Introduction
Pancreatic cancer is one of the most common human
gastrointestinal malignancies and the fourth leading cause of
cancer-associated cases of mortality worldwide (1). In China, morbidity of pancreatic cancer
ranks seventh among malignancies and pancreatic cancer is the sixth
leading cause of mortality among all cancer types (2). Furthermore, the incidence of pancreatic
cancer has demonstrated an upward trend in recent years (3). Primary characteristics of pancreatic
cancer include late diagnosis, strong local invasion, early
metastasis, high mortality rate, poor prognosis and low long-term
survival (4). Compared with other
common treatments, including chemotherapy, radiotherapy and
biological therapy (5–7), surgical excision is considered the most
effective option at present, but only 10–15% of patients undergo
complete tumor resection (8).
Despite the available therapies, the 5-year survival rate is ~5%
(8). In addition, within 7 years
following surgery of pancreatic cancer the mortality rate among
patients is ~100% (9,10). Therefore, it is necessary to develop
a more effective treatment for pancreatic cancer.
As previously demonstrated, overexpression of the
prostaglandin-endoperoxide synthase 2 (COX-2) gene may be
associated with tumorigenesis and progression of breast, prostate
and lung cancer (11–13). Furthermore, COX-2 is considered a
therapeutic target for prevention of pancreatic cancer (14,15).
Nimesulide is a selective COX-2 inhibitor that could delay the
progression of pancreatic cancer precursor lesions, inhibit cell
proliferation and induce apoptosis (16–18).
Phosphatase and tensin homolog (PTEN) is a lipid phosphatase that
serves a role in tumor suppression (19). However, the effect of COX-2
inhibitors on PTEN in the context of pancreatic cancer remains to
be elucidated.
In the present study, the effects of nimesulide on
proliferation and apoptosis of pancreatic cancer cells were
investigated with the aim of elucidating the potential
PTEN-associated effect of nimesulide on pancreatic cancer.
Materials and methods
Reagents and cell culture
Nimesulide was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Dimethyl sulfoxide (DMSO), MTT and
Annexin V/Dead Cell Apoptosis kit were purchased from Hangzhou
Multi Sciences Biotech Co., Ltd. (Hangzhou, China). Primary
antibodies at a dilution of 1:1,000 against cleaved-caspase-3 (cat.
no. AC033), pro-caspase-3 (cat. no. AF1261), PTEN (cat. no.
AF1426), COX-2 (cat. no. AF1924) and vascular endothelial growth
factor (VEGF; cat. no. AF1309), Bcl-2 (cat. no. AB112), Bcl-2
associated protein X (Bax; cat. no. AB026) and β-actin (cat. no.
AA128) were utilized in the present study. The following secondary
antibodies (Horseradish peroxidase conjugated Goat Anti-Rabbit
Immunoglobulin G, 1:5,000; cat. no. A0208; Horseradish peroxidase
conjugated Goat Anti-Mouse immunoglobulin G; 1:5,000; cat. no.
A0216) were also utilized. All antibodies were supplied by Beyotime
Institute of Biotechnology (Haimen, China) Human pancreatic cancer
cell line PANC-1 was obtained from the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere at 37°C with 5%
CO2. DMEM, FBS and 0.25% Trypsin-EDTA were purchased
from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell proliferation assay
Viability of PANC-1 cells following treatment with
nimesulide was evaluated using an MTT assay, as previously
described (20). Briefly,
5×103 cells/well (100 µl) were seeded in 96-well plates
with different concentrations of nimesulide (0, 25, 50, 100, 200
and 400 µmol/l). DMSO was used as the control treatment. Following
incubation at 37°C for 48 h, 20 µl MTT (5 mg/ml) was added to each
well followed by incubation at 37°C for 4 h. DMSO was utilized to
dissolve the purple formazan and the absorbance was measured at a
wavelength of 490 nm using a microplate reader (Synergy HTX; BioTek
Instruments, Inc., Winooski, VT, USA). The results are expressed as
inhibition rates according to the following formula: Inhibition
rate (%)=1-(OD treatment-OD blank)/(OD control-OD blank) ×100%.
DNA laddering analysis
Cells were collected following treatment with
different concentrations of nimesulide at 37°C for 48 h. The
supernatant was discarded following centrifugation at a speed of
1,000 × g for 5 min at room temperature and the pellet was washed
with PBS (0.01 M, pH 7.4). Cells were incubated with 500 µl lysis
buffer [0.5 M Tris-HCl (pH 8.0), 0.02 mmol/l EDTA and 1% NP-40] in
a water bath at 55°C for 16 h. The solutions were centrifuged at a
speed of 12,000 × g for 5 min at 4°C and treated with RNase A
(final concentration, 20 mg/l; cat. no. R6148; Sigma-Aldrich) with
1% SDS and proteinase K (final concentration, 20 mg/l; cat. no.
P2308; Sigma-Aldrich). A total of 60 µl 3 M sodium acetate and 600
µl ice-cold absolute ethanol was added, and samples were incubated
at −20°C for at least 1 h, followed by centrifugation at a speed of
12,000 × g for 20 min at 4°C. Resulting DNA pellets were dissolved
in TE buffer (10 mM Tris-HCl, 1 mM EDTA at pH 7.4) and the DNA
ladder was separated by electrophoresis on a 2% agarose gel
(21).
Apoptosis assay
Apoptosis of PANC-1 cells were detected using the
aforementioned Annexin V/propidium iodide (PI) Apoptosis Detection
kit. Briefly, cells were exposed to various concentrations (50,
100, 200 and 400 µmol/l) of nimesulide for 48 h at 37°C. Control
cells were treated with DMSO. Cells were collected and washed twice
with PBS. A total of 5×105 cells/ml were re-suspended in
400 µl binding buffer with 5 µl Annexin V-fluorescein
isothiocyanate (FITC) and 1 µl PI (100 µg/ml) in the dark.
Following incubation at 37°C for 15 min, cell apoptosis was
detected by flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA) and was analyzed using CellQuest 3.3 software (BD
Biosciences).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
lysate (Beyotime Institute of Biotechnology) to extract the total
protein. The concentration of total protein was then quantitated
using a BCA protein assay kit (Beyotime Institute of
Biotechnology). Following this, 40 µg protein was loaded and
separated on 12% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were blocked with 5% non-fat milk for 1 h
at 37°C and probed with specific primary antibodies against COX-2,
Bcl-2, Bax, VEGF, cleaved-caspase-3, pro-caspase-3, PTEN and
β-actin at 4°C overnight. Subsequently, the membranes were
incubated at 37°C with their corresponding secondary antibodies for
1 h. Target bands were visualized using an enhanced
chemiluminescence solution (Qihai Biotec, Shanghai, China) and the
Gel-Pro-Analyzer software (Bethesda, MD, USA) was employed to
measure relative band intensities. Each target protein was
normalized to the corresponding β-actin band. Protein from
untreated cells were loaded onto each gel for comparison.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA). Data are presented as the mean ± standard deviation (n≥3).
One way analysis of variance followed by Tukey's multiple
comparisons test was used to compare differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Nimesulide inhibits proliferation of
PANC-1 cells
The results of MTT assays indicated that the
inhibitory effect of nimesulide on the proliferation of PANC-1
cells could be observed from a dose of 50–400 µmol/l (Table I). The inhibitory effect occurred in
a concentration-dependent manner.
 | Table I.Nimesulide inhibits proliferation of
PANC-1 cells (n=3). |
Table I.
Nimesulide inhibits proliferation of
PANC-1 cells (n=3).
| Nimesulide
(µmol/l) | Absorbance | Inhibition rate
(%) |
|---|
| 0 | 1.046±0.032 | 0 |
| 25 | 1.005±0.029 | 3.5±0.92 |
| 50 |
0.912±0.025a, b |
12.7±3.29a, b |
| 100 |
0.677±0.036a–c |
35.2±4.21a–c |
| 200 |
0.532±0.019a–d |
49.1±3.75a–d |
| 400 |
0.328±0.016a–e |
68.3±2.87a–e |
Nimesulide induces apoptosis of PANC-1
cells
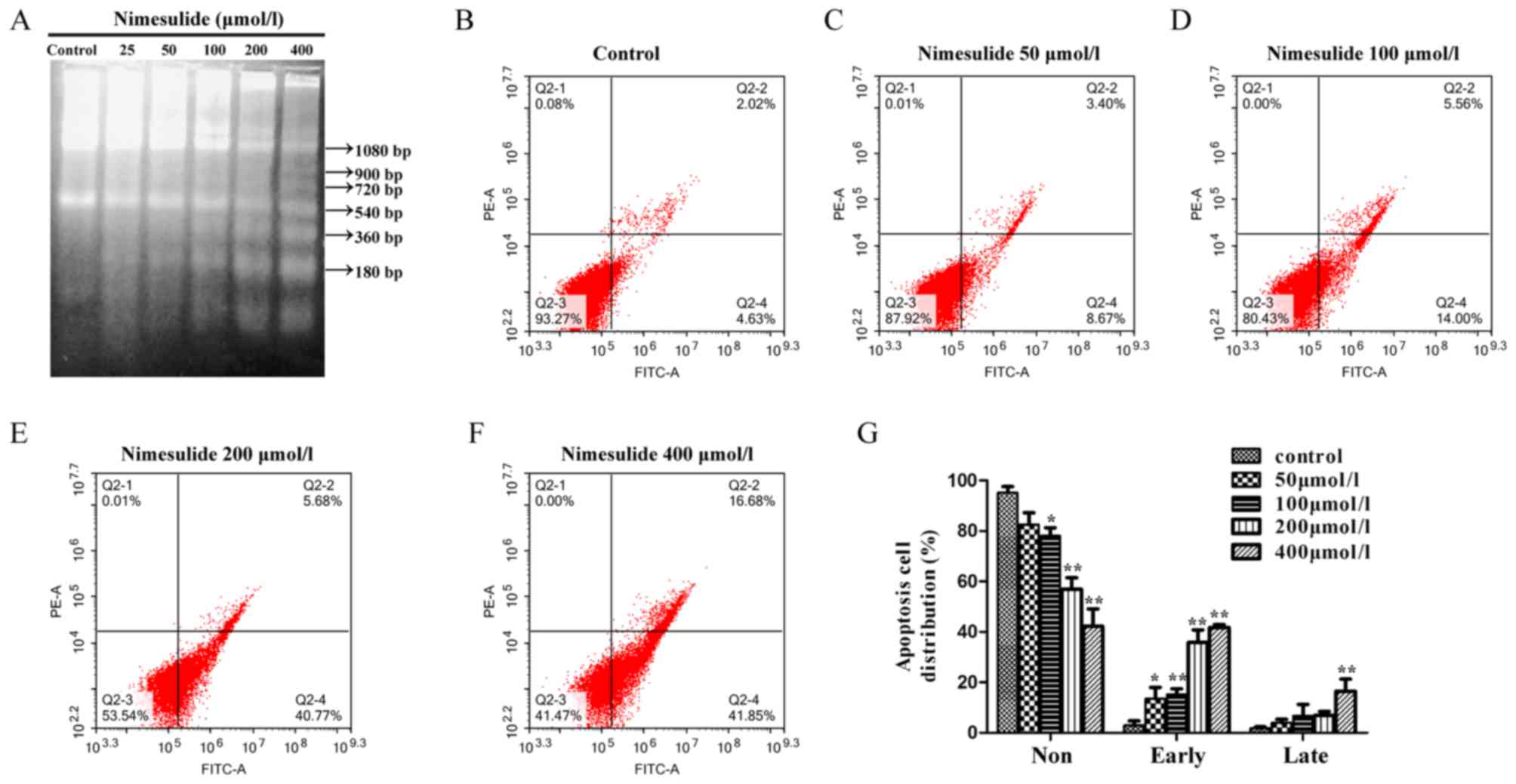
DNA laddering demonstrated that characteristics of
apoptosis occurred following a 48 h treatment with nimesulide from
the concentration of 50–400 µmol/l (Fig.
1A). The results of flow cytometry demonstrated that treatment
with 200 and 400 µmol/l nimesulide for 48 h significantly increased
early apoptosis of PANC-1 cells, compared with control cells
(Fig. 1B-G). The above results
indicated that nimesulide could induce early and late apoptosis of
PANC-1 cells. To further investigate the mechanisms underlying
nimesulide-induced apoptosis in PANC-1 cells, downstream mediators
in the apoptotic cascade were analyzed by western blotting
(Fig. 2). Following treatment with
100 and 200 µmol/l nimesulide for 48 h, increased expressions of
Bax and cleaved caspase-3 was observed, respectively. Expression of
pro-caspase-3 and Bcl-2 decreased following treatment with 100 and
50–200 µmol/l nimesulide, respectively.
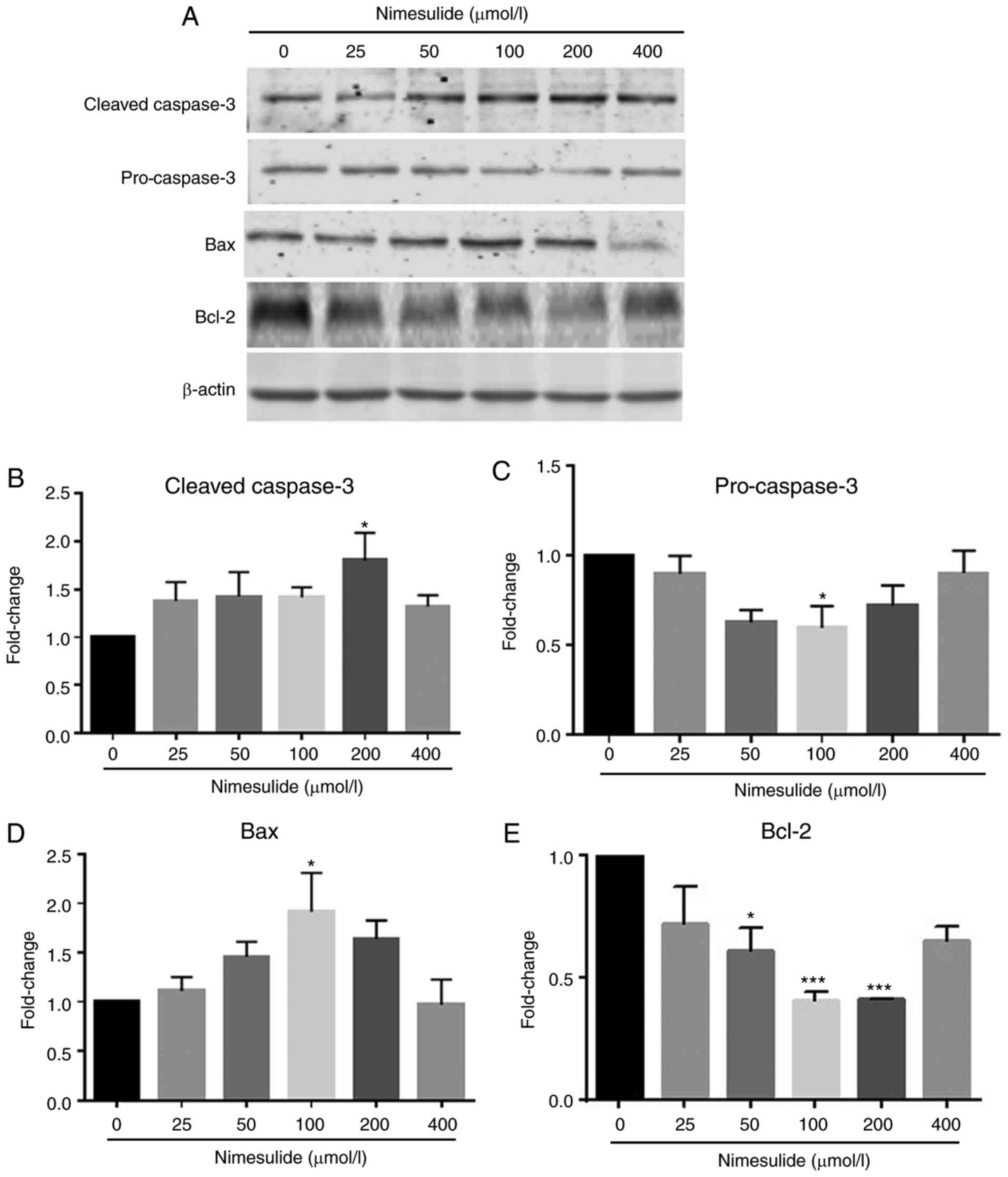 | Figure 2.Effects of nimesulide on expression
of cleaved-caspase-3, pro-caspase-3, Bax and Bcl-2 in PANC-1 cells.
(A) PANC-1 cells were treated with different concentrations of
nimesulide (0, 25, 50, 100, 200 and 400 µmol/l) for 48 h and
protein expression was determined by western blotting. Expression
of (B) cleaved caspase-3, (C) pro-caspase-3, (D) Bax and (E) Bcl-2
was analyzed. *P<0.05 and ***P<0.001 vs. the control group.
Bax, Bcl-2 associated protein X; Bcl-2, B-cell lymphoma 2. |
Nimesulide decreases expression of
COX-2 in PANC-1 cells
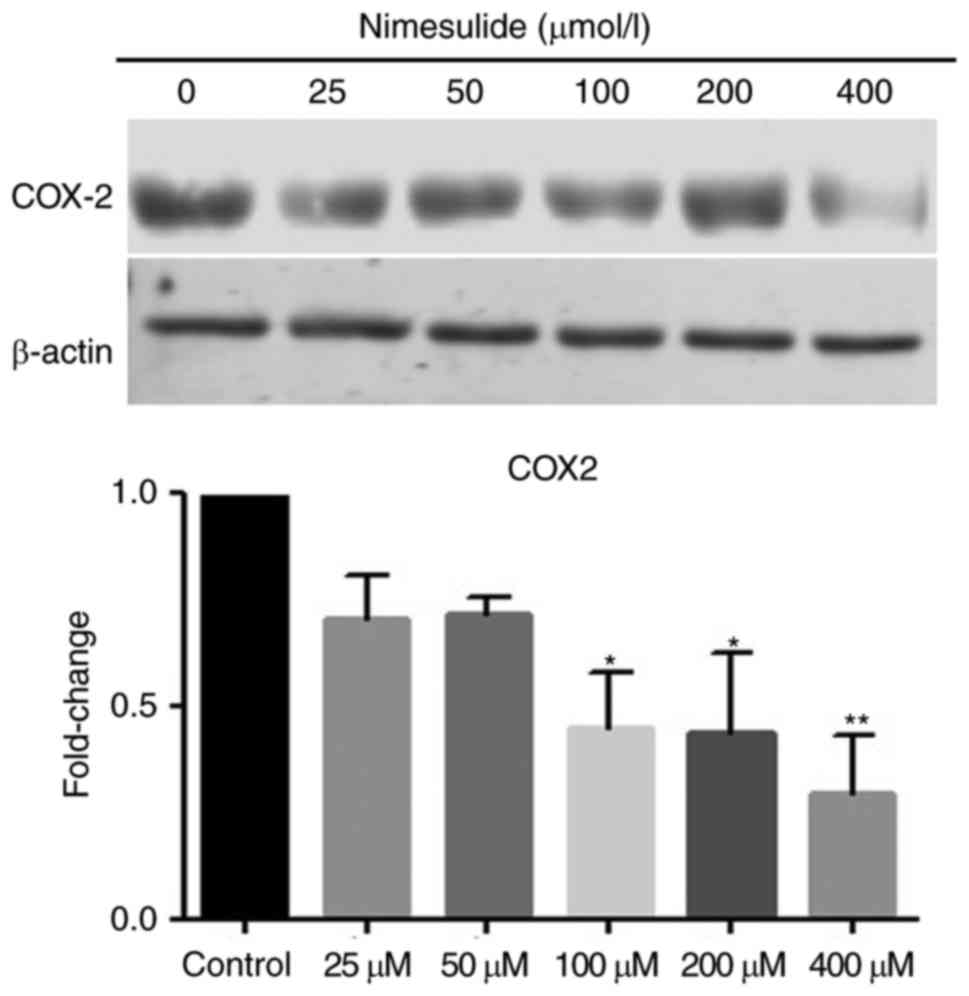
Protein expression of COX-2 in PANC cells was
downregulated following treatment with nimesulide. Following
treatment with 100, 200 or 400 µmol/l nimesulide for 48 h, cells
demonstrated significantly lower expression of COX-2 protein
compared with the untreated control cells (Fig. 3). Therefore, nimesulide suppressed
expression of COX-2 in PANC-1 cells. The above results suggest the
nimesulide may function as a COX-2 inhibitor in pancreatic cancer
cells.
Nimesulide enhances expression of PTEN
and downregulates expression of VEGF in PANC-1 cells
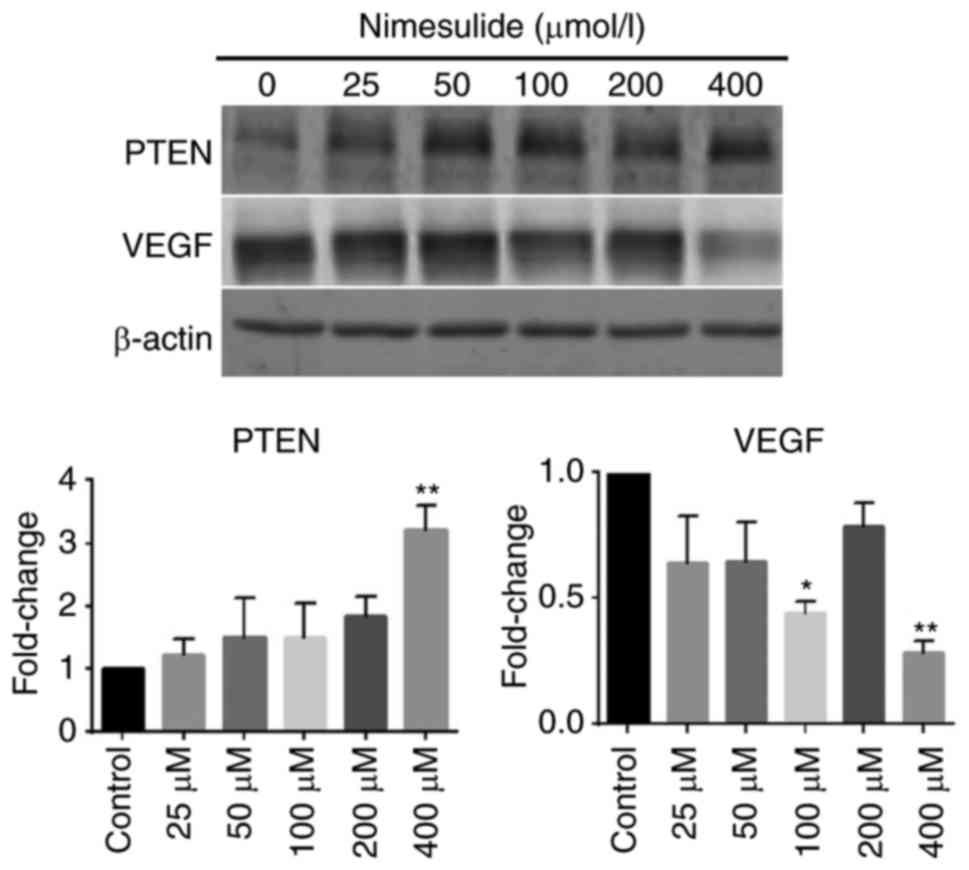
To elucidate the mechanism underlying the
anti-proliferative and pro-apoptotic effects of nimesulide, protein
expression of PTEN and VEGF were determined (Fig. 4). Following treatment with 400 µmol/l
nimesulide for 48 h, expression of PTEN increased, compared with
the control group. Expression of VEGF decreased significantly
following treatment with 100 and 400 µmol/l nimesulide. These
results indicated that PTEN and VEGF may be involved in the
anti-proliferative and pro-apoptotic effects of nimesulide in PANC
cells. However, the expression of VEGF was not significant
following treatment with 200 µmol/l nimesulide. This may have been
due to experimental error, but further study is required for
clarification.
Discussion
Pancreatic cancer is an aggressive malignant disease
and is one of the tumor types that is intrinsically resistant to
chemotherapy (22,23). Apoptosis, also known as programmed
cell death, serves a role in maintaining homeostasis of both normal
and neoplastic cells (24).
Suppression of cancer cell apoptosis is considered to contribute to
development and progression of carcinomas by triggering gene
mutations and promoting resistance to immune-based cytotoxicity
(25). Previous studies have
demonstrated that nimesulide promotes apoptosis of KOSC-2 oral
squamous carcinoma cells (26).
Nimesulide can also induce apoptosis by inactivating the Janus
kinase 2/signal transducer and activator of transcription 3 pathway
in Eca-109 cells (21). Consistent
with the aforementioned studies, the present study demonstrated
that nimesulide can induce apoptosis of PANC-1 cells as
demonstrated by DNA laddering and Annexin V-FITC/PI experiments.
Bax is a pro-apoptotic protein, whereas Bcl-2 is an anti-apoptotic
protein (27). It has been
previously demonstrated that downregulation of Bcl-2 enables
oligomerized Bax to insert into the outer mitochondrial membrane
and promote apoptosis (28). The
present study demonstrated that nimesulide could decrease
expression levels of Bcl-2 and increase expression levels of Bax in
PANC-1 cells, which suggested that apoptosis induced by nimesulide
treatment of PANC-1 cells may be due to the activation of
mitochondrial apoptotic pathways.
It has been previously reported that PTEN can
regulate angiogenesis of human pancreatic cancer cells and that it
is a suppressor of pancreatic ductal adenocarcinoma (29,30).
Therefore, enhanced expression of PTEN in PANC-1 cells following
treatment with nimesulide indicated a possible novel role of
nimesulide in the treatment of pancreatic cancer in addition to
inhibition of COX-2. The major substrate, with which PTEN
interacts, is phosphatidylinositol (3,4,5)-trisphosphate, which is produced by the
action of phosphoinositide-3-kinases (PI3Ks) (19). The PI3K/RAC-alpha
serine/threonine-protein kinase (Akt) signaling pathway serves a
role in the development of resistance to carcinoma therapy, and
inhibition of the PI3K/Akt signaling pathway may suppress cancer
cell growth and induce apoptosis in various cancer types (31–33).
However, effects of upregulation of PTEN by nimesulide on PI3K/Akt
signaling-mediated apoptosis of PANC-1 cells remains to be
elucidated. Activation of peroxisome proliferator-activated
receptor γ in human pancreatic cancer cells has been demonstrated
to be associated with enhanced expression of PTEN and apoptosis
(34), which suggests that there may
be a PI3K/Akt-independent mechanism underlying the anti-apoptotic
and PTEN-enhancing effect of nimesulide in PANC-1 cells.
VEGF, a selective mitogen of vascular endothelial
cells, serves a role in angiogenesis (35). In endothelial cells, PTEN antagonizes
PI3K signalling, which mediates VEGF expression and angiogenesis
(36). Overexpression of PI3K and
Akt could induce transcription of VEGF and promote the formation of
new blood vessels (37).
Furthermore, following inhibition of PTEN, PI3K/Akt is activated,
resulting in cell division, increased cell volume, apoptosis and
tumor angiogenesis (38,39). In the present study, the results
indicated that nimesulide increased PTEN expression but decreased
expression levels of VEGF, which suggested that nimesulide may
inhibit angiogenesis of PANC-1 cells.
The carcinogenic role of COX-2 overexpression has
been demonstrated in a number of human malignancies, including
pancreatic cancer (40).
Overexpression of COX-2 is associated with tumor aggressiveness and
growth in cancer biology (41,42).
However, a previous study reported that nimesulide induces
apoptosis in MIA PaCa-2 cells (no COX-2 protein expression) and
BxPC-3 cells (high COX-2 protein expression), which suggested that
the effect of nimesulide may be independent of COX-2 protein
expression (16). In the present
study, the results demonstrated that nimesulide decreases the
expression of COX-2 and increases the expression of PTEN, but also
results in inhibition of proliferation of PANC-1 cells.
Furthermore, COX-2 positively regulates Akt signalling by
suppressing the activity of PTEN (43) and prostaglandin E2 (44). In a previous study, In cells
transformed with erb-b2 receptor tyrosine kinase 2, the activation
or inhibition of mitogen-activated protein kinase and PI3K/Akt
cascades resulted in the up- and downregulation of COX-2,
respectively (45). In summary, the
anti-cancer effect of nimesulide in PANC-1 cells may be associated
with the interaction between PTEN and COX-2.
In conclusion, the results of the present study
demonstrated that nimesulide induced an anti-cancer effect on
PANC-1 cells. Specifically, nimesulide inhibited proliferation and
promoted apoptosis of PANC-1 cells via enhancement of expression of
PTEN. Furthermore, the results of the present study suggest that
nimesulide may prevent tumor angiogenesis by inhibiting expression
of VEGF. The regulatory effects of nimesulide on PANC-1 cells may
be associated with interactions between PTEN and COX-2 through the
PI3K/Akt signalling pathway. However, this hypothesis requires
further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants obtained
from the National Natural Science Foundation of China (grant no.
81772232), a project funded by Zhejiang Medical College Youth Dr.
start-up (grant. no. 2015B07), a project funded by the Education
Department Foundation of Zhejiang Province (grant no. Y201636954)
and a project funded by Zhejiang Medicine Health Science and
Technology Funding (grant no. 2013KYAO47).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and AS conceived and designed the study. MC, TW
and YC performed the experiments. YC wrote the paper. YC, MC and TW
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He Y, Zheng R, Li D, Zeng H, Zhang S and
Chen W: Pancreatic cancer incidence and mortality patterns in
China, 2011. Chin J Cancer Res. 27:29–37. 2015.PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei X, Wang W, Wang L, Zhang Y, Zhang X,
Chen M, Wang F, Yu J, Ma Y and Sun G: MicroRNA-21 induces
5-fluorouracil resistance in human pancreatic cancer cells by
regulating PTEN and PDCD4. Cancer Med. 5:693–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neoptolemos JP, Dunn JA, Stocken DD,
Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P,
Dervenis C, et al: Adjuvant chemoradiotherapy and chemotherapy in
resectable pancreatic cancer: A randomised controlled trial.
Lancet. 358:1576–1585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Laethem JL, Hammel P, Mornex F, Azria
D, Van Tienhoven G, Vergauwe P, Peeters M, Polus M, Praet M, Mauer
M, et al: Adjuvant gemcitabine alone versus gemcitabine-based
chemoradiotherapy after curative resection for pancreatic cancer: A
randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J
Clin Oncol. 28:4450–4456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sutton JM and Abbott DE: Neoadjuvant
therapy for pancreas cancer: Past lessons and future therapies.
World J Gastroenterol. 20:15564–15579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miglietta A, Toselli M, Ravarino N, Vencia
W, Chiecchio A, Bozzo F, Motta M, Torchio B and Bocca C: COX-2
expression in human breast carcinomas: Correlation with
clinicopathological features and prognostic molecular markers.
Expert Opin Ther Targets. 14:655–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Richardsen E, Uglehus RD, Due J, Busch C
and Busund LT: COX-2 is overexpressed in primary prostate cancer
with metastatic potential and may predict survival. A comparison
study between COX-2, TGF-beta, IL-10 and Ki67. Cancer Epidemiol.
34:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang J, Xue M, Yang S, Yao B, Zhang B,
Chen X, Pozzi A and Zhang MZ: Inhibition of 11β-Hydroxysteroid
Dehydrogenase type II suppresses lung carcinogenesis by blocking
tumor COX-2 expression as well as the ERK and mTOR signaling
pathways. PLoS One. 10:e01270302015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakstaite A, Maziukiene A, Silkuniene G,
Kmieliute K, Gulbinas A and Dambrauskas Z: HuR mediated
post-transcriptional regulation as a new potential adjuvant
therapeutic target in chemotherapy for pancreatic cancer. World J
Gastroenterol. 21:13004–13019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Gu Z, Xiao Z, Zhou T, Li J and Sun
K: Anti-tumor effect and mechanism of cyclooxygenase-2 inhibitor
through matrix metalloproteinase 14 pathway in PANC-1 cells. Int J
Clin Exp Pathol. 8:1737–1742. 2015.PubMed/NCBI
|
|
16
|
Eibl G, Reber HA, Wente MN and Hines OJ:
The selective cyclooxygenase-2 inhibitor nimesulide induces
apoptosis in pancreatic cancer cells independent of COX-2.
Pancreas. 26:33–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Funahashi H, Satake M, Dawson D, Huynh NA,
Reber HA, Hines OJ and Eibl G: Delayed progression of pancreatic
intraepithelial neoplasia in a conditional Kras(G12D) mouse model
by a selective cyclooxygenase-2 inhibitor. Cancer Res.
67:7068–7071. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eibl G, Takata Y, Boros LG, Liu J, Okada
Y, Reber HA and Hines OJ: Growth stimulation of COX-2-negative
pancreatic cancer by a selective COX-2 inhibitor. Cancer Res.
65:982–990. 2005.PubMed/NCBI
|
|
19
|
Kishimoto H, Hamada K, Saunders M, Backman
S, Sasaki T, Nakano T, Mak TW and Suzuki A: Physiological functions
of Pten in mouse tissues. Cell Struct Funct. 28:11–21. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J,
Zhu G, Wang X, Chang LS, He D and Li L: Kaempferol suppresses
bladder cancer tumor growth by inhibiting cell proliferation and
inducing apoptosis. Mol Carcinog. 54:831–840. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu JR, Wu WJ, Liu SX, Zuo LF, Wang Y,
Yang JZ and Nan YM: Nimesulide inhibits the growth of human
esophageal carcinoma cells by inactivating the JAK2/STAT3 pathway.
Pathol Res Pract. 211:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su M, Mei Y and Sinha S: Role of the
crosstalk between autophagy and apoptosis in cancer. J Oncol.
2013:1027352013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satoh K, Kaneko K, Hirota M, Masamune A,
Satoh A and Shimosegawa T: Expression of survivin is correlated
with cancer cell apoptosis and is involved in the development of
human pancreatic duct cell tumors. Cancer. 92:271–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan Z, Chen D, Chen X and Wei Y: Novel
combination of Vincristine and COX-2 Inhibitor Nimesulide provides
synergistic anti-proliferative and pro-apoptotic effects in KOSC-2
oral squamous carcinoma cells. Int J Clin Exp Me. 9:877–887.
2016.
|
|
27
|
Kumar S, Eroglu E, Rd SJ, Stokes JA III,
Scissum-Gunn K, Saldanha SN, Singh UP, Manne U, Ponnazhagan S and
Mishra MK: Resveratrol induces mitochondria-mediated,
caspase-independent apoptosis in murine prostate cancer cells.
Oncotarget. 8:20895–20908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhola PD and Letai A: Mitochondria-judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma J, Sawai H, Ochi N, Matsuo Y, Xu D,
Yasuda A, Takahashi H, Wakasugi T and Takeyama H: PTEN regulates
angiogenesis through PI3K/Akt/VEGF signaling pathway in human
pancreatic cancer cells. Mol Cell Biochem. 331:161–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ying H, Elpek KG, Vinjamoori A, Zimmerman
SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W,
et al: PTEN is a major tumor suppressor in pancreatic ductal
adenocarcinoma and regulates an NF-κB-cytokine network. Cancer
Discov. 1:158–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng TC, Lai CS, Chung MC, Kalyanam N,
Majeed M, Ho CT, Ho YS and Pan MH: Potent anti-cancer effect of
3′-hydroxypterostilbene in human colon xenograft tumors. PLoS One.
9:e1118142014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gowda R, Madhunapantula SV, Desai D, Amin
S and Robertson GP: Simultaneous targeting of COX-2 and AKT using
selenocoxib-1-GSH to inhibit melanoma. Mol Cancer Ther. 12:3–15.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hodgson MC, Deryugina EI, Suarez E, Lopez
SM, Lin D, Xue H, Gorlov IP, Wang Y and Agoulnik IU: INPP4B
suppresses prostate cancer cell invasion. Cell Commun Signal.
12:612014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farrow B and Evers BM: Activation of
PPARgamma increases PTEN expression in pancreatic cancer cells.
Biochem Biophys Res Commun. 301:50–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang D, Chang JR, Chin AJ, Smith A, Kelly
C, Weinberg ES and Ge R: The role of vascular endothelial growth
factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in
zebrafish development. Mech Dev. 108:29–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wen S, Stolarov J, Myers MP, Su JD, Wigler
MH, Tonks NK and Durden DL: PTEN controls tumor-induced
angiogenesis. Proc Natl Acad Sci USA. 98:4622–4627. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
An X, Lv H, Tian J, He X and Ling N: Role
of the PTEN/PI3K/VEGF pathway in the development of Kawasaki
disease. Exp Ther Med. 11:1318–1322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carracedo A and Pandolfi PP: The PTEN-PI3K
pathway: Of feedbacks and cross-talks. Oncogene. 27:5527–5541.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song Z, Bhagat G, Quante M, Baik GH,
Marrache F, Tu SP, Zhao CM, Chen D, Dannenberg AJ and Wang TC:
Potential carcinogenic effects of cigarette smoke and Swedish moist
snuff on pancreas: A study using a transgenic mouse model of
chronic pancreatitis. Lab Invest. 90:426–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Juuti A, Louhimo J, Nordling S, Ristimäki
A and Haglund C: Cyclooxygenase-2 expression correlates with poor
prognosis in pancreatic cancer. J Clin Pathol. 59:382–386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ristimäki A, Sivula A, Lundin J, Lundin M,
Salminen T, Haglund C, Joensuu H and Isola J: Prognostic
significance of elevated cyclooxygenase-2 expression in breast
cancer. Cancer Res. 62:632–635. 2002.PubMed/NCBI
|
|
43
|
Li CJ, Chang JK, Wang GJ and Ho ML:
Constitutively expressed COX-2 in osteoblasts positively regulates
Akt signal transduction via suppression of PTEN activity. Bone.
48:286–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vo BT, Morton D Jr, Komaragiri S, Millena
AC, Leath C and Khan SA: TGF-β effects on prostate cancer cell
migration and invasion are mediated by PGE2 through activation of
PI3K/AKT/mTOR pathway. Endocrinology. 154:1768–1779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Subbaramaiah K, Norton L, Gerald W and
Dannenberg AJ: Cyclooxygenase-2 is overexpressed in
HER-2/neu-positive breast cancer: Evidence for involvement of AP-1
and PEA3. J Biol Chem. 277:18649–18657. 2002. View Article : Google Scholar : PubMed/NCBI
|