Introduction
Due to improvements in living standards, the number
of patients with type 2 diabetes has been increasing; in 1990
3.8/100,000 individuals were diabetic, however in 2010 4.9/100,000
were diabetic (1,2). According to a survey released by The
New England Journal of Medicine in March 2014, the morbidity rate
of individuals aged >20 years old with diabetes in China was
~9.1% (2). In addition, ~140 million
individuals are classed as pre-diabetic patients in China (3). Diabetic retinopathy (DR) is one of the
primary microvascular complications of diabetes and the majority of
patients with diabetes suffer from a certain level of DR (4). Previous studies have demonstrated that
the morbidity rate of DR is high and leads to severely impaired
eyesight (1,4). In order to inhibit the progression of
diabetes, it is essential to diagnose the condition early and
control blood glucose levels. There is currently no cure for DR;
therefore, further studies investigating the pathogenesis of DR are
required (1).
As a small single-stranded RNA molecule with a
length of 21–25 nucleotides, microRNA (miRNA) participates in
regulating molecular growth, differentiation, proliferation and
apoptosis. miRNA binds to target mRNA molecules through
complementary base paring (5).
Increasing amounts of evidence demonstrates that miRNA serves an
important role in regulating diabetes and its complications
(5). Furthermore, miRNA regulates DR
through a number of biological signaling pathways, including
phosphatidylinositol 3-kinase/RAC-alpha serine/threonine-protein
kinase, transforming growth factor β-1 and Wnt (6).
DR is a disease that may lead to blindness in
individuals between 20 and 70 years of age, and is therefore an
important area of research (7). A
number of previous studies have suggested that different
pro-inflammatory factors participate in the occurrence and
progression of DR (8,9). This provides novel directions for
future research investigating the pathogenesis of DR and for
potential treatments for DR using anticytokine agents.
Diabetes is a chronic metabolic disease
characterized by hyperglycemia resulting from defects in insulin
production or insulin resistance, and is associated with a high
morbidity rate and a number of complications (7). Previous studies have revealed that type
1 and 2 diabetes present as mild systemic inflammation, resulting
from the production and secretion of proinflammatory cytokines
following the stimulation of innate immune cells by endogenous and
exogenous ligands (10,11). For example, free fatty acids and
glycosylated terminal products induce an increase in leukocyte
adhesion molecules, proinflammatory cytokines and haemopoietic
factors by combining with Toll-like receptors (TLRs) to activate
nuclear factor (NF)-κB-associated signaling pathways (12). As a state of mild systemic
inflammation, diabetes is characterized by an increase in numerous
proinflammatory cytokines, including tumor necrosis factor (TNF)-α,
interleukin (IL)-6, IL-1β, C-reactive protein, plasminogen
activator inhibitor-1 and adiponectin (13).
Materials and methods
Cell culture
Human retinal pigment epithelial (RPE)-1 cells were
purchased from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China) and maintained in Dulbecco's
modified Eagle's medium: Nutrient Mixture F-12 (DMEM-F12)
supplemented with 10% fetal bovine serum, 100 units/ml penicillin
and 100 µg/ml streptomycin (all Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in a tissue culture incubator at 37°C with
5% CO2.
Cell viability assay
Cell viability was determined using MTT reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RPE-1 cells
(1×103 cell/ml) were incubated with different doses of
glucose (0, 1, 5, 10, 20, 50 and 100 mM; Guoyao Group Chemical
Reagent Co., Ltd., Shanghai, China) in DMEM-F12 for 12 or 24 h at
4°C. Subsequently, 0.5 mg/ml MTT solution was added and the cells
were incubated for 4 h at 37°C. Then, the supernatants were removed
and dimethyl sulfoxide was added for 20 min at 37°C. Cell viability
was measured at 490 nm using a microplate reader
(Multiskan® EX; Thermo Fisher Scientific, Inc.).
miRNA reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from RPE-1 cells using the
TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Total RNA was
reverse-transcribed into cDNA using the PrimeScript® RT
Master Mix (Perfect Real Time) assay kit (Takara Bio, Inc., Shiga,
Japan) according to the manufacturer's protocol. RT-qPCR was
performed using a SYBR® Premix Ex Taq™ kit
(Takara Bio, Inc.) according to the manufacturer's protocol. qPCR
was completed using 500 ng of cDNA and the SYBR-Green Real-Time PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions for PCR included a total of 40 cycles
as follows: 5 min at 95°C; 30 sec at 95°C; 60 sec at 30°C; and 30
min at 72°C. The following primer sequences were used: miRNA27a
forward, 5′-ACAGGCTAGCGCCGCCTAAC-3′ and reverse,
5′-CCTTAAGGCCCAAGATTACG-3′; and U6 forward,
5′-TCGCTTCGGCAGCACATATAC-3′ and reverse
5′-TATGGAACGCTTCACGAATTTG-3′. Expression levels were quantified
using the 2−ΔΔCq method (14).
Small interfering (si)RNA and
immunostimulatory (is)RNA transfection
The sequences of siRNA directed against miRNA27a
(si-miRNA27a; GenePharma Co., Ltd., Shanghai, China) were as
follows: Sense, 5′-GCGGAACUUAGCCACUGUGAA-3′ and antisense,
5′-CAGUACUUUUGUGUAGUACAA-3′. The sequences of isRNA directed
against TLR4 (isRNA-TLR4; GenePharma Co., Ltd.) were as follows:
Sense, 5′-GGACUUGAAAGACCUUGGATT-3′ and antisense,
5′-UCCAAGGUCUUUCAAGUCCTC-3′. RPE-1 cells (1×105 cell/ml)
were transfected with 200 ng of si-miRNA27a and/or isRNA-TLR4 using
a Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Transfected RPE cells were incubated with glucose for 24 h prior to
subsequent assays.
Caspase-3/9 activity assay
A total of 1×106 transfected RPE-1 cells
were washed with ice-cold PBS and protein extracted using
radioimmunoprecipitation assay (RIPA) buffer (Promega Corporation,
Madison, WI, USA). The amount of total protein was quantified using
a BCA Protein assay kit (Pierce Biotechnology; Thermo Fisher
Scientific, Inc.). Total protein (20 µg) was incubated with
Ac-DEVD-pNA and Ac-LEHD-pNA (both Beyotime Institute of
Biotechnology, Haimen, China) for 1 h at 37°C, substrates for
Caspase-3 and 9, respectively. Caspase-3/9 activity was
subsequently measured at 405 nm using a microplate reader
(Multiskan EX).
Western blot analysis
Transfected RPE cells were washed with ice-cold PBS
and protein extracted using RIPA buffer. The amount of total
protein was quantified using a BCA Protein assay kit (Pierce
Biotechnology; Thermo Fisher Scientific, Inc.). Total protein (50
µg) was separated by 8–12% SDS-PAGE and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membrane was blocked with 5% skimmed milk-Tris-buffered
saline-Tween-20 for 1 h at 37°C. The membrane was then incubated
with rabbit polyclonal primary antibodies directed against B-cell
lymphoma 2-associated X protein (Bax; cat. no. sc-6236), TLR4 (cat.
no. sc-10741) (both 1:500; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and GAPDH (cat. no. sc-25778; 1:2,000; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) overnight at 4°C, followed by
incubation with horseradish peroxidase-conjugated secondary
antibodies (cat. no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature. The blots were visualized using
enhanced chemiluminescence reagent (Boster Biological Technology,
Ltd., Wuhan, China) and analysed using Image Lab software (version
3.0; Bio-Rad Laboratories, Inc.).
ELISA to assess the expression of
IL-6, IL-1β and TNF-α
Transfected RPE-1 cells (1×105 cell/ml)
were washed with ice-cold PBS and centrifuged at 12,000 × g for 15
min at 4°C. The expression of IL-6 (cat. no. D6050), IL-1β (cat.
no. DLB50) and TNF-α (cat. no. DTA00C) in the cell culture
supernatant were measured using ELISA kits (R&D Systems Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol. The
ELISA plates were then measured at 450 nm using a microplate reader
(Multiskan® EX) to determine the expression of IL-6,
IL-1β and TNF-α.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Two-tailed Student's t-tests were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Glucose suppresses RPE cell viability
and mRNA27a expression
In the current study, RPE cells were treated with a
series of glucose concentrations (0, 1, 5, 10, 20, 50 and 100 mM)
for 12 or 24 h, after which the expression of mRNA27a was measured.
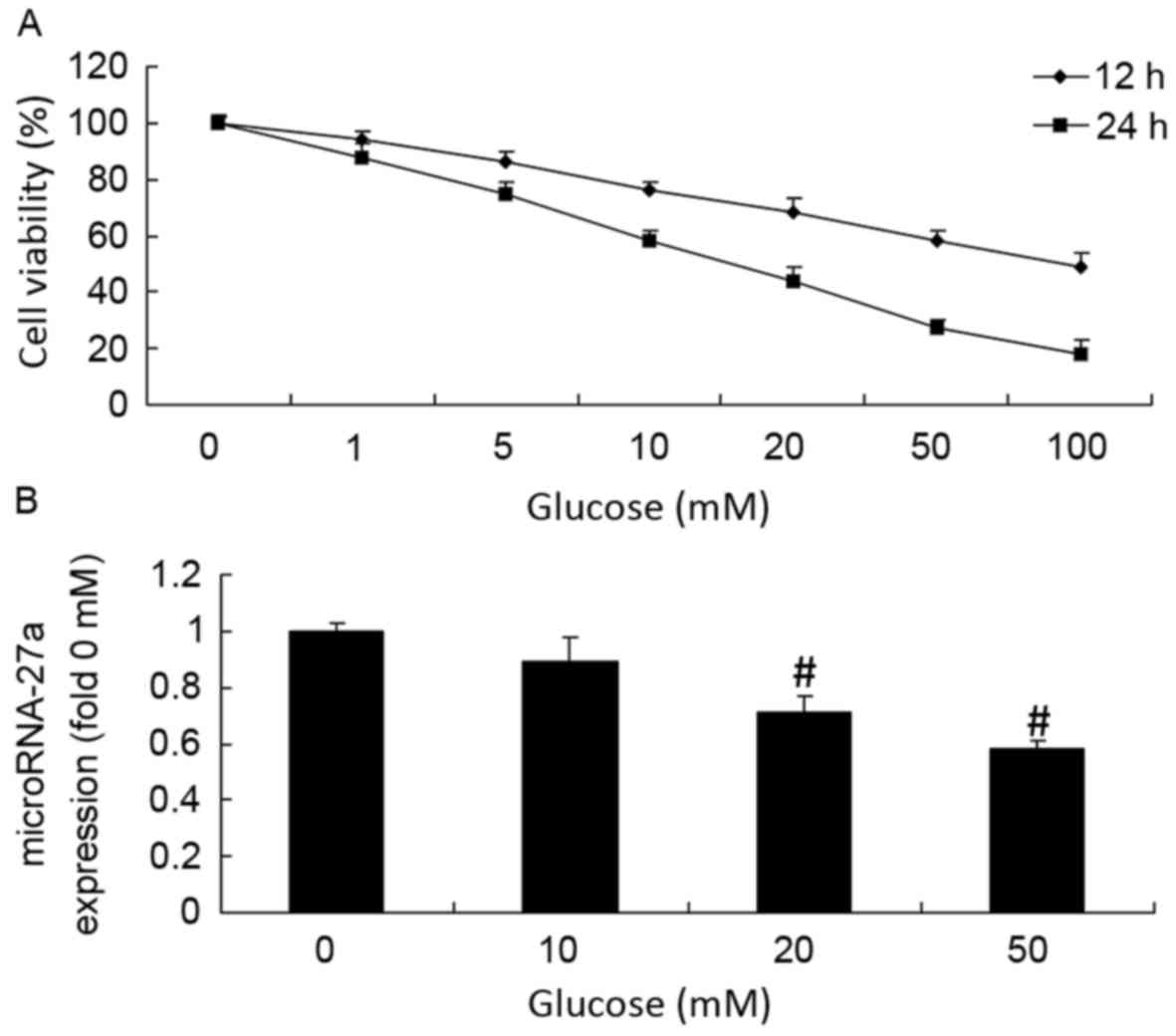
Glucose treatment was demonstrated to reduce the RPE cell viability
in a dose- and time-dependent manner (Fig. 1A). Notably, 20 mM glucose reduced the
number of viable cells to 50% after 24 h (Fig. 1A). Glucose treatment also decreased
the amount of miRNA27a in a dose-dependent manner for 24 h
(Fig. 1B). A total of 20 and 50 mM
glucose was also able to significantly inhibit the expression of
mRNA27a in RPE cells compared with the control (0 mM glucose)
(P<0.01; Fig. 1B).
Inhibition of miRNA-27a suppresses the
viability of RPE cells under high glucose conditions
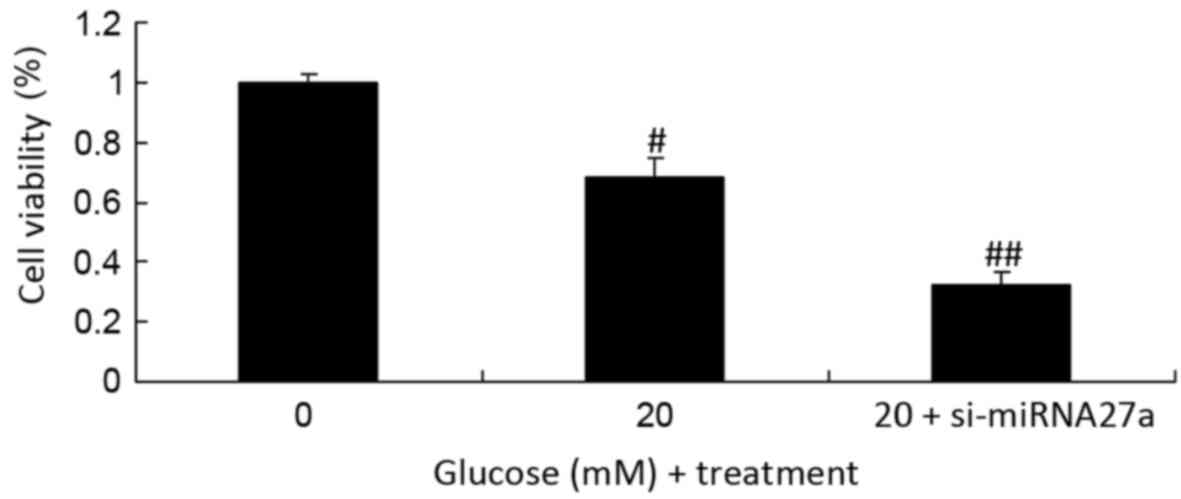
Due to the results of the initial experiment, 20 mM
glucose was selected as the high concentration of glucose and used
in subsequent experiments. In addition to glucose treatment,
miRNA27a was inhibited via the transfection of si-miRNA27a. The
results of this assay indicated that the inhibition of miRNA27a and
treatment with high glucose significantly inhibited the viability
of RPE cells compared with the untransfected RPE cells cultured
under high glucose conditions (P<0.01; Fig. 2).
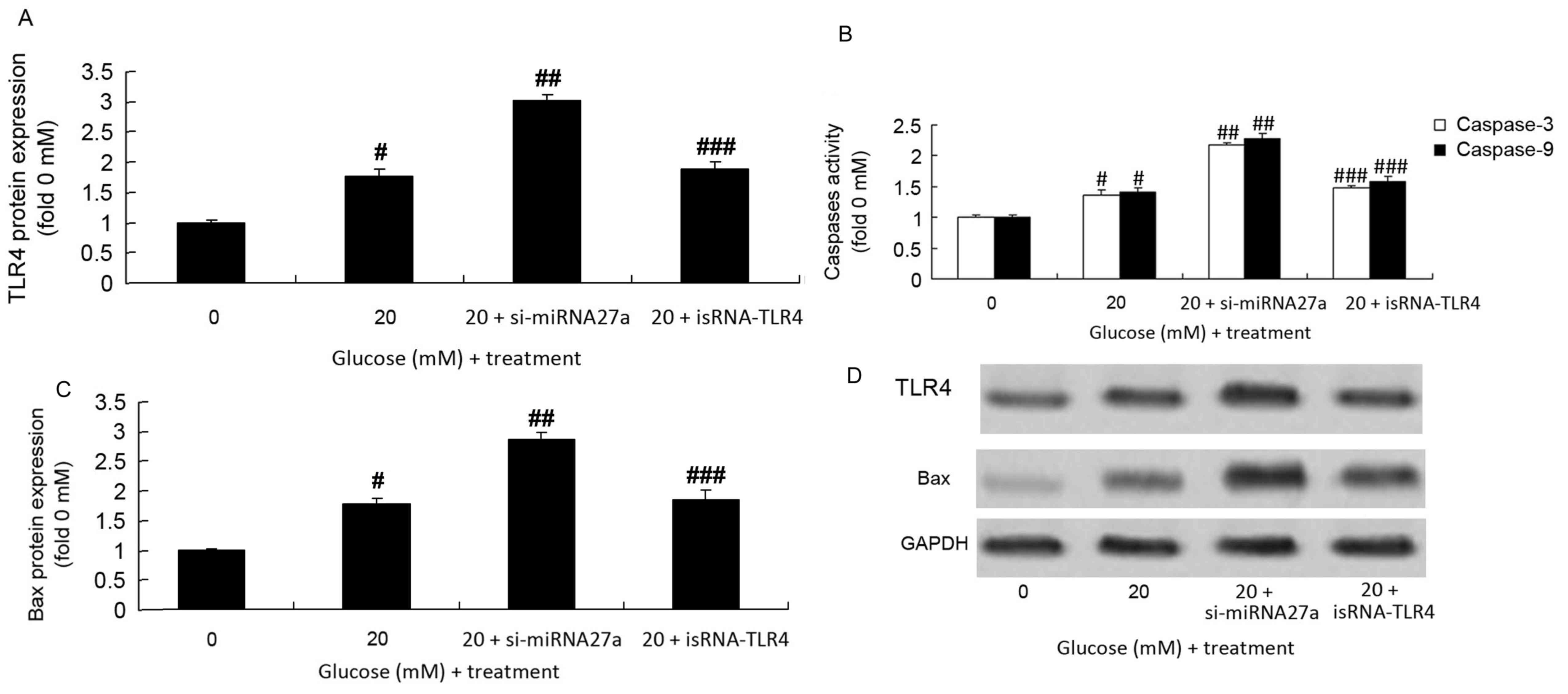
Inhibition of mRNA27a increases
caspase-3/9 activity, and Bax and TLR4 protein expression in RPE
cells subjected to high glucose
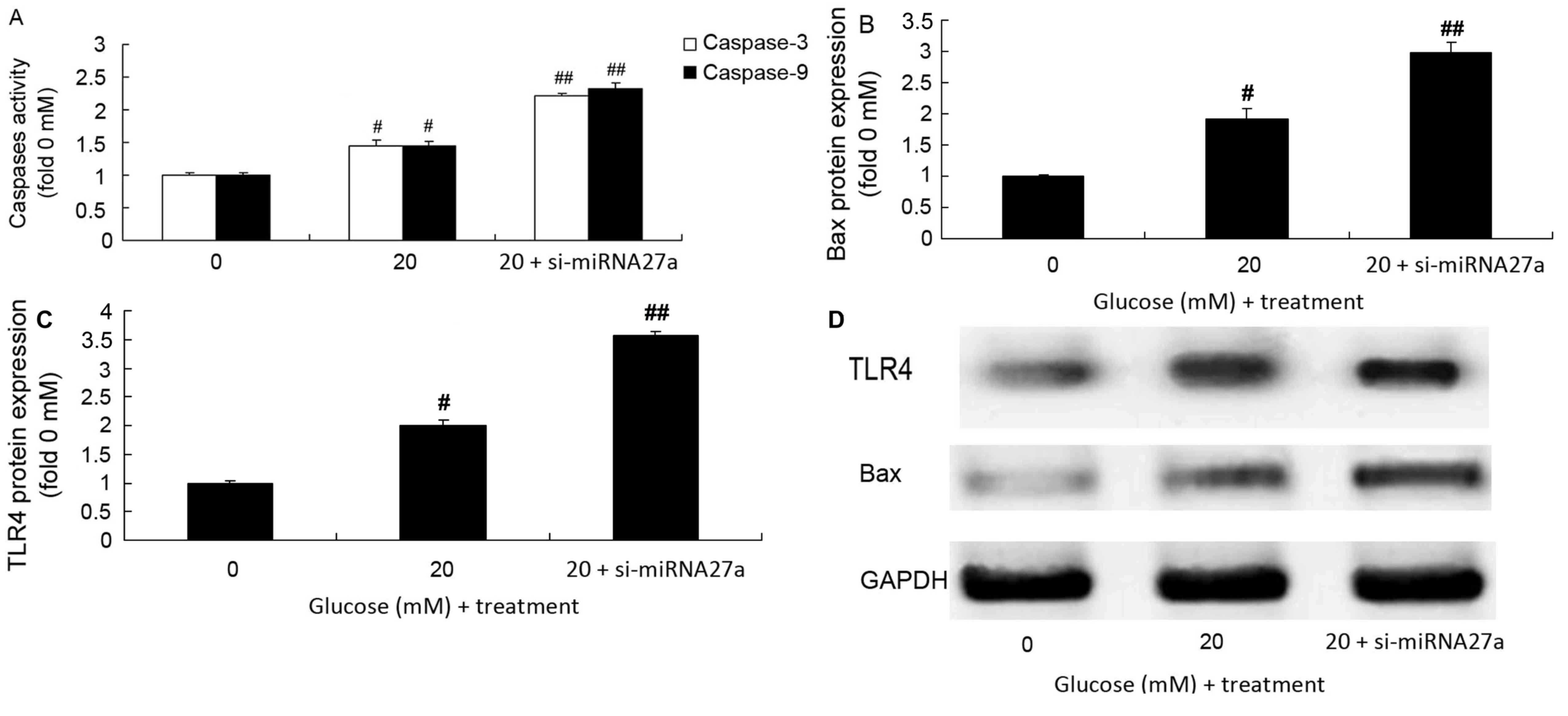
The role of mRNA27a in regulating caspase-3/9
activity, Bax and TLR4 protein expression in RPE cells under high
glucose conditions was assessed. Caspase-3/9 activity (Fig. 3A), and Bax and TLR4 protein
expression (Fig. 3B-D) in RPE cells
treated with high glucose significantly increased following the
inhibition of miRNA27a compared with the group treated with high
glucose alone (P<0.01; Fig.
3).
Inhibition of mRNA27a increases IL-6,
IL-1β and TNF-α expression in RPE cells subjected to high
glucose
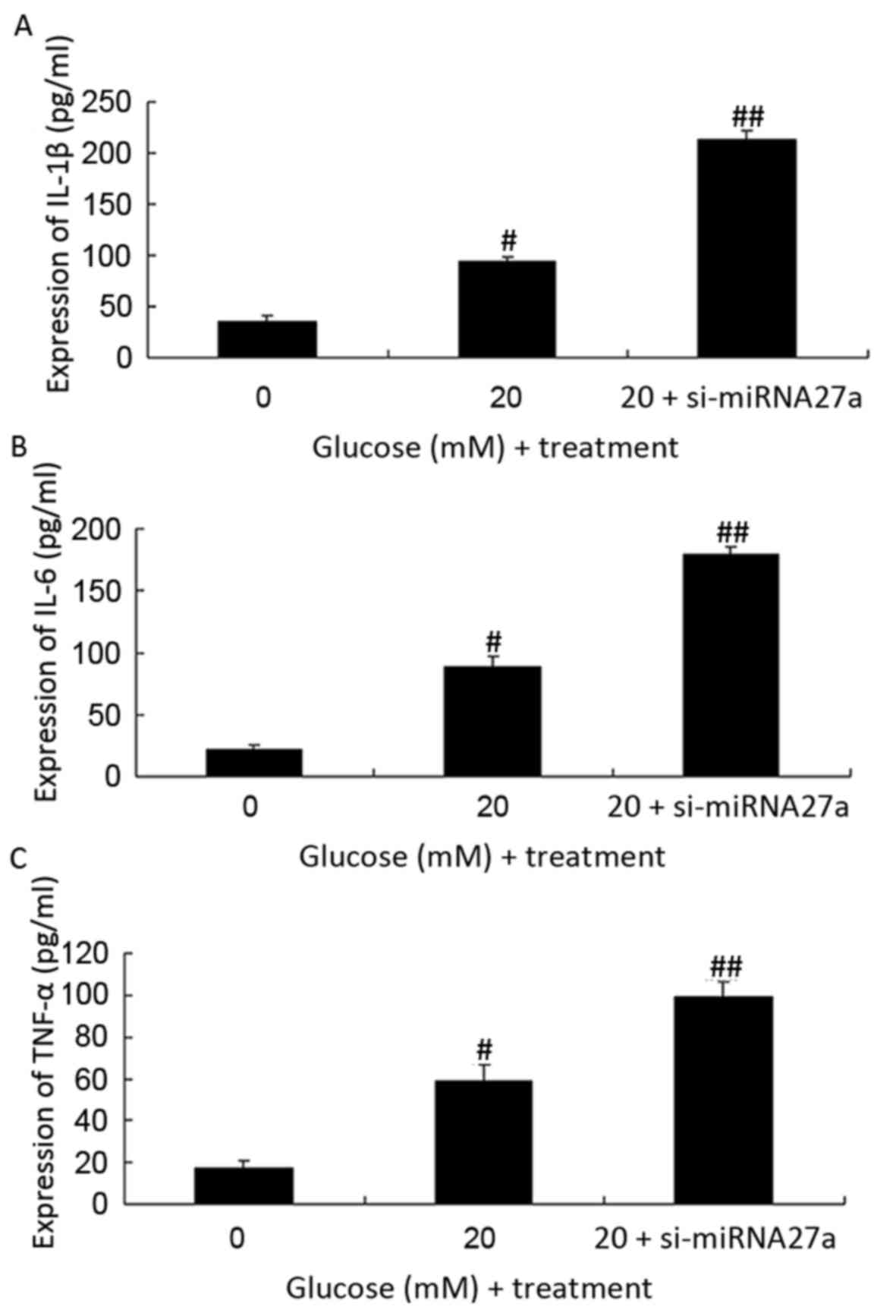
The expression of IL-6, IL-1β and TNF-α was measured
in RPE cells transfected with si-miRNA27a and cultured under high
glucose conditions using ELISAs. When compared with the
untransfected RPE cells cultured with 20 mM glucose, the expression
of IL-6, IL-1β and TNF-α was significantly increased in RPE cells
treated with si-miRNA27a and high glucose (P<0.01; Fig. 4).
TLR4 expression is inhibited in
si-miRNA27a-transfected RPE cells subjected to high glucose
following the transfection of isRNA-TLR4
The present study assessed the effect of isRNA-TLR4
on the expression of TLR4 and Bax protein, and caspase-3/9 activity
in RPE cells subjected to high glucose following the inhibition of
mRNA27a. isRNA-TLR4 significantly suppressed the expression of TLR4
and Bax protein, and caspase-3/9 activity in RPE cells treated with
high glucose and transfected with si-miRNA27a, compared with RPE
cells treated with high glucose and transfected with si-miRNA27a
(P<0.01; Fig. 5).
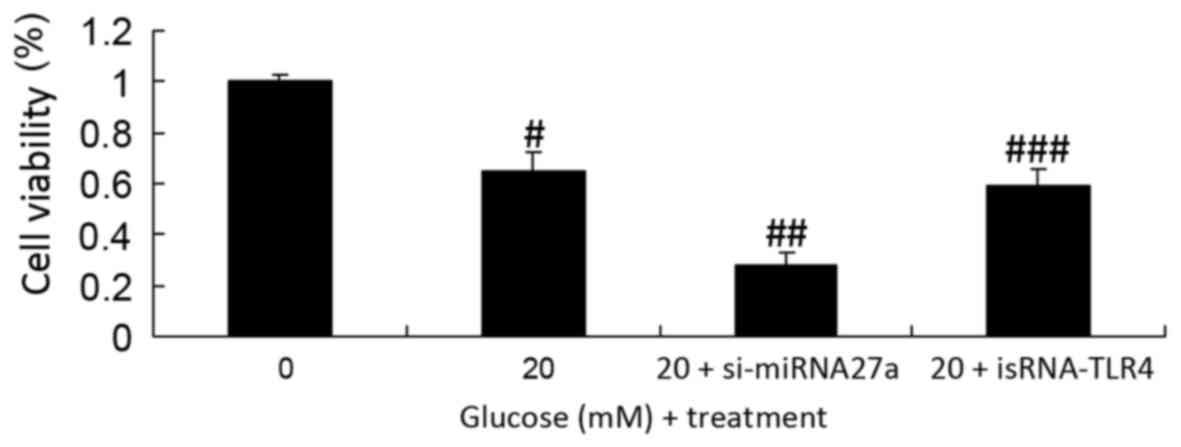
Inhibition of TLR4 increases the
viability of si-miRNA27a-transfected RPE cells subjected to high
glucose
The effect of TLR4 on the viability of RPE cells
subjected to high glucose following the inhibition of miRNA27a
expression was investigated. Inhibition of TLR4 significantly
increased the viability of si-miRNA27a-transfected RPE cells under
high glucose conditions, compared with RPE cells treated with high
glucose and transfected with si-miRNA27a (P<0.01; Fig. 6).
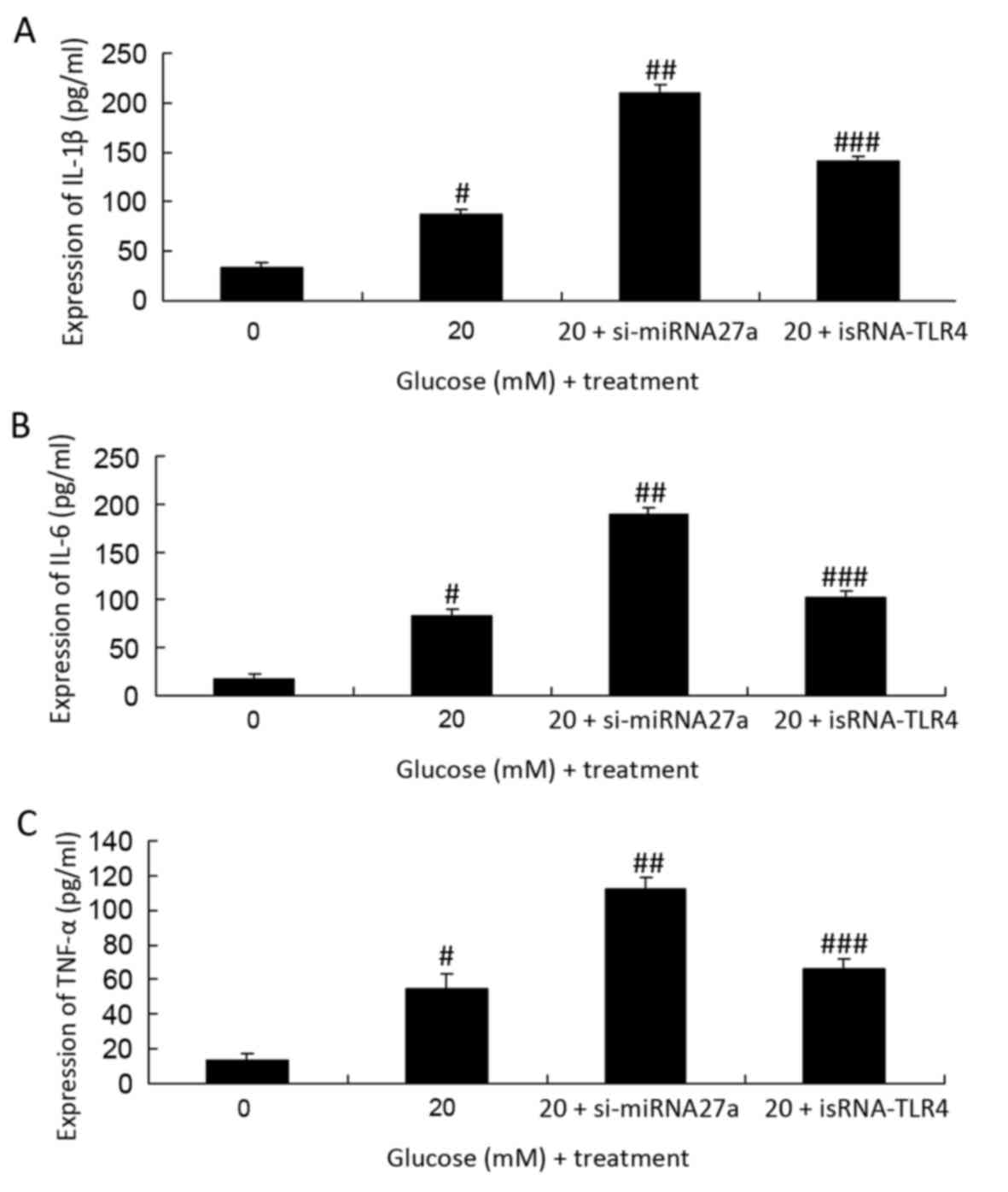
Inhibition of TLR4 decreases the
expression of IL-6, IL-1β and TNF-α in miRNA27a-transfected RPE
cells subjected to high glucose
To assess the effect of TLR4 inhibition on the
expression of IL-6, IL-1β and TNF-α in RPE cells subjected to high
glucose following the inhibition of miRNA-27a was investigated. The
expression of IL-6, IL-1β and TNF-α in miRNA27a-transfected RPE
cells subjected to high glucose was significantly suppressed
following TLR4 inhibition, compared with RPE cells treated with
high glucose and transfected with si-miRNA27a (P<0.01; Fig. 7).
Discussion
Diabetes is the third most frequently chronic
non-infectious disease diagnosed globally, following tumors and
cardiovascular/cerebrovascular diseases (1). In developed countries, DR is the
primary cause of blindness in adults (1). miRNA, which exists in nematodes,
drosophila, mammals, plants and viruses, interacts with and
inhibits the transcription of target mRNA molecules (15). miRNA genes are identified in the
genome as single copies, multiple copies or gene clusters, the
majority of which are located in intergenic regions (6). The transcription of miRNAs is
independent of other genes and remains highly conserved throughout
evolution. miRNA has spatial and temporal specificity (6). In addition, miRNA serves an important
role in vital movements, such as transcription (6,16). The
results of the present study revealed that a high concentration of
glucose was able to inhibit the expression of miRNA27a in RPE
cells.
Previous studies from a number of institutions have
revealed that diabetes may induce an increase in the expression of
TLRs. A previous study demonstrated that the expression of TLR4 in
the peripheral blood mononuclear cells of patients with diabetes
was higher compared with those of individuals without diabetes
(13). In addition, the expression
of TLR4 is positively associated with the level of glycated
hemoglobin, IL-lβ and TNF-α (16).
Another previous study demonstrated that the expression of TLR2 and
TLR4 is increased in the peripheral blood mononuclear cells of
patients with diabetes who also present with microvascular
complications (17). Culturing these
monocytes in vitro in a hyperglycemic state promoted the
expression of TLR2 and TLR4 in a time- and dose-dependent manner
(18). In addition, the expression
of TLR4 was demonstrated to be significantly increased in the
adipose tissues of obese db/db mice (18). Previous studies have revealed that
the expression of TLR2 in the skeletal muscle and white fat of
obese mice is increased (13,19). The
inhibition of TLR2 expression may suppress the activities of
inhibitor of NF-κB subunit β and mitogen-activated protein kinase
8, indicating that TLR2 is a key regulator of inflammation and
metabolism (18). It has also been
demonstrated that in patients with type 2 diabetes the expression
of TLR4 protein is associated with the severity of insulin
resistance (18). The results of the
present study indicated that the inhibition of miRNA27a increases
TLR4 protein expression in RPE cells subjected to high glucose. Lv
et al (20) suggested that
miRNA27a is associated with the inflammatory response by targeting
TLR4.
The inflammatory response is a self-protective
mechanism produced by the innate immune system when organisms are
exposed to foreign antibodies or microorganisms (21). Molecules produced by pathogens are
recognized by pattern recognition receptors, which stimulates the
generation of TNF-α and IL-1β, and proinflammatory proteins,
including cyclooxygenase-2 and inducible nitric oxide synthase
(1). Animal models of early stage DR
demonstrate the production of these proteins, which may function to
prevent the progression of retinopathy (22). The results of the present study
indicated that inhibition of miRNA27a increases the expression of
IL-6, IL-1β and TNF-α in RPE cells subjected to high glucose.
Inhibition of TLR4 was demonstrated to decrease the expression of
IL-6, IL-1β and TNF-α in si-miRNA27a-transfected RPE cells
subjected to high glucose. Jennewein et al (23) reported that miRNA27b contributes to
the progression of lipopolysaccharide-associated inflammatory
diseases. The inhibition of miRNA-27a in RPE cells subjected to
high glucose has an effect on inflammation and apoptosis,
suggesting that the level of miRNA-27a may be used as a biomarker
for DR.
DR, the most common and severe complication of
diabetes, is a major cause of blindness in developed countries
(24). A previous study revealed
that in the early stage of diabetes, patients present with retinal
microangiopathy and inhibited neuronal function, which are
associated with apoptosis (25). A
previous study investigating the expression of apoptotic markers
indicated that the expression of certain markers, including
caspase-3, fatty acid synthase (Fas) and Bax, was significantly
increased in patients with diabetes compared with individuals
without diabetes (26). In patients
with diabetes, retinal ganglion cells that express caspase-3, Fas
and Bax indicate cytoplasmic immunoreactivity. The apoptosis of
pericytes results in the destruction of the retinal barrier and the
apoptosis of retinal ganglion cells leads to decreasing visual
function (27). Genes associated
with apoptosis are members of the Bax protein family (28). In the present study, inhibition of
miRNA27a was demonstrated to reduce the viability, and increase the
caspase-3/9 activity and Bax protein expression of RPE cells
subjected to high glucose. Tian et al (29) demonstrated that miRNA27a promotes the
proliferation and suppresses the apoptosis of laryngeal carcinomas.
These results suggest that the inhibition of miRNA27a has a
protective effect on the high glucose-induced apoptosis of human
RPE cells.
The results of the present study demonstrated that
the inhibition of miRNA27a reduced the viability, and increased the
caspase-3/9 activity and Bax protein expression of RPE cells
subjected to high glucose concentrations through suppression of
inflammation by targeting TLR4. In conclusion, the present study
provides a useful insight into the role of mRNA27a in the
regulation of high glucose-induced apoptosis and inflammatory
mediators in DR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT, XW, JZ and GL designed the research study. YD
performed the research. YD and XT analyzed the data. YD wrote the
paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jeon S and Lee WK: Intravitreal
bevacizumab increases intraocular interleukin-6 levels at 1 day
after injection in patients with proliferative diabetic
retinopathy. Cytokine. 60:535–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ambresin A, Strueven V and Pournaras JA:
Painless indirect argon laser in high risk proliferative diabetic
retinopathy. Klin Monbl Augenheilkd. 232:509–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ribeiro L, Bandello F, Tejerina AN,
Vujosevic S, Varano M, Egan C, Sivaprasad S, Menon G, Massin P,
Verbraak FD, et al: Characterization of retinal disease progression
in a 1-year longitudinal study of eyes with mild nonproliferative
retinopathy in diabetes type 2. Invest Ophthalmol Vis Sci.
56:5698–5705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansberger SL, Sheppler C, Barker G,
Gardiner SK, Demirel S, Wooten K and Becker TM: Long-term
comparative effectiveness of telemedicine in providing diabetic
retinopathy screening examinations: A randomized clinical trial.
JAMA Ophthalmol. 133:518–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qing S, Yuan S, Yun C, Hui H, Mao P, Wen
F, Ding Y and Liu Q: Serum miRNA biomarkers serve as a fingerprint
for proliferative diabetic retinopathy. Cell Physiol Biochem.
34:1733–1740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zampetaki A, Willeit P, Burr S, Yin X,
Langley SR, Kiechl S, Klein R, Rossing P, Chaturvedi N and Mayr M:
Angiogenic microRNAs linked to incidence and progression of
diabetic retinopathy in type 1 diabetes. Diabetes. 65:216–227.
2016.PubMed/NCBI
|
|
7
|
Mohamed QA, Fletcher EC and Buckle M:
Diabetic retinopathy: Intravitreal vascular endothelial growth
factor inhibitors for diabetic macular oedema. BMJ Clin Evid.
2016:pii: 0702. 2016.PubMed/NCBI
|
|
8
|
Scott IU, Jackson GR, Quillen DA, Larsen
M, Klein R, Liao J, Holfort S, Munch IC and Gardner TW: Effect of
doxycycline vs placebo on retinal function and diabetic retinopathy
progression in patients with severe nonproliferative or
non-high-risk proliferative diabetic retinopathy: A randomized
clinical trial. JAMA Ophthalmol. 132:535–543. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaštelan S, Tomić M, Antunica Gverović A,
Salopek Rabatić J and Ljubić S: Inflammation and pharmacological
treatment in diabetic retinopathy. Mediators Inflamm.
2013:2131302013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen ZH, Wu YF, Wang PL, Wu YP, Li ZY,
Zhao Y, Zhou JS, Zhu C, Cao C, Mao YY, et al: Autophagy is
essential for ultrafine particle-induced inflammation and mucus
hyperproduction in airway epithelium. Autophagy. 12:297–311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin WD, Liu GL, Wang J, Wang H, Zhang JN,
Zhang F, Ma Y, Ji XY, Li C and Zhang MX: Poly(ADP-ribose)
polymerase 1 inhibition protects cardiomyocytes from inflammation
and apoptosis in diabetic cardiomyopathy. Oncotarget.
7:35618–35631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Sun CY, Zhang YB, Guo HZ, Feng
XX, Peng SZ, Yuan J, Zheng RB, Chen WP, Su ZR and Huang XD: Kegan
Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung
injury through inhibition of TLR4-mediated NF-κB signaling pathway
and MMP-9 expression. J Ethnopharmacol. 186:91–102. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh K, Kant S, Singh VK, Agrawal NK,
Gupta SK and Singh K: Toll-like receptor 4 polymorphisms and their
haplotypes modulate the risk of developing diabetic retinopathy in
type 2 diabetes patients. Mol Vis. 20:704–713. 2014.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bressler SB, Almukhtar T, Aiello LP,
Bressler NM, Ferris FL III, Glassman AR and Greven CM: Diabetic
Retinopathy Clinical Research Network: Green or yellow laser
treatment for diabetic macular edema: Exploratory assessment within
the diabetic retinopathy clinical research network. Retina.
33:2080–2088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin WJ and Yeh WC: Implication of
Toll-like receptor and tumor necrosis factor alpha signaling in
septic shock. Shock. 24:206–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XL, Zhang XD, Li YY, Chen XM, Tang DR
and Ran RJ: Involvement of HMGB1 mediated signalling pathway in
diabetic retinopathy: Evidence from type 2 diabetic rats and
ARPE-19 cells under diabetic condition. Br J Ophthalmol.
97:1598–1603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajamani U and Jialal I: Hyperglycemia
induces Toll-like receptor-2 and −4 expression and activity in
human microvascular retinal endothelial cells: Implications for
diabetic retinopathy. J Diabetes Res. 2014:7909022014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berger EA, Carion TW, Jiang Y, Liu L,
Chahine A, Walker RJ and Steinle JJ: β-Adrenergic receptor agonist,
compound 49b, inhibits TLR4 signaling pathway in diabetic retina.
Immunol Cell Biol. 94:656–661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv YN, Ou-Yang AJ and Fu LS: MicroRNA-27a
negatively modulates the inflammatory response in
lipopolysaccharide-stimulated microglia by targeting TLR4 and
IRAK4. Cell Mol Neurobiol. 37:195–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H and Chen M: Targeting the complement
system for the management of retinal inflammatory and degenerative
diseases. Eur J Pharmacol. 787:94–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wohlfart P, Lin J, Dietrich N, Kannt A,
Elvert R, Herling AW and Hammes HP: Expression patterning reveals
retinal inflammation as a minor factor in experimental retinopathy
of ZDF rats. Acta Diabetol. 51:553–558. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jennewein C, von Knethen A, Schmid T and
Brune B: MicroRNA-27b contributes to lipopolysaccharide-mediated
peroxisome proliferator-activated receptor gamma (PPARgamma) mRNA
destabilization. J Biol Chem. 285:11846–11853. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong Z, Tao X, Fu X, Wang H, Wang D and
Zhang T: Protective effects of Purendan superfine powder on retinal
neuron apoptosis in a rat model of type 2 diabetes mellitus. Neural
Regen Res. 7:202–206. 2012.PubMed/NCBI
|
|
25
|
Kowluru RA: Diabetic retinopathy:
Mitochondrial dysfunction and retinal capillary cell death.
Antioxid Redox Signal. 7:1581–1587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khalfaoui T, Basora N and Ouertani-Meddeb
A: Apoptotic factors (Bcl-2 and Bax) and diabetic retinopathy in
type 2 diabetes. J Mol Histol. 41:143–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kern TS, Du Y, Miller CM, Hatala DA and
Levin LA: Overexpression of Bcl-2 in vascular endothelium inhibits
the microvascular lesions of diabetic retinopathy. Am J Pathol.
176:2550–2558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan Y, Liu K, Wang Q, Ruan Y, Zhang Y and
Ye W: Exendin-4 protects retinal cells from early diabetes in
Goto-Kakizaki rats by increasing the Bcl-2/Bax and Bcl-xL/Bax
ratios and reducing reactive gliosis. Mol Vis. 20:1557–1568.
2014.PubMed/NCBI
|
|
29
|
Tian Y, Fu S, Qiu GB, Xu ZM, Liu N, Zhang
XW, Chen S, Wang Y, Sun KL and Fu WN: MicroRNA-27a promotes
proliferation and suppresses apoptosis by targeting PLK2 in
laryngeal carcinoma. BMC Cancer. 14:6782014. View Article : Google Scholar : PubMed/NCBI
|