Introduction
Acute myocardial infarction (AMI) is one of the most
common cardiovascular diseases and attributed to occlusion of the
epicardial coronary artery, ultimately leading to progressive
chronic heart failure. The therapeutic approaches available for AMI
injury and myocardial infarction are timely myocardial reperfusion
using either thrombolytic therapy or primary percutaneous coronary
intervention (1). However,
reperfusion to rescue ischemic myocardium may bring a high risk of
cardiomyocyte death, namely myocardial ischemia-reperfusion (IR)
injury, which includes the following forms: Reperfusion-induced
arrhythmias, myocardial stunning, microvascular obstruction and
lethal myocardial reperfusion injury (2–7).
Sphingosine 1-phosphate (S1P) is a lysophospholipid
mediating a series of cell functions, including cell motility, cell
proliferation and differentiation, immune system surveillance,
vascular permeability, cytoskeletal organization and viral
infections (8). S1P receptors
(S1PRs) are a group of G-protein coupled receptors responsible for
S1P functions that are referred to as S1PR1-5. However, the five
S1PRs differ in their distribution. S1PR1, S1PR2 and S1PR3 are
widely expressed, whereas S1PR4 and S1PR5 mainly exist in the
immune and nervous systems (9–11). S1P
has been reported to have a critical role in the protection of
cardiomyocytes and heart function from IR injury in vitro
and in vivo (12). For
instance, high-density lipoprotein and its lipid component S1P are
known to attenuate IR injury (13).
S1P has been demonstrated to protect cardiomyocytes of neonatal
rats and the heart from ischemic damage in perfused rabbits and
mice (14–16). Furthermore, the S1P/S1PR1 pathway was
reported to induce hypertrophy of cardiomyocytes and reduce
mortality of hypoxic cardiomyocytes in vitro (17,18).
TGF-β is a group of structurally associated proteins
regulating a number of critical cellular processes, including
apoptosis, tumor occurrence and IR (19–21). The
biological functions of TGF-β are initiated by binding to two types
of transmembrane receptor: TGF-β receptor type I (TGFβR1) and
TGFβR2. Activation of TGFBR2 leads to phosphorylation of TGFBR1,
which triggers activation of Smad3 and forces Smad3 to translocate
into the nucleus (22,23). In a previous study, Vivar et
al (24) revealed that TGF-β
blocked the IR-induced apoptosis of cardiac fibroblasts through the
Smad3, extracellular signal-regulated kinase (ERK)1/2 and Akt
signaling pathways. Furthermore, TGF-β participated in the
cross-talk of ERK1/2 and Akt with the Smad2/3 signaling pathway;
however, these signaling pathways appear to have independent
roles.
Although S1P/S1PR1 and TGF-β/Smad3 were all
demonstrated to be actively implicated in IR injury of myocardial
cells, their association has remained to be fully elucidated. The
present study reported that exogenous TGF-β significantly increased
the levels of S1PR1 compared with those of S1PR2-5. In addition,
the results suggested that TGF-β/Smad3 contributed to the
cardioprotective effect of S1P/S1PR1 in an established in
vitro IR model.
Materials and methods
Reagents
All treatments were administered at a concentration
of 10 mM for 48 h of treatment at 37°C. S1P, W146, SB-431542 (SB4),
SIS3 and TGF-β were all purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The primary anti-S1PR1, anti-TGF-β and
anti-Smad3 antibodies (Cat. no. ab72806, ab31013, ab40854,
respectively) were acquired from Abcam (Cambridge, UK). Fetal
bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM)
were from Hyclone (GE Healthcare, Little Chalfont, UK). The
secondary antibodies conjugated to horseradish peroxidase (Cat. no.
ab6789) were purchased from Zhongshan Goldenbridge Bio (Beijing,
China). The enhanced chemiluminescent (ECL) kit was obtained from
Thermo Fisher Scientific (Shanghai, China). For
Reverse-transcription quantitative polymerase chain reaction
(RT-qPCR), the MagExtractor-RNA kit and ReverTra Ace qPCR RT Master
Mix with gDNA Remover kit were all from Toyobo (Cat. no. FSQ-301,
Tokyo, Japan). The lactate dehydrogenase (LDH) detection activity
assay kit and the commercialized caspase-3 assay kit were purchased
from Sigma-Aldrich (cat. no. CASP3F-1KT, Merck KGaA) and Biovision,
Inc. (cat. no. 1533-100, Milpitas, CA, USA), respectively.
Animals
Neonatal mice were purchased from SLRC Laboratory
Animal (Changsha, China). Animals were provided with standard
rodent chow and water ad libitum. All animal procedures were
approved by the Institutional Animal Care and Use Committee of
Jining No.1 People's Hospital (Jining, China). All protocols
conformed to the National Research Council's Guide for the Care and
Use of Laboratory Animals.
Isolation and cultivation of
cardiomyocytes
Animals were anesthetized with ether to remove the
hearts, which were put in pre-cooled D-hanks medium (Procell,
Wuhan, China). The heart tissues were immersed in 0.1% trypsin
(Procell) and oscillated overnight at 4°C. Complete medium was
added to terminate digestion by incubation at 37°C for 10 min.
After discarding the supernatant, the remnant was incubated with
0.08% collagenase II (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) at 37°C for 10 min and the supernatants were pooled. The
combined supernatants were centrifuged at 250 × g for 5 min, and
DMEM containing 20% FBS was used to re-suspend the precipitates.
The cells were then inoculated in a culture dish and incubated for
50 min at 37°C in a humidified atmosphere containing 5%
CO2. The non-adherent cells were aspirated to be
re-suspended for a second adherence culture. The final
concentration of the cells was adjusted to 5×105
cells/ml and 10 ml cell suspension was mixed with 100 µl
bromodeoxyuridine to inhibit the proliferation and differentiation
of non-fibroblasts but without any obvious effect on the
proliferation of the fibroblasts (25). The cells were cultured under the
abovementioned conditions and the medium was changed once every 2–3
days. The growth and morphological changes of cardiomyocytes were
observed and recorded every day.
In vitro ischemia-reperfusion (IR)
model
The IR model was established and evaluated as
described previously with minor modifications (26). In brief, the culture medium with 20%
FBS was centrifuged at 250 × g for 5 min and resuspended in D-hanks
medium with a gas mixture of 95% O2-5% CO2
for 30 min of incubation prior to hypoxia. To simulate ischemia,
the culture plate was transferred into an anoxic incubator for 2 h
of incubation with a gas mixture of 95% N2-5%
CO2. For the reperfusion process, the D-hanks medium was
replaced with DMEM containing 20% FBS for 24 h of incubation at
37°C with a gas mixture of 95% O2-5% CO2. The
established IR model was evaluated by inspecting apoptosis, LDH
release and caspase activity. For the apoptosis assay,
cardiomyocytes were collected and centrifuged at 250 × g for 5 min.
The cells were inoculated in 6-well flat-bottom plates and digested
with 0.3 ml 1X trypsin-EDTA in PBS (37°C). The cells were
immediately stained in the dark for 30 min according to the
instructions of the Annexin V-FITC/PI apoptosis detection kit (cat.
no. 88-8005-72, Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Finally, cellular apoptosis was determined by flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) within 1 h.
The measurements of LDH release and caspase-3 activity were
performed using respective commercial kits based on the
manufacturer's instructions.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cardiomyocytes treated
with 1 µM S1P alone or in combination with 0.4 µM W146 using a
MagExtractor-RNA kit. The extracted total RNA was
reverse-transcribed into complementary DNA using ReverTra Ace qPCR
RT Master Mix with gDNA Remover kit. The primers, which were
synthesized by Sangon Biotech (Shanghai, China), had the following
sequences: S1PR1 forward, 5′-AACTACACAACGGCAGCAAC-3′ and reverse,
5′-GCAGGCAATGAAGACACTCA-3′; S1PR2 forward,
5′-GGCTCTGTTCCCTGTATTG-3′ and reverse, 5′-GGGCTCACTTTGCTCCTC-3′;
S1PR3 forward, 5′-AAATGGCTGCCTTGGAC-3′ and reverse,
5′-CCCATCGGTTTGGTGCT-3′; S1PR4 forward, 5′-ACGATAGGTGCTGTTAGT-3′
and reverse, 5′-CAGATATGCTGCTTCTTT-3′; S1PR5 forward,
5′-TGGTGGTCCTCATCGTCG-3′ and reverse, 5′-GGAGAAGGTGGCAGTGGTAA-3′;
TGF-β forward, 5′-GACTACTACGCCAAGGAGGTC-3′ and reverse,
5′-GAGAGCAACACGGGTTCAG-3′; Smad3 forward, 5′-TGTTGGTGGAGGGTGTAG-3′
and reverse, 5′-AGCAGCAGTGAAGGTGAG-3′; β-actin forward,
5′-ACTCTTCCAGCCTTCCTTC-3′ and reverse, 5′-ATCTCCTTCTGCATCCTGTC-3′.
RT-qPCR was performed on an ABI7000 fluorescent quantitative PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with
the following thermocycling procedure: 95°C for 60 sec, followed by
40 cycles of 95°C for 15 sec, 55°C for 15 sec and 72°C for 45 sec.
All data were normalized to the housekeeping gene β-actin used as a
reference. The relative expression of the target genes was
calculated using the 2−ΔΔCq method (27).
Western blot analysis
Cardiomyocytes were incubated with 200 ml lysis
buffer (25 mM MgCl2, 5 mM KCl, 20 mM
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, 0.5% (v/v)
complete protease inhibitor and Triton X-100). Protein
concentrations were determined using a BCA Protein Quantification
kit (Vazyma, Nanjing, China) according to the manufacturer's
instructions. Cellular protein (50 mg) was separated using 12%
SDS-PAGE prior to transfer onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The protein bands
were blocked for 1 h in blocking buffer at room temperature.
Antibodies (monoclonal rabbit anti-S1PR1, TGF-β and Smad3; 1:500
dilution) were incubated with the membranes overnight at 4°C.
Secondary antibodies conjugated to horseradish peroxidase (1:2,000
dilution) were incubated with the membranes for 1 h at room
temperature, followed by an ECL assay. The bands were imaged with
the ChemiDoc™ XRS Gel image system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and their intensities were measured using
Quantity one v4.62 software (Bio-Rad Laboratories, Inc.).
S1P measurement by liquid
chromatography tandem mass spectrometry (LC-MS/MS)
The S1P content was measured by LC-MS/MS according
to previously described procedures (28).
Statistical analysis
Each experiment was performed in triplicate on three
independent occasions. Values are expressed as the mean ± standard
deviation and analyzed using one-way analysis of variance with the
Least Significant Difference post hoc test. The statistical
analyses were performed using SPSS 11.5 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment and evaluation of in
vitro IR injury model
The isolated myocardial cells were used for the
establishment of an in vitro IR injury model. In the
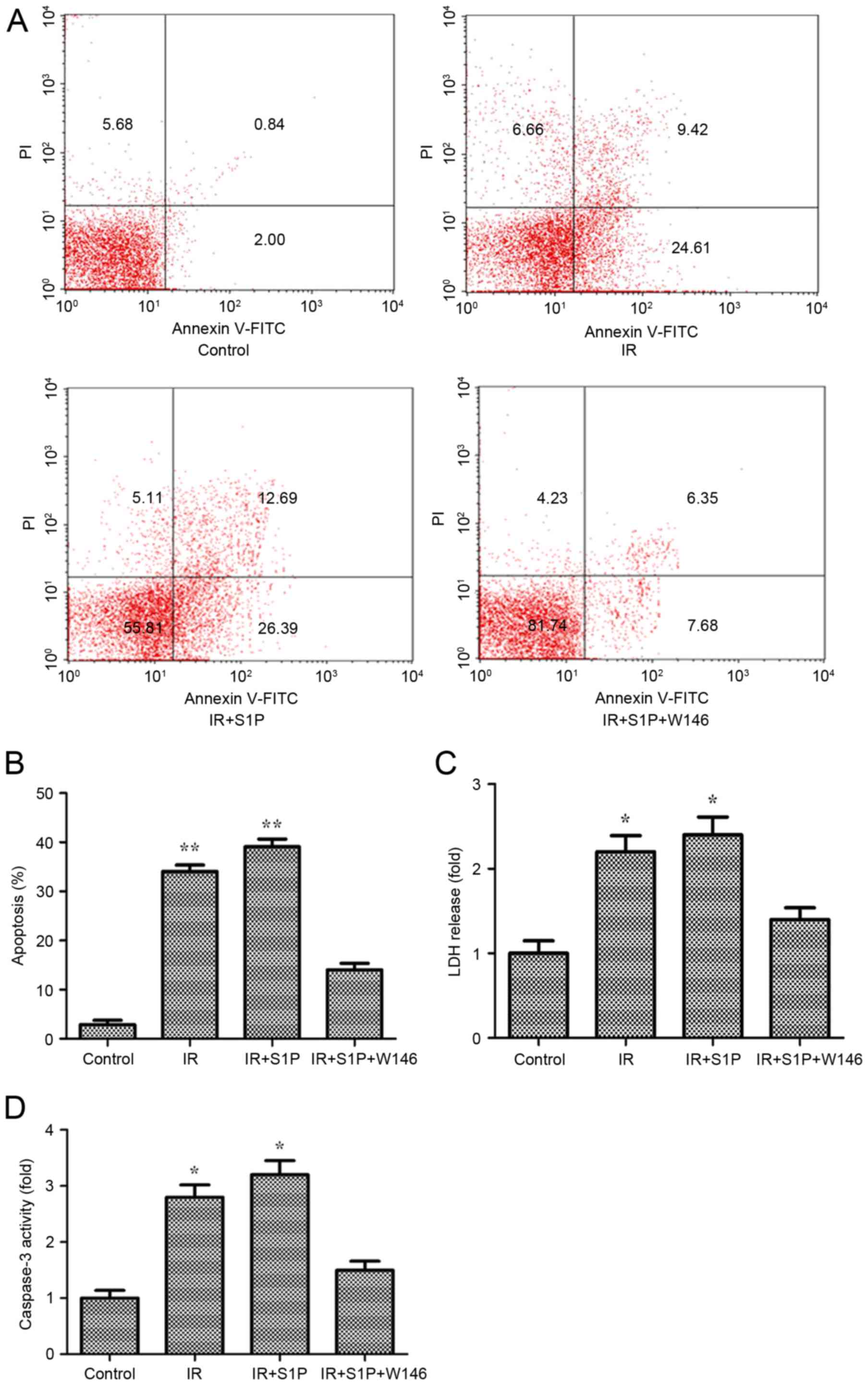
established IR model, the apoptotic rate was ~34% (P<0.01 vs.
control). Addition of S1P resulted in a further increase of cell
apoptosis up to ~39% (P<0.01). However, exogenous W146 inhibited
the increase of cell apoptosis caused by S1P, resulting in an
apoptotic rate that was significantly different from that of the
control (Fig. 1A and B). The LDH
levels and caspase-3 activity were also measured due to the
stability of LDH in dead cells and the critical role of caspase-3
in apoptosis. The results demonstrated that in IR-treated cells,
LDH levels and caspase3 activity increased by 2.2- and 2.8-fold,
respectively (P<0.05), and were further enhanced in
IR+S1P-treated cells, resulting in 2.4- and 3.2-fold increases,
respectively, compared with the control (P<0.05). However,
introduction of W146 inhibited the increases of LDH levels and
caspase3 activity caused by S1P with the resulting values being not
significantly different from those in the control group (Fig. 1C and D).
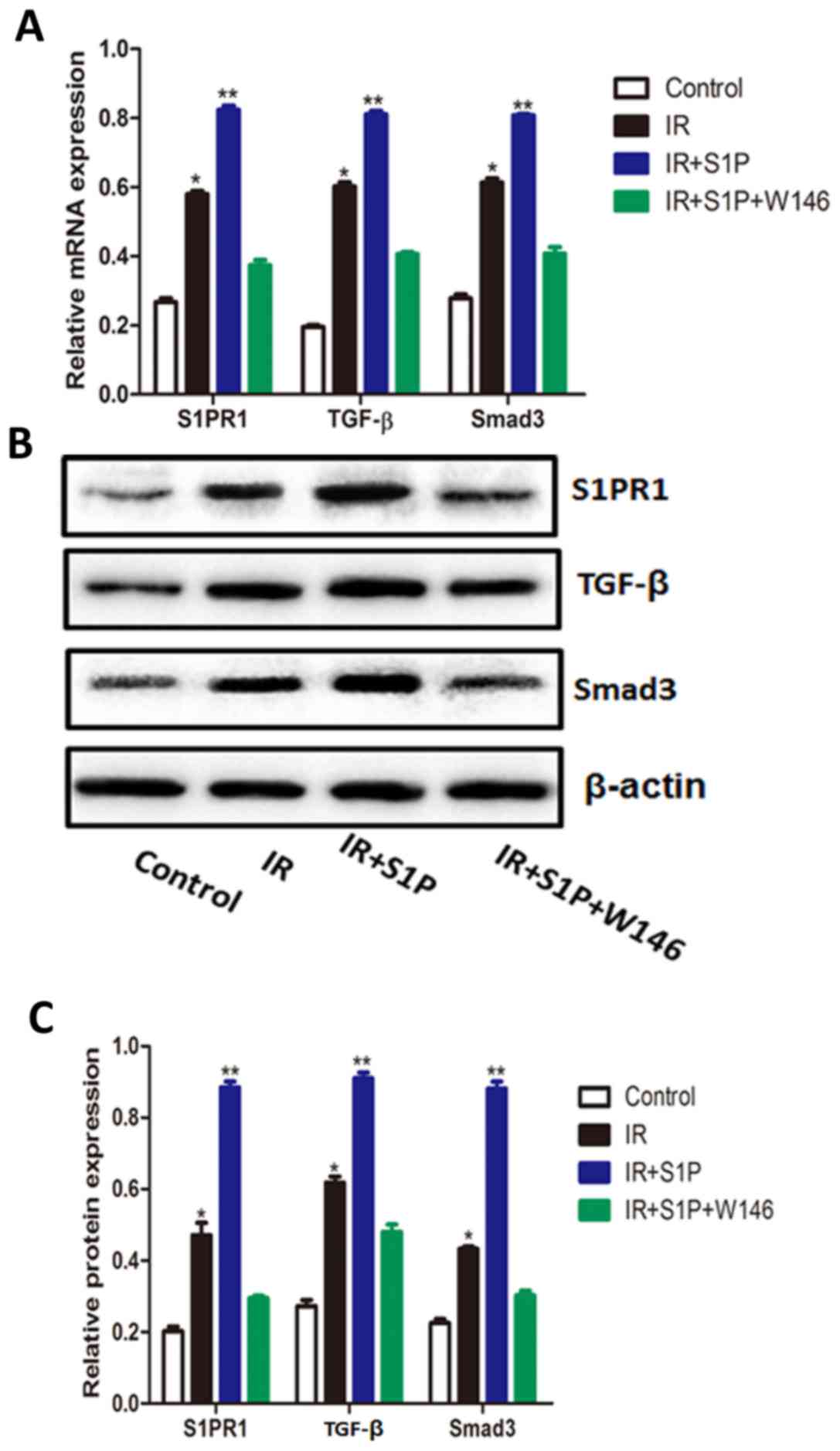
S1P increases the mRNA and protein
levels of S1PR1, TGF-β and Smad3 in an in vitro IR model
After IR injury, the mRNA and protein levels of
S1PR1, TGF-β and Smad3 were significantly increased (P<0.01).
Exogenous S1P further increased the mRNA and protein expression of
S1PR1, TGF-β and Smad3 (P<0.001). In comparison, W146 abolished
the stimulatory effects of S1P on S1PR1, TGF-β and Smad3 mRNA and
protein expression, resulting in levels that were comparable to
those of the control group (Fig.
2).
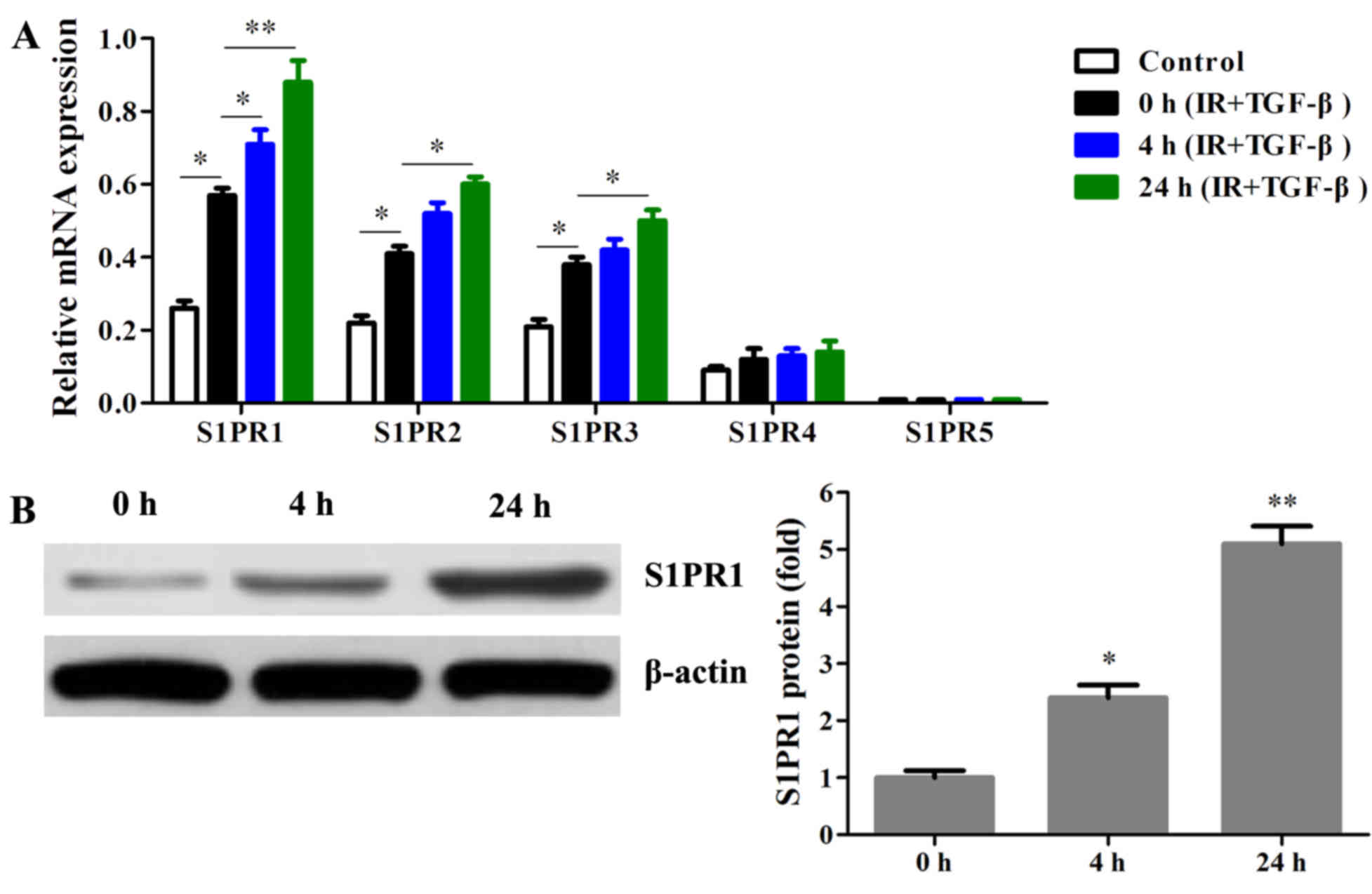
TGF-β/Smad3 pathway activation
stimulates S1P/S1PR1 in IR injury model
Induction of IR resulted in upregulation of S1PR1-3
in myocardial cells (P<0.05 or P<0.01). Pretreatment with
TGF-β caused a significant increase of S1PR1 mRNA at 4 h
(P<0.05). After 24 h of treatment with TGF-β, the levels of
S1PR1-3 mRNA were all significantly stimulated (P<0.05 or
P<0.01). The expression of S1PR5 mRNA was not detectable, while
S1PR4 mRNA appeared to not be significantly affected by TGF-β
(Fig. 3A). The protein levels of
S1PR1 were also increased by pretreatment with TGF-β for 0, 4 and
24 h (P<0.05 or P<0.01; Fig.
3B). These results suggested that S1PR1 mRNA was more affected
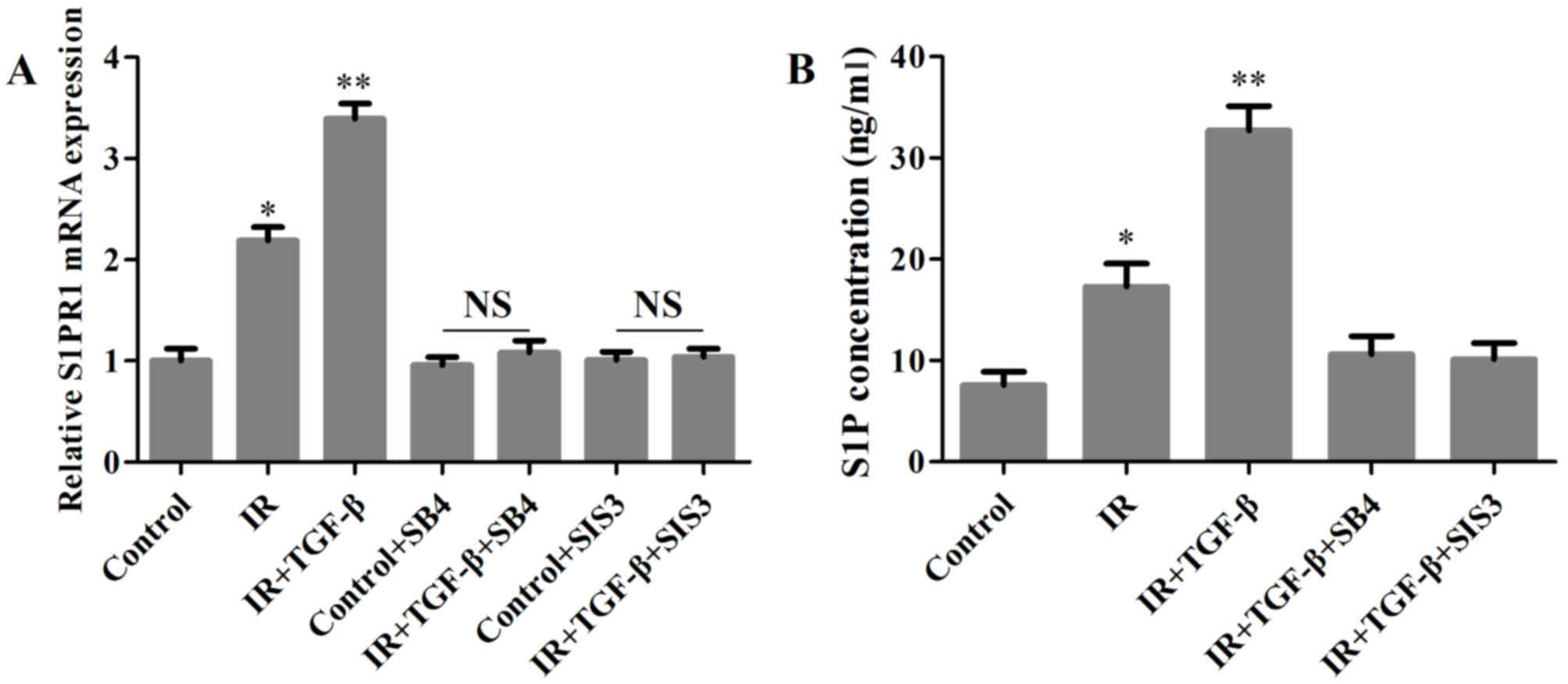
by TGF-β than S1PR2 and S1PR3 mRNA. By using TGFβR1 inhibitor SB4
and Smad3 inhibitor SIS3, the stimulatory effects of TGF-β on S1PR1
and S1P were abolished (Fig. 4A and
B).
Discussion
In the present study, an in vitro IR model
was successfully established in myocardial cells and evaluated by
analysis of apoptosis, LDH release and caspase-3 activity. It was
observed that extraneous TGF-β induced the most significant
increase of S1PR1 among the five S1P receptors (S1PR1-5) within 24
h. External S1P caused elevated S1PR1, TGF-β and Smad3, while W146,
a specific S1PR1 antagonist (29),
abolished the effects of S1P. It was also revealed that SB4 (TGFβR1
inhibitor) and SIS3 (Smad3 inhibitor) offset the stimulatory effect
of TGF-β on the levels of S1PR1 mRNA and S1P. These results
suggested an intimate association of S1P/S1PR1 with
TGF-β/Smad3.
As mentioned above, the levels of S1P rose following
IR, which was indicative of the protection of myocardial cells from
IR (14–16). The inherent TGF-β levels increased
when IR occurred and autoinduction or exogenous addition of TGF-β
also protected the heart from IR to a large extent, suggesting a
potentially cardioprotective role of TGF-β against IR in
cardiomyocytes (30–33). The results of the present study were
consistent with those of these previous studies on IR. The present
study also observed that replenishment of S1P further promoted the
mRNA and protein expression of S1PR1, TGF-β and Smad3 after IR.
These results did not only suggest a protective effect of S1P
against IR, but also the close association of S1P/S1PR1 with
TGF-β/Smad3. The present results also demonstrated that the
increases of S1PR1, TGF-β and Smad3 were almost reversed by the
addition of the S1PR1 antagonist W146, which was consistent with a
previous study (34). From these
results, it may be deduced that the abolishment of the protective
effect caused by W146 was mainly due to the disruption of the
ligation between S1P and S1PR1, resulting in the interruption of
the association of S1P/S1PR1 with TGF-β/Smad3.
As is known, differential expression patterns of
S1PR subtypes are important for subsequent cellular responses
(35). Although S1PR1-5 are widely
distributed in numerous tissue types, the present results indicated
that the expression of S1PR1-3 mRNA was significantly upregulated
after induction of IR, while the expression of S1PR4 and 5 was
generally low and not affected, suggesting that S1PR1-3 may have a
more important role in cardioprotection than S1PR4 and −5. However,
the relative expression of S1PR1-3 in cardiac myocytes is still
under debate. For instance, Forrest et al (36) reported that S1PR1 was not detected in
myocytes in adult rat and mouse heart sections with
subtype-selective antibodies against S1PR1 and S1PR3 compared with
marked staining with S1PR3 antibody, indicating that S1PR3, but not
S1PR1, may be involved in the protective effect of S1P on cardiac
myocytes. However, Robert et al (17) demonstrated that S1PR1 existed in
neonatal rat heart homogenates and membranes of neonatal
cardiomyocytes detected with polyclonal antibodies against a S1PR1
domain that is highly homogenous across multiple mammalian species.
A similar conclusion that S1PR1 resided in human ventricular
myocytes as well as coronary artery endothelial cells was also
drawn using the same antibodies (37). Growing evidence appears to reach a
consensus that S1PR1 levels are relatively high in cardiomyocytes
throughout development (18,35,38–40).
As S1PR2 and S1PR3 mRNA are expressed in myocardial
cells, their roles in TGF-β-mediated cardioprotection via S1P/S1PR1
were also investigated in the present study. It was revealed that
extraneous TGF-β increased the levels of S1PR1 mRNA but not those
of S1PR2 and S1PR3 mRNA after 4 h of incubation. The results also
suggested that the stimulatory effect of TGF-β on the expression of
S1PR1 mRNA was more prominent than on that of S1PR2 and S1PR3 mRNA,
implying a more critical role of S1PR1 in the treatment of IR. The
wide-spectrum use of inhibitors vastly facilitates the study of the
TGF-β/Smad3 pathway (28,41,42). The
present results demonstrated that either TGFβR1 inhibitor SB4 or
Smad3 inhibitor SIS3 was able to abolish the enhancement of the
cardioprotective effect of S1P/S1PR1 by TGF-β.
In conclusion, the present study suggested that the
TGF-β/Smad3 pathway mediates the protection of myocardial cells
from IR injury via the stimulation of S1P/S1PR1. However, the TGF-β
pathway may be either Smad-dependent or -independent (43), and the mRNA levels of S1PR2 and S1PR3
were also significantly affected by TGF-β. Further study is
required to elucidate the underlying cardioprotective mechanisms
for IR treatment and the association of S1P/S1PRs with
TGF-β-mediated pathways.
References
|
1
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braunwald E and Kloner RA: Myocardial
reperfusion: A double-edged sword? J Clin Invest. 76:1713–1719.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piper HM, Garcia-Dorado D and Ovize M: A
fresh look at reperfusion injury. Cardiovasc Res. 38:291–300. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hearse DJ and Tosaki A: Free radicals and
reperfusion-induced arrhythmias: Protection by spin trap agent PBN
in the rat heart. Circ Res. 60:375–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kloner RA, Bolli R, Marban E, Reinlib L
and Braunwald E: Medical and cellular implications of stunning,
hibernation, and preconditioning: An NHLBI workshop. Circulation.
97:1848–1867. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krug A, Du Mesnil de Rochemont and Korb G:
Blood supply of the myocardium after temporary coronary occlusion.
Circ Res. 19:57–62. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen H, Sanna Germana M, Gonzalez-Cabrera
PJ and Roberts E: The organization of the sphingosine 1-phosphate
signaling system. Curr Top Microbiol Immunol. 378:1–21.
2014.PubMed/NCBI
|
|
9
|
Ishii I, Fukushima N, Ye X and Chun J:
Lysophospholipid receptors: Signaling and biology. Annu Rev
Biochem. 73:321–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Im DS, Heise CE, Ancellin N, O'Dowd BF,
Shei GJ, Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, et
al: Characterization of a novel sphingosine 1-phosphate receptor,
Edg-8. J Biol Chem. 275:14281–14286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gräler MH, Grosse R, Kusch A, Kremmer E,
Gudermann T and Lipp M: The sphingosine 1-phosphate receptor S1P4
regulates cell shape and motility via coupling to Gi and G12/13. J
Cell Biochem. 89:507–519. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knapp M: Cardioprotective role of
sphingosine-1-phosphate. J Physiol Pharmacol. 62:601–607.
2011.PubMed/NCBI
|
|
13
|
Theilmeier G, Schmidt C, Herrmann J, Keul
P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S,
Stypmann J, et al: High-density lipoproteins and their constituent,
sphingosine-1-phosphate, directly protect the heart against
ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid
receptor. Circulation. 114:1403–1409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin ZQ, Goetzl EJ and Karliner JS:
Sphingosine kinase activation mediates ischemic preconditioning in
murine heart. Circulation. 110:1980–1989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin ZQ, Zhou HZ, Zhu P, Honbo N,
Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS and Gray MO:
Cardioprotection mediated by sphingosine-1-phosphate and
ganglioside GM-1 in wild-type and PKC epsilon knockout mouse
hearts. Am J Physiol Heart Circ Physiol. 282:H1970–H1977. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karliner JS, Honbo N, Summers K, Gray MO
and Goetzl EJ: The lysophospholipids sphingosine-1-phosphate and
lysophosphatidic acid enhance survival during hypoxia in neonatal
rat cardiac myocytes. J Mol Cell Cardiol. 33:1713–1717. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robert P, Tsui P, Laville MP, Livi GP,
Sarau HM, Bril A and Berrebi-Bertrand I: EDG1 receptor stimulation
leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell
Cardiol. 33:1589–1606. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang J, Honbo N, Goetzl EJ, Chatterjee K,
Karliner JS and Gray MO: Signals from type 1 sphingosine
1-phosphate receptors enhance adult mouse cardiac myocyte survival
during hypoxia. Am J Physiol Heart Circ Physiol. 293:H3150–H3158.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toledo-Pereyra LH, Toledo AH, Walsh J and
Lopez-Neblina F: Molecular signaling pathways in
ischemia/reperfusion. Exp Clin Transplant. 2:174–177.
2004.PubMed/NCBI
|
|
20
|
Truty MJ and Urrutia R: Basics of TGF-beta
and pancreatic cancer. Pancreatology. 7:423–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Li D, Saldeen T and Mehta JL:
TGF-β1 attenuates myocardial ischemia-reperfusion injury via
inhibition of upregulation of MMP-1. Am J Physiol Heart Circ
Physiol. 284:H1612–H1617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng XH and Derynck R: Specificity and
versatility in TGF-β signaling through Smads. Annu Rev Cell Dev
Biol. 21:659–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massagué J, Seoane J and Wotton D: Smad
transcription factors. Genes Dev. 19:2783–2810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vivar R, Humeres C, Ayala P, Olmedo I,
Catalán M, García L, Lavandero S and Díaz-Araya G: TGF-β1 prevents
simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis
by activation of both canonical and non-canonical signaling
pathways. Biochim Biophys Acta. 1832:754–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rutter WJ, Pictet RL and Morris PW: Toward
molecular mechanisms of developmental processes. Annu Rev Biochem.
42:601–646. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Z, Qi Y and Gao C: Cardiac
myocyte-protective effect of microRNA-22 during ischemia and
reperfusion through disrupting the caveolin-3/eNOS signaling. Int J
Clin Exp Pathol. 8:4614–4626. 2015.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Liu J, Lee JF, Zhang W, Kandouz M,
VanHecke GC, Chen S, Ahn YH, Lonardo F and Lee MJ: TGF-β/SMAD3
pathway stimulates Sphingosine-1 phosphate receptor 3 Expression:
IMPLICATION OF SPHINGOSINE-1 PHOSPHATE RECEPTOR 3 IN LUNG
ADENOCARCINOMA PROGRESSION. J Biol Chem. 291:27343–27353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ham A, Kim M, Kim JY, Brown KM, Fruttiger
M, D'Agati VD and Lee HT: Selective deletion of the endothelial
sphingosine-1-phosphate 1 receptor exacerbates kidney
ischemia-reperfusion injury. Kidney Int. 85:807–823. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lefer AM, Ma XL, Weyrich AS and Scalia R:
Mechanism of the cardioprotective effect of transforming growth
factor beta 1 in feline myocardial ischemia and reperfusion. Proc
Natl Acad Sci USA. 90:1018–1022. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lefer AM, Tsao P, Aoki N and Palladino MA
Jr: Mediation of cardioprotection by transforming growth
factor-beta. Science. 249:61–64. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mehta JL, Yang BC, Strates BS and Mehta P:
Role of TGF-beta1 in platelet-mediated cardioprotection during
ischemia-reperfusion in isolated rat hearts. Growth Factors.
16:179–190. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moses HL, Yang EY and Pietenpol JA: TGF-β
stimulation and inhibition of cell proliferation: New mechanistic
insights. Cell. 63:245–247. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsukada YT, Sanna MG, Rosen H and Gottlieb
RA: S1P1-selective agonist SEW2871 exacerbates reperfusion
arrhythmias. J Cardiovasc Pharmacol. 50:660–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Means CK and Brown JH:
Sphingosine-1-phosphate receptor signalling in the heart.
Cardiovasc Res. 82:193–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forrest M, Sun SY, Hajdu R, Bergstrom J,
Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, et al:
Immune cell regulation and cardiovascular effects of sphingosine
1-phosphate receptor agonists in rodents are mediated via distinct
receptor subtypes. J Pharmacol Exp Ther. 309:758–768. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mazurais D, Robert P, Gout B,
Berrebi-Bertrand I, Laville MP and Calmels T: Cell type-specific
localization of human cardiac S1P receptors. J Histochem Cytochem.
50:661–670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Wada R, Yamashita T, Mi Y, Deng CX,
Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, et al: Edg-1,
the G protein-coupled receptor for sphingosine-1-phosphate, is
essential for vascular maturation. J Clin Invest. 106:951–961.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nakajima N, Cavalli AL, Biral D,
Glembotski CC, McDonough PM, Ho PD, Betto R, Sandona D, Palade PT,
Dettbarn CA, et al: Expression and characterization of Edg-1
receptors in rat cardiomyocytes: Calcium deregulation in response
to sphingosine 1-phosphate. Eur J Biochem. 267:5679–5686. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang G, Contos JJ, Weiner JA, Fukushima N
and Chun J: Comparative analysis of three murine G-protein coupled
receptors activated by sphingosine-1-phosphate. Gene. 227:89–99.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu R, Das B, Xiao W, Li Z, Li H, Lee K
and He JC: A Novel Inhibitor of Homeodomain Interacting protein
Kinase 2 mitigates kidney fibrosis through inhibition of the
TGF-β1/Smad3 pathway. J Am Soc Nephrol. 28:2133–2143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan YM, Ai J, Shi YN, Zuo ZL, Hou B, Luo J
and Cheng YX: (+/−)-Aspongamide A, an N-acetyldopamine trimer
isolated from the insect Aspongopus chinensis, is an inhibitor of
p-Smad3. Org Lett. 16:532–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kiyono K, Suzuki HI, Matsuyama H,
Morishita Y, Komuro A, Kano MR, Sugimoto K and Miyazono K:
Autophagy is activated by TGF-β and potentiates TGF-β-mediated
growth inhibition in human hepatocellular carcinoma cells. Cancer
Res. 69:8844–8852. 2009. View Article : Google Scholar : PubMed/NCBI
|