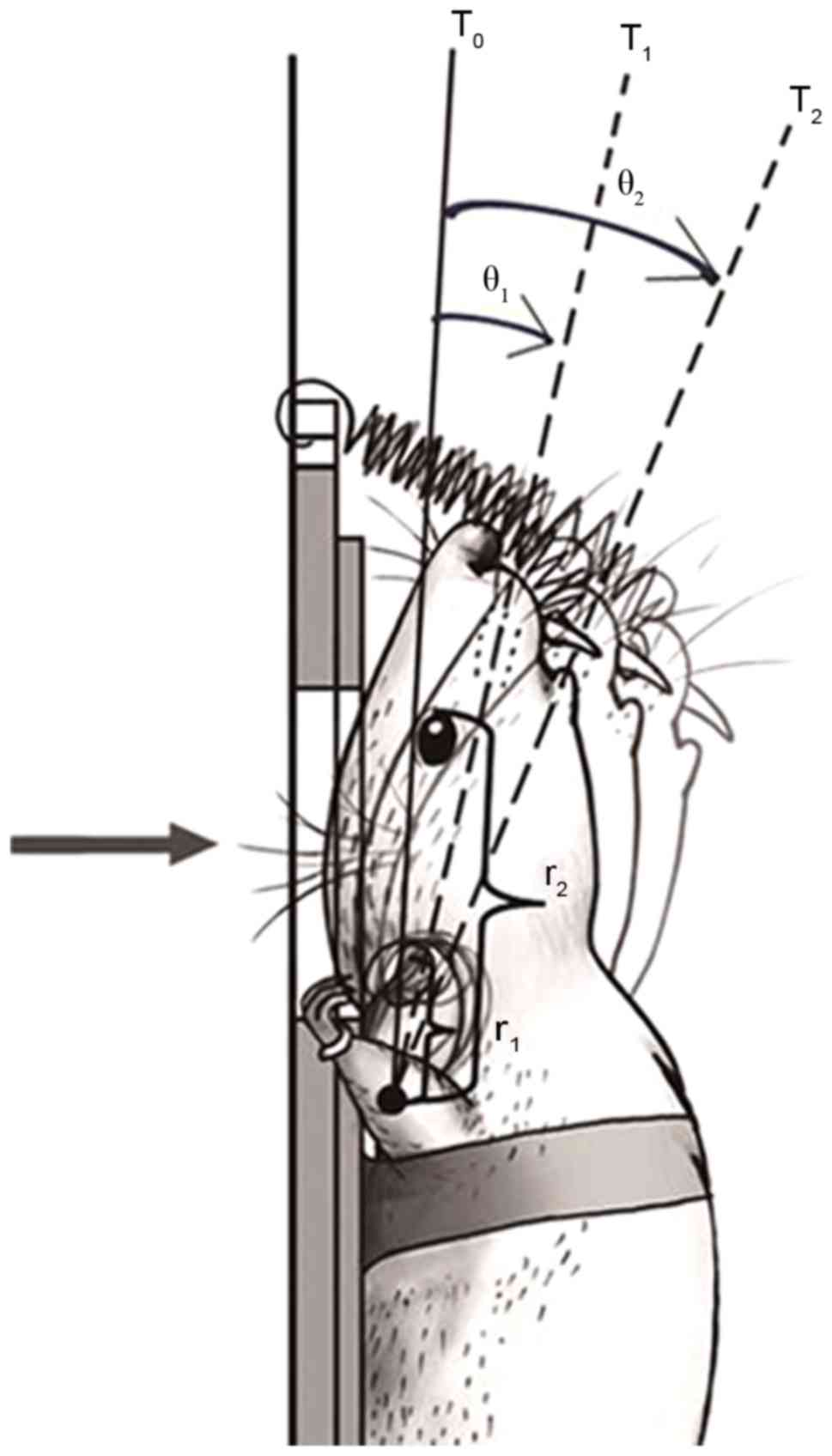 | Figure 2.Schematic diagram of the head
fixation and head swing. A self-made spring hanging hook was fixed
to the top of the device at one end, the other end was used to hook
the rat incisor. The solid line indicates the initial position of
the explosion, the dotted line indicates the head position at 1 and
2 msec after explosion. The arrow shows the direction of the blast
wave, and the curved arrow demonstrates the corresponding swing
angle of each time period. r1, the distance between the external
auditory foramen and the axis of swing; r2, distance between the
inner canthus and the axis of swing; T0, explosion;
T1, 1 msec after explosion; T2, 2 msec after
explosion; θ1, the swing angle within 1 msec;
θ2, the swing angle within 2 msec. |
Measurement of blast waves
Based on previous experimental data (22), the distance between the piezoelectric
pressure sensors, electric detonators and the exposed head of the
rat were all adjusted to 10 cm, and the sensors were at the same
height as the head; the sensor signals were connected to the
Wavebook/516A data acquisition system. Following initiation of the
electric detonator, the blast overpressure was recorded using a
data acquisition system, and subsequently filtered and analysed
using Origin 7.0 software (OriginLab Corporation, Northampton, MA,
USA).
Measurement of biomechanical
parameters of the sagittal head swing using a high-speed
camera
High-speed filming was conducted perpendicular to
the sagittal plane of the rats, with an image capture frequency of
1,000 times/sec, i.e., one sampling per 1 msec. According to the
results of preliminary experiments, the explosion caused repeated
back-forth head swinging. The initial amplitude of the swing was
the largest, and it took ~5 msec to achieve the maximum swing
displacement (data not shown). Due to the limited speed of camera
sampling, the sample sizes of the data were relatively small, and
the credibility of the curve-fitting equation was not high; thus,
it was difficult to accurately describe the head swing during
explosion. Therefore, the head swinging process was divided into
five successive stages (T1-T5), presented in
chronological order, assuming that each movement within 1 msec was
a uniformly accelerated swing, and a separate examination was
conducted to obtain a general understanding of the entire process.
Five consecutive time periods T1-T5 after the
explosion were selected for parametric analysis. The five captured
pictures were completely overlapped, and a line connecting the
supraorbital rim with the external auditory foramen was set as the
calibration line and marked as five straight lines, where the
intersection of the straight lines represented the swing axis
(Fig. 2). It was initially assumed
that each movement within 1 msec was a uniformly accelerated swing,
and the swing angle was measured as θn (n=1–5), and
according to the formulae, the equations of motion were calculated
as follows:
ωn=(θn-θn-1)xπx103/180(red/sec)(θ0=0)αn=(ωn-ωn-1)x103(red/sec2)(ω0=0)V1n=ωnxr1(m/sec)V2n=ωnxr2(m/sec)
ωn is the mean angular velocity of each
time period; αn is the angular acceleration of each time
period; v1n is the line speed at the external auditory
foramen; v2n is the speed at the internal canthus;
r1 is the distance between external auditory foramen and
the axis of swing; and r2 is the distance between the
inner canthus and the axis of swing. The swing of the external
auditory foramen and that of the middle brainstem exhibited the
same radius and similar linear speed, which may therefore be
considered as the indirect examination of the swing of the brain
stem. Similarly, the inner canthus was adopted as the reference for
prefrontal movements.
Observation of clinical symptoms
The rats lost their corneal reflex when they were
anesthetized. The duration of the suppression of the corneal reflex
was used as an index of traumatic unconsciousness. The duration of
the recovery of other reflexes was also assessed following
anaesthesia. The reflexes were assessed according to the
methodology of Fijalkowski et al (29). The following changes were also
assessed: Number of cases of respiratory arrest, spontaneous
breathing recovery time, number of cases of seizures or convulsions
in the limbs after injury.
MRI T2 sequence scanning of the
head
MRI of the head was conducted four times at 2, 12,
24 and 48 h after injury. Each rat was anesthetized with isoflurane
(2–3%) in a small container and maintained with a mixture of 100%
oxygen and isoflurane (1–2%) during the MRI scan. The body
temperature was kept constant using a heating blanket at 37°C
monitored with a rectal temperature probe. Each animal was placed
prone in a surface coil. The ECG signal was obtained from two
subcutaneous copper needles loaded in the left forelimb and hind
limb. Respiration signals were acquired from a respiratory pillow
(SA Instruments Inc., Stony Brook, NY, USA) under the rat. Images
were recorded using a 7T MRI scanner with a volume coil (outer
diameter, 44 mm; inner diameter, 23 mm) and ParaVision 5.0 software
(Bruker Corporation). The MRI sequences included RARE-T2
(repetition time, 3,000 msec; echo time, 45 msec; slice thickness,
1 mm; field of view, 18 mm; matrix, 256×256).
Pathological examination of brain
tissue
The rats were decapitated while still anesthetized
immediately following the final MRI examination at 48 h, and a
rapid craniotomy was performed to assess the general traumatic
craniocerebral injury. Specimens of brain stem tissue were
extracted and subjected to 10% formalin-fixation at room
temperature for 48 h, dehydration with different concentrations of
ethanol, vitrification with xylene and embedding in paraffin.
Subsequently, 5-µm slices were obtained and subjected to
hematoxylin staining for 5 min at room temperature and eosin
staining for 20 sec at room temperature and observed using light
microscopy.
Statistical analysis
All statistical analyses were performed using SPSS
version 16.0 software (SPSS, Inc., Chicago, IL, USA). Continuous
variables are presented as the mean ± standard deviation and
categorical variables are presented as absolute value with the
percentage in parentheses. Fisher's exact test was used to compare
the rates of respiratory arrest and short convulsions in the limbs
among the three groups. One-way analysis of variance followed by
least significant difference tests were used to compare the
different study groups with regard to continuous variables,
including the peak pressure and rise time. The frequencies of
categorical variables were compared using Pearson χ2 or
Fisher's exact test, when appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
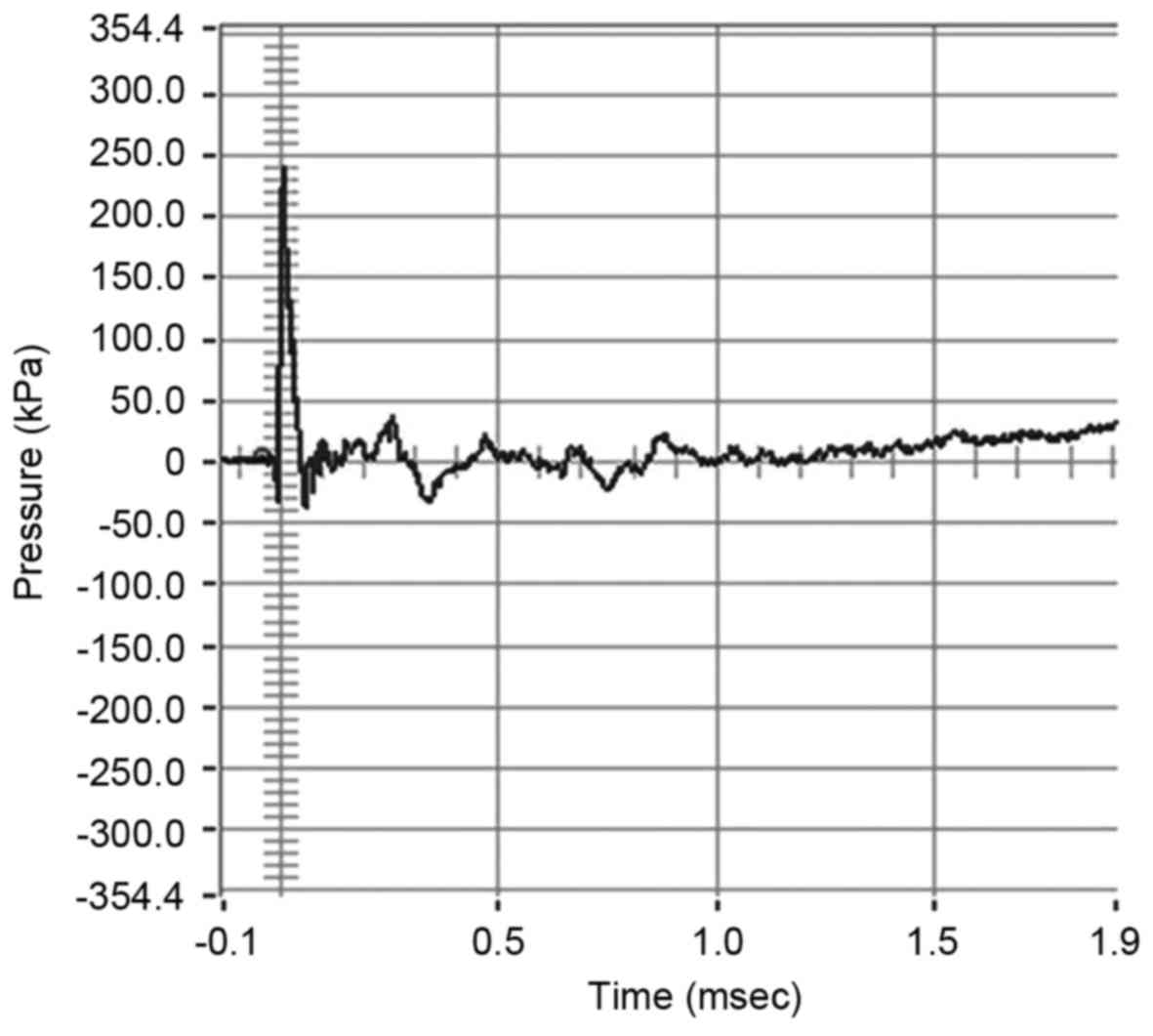
Test of detonator blast
The waveform and composition of the blast waves is
shown in Fig. 3. Analysis of the
results demonstrated that the parameters of blast exposure,
including peak pressure and blast impulse were relatively constant
among the three groups (P>0.05; Table
I).
 | Table I.Parameters of blast overpressure. |
Table I.
Parameters of blast overpressure.
| Groups | Peak pressure
(kPa) | Rise time
(msec) | Duration
(msec) | Blast impulse
(Pa.sec) |
|---|
| Control | 238.86±18.34 | 0.014±0.004 | 0.048±0.004 | 32.67±1.59 |
| Sham control | 239.16±13.25 | 0.012±0.004 | 0.039±0.002 | 31.23±1.39 |
| Experimental | 240.52±17.54 | 0.013±0.005 | 0.051±0.003 | 30.55±2.09 |
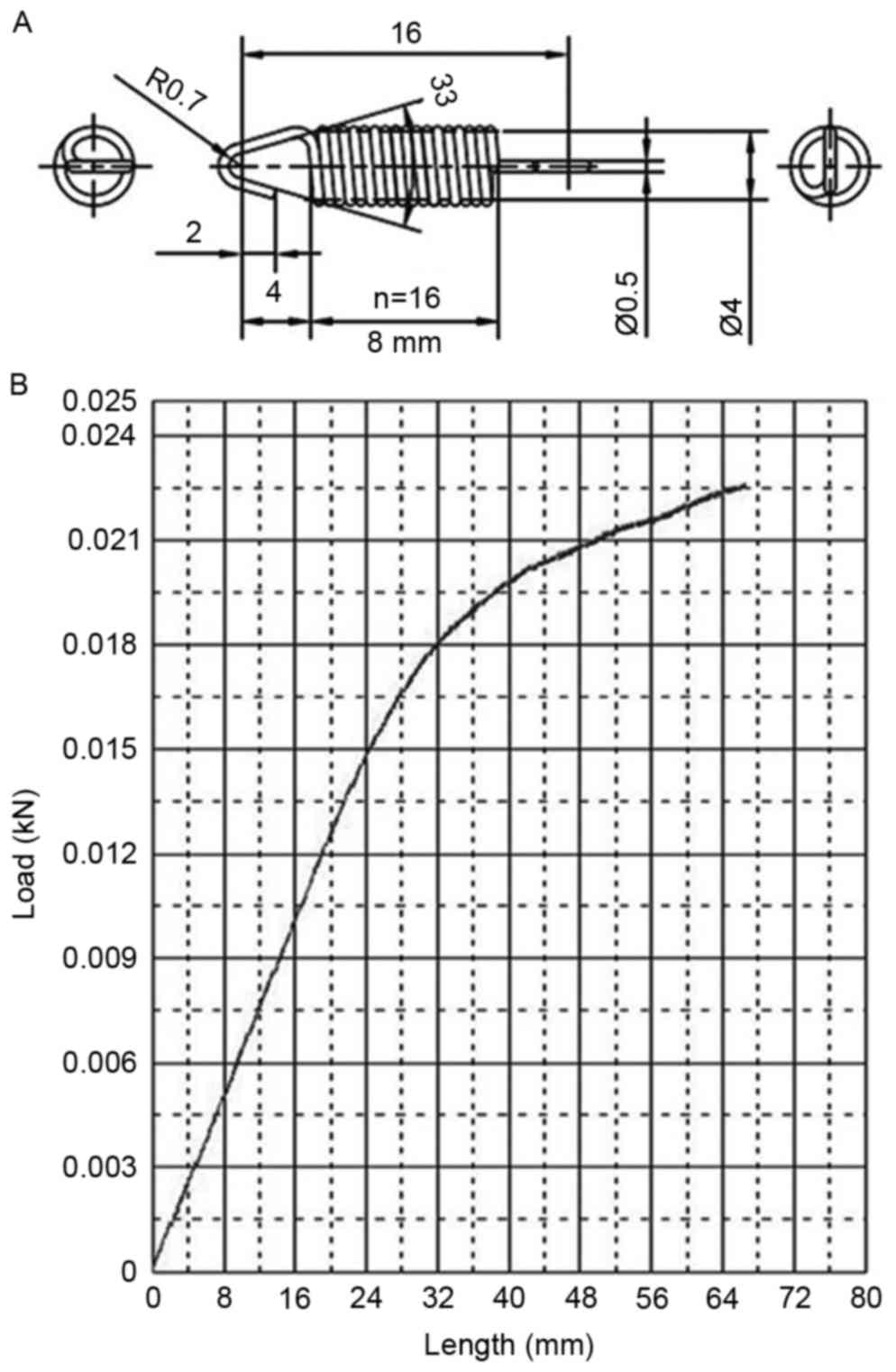
Spring parameters
The detailed parameters of the spring and the
elastic are presented in Fig. 4. The
stretching length of the spring was in the 0–24 mm range at
explosion, and this distance in the load-stroke curves were almost
in a straight line, indicating a stable and reliable spring
tension.
Biomechanical parameters of the
sagittal head swing
No obvious head swing was noted in the control group
at the instantaneous moment of explosion; a curved head swing was
observed at the sagittal plane in the control group, and the swing
axis was roughly at the cervical-thoracic junction (Fig. 2). The five successive stages
(T1-T5) of the head swinging process were
analyzed. The mean angular velocity, angular acceleration and line
speed at the external auditory foramen are presented in Table II. The measurement of the
biomechanical parameters of the head swings during the explosion
demonstrated that the swing went through an
acceleration-deceleration cycle. The momentary head swing caused
the non-linear acceleration-deceleration of brain tissues, inducing
shear stress perpendicular to the swing axis. The amount of the
shear stress is associated with the swing radius, the weight and
density of the brain tissue. This hypothesis was confirmed through
measurements of the line speed at the external auditory foramen and
inner canthus, which revealed differences in the swing radius. The
line speeds of the middle brain stem (the external auditory
foramen) and prefrontal area (the internal canthus) were
significantly different, with the latter speed ~3 times that of the
former (Table II). Using time as
the abscissa and the mean angular velocity and angular acceleration
as the vertical axis, a Cartesian coordinate system was established
and used to monitor the corresponding data points.
 | Table II.Measurement of the biomechanical
parameters of the head swing in the experimental group (n=16). |
Table II.
Measurement of the biomechanical
parameters of the head swing in the experimental group (n=16).
| Time period | T1 | T2 | T3 | T4 | T5 |
|---|
| Mean angular
velocity (rad/sec) | 160.2±4.3 | 240.3±3.8 | 236.8±4.1 | 168.2±3.7 | 10.3±2.2 |
| Mean angular
acceleration (krad/sec2) | 161.7±6.5 | 60.9±4.9 | −58.9±5.1 | −115.4±5.4 | −202.3±9.5 |
| Line speed at the
external auditory foramen (m/sec)a | 4.038±0.015 | 6.274±0.021 | 5.978±0.019 | 4.286±0.023 | 0.233±0.011 |
| Speed at the
internal canthus (m/sec)b | 12.836±0.034 | 19.154±0.023 | 17.648±0.017 | 11.197±0.018 | 0.522±0.013 |
Clinical observation
The number of cases of brief respiratory arrest,
mean recovery time of spontaneous breathing, number of cases of
short convulsions in the limbs, and mean duration from anaesthesia
to the recovery of the corneal reflex of the rats in the
experimental group were significantly higher than those of the rats
in the control group (P<0.05; Table
III).
 | Table III.Clinical observation of rats. |
Table III.
Clinical observation of rats.
| Group | Respiratory arrest,
n (%) | Mean recovery time
of spontaneous breathing (sec) | Short convulsions
in the limbs, n (%) | Recovery time of
corneal reflex from anaesthesia (h) |
|---|
| Control | 1 (10) | 2.2±0.1 | 1 (10) | 1.2±0.2 |
| Sham control | 0 (0) | 0 | 0 (0) | 1.3±0.1 |
| Experimental | 13
(81.3)a,b |
8.6±0.2a,b | 12
(75)a,b |
12.5±0.2a,b |
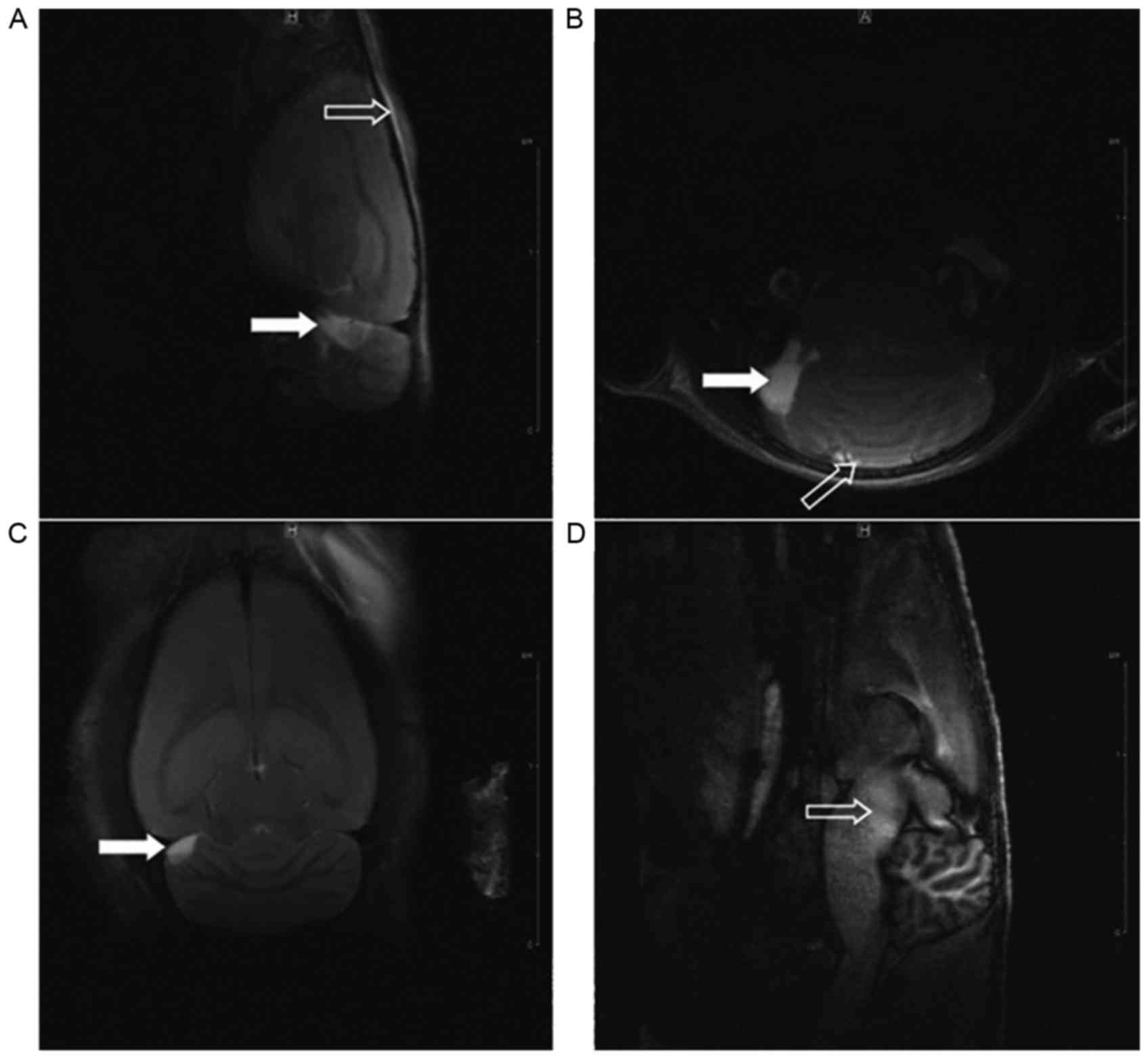
MRI T2 sequence scanning of rat
heads
Complete skulls were observed in the three groups,
with no evident fracture, displacement or compression. At 2 h after
injury, abnormal brain signals in the injured regions of rats in
the experimental group were detected, and the signal change was
most evident at 24 h after injury; however, no marked difference
was observed between 48 and 24 h after injury in the experimental
group. The results at 24 h indicated 14 cases of mixed signals at
the upper dorsolateral brainstem in the experimental group, of
which 6 cases exhibited contusions at the frontal cortex and top
ventrolateral cerebellum (Fig. 5);
furthermore, 1 case of a contusion at the frontal cortex was
observed in the control group.
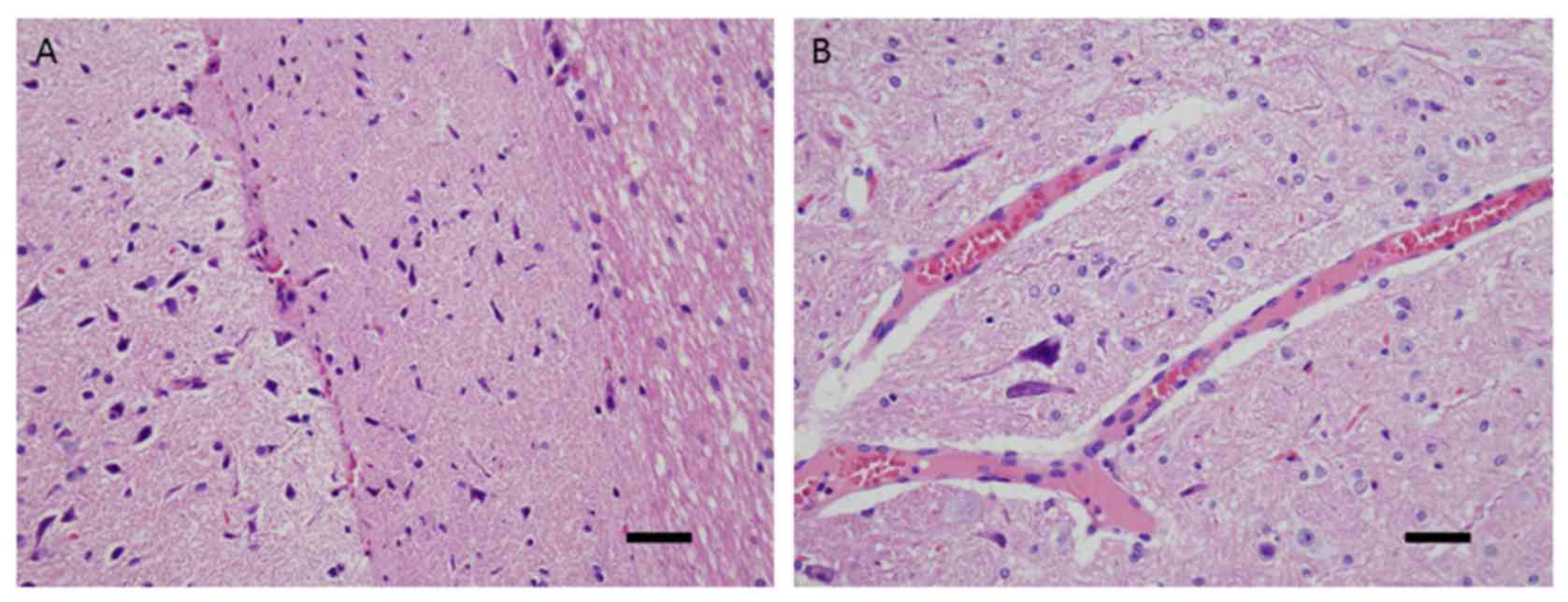
Histopathological examination of rat
brain stems
The pathological results obtained in the
experimental group were 2 cases of basal cell subarachnoid
haemorrhage, 6 cases of focal brain contusions and 14 cases of
diffuse swelling and congestion of the brain stem, with marked
regional damage in the upper dorsolateral brainstem. The following
morphological changes were observed using microscopy: Triangular,
hyperchromatic nerve cell nuclei in the brainstem injury zone, with
a thin cell body (Fig. 6A); and
capillary congestion and expansion, sedimentation of erythrocytes
and peripheral oedema (Fig. 6B).
Conversely, the brain stems obtained from the control and sham
control groups were normal.
Discussion
Although there are a variety of animal models of
DAI, it is widely accepted that DAI is caused through shear stress,
and this injury occurs almost ubiquitously throughout bTBI, which
suggests an association of bTBI with DAI (30). Based on this understanding, the
present study improved upon a previously investigated rat brain
blast injury model (22) that caused
rat head swinging with a detonator explosion shock wave in order to
generate shearing stress, and subsequently induce DAI.
The present study model included a range of
characteristics. Firstly, the model allowed for simulation of the
real clinical traumatic brain blast injury. In the previous DAI
models, the instantaneous rotation, hydraulic shock, weight drop,
rotation and pounding, acceleration or deceleration injury were
unable to completely reproduce traumatic brain blast injury, which
made it difficult to conduct research on the occurrence of DAI at
the moment of craniocerebral blast injury. Therefore, in the
present study, the explosion of an electric detonator was used to
cause injury. Furthermore, the present study presented a simplified
model for evaluating the complex mechanisms of injury. The
initiation of the electric detonator ensured that no
fragment-induced injuries occurred within the effective injury
distance, which minimized the possibility of secondary injury.
Additionally, fixation of the limbs of the experimental animals
avoided ternary injury, and the masking exposure method, where only
the head was exposed to injury, effectively protected other parts
of the rat, thus avoiding the possibility of brain injury induced
through the chest, abdominal squeezing or damage to other organs,
and reduced quaternary injury. In addition, reproduction of the
present study model is simple and economical, and the modelling
apparatus itself is removable and easily assembled.
Shearing force has an important role in the
development of DAI and is associated with acceleration, which
varies according to the shape and weight of the animal. Due to rat
anatomical features, including a short neck and low weight, the
shearing force from whole exposure injury on rats is far less
compared with the exposure to humans (17). Thus, certain DAI models have been
obtained using tightly coupled repetitive blast-induced traumatic
brain injury (11). Head swinging
was used in the present study to increase the destructive effect of
the shearing force and to imitate DAI in humans, with a spring to
reduce excess swing amplitude in order to avoid and reduce cervical
injury.
According to previous experimental results for the
rat brain blast injury model (22),
a detonation distance of 10 cm was set; the blast overpressure over
this distance was ~238.86±18.34 kPa, which is able to cause stable
bTBI in adult rats. The tension-elastic parameters of the self-made
spring were measured to obtain a reference for replication of the
model. Due to the anatomical features of the brain stem, a wide
radius range was expected for swinging in the sagittal plane, with
an increased line speed causing an increase in the corresponding
generated shear stress. Furthermore, the direction of loading
(i.e., the direction of the force and the swing) was perpendicular
to the longitudinal axis of the vast majority of the brain stem
fibres, suggesting that the brain stem is susceptible to
acceleration-deceleration movement. In addition, the brain stem and
the distal brain have different compositions, resulting in large
differences in their weight and density (29); thus, when the brain stem and distal
brain were subjected to identical external forces, different speeds
would result, thus generating shear stress. These findings indicate
that shear stress was an important factor for injury in the present
DAI model.
To the best of our knowledge, no recorded data
concerning DAI caused by sagittal swing exists. In the present
study, injury was induced through head tilt and biomechanical
parameters were compared. However, Margulies et al (31) measured the threshold of the
mechanical parameters of baboon head tilt-induced DAI as an angular
velocity of 260 rad/sec and an angular acceleration of 100
krad/sec2, which suggested that the lower the weight of
the brain tissue, the higher the speed of the angular velocity and
angular acceleration required to cause injury. In addition, Maxwell
et al (32) measured the
angular acceleration of DAI in the baboon model as 100–200
krad/sec2. Furthermore, using the DAI model established
through rat head tilt, with an angular velocity of 801.27 rad/sec
and an angular acceleration of 204.4 krad/sec2,
Xiao-Sheng et al (21)
measured the brain surface speed as 6.010±0.078 m/sec, with a
turning radius of ~0.0075 m. The maximum angular velocity of the
present model was 248 rad/sec, and the maximum angular acceleration
was ~212 krad/sec2. As the swing radius at the sagittal
plane was larger than the radius of the tilt swing, and the
intensity of the swing was no less than that observed in the models
described above, the present established model may be considered
reliable.
As no clear clinical diagnostic criteria of DAI
exist at present, assessment of the injury in the present study was
based on the observation of three relevant indicators of rats after
injury: Clinical manifestations, MRI imaging and the pathological
examination of brain tissue. Regarding the clinical manifestations,
in addition to the manifestations of bTBI, such as respiratory
arrest and convulsions in the limbs, there were 12 cases (75%) in
the experimental group in which the duration from the beginning of
anaesthesia to the recovery of the corneal reflex was >12 h,
which are characteristic symptoms consistent with the clinical
manifestations of DAI (30). MRI
imaging results of rat brains in the experimental group indicated
two major types of injury, namely cortical contusions and brain
stem injury. The former may reflect the conductance of the blast
shock wave through the scalp and skull to reach the brain tissue,
thereby causing injury. A contrecoup injury could also be observed.
In the present study, brain stem injury was the primary basis for
the clinical diagnosis of DAI, which was characterized by mixed
signals on the upper brain stem and clear turns in the fibres
during acceleration or deceleration, which generated shearing
stress and resulted in the injury and fracture of fibres. The test
results obtained at different time points revealed abnormal brain
signals in the injured area at 2 h after injury; however, signal
changes were most evident at 24 h after injury, and no significant
changes were observed between 24 and 48 h after injury. The present
results revealed that changes in the signal intensity in the
injured area peaked at 24 h after the injury, with no fading, even
at 48 h after the injury, which was consistent with the clinical
congestion and swelling of the brain tissue affected by DAI. Brain
pathology results at 48 h after injury demonstrated characteristic
pathological changes of DAI. In conclusion, 14 cases (87.5%) in the
experimental rat group were indicated to exhibit blast DAI, and no
DAI was observed in the rats from the control group. The
frequencies of categorical variables were compared using Pearson
χ2 or Fisher's exact test, when appropriate. A value of
P<0.05 was considered significant. The experimental design
provided a stable model of rat blast DAI.
Several disturbance factors, including individual
differences among animals, the blast environment and the impact of
anaesthesia, existed in this model. To increase the credibility of
the results, rats that were the same age, a fixed weight, and had a
fixed feeding method were used. Standard atmospheric pressure and
room temperature (22°C), with no noise disturbances, and a
reduction of the influence of other controllable factors including,
light, humidity and airflow, were set as a unified environment for
the blast. Although intraperitoneal anaesthesia may affect result,
the application of anaesthesia is essential to ensure the accurate
exposure of the injury site to the blast at a specific distance and
direction.
Some potential reasons for variability of the brain
damage include i) uneven local pressure, ii) thermal damage and
iii) fine fragment injury. The present model may be improved in two
ways: The addition of another stable shock wave-generating source,
with adjustable pressure and temperature settings, to replace the
electric detonators used in this study, and the use of a special
helmet to partially protect the head of the rat.
In conclusion, although previous studies of bTBI
have demonstrated that DAI is a significant outcome, and various
animal models have been established in accordance with the
complicated mechanism of DAI, these models are not widely accepted
in the field of neurology. Currently, there is no standard animal
model for DAI. Although it does not represent simple DAI injury,
the model developed in the present study may be useful for research
regarding bTBI and DAI. Under the appropriate protection, the DAI
occurrence rate remained high (87.5%) in the present model, and
bTBI is likely to occur with DAI in real-life settings. In further
research, remote craniocerebral and systemic exposure injuries with
partial protection of the head and exposure of the trunk will be
examined to compare brain electrical activity, cerebral blood flow,
brain water content, intracranial pressure changes and
immunohistochemical changes to further explore the specific
mechanisms of brain blast injury and improve understanding of the
blast characteristics of DAI. The present findings may have useful
applications in clinical diagnosis and treatment, and the
development of protective equipment.
Acknowledgements
The authors would like to thank Mr Shoucheng Huang
(Chengdu New Innovation Sci-Tech Co., Ltd., Chengdu, China),
Professor Lu Min, Dr Li Yunming (General Hospital of People's
Liberation Army Chengdu Military Region, Chengdu, China) and Dr
Kang Jianyi (The Sixth Research Center of the Institute of Surgery
Research, Third Military Medical University, Chongqing, China) for
their excellent technical support.
Funding
The present study was supported by the Key Medical
Grant of 11th five years' plan from The Chinese People's Liberation
Army (grant no. 08Z011), the National Basic Research Program of
China (grant no. 2011CB935800), the Public Health Training Program
of the Capital (grant no. Z151100003915125) and the National
Natural Science Foundation of China (grant no. 31770386).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHZ performed the model preparation and was a major
contributor in writing the manuscript. JWG designed and supervised
the experiment. BCL performed blast waves measurement. FBG
performed magnetic resonance imaging T2 sequence head scanning. XML
measured the biomechanical parameters of the sagittal head swing.
SJC performed pathological examinations of the brain tissue. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal use protocol was reviewed and approved by
the Institutional Animal Care and Use Committee (IACUC) of Sichuan
University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interests.
References
|
1
|
Benzinger TL, Brody D, Cardin S, Curley
KC, Mintun MA, Mun SK, Wong KH and Wrathall JR: Blast-related brain
injury: Imaging for clinical and research applications: Report of
the 2008 st. Louis workshop. J Neurotrauma. 26:2127–2144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Y and Wang ZG: Blast-induced
traumatic brain injury: A new trend of blast injury research. Chin
J Traumatol. 18:201–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinzelmann M, Reddy SY, French LM, Wang
D, Lee H, Barr T, Baxter T, Mysliwiec V and Gill J: Military
personnel with chronic symptoms following blast traumatic brain
injury have differential expression of neuronal recovery and
epidermal growth factor receptor genes. Front Neurol. 5:1982014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valiyaveettil M, Alamneh YA, Miller SA,
Hammamieh R, Arun P, Wang Y, Wei Y, Oguntayo S, Long JB and Nambiar
MP: Modulation of cholinergic pathways and inflammatory mediators
in blast-induced traumatic brain injury. Chem Biol Interact.
203:371–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtney A and Courtney M: The complexity
of biomechanics causing primary blast-induced traumatic brain
injury: A review of potential mechanisms. Front Neurol. 6:2212015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edwards MJ, Lustik M, Carlson T, Tabak B,
Farmer D, Edwards K and Eichelberger M: Surgical interventions for
pediatric blast injury: An analysis from Afghanistan and Iraq 2002
to 2010. J Trauma Acute Care Surg. 76:854–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YC, Smith DH and Meaney DF: In-vitro
approaches for studying blast-induced traumatic brain injury. J
Neurotrauma. 26:861–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wei Y, Oguntayo S, Wilkins W, Arun
P, Valiyaveettil M, Song J, Long JB and Nambiar MP: Tightly coupled
repetitive blast-induced traumatic brain injury: Development and
characterization in mice. J Neurotrauma. 28:2171–2183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Svetlov SI, Prima V, Kirk DR, Gutierrez H,
Curley KC, Hayes RL and Wang KK: Morphologic and biochemical
characterization of brain injury in a model of controlled blast
overpressure exposure. J Trauma. 69:795–804. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling G, Bandak F, Armonda R, Grant G and
Ecklund J: Explosive blast neurotrauma. J Neurotrauma. 26:815–825.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Risling M, Plantman S, Angeria M, Rostami
E, Bellander BM, Kirkegaard M, Arborelius U and Davidsson J:
Mechanisms of blast induced brain injuries, experimental studies in
rats. Neuroimage. 54 Suppl 1:S89–S97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bell MK: Standardized model is needed to
study the neurological effects of primary blast wave exposure. Mil
Med. 173:v–viii. 2008.PubMed/NCBI
|
|
13
|
Zhao Y, Zhao Y, Zhang M, Zhao J, Ma X,
Huang T, Pang H, Li J and Song J: Inhibition of TLR4
signalling-induced inflammation attenuates secondary injury after
diffuse axonal injury in rats. Mediators Inflamm. 2016:47069152016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gennarelli TA, Thibault LE, Tipperman R,
Tomei G, Sergot R, Brown M, Maxwell WL, Graham DI, Adams JH, Irvine
A, et al: Axonal injury in the optic nerve: A model simulating
diffuse axonal injury in the brain. J Neurosurg. 71:244–253. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon CE, Lyeth BG, Povlishock JT,
Findling RL, Hamm RJ, Marmarou A, Young HF and Hayes RL: A fluid
percussion model of experimental brain injury in the rat. J
Neurosurg. 67:110–119. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marmarou A, Foda MA, van den Brink W,
Campbell J, Kita H and Demetriadou K: A new model of diffuse brain
injury in rats. Part I: Pathophysiology and biomechanics. J
Neurosurg. 80:291–300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HC, Duan ZX, Wu FF, Xie L, Zhang H
and Ma YB: A new rat model for diffuse axonal injury using a
combination of linear acceleration and angular acceleration. J
Neurotrauma. 27:707–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishimoto T and Murakami S: Relation
between diffuse axonal injury and internal head structures on blunt
impact. J Biomech Eng. 120:140–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garman RH, Jenkins LW, Switzer RC III,
Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE,
Clark RS, et al: Blast exposure in rats with body shielding is
characterized primarily by diffuse axonal injury. J Neurotrauma.
28:947–959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Säljö A, Bao F, Haglid KG and Hansson HA:
Blast exposure causes redistribution of phosphorylated
neurofilament subunits in neurons of the adult rat brain. J
Neurotrauma. 17:719–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao-Sheng H, Sheng-Yu Y, Xiang Z, Zhou F
and Jian-ning Z: Diffuse axonal injury due to lateral head rotation
in a rat model. J Neurosurg. 93:626–633. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Gu J, Ma Y, Yang T, Kuang Y, Li B
and Kang J: Development of a rat model for studying blast-induced
traumatic brain injury. J Neurol Sci. 294:23–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elder GA, Sosa Gama MA, De Gasperi R,
Stone JR, Dickstein DL, Haghighi F, Hof PR and Ahlers ST: Vascular
and inflammatory factors in the pathophysiology of blast-induced
brain injury. Front Neurol. 6:482015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huo J, Liu J, Wang J, Zhang Y, Wang C,
Yang Y, Sun W and Xu S: Early hyperbaric oxygen therapy inhibits
aquaporin 4 and adrenocorticotropic hormone expression in the
pituitary gland of rabbits with blast-induced craniocerebral
injury. Neural Regen Res. 7:1729–1735. 2012.PubMed/NCBI
|
|
25
|
Gennarelli TA, Thibault LE, Adams JH,
Graham DI, Thompson CJ and Marcincin RP: Diffuse axonal injury and
traumatic coma in the primate. Ann Neurol. 12:564–574. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meythaler JM, Peduzzi JD, Eleftheriou E
and Novack TA: Current concepts: Diffuse axonal injury-associated
traumatic brain injury. Arch Phys Med Rehabil. 82:1461–1471. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hill CS, Coleman MP and Menon DK:
Traumatic axonal injury: Mechanisms and translational
opportunities. Trends Neurosci. 39:311–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siedler DG, Chuah MI, Kirkcaldie MT,
Vickers JC and King AE: Diffuse axonal injury in brain trauma:
Insights from alterations in neurofilaments. Front Cell Neurosci.
8:4292014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fijalkowski RJ, Stemper BD, Pintar FA,
Yoganandan N, Crowe MJ and Gennarelli TA: New rat model for diffuse
brain injury using coronal plane angular acceleration. J
Neurotrauma. 24:1387–1398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma J, Zhang K, Wang Z and Chen G: Progress
of research on diffuse axonal injury after traumatic brain injury.
Neural Plast. 2016:97463132016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Margulies SS, Thibault LE and Gennarelli
TA: Physical model simulations of brain injury in the primate. J
Biomech. 23:823–836. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maxwell WL, Watt C, Graham DI and
Gennarelli TA: Ultrastructural evidence of axonal shearing as a
result of lateral acceleration of the head in non-human primates.
Acta Neuropathol. 86:136–144. 1993. View Article : Google Scholar : PubMed/NCBI
|