Introduction
Recently rehabilitation of heart failure patients
has received increased attention in China.
Evidence exists on the effects of exercise training
for adults with heart failure (1).
According to Psaltis et al (2), because of ischemia, coronary heart
disease (CHD) can largely damage heart function and cause heart
failure. However, in heart failure complicated with CHD, or in
older adults, normal aerobic exercise is not possible because of
poor physical fitness.
A new physiological ischemic training (PIT)
programme for older adults has been described by Ni and co-workers
(3). It refers to reversible
ischemia training of normal skeletal muscles by using a tourniquet
or isometric contraction to cause physiologic ischemia for
approximately 4 weeks to trigger molecular and cellular mechanisms
to promote angiogenesis and the formation of collateral vessels and
protect remote ischemia areas. Physiological ischemia training
therapy augments angiogenesis in the ischemic myocardium by
inducing the differential expression of proteins involved in energy
metabolism, cell migration, protein folding, and generation. The
programme can cause vascular endothelial growth factor (VEGF) and
endothelial progenitor cells (EPCs) to increase in peripheral
blood, and retrohoming to heart and promote collateral circulation
(4). Our previous study indicated
that isometric handgrip exercise-induced physical ischemia training
may promote remote collateral growth in CAD patients through EPCs
and VEGF release (3), and the
segment score of ischemia area of single-photon emission computed
tomography (SPECT) reduced significantly.
The aim of the present study was to evaluate the
effects of a 12-week PIT programme in older patients with CHD
complicated with heart failure, with regards to safety of this
training to these patients, VEGF of peripheral blood and quality of
life (QOL).
Materials and methods
Patients
This is a prospective, randomized clinical trial
with a 12-week follow-up. There were initially 49 subjects included
in the study. Of these, 13 did not meet the inclusion criteria;
thus, 36 older adults were included in the study. Of these, 19
subjects were randomized to the PIT group and 17 subjects to the
control group. Participants were recruited in the clinic at our
Department at Xuzhou Central Hospital (Xuzhou, China). The
inclusion criteria were diagnosis of CHD combined with heart
failure, diagnostic criteria for heart failure according to
American heart failure diagnosis and treatment guidelines (5), clinical symptoms and signs stable for
>1 month, New York cardiac function class II–III, and no formal
history of exercise training. The exclusion criteria were unstable
angina pectoris and acute myocardial infarction, malignant
arrhythmia and high atrioventricular block, hemodynamic instability
and uncontrolled hypertension; acute pericarditis, severe valvular
heart disease; chronic obstructive pulmonary disease, pulmonary
heart disease or pulmonary vascular disease; a thrombophlebitis or
intracardial thrombus, intermittent claudication, lower limb
instability disease. At baseline, testing subjects were randomized
to either the intervention or control group by the random number
table method.
Eventually, 30 participants followed through with
the study. Four of the PIT group were lost to follow-up due to low
compliance (n=2), and disease exacerbation (n=2). Two of the
control group were lost to follow-up due to low compliance (n=2).
The remaining 30 participants were included in the PIT group (mean
age, 66.4±12.1; male to female ratio, 8/7) and the control group
(mean age, 67.1±12.8; male to female ratio, 9/6). This study was
approved by the Ethics Committee of Xuzhou Central Hospital.
Informed consents were signed by the patients that participated in
the study.
Method
The Minnesota Living with Heart Failure
Questionnaire (MLHFQ) was completed at baseline and at the 12-week
follow-up by interviewer-administered, self-assessment.
Test leaders were experienced nurses, and were
blinded to group allocation at baseline but not at follow-up.
The intervention study was a 12-week programme of
progressive and individually PIT as described in our previous study
(3). Training was isometric handgrip
exercise-induced physical ischemia training. It was carried out 5
times a week, for 12 weeks; in the course of training, a patient
was required to hold a grip and attempt to keep clenching with
subjective maximum effort, and each time for 1 min, relaxed 1 min
and repeated 10 times for 1 group, prior to repeating the process
on the other hand. There were 4 groups every day, in a.m. for 2
groups and p.m. for 2 groups. The exercise required that the
patient keep breathing naturally and avoid holding their
breath.
Participants in the control group were encouraged to
continue living as before (the same activity level).
The participants underwent peripheral blood VEGF
test at baseline and at 12-week follow-up. The VEGF was tested
using ELISA. The ELISA kits (R&D Systems GmBH, Wiesbaden,
Germany) were used to measure serum VEGF level.
Statistical analysis
Statistical analyses were performed using SPSS 10.0
software. Measurement data were expressed as mean ± standard
deviation (SD) and comparisons were performed using t-test.
Analysis of variance was used for comparison among multiple groups
and the post hoc test was Dunnett test. Enumeration data were
expressed as a percentage. P<0.05 was regarded as significant
difference.
Results
Patient data
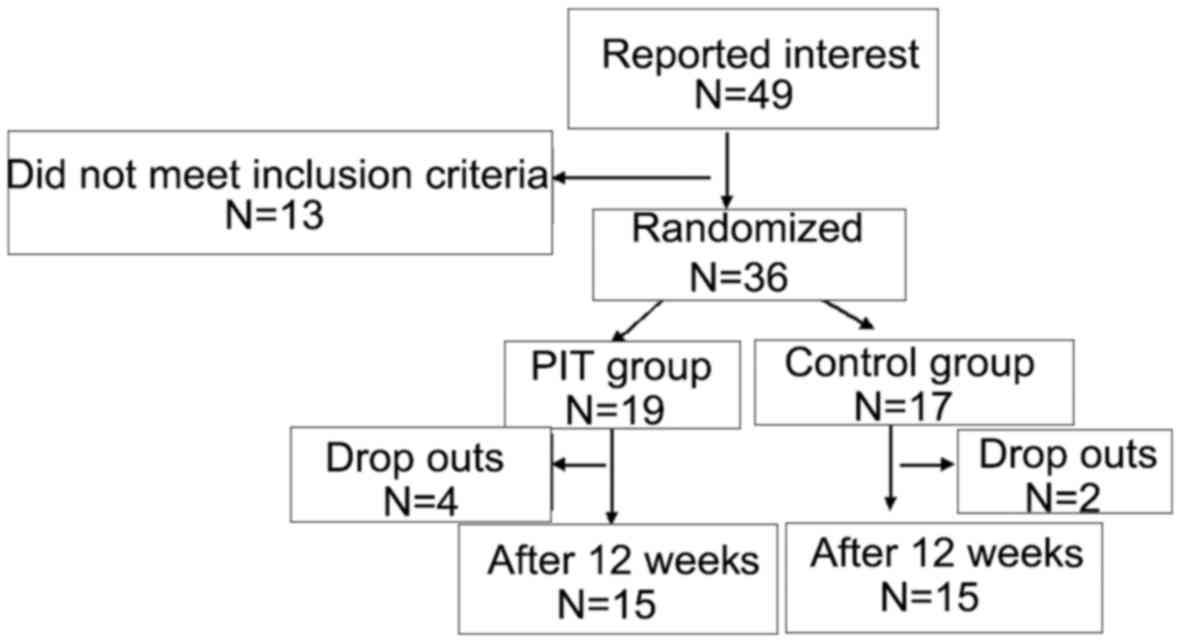
There were 49 responses in the clinic. Out of these,
13 did not meet the inclusion criteria, thus, 36 older adults were
included in the study. Of these, 19 were randomized to the PIT
group and 17 to the control group (Fig.
1). Demographic data of the participants are presented in
Table I. There were no differences
between the two groups at baseline regarding age, sex, medical
history, medication (Table I).
 | Table I.Demographic data of participants in
PIT and control group at the time of randomization. |
Table I.
Demographic data of participants in
PIT and control group at the time of randomization.
| Variables | PIT group (n=19) | Control group
(n=17) | P-value |
|---|
| Age (years) | 66.4±12.1 | 67.1±12.8 | 0.872 |
| Sex
(male/female) | 8/7 | 9/6 |
|
| Systolic pressure
(mmHg) | 128.9±16.2 | 133.5±12.1 | 0.138 |
| Diastolic pressure
(mmHg) | 69.73±5.3 | 73.4±6.1 | 0.389 |
| LVEF (%) | 39.7±3.2 | 38.7±4.6 | 0.128 |
| NYHA (1/2/3/4) | 4/8/3/0 | 4/9/2/0 |
|
| Medical history (n,
%) |
|
|
|
|
Hypertension | 12 (80) | 10 (67) |
|
| Diabetes
mellitus | 5
(33) | 4
(27) |
|
|
Hyperlipidemia | 5
(33) | 5
(33) |
|
|
Smoke | 4
(27) | 6
(40) |
|
|
Stroke | 12 (80) | 10 (67) |
|
| Medication (n,
%) |
|
|
|
|
Aspirin | 15
(100) | 15
(100) |
|
|
Clopidogrel | 10 (67) | 10 (67) |
|
|
ACEI/ARB | 6
(40) | 7
(47) |
|
| CCB | 5
(33) | 5
(33) |
|
| β
blocker | 7
(47) | 6
(40) |
|
A total of 30 participants followed
through with the study
Four of the PIT group were lost to follow-up due to
low compliance (n=2), and disease exacerbation (n=2). Two of the
control group were lost to follow-up due to low compliance
(n=2).
Training programme, VEGF and MLHFQ
scores
During PIT training, there was significant increase
in SBP and DBP in training group (P<0.05 both); although heart
rate increased, there was no significant difference. The above
indexes recovered to resting levels after training for 10 min.
Results are shown in Table II.
During 12-weeks PIT, there were no ECG and clinical manifestations
of myocardial ischemia in training group.
 | Table II.PIT group before and after isometric
handgrip exercise, the changes of heart rate and blood
pressure. |
Table II.
PIT group before and after isometric
handgrip exercise, the changes of heart rate and blood
pressure.
|
| Before PIT | During PIT | 10 min after PIT | P-value |
|---|
| Heart rate (bpm) | 75.2±14.5 | 78.9±14.9 | 74.7±15.3 | 0.517 |
| Systolic pressure
(mmHg) | 128.9±16.2 |
137.4±18.4a |
129.6±16.3b | 0.005 |
| Diastolic pressure
(mmHg) | 69.73±5.3 |
78.9±12.8a | 74.4±7.8b | 0.003 |
There was no significant difference in blood VEGF
concentration between two groups before treatment, P>0.05.
Compared with baseline level, there was significant increase in
VEGF concentration 81.66±17.1 pg/ml after 12-weeks PIT in training
group, P<0.05. There was no significant difference in the above
index in control group before and after treatment, P>0.05. The
12-week training 1evels of the above index in training group were
significantly higher than that of control group, P<0.05
(Table III).
 | Table III.Changes of blood VEGF concentration in
the two groups before and 12-weeks after treatment (SD). |
Table III.
Changes of blood VEGF concentration in
the two groups before and 12-weeks after treatment (SD).
| VEGF (pg/ml) | Before training | After training | P-value |
|---|
| PIT group (n=15) | 48.89±15.8 |
81.66±17.1a | <0.001 |
| Control group
(n=15) | 48.42±16.3 | 50.5±16.4 | 0.860 |
The MLHFQ scores in PIT and control group had no
differences at baseline. After 12-weeks the MLHFQ scores, the
emotional, physical, and total scores, were improved in PIT group
compared to baseline (P<0.05), with better QOL. But after
12-weeks in control group there was no significant difference
compared to baseline (P>0.05) (Table
IV).
 | Table IV.Comparison of the two groups of MLHFQ
before and after 12-weeks (SD). |
Table IV.
Comparison of the two groups of MLHFQ
before and after 12-weeks (SD).
| Variable | Before
training | After training | P-value |
|---|
| PIT group
(n=15) |
|
Physical | 31.2±4.52 | 23.7±7.58 | 0.037 |
|
Emotional | 9.5±4.36 | 5.92±2.84 | 0.009 |
|
Total | 47.5±18.24 | 39.1±8.57 | 0.025 |
| Control group
(n=15) |
|
Physical | 30.7±5.13 | 28.4±4.34 | 0.398 |
|
Emotional | 9.6±5.27 | 8.6±4.68 | 0.614 |
|
Total | 47.35±17.67 | 46.6±17.11 | 0.953 |
Discussion
About 23 million individuals suffer from heart
failure worldwide, giving rise to heavy global health and economic
burdens (6,7). Congestive heart failure (CHF) is among
the most common causes of hospital admissions and readmissions in
the Western world (8). In the face
of high morbidity and mortality, cardiopulmonary rehabilitation,
can improve the QOL, increase the effect of exercise endurance
(9). Cardiac rehabilitation is an
important content of secondary prevention, has become more and more
respected by clinicians, exercise rehabilitation is its core
content (10,11). Exercise rehabilitation prescription
formulation is a key link in the process of rehabilitation, the
heart movement in elderly patients with CHD is usually due to a
variety of complications, such as muscle mass reducing, myocardial
infarction, heart failure, unstable angina and sudden cardiac
arrest, malnutrition, mood, sleep disorders, severe osteoporosis,
balance coordination fall (12,13),
caused the general aerobic and resistance training to have
difficulty in implementation.
In previous studies, we demonstrated that skeletal
muscle ischemia can induce ischemic myocardial collateral
circulation (14,15) and reduce the area (16). Previous findings have confirmed that
the isometric contractions in 40–50% of the largest independent
contraction [maximal voluntary contraction (MVC)] intensity can
almost completely block blood flow (17); therefore, the movement form can be
used as a peripheral controllable physical model of ischemia. Our
task group named this exercise as the PIT. Physiological mechanisms
of ischemia training are as follows: peripheral skeletal muscle
training in brief, periodic, long cycle of ischemic training,
produced by peripheral blood VEGF and vascular EPCs through homing
mechanism into the heart, reach the biological bypass, improve the
collateral circulation, and improve myocardial blood flow (7,14,16,18–20).
In patients with heart failure, vascular endothelial function was
impaired and endothelial cell dysfunction provided a potential
pathophysiological relationship between normal endothelial function
loss and heart failure (21). CHD is
the main cause of heart failure. Due to hypoxia of myocardial
ischemia, heart failure can be induced, and heart failure can
further affect the cardiac blood supply of CHD (2). Based on previous research, this study
suggests that PIT training can improve the endothelial function of
blood vessels, and provides the possibility to delay the process of
ischemic heart failure caused by CHD. The present findings show
that, before, during and after PIT training in patients with CHD,
VEGF concentrations were significantly higher than before.
Furthermore, movement training improved the patient's coronary
collateral circulation. Both of these constitute possible reasons
for the movement to promote peripheral blood VEGF concentration
increases, homing to the heart, to improve the patient's collateral
circulation. These results are in agreement with those of Lin et
al on physiologic ischemia of CHD training (19), which further improves myocardial
ischemia in patients with heart failure.
There is a certain cardiovascular risk in
rehabilitation training for patients with heart failure, so it is
necessary to assess the risk of rehabilitation training properly.
In this study, during PIT training, the systolic pressure, and
diastolic blood pressure compared with before PIT training (rest)
was increased, and the difference was statistically significant. In
addition, PIT training blocked the blood flow to part of the
skeletal muscle, causing a change in blood pressure and
hemodynamics, and even increased the shear force of blood vessels.
This increase in blood pressure was relatively high, and patients
could tolerate it, and there were fewer opportunities for ST-T
changes and arrhythmias, results that are similar to those of Olher
et al (22). This change of
blood pressure can cause transient ischemia of skeletal muscle and
improve the preadaptability of ischemia, as also reported by Lin
et al (19). During and after
PIT training, the change of heart rate was not statistically
significant, and the training effect on the heart rate was
relatively small, probably because of heart failure patients with
widespread use of β blockers, and relative amount was larger,
myocardial contraction in patients with heart failure ability was
relatively weak, an exercise that is less likely to cause a rapid
increase in the heart rate. The results show that PIT training is
safe for patients with CHD and heart failure.
In patients with heart failure, the relative
activity endurance decreases (23),
the heart failure disease restricts its daily life activities, and
seriously affects QOL (24). Elderly
patients with CHD (ADL) and QOL are the primary target (13). In this study, the effect of PIT
training on the QOL was emphasized because of the selection of
patients with coronary heart failure. The Minnesota heart failure
scale is a recognized indicator of cardiac failure (25). In addition, we found that the
Minnesota heart failure scale, the scores of the three aspects are
also lower before treatment, and shows that the quality of patient
life greatly improved, instructions for the intervention of the
heart failure can retard the disease process, improving the
QOL.
To sum up, PIT is effective for patients with
coronary heart failure and patients with heart failure. The overall
patient's motor ability and QOL were improved. This investigation
of the CHD with mild or moderate heart failure patients the sample
size was small. In future the sample size will be increased in
order to observe the changes of peripheral blood in patients with
severe heart failure VEGF concentration, and cardiopulmonary
exercise testing response, to provide theoretical basis for sports
rehabilitation in patients with heart failure.
Acknowledgements
Not applicable.
Funding
This study is funded by the 2016 Xuzhou Municipal
Science and Technology Program (KC16SH045). The name of this
program is: The therapeutic effect and mechanism of physiological
ischemia training in patients with coronary heart disease combined
with heart failure.
Availability of data and materials
The datasets analyzed during the current study are
not publicly available due to the protection of patient privacy but
are available from the corresponding author on reasonable
request.
Authors' contributions
MG and JL were responsible for the Minnesota Living
with Heart Failure Questionnaire; XL and WC collected the patient
clinical information and follow-up; GHX, YZ and RY performed ELISA.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Xuzhou Central Hospital (Xuzhou, China). Informed consents were
signed by the patients that participated in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirai DM, Musch TI and Poole DC: Exercise
training in chronic heart failure: Improving skeletal muscle
O2 transport and utilization. Am J Physiol Heart Circ
Physiol. 309:H1419–H1439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Psaltis PJ, Schwarz N, Toledo-Flores D and
Nicholls SJ: Cellular therapy for heart failure. Curr Cardiol Rev.
12:195–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ni J, Lu H, Lu X, Jiang M, Peng Q, Ren C,
Xiang J, Mei C and Li J: The evolving concept of physiological
ischemia training vs. ischemia preconditioning. J Biomed Res.
29:445–450. 2015.PubMed/NCBI
|
|
4
|
Wang S, Chen Z, Tang X, Liu H, Yang L and
Wang Y: Transplantation of vascular endothelial growth factor
165-transfected endothelial progenitor cells for the treatment of
limb ischemia. Mol Med Rep. 12:4967–4974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaudhry SI, McAvay G, Chen S, Whitson H,
Newman AB, Krumholz HM and Gill TM: Risk factors for hospital
admission among older persons with newly diagnosed heart failure:
Findings from the Cardiovascular Health Study. J Am Coll Cardiol.
61:635–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bui AL, Horwich TB and Fonarow GC:
Epidemiology and risk profile of heart failure. Nat Rev Cardiol.
8:30–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McMurray JJ, Petrie MC, Murdoch DR and
Davie AP: Clinical epidemiology of heart failure: Public and
private health burden. Eur Heart J. 19 Suppl P:P9–P16.
1998.PubMed/NCBI
|
|
8
|
Bash LD, Weitzman D, Blaustein RO, Sharon
O, Shalev V and Chodick G: Comprehensive healthcare resource use
among newly diagnosed congestive heart failure. Isr J Health Policy
Res. 6:262017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitzman DW, Brubaker PH, Herrington DM,
Morgan TM, Stewart KP, Hundley WG, Abdelhamed A and Haykowsky MJ:
Effect of endurance exercise training on endothelial function and
arterial stiffness in older patients with heart failure and
preserved ejection fraction: A randomized, controlled, single-blind
trial. J Am Coll Cardiol. 62:584–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heran BS, Chen JM, Ebrahim S, Moxham T,
Oldridge N, Rees K, Thompson DR and Taylor RS: Exercise-based
cardiac rehabilitation for coronary heart disease. Cochrane
Database Syst Rev. 7:CD0018002011.
|
|
11
|
Edwards K, Jones N, Newton J, Foster C,
Judge A, Jackson K, Arden NK and Pinedo-Villanueva R: The
cost-effectiveness of exercise-based cardiac rehabilitation: A
systematic review of the characteristics and methodological quality
of published literature. Health Econ Rev. 7:372017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris DR, Moxon JV, Biros E, Krishna SM
and Golledge J: Meta-analysis of the association between
transforming growth factor-beta polymorphisms and complications of
coronary heart disease. PLoS One. 7:e378782012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maneerat Y, Prasongsukarn K,
Benjathummarak S, Dechkhajorn W and Chaisri U: Increased
alpha-defensin expression is associated with risk of coronary heart
disease: A feasible predictive inflammatory biomarker of coronary
heart disease in hyperlipidemia patients. Lipids Health Dis.
15:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Y, Lu X, Li J, Zhang Q and Reinhardt
JD: Impact of remote physiological ischemic training on vascular
endothelial growth factor, endothelial progenitor cells and
coronary angiogenesis after myocardial ischemia. Int J Cardiol.
177:894–901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Shen M, Guo X, Li X and Li J:
Proteomic mechanism of myocardial angiogenesis augmented by remote
ischemic training of skeletal muscle in rabbit. Cardiovasc Ther.
29:199–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin A, Li J, Zhao Y, Xiao M, Xiao B, Lu X
and Wan C: Effect of physiologic ischemic training on protection of
myocardial infarction in rabbits. Am J Phys Med Rehabil. 90:97–105.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown SP, Miller WC and Eason JM: Exercise
Physiology: Basis of Human Movement in Health and Disease.
Lippincott Williams & Wilkins; Baltimore, MD: pp. 187–188.
2006
|
|
18
|
Wan C, Li J and Yi L: Enhancement of
homing capability of endothelial progenitor cells to ischaemic
myocardium through physiological ischaemia training. J Rehabil Med.
43:550–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin S, Lu X, Chen S, Ye F, Zhang J, Ma Y
and Li J: Human coronary collateral recruitment is facilitated by
isometric exercise during acute coronary occlusion. J Rehabil Med.
44:691–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu J, Wang Y, Li J, Wang J and Jin T:
Proteomic analysis of left ventricular tissues following
intermittent myocardial ischemia during coronary collateralization
in rabbits. Int J Cardiol. 131:326–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Testa M, Yeh M, Lee P, Fanelli R,
Loperfido F, Berman JW and LeJemtel TH: Circulating levels of
cytokines and their endogenous modulators in patients with mild to
severe congestive heart failure due to coronary artery disease or
hypertension. J Am Coll Cardiol. 28:964–971. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rdos Olher R, Bocalini DS, Bacurau RF,
Rodriguez D, Figueira A Jr, Pontes FL Jr, Navarro F, Simões HG,
Araujo RC and Moraes MR: Isometric handgrip does not elicit
cardiovascular overload or post-exercise hypotension in
hypertensive older women. Clin Interv Aging. 8:649–655.
2013.PubMed/NCBI
|
|
23
|
Zhang Z, Sun X, Xi J, Ge W, Li H, Liu Y,
Feng J, Jiang L and Gao H: The role of cardiopulmonary exercise
testing in the formulation of high-intensity individualized
rehabilitation exercise prescription and exercise rehabilitation
effects evaluation among patients with chronic heart failure. Chin
Gen Pract. 17:2061–2067. 2016.(In Chinese).
|
|
24
|
Zhang Y and Chen S: The improvement of
cardiac function and quality of life in elderly patients with
chronic heart failure. Zhongguo Laonianxue Zazhi. 33:5307–5308.
2013.
|
|
25
|
Dunbar SB, Reilly CM, Gary R, Higgins MK,
Culler S, Butts B and Butler J: Randomized clinical trial of an
integrated self-care intervention for persons with heart failure
and diabetes: Quality of life and physical functioning outcomes. J
Card Fail. 21:719–729. 2015. View Article : Google Scholar : PubMed/NCBI
|