Introduction
Preeclampsia is one of the main causes of perinatal
maternal and infant mortality, with an incidence of 1–5% (1,2).
Pathophysiological changes begin in early pregnancy; however
symptoms usually become evident after 20 weeks gestation (3). The main symptoms of preeclampsia
include hypertension, proteinuria, edema, full body spasms and in
severe cases, multiple-organ dysfunction (3). Preeclampsia is a condition associated
with the placenta; clinical practice has demonstrated that
disease-associated symptoms are rapidly relieved following
placental delivery (4). Decreased
trophoblast invasion, required to establish pregnancy, contributes
to the development of preeclampsia (5) and inhibits vascular reconstruction in
the uterine spiral arteries (6).
Preeclampsia is a complex disease and its course is difficult to
predict, therefore the termination of pregnancy may be recommended
as a method of controlling disease progression (7). The etiology and pathogenesis of
preeclampsia remains unclear.
Forkhead box protein M1 (FOXM1) is a protein
expressed in a number of mammalian cells and serves important roles
in physiological functions and pathological conditions, including
mitosis, cell proliferation, differentiation, senescence,
organogenesis, DNA repair and cancer invasion (8–10). FOXM1
in villous and decidual tissue is expressed in the cytotrophoblast
during early pregnancy, and may affect embryo implantation and
early placental formation (11–13). It
has been demonstrated that the vascular endothelial growth factor
(VEGF) signaling pathway is involved in the function of
extravillous trophoblasts and trophoblast invasion (14,15).
Furthermore, VEGF promotes endothelial cell division, vascular
endothelial cell proliferation and migration, and tubular structure
formation during neovascularization. It also enhances the
permeability of vascular endothelial cells (16). Therefore, the current study
investigated whether FOXM1 involvement during preeclampsia occurs
via the VEGF signaling pathway.
In the present study, the mRNA and protein
expression of FOXM1 in the placenta of patients with or without
preeclampsia were measured by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting. In
vitro function experiments were performed in HTR8/SVneo
trophoblast cells. The association between FOXM1 and VEGF was
investigated to explore the role of FOXM1 in preeclampsia.
Patients and methods
Patients
A total of 28 pregnant patients with preeclampsia
and 30 patients without preeclampsia who underwent cesarean
sections at the Affiliated Hospital of Qingdao University between
June 2013 and September 2016 were enrolled in the present study.
The patients without preeclampsia were used as controls.
Participants were all female, with a median age of 33 years (range,
21–41 years) in the preeclampsia group and 35 years (range, 20–42
years) in the control group. The diagnosis of preeclampsia was made
by professors from the Department of Pathology of the Affiliated
Hospital of Qingdao University (Qingdao, China). This diagnosis was
confirmed by serological tests and other clinical examinations,
including blood pressure assessment, routine laboratory evaluation
(the measurement of urine volume, creatinine, alanine
aminotransferase or aspartate aminotransferase, and platelets),
fetal status assessment and proteinuria detection. The mean weights
of infants from the control and preeclampsia groups were 3.15±0.97
and 2.79±1.03 kg, respectively. The blood pressure of infants was
not measured. Placental tissue samples (500 mg) were collected from
each participant during cesarean section and stored in liquid
nitrogen at −196°C. The protocol of the present study was approved
by the Ethical Review Board of the Affiliated Hospital of Qingdao
University and written informed consent was obtained from all
patients.
Reagents and equipment
SuperReal PreMix (SYBR-Green; cat. no. FP204) and
the TIANScript II RT kit (cat. no. KR107) were purchased from
Tiangen Biotech Co., Ltd. (Beijing, China). iQ™ and
Image Lab™ software (version 3.0) were purchased from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA) for RT-qPCR. The BCA
protein assay kit (cat. no. RTP7102) was purchased from Real-Times
(Beijing) Biotechnology Co., Ltd. (Beijing, China). Cell
preservation solution was purchased from Ruikangdi Co., Ltd.
(Beijing, China). Enhanced chemiluminscence (ECL) solution (cat.
no. ab65623) was purchased from Abcam (Cambridge, MA, USA). All
plasmids were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China). Cell proliferation and cytotoxicity test kits
for the MTT assay were purchased from JRDUN Biotechnology Co., Ltd.
(Shanghai, China). Cell cycle and apoptosis detection kits were
purchased from Beyotime Institute of Biotechnology (Jiangsu,
China). The Annexin V-FITC apoptosis detection kit was purchased
from Vazyme Biotech, Co., Ltd. (Nanjing, China).
RT-qPCR
Placental tissues were pulverized with liquid
nitrogen. A total of 1 ml TRIzol (cat. no. 15596026; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was added to each
100 mg tissue for adequate lysis and RNA was extracted using the
phenol chloroform method as previously described (17). The integrity of the RNA bands was
detected using agarose gel electrophoresis as previously described
(18). RNA purity was assessed by
measuring the 260/280 ratio using a spectrophotometer. RT was
performed using 1 µg total RNA using a TIANScript II RT kit.
Template cDNA was stored at −20°C. qPCR was performed using the
SuperReal PreMix (SYBR-Green) and specific primers. The primers for
FOXM1 were as follows: Forward, 5′-GGCTCCCGCAGCATCAAGCA-3′ and
reverse, 5′-TGTTCCGGCGGAGCTCTGGA-3′. The primers for β-actin, which
was used as the internal reference gene, were as follows: Forward,
5′-ATCTGGCACCACACCTTCACAATGAGCTGCG-3′ and reverse,
5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′. The primers for VEGF were
as follows: Forward, 5′-TTGCCTTGCTGCTCTACCTC-3′ and reverse,
5′-AAATGCTTTCTCCGCTCTGA-3′. The qPCR reaction conditions were as
follows: Initial denaturation for 2 min at 94°C, followed by 35
cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at
55°C and extension for 1 min at 71°C, and final extension for 2 min
at 71°C. Relative gene expression of FOXM1 and VEGF was calculated
using the 2−ΔΔCq method (19). All experiments were performed in
triplicate.
Western blotting
The expression of FOXM1 and VEGF proteins was
determined by western blotting. Total proteins were extracted with
radioimmunoprecipitation lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology) following the supplier protocol.
Protein concentration was determined using a BCA kit. Proteins (50
µg per lane) were separated using 10% SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% skimmed milk in Tween-20 in
Tris buffer solution at room temperature for 1 h. Proteins were
then incubated with rabbit anti-human primary antibodies against
FOXM1 (cat. no. ab175798; 1:2,000), VEGF (cat. no. ab46154;
1:1,000), BCL-2 (cat. no. ab32124; 1:1,000) and the internal
reference protein β-actin (cat. no. ab129348; 1:5,000; all Abcam)
overnight at 4°C. Proteins were subsequently incubated with
horseradish-conjugated secondary antibodies (goat anti-rabbit;
ab6721; 1:3,000; Abcam) at room temperature for 1 h. The membrane
was exposed to the ECL solution and then exposed to the Gel Doc XR
gel imaging and analysis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The image signal was analyzed and quantified by
Image Lab software version 3.0 (Bio-Rad Laboratories, Inc.). The
relative content of the target protein was calculated by the ratio
of the gray value of the target protein band to that of the
internal reference band. All experiments were performed in
triplicate.
Immunohistochemistry
Placental tissues were fixed in 10% formaldehyde at
4°C for 24 h, embedded in paraffin and cut into 5-µm-thick
sections. Then, sections were dewaxed using xylene and rehydrated
in a descending alcohol series. Antigen retrieval was achieved by
incubation with sodium citrate solution (Beyotime Institute of
Biotechnology) and the sample was heated in the microwave at 97°C
for 12 min. Following washing of the sections, they were incubated
with 3% hydrogen peroxide at room temperature for 10 min. Following
blocking with 10% goat serum (Beyotime Institute of Biotechnology)
at room temperature for 1 h, sections were incubated with rabbit
anti-human FOXM1 primary antibodies (cat. no. ab83097; 1:50; Abcam)
overnight at 4°C. Samples were then washed with PBS, incubated with
the goat anti-rabbit horseradish peroxidase conjugated secondary
antibodies (cat. no. ab6721; 1:1,000; Abcam) for 1 h at room
temperature and washed with PBS. Finally, sections were developed
with chromogenic reagent 3′3-diaminobenzidine for ~10 sec at room
temperature, counterstained with hematoxylin for 20 sec at room
temperature, mounted with neutral gum and observed using a Nikon
TS100-F optical microscope (Nikon Corporation, Tokyo, Japan;
magnification, ×500).
Human HTR8/SVneo transfection
HTR8/SVneo cells were obtained from American Type
Culture Collection (Manassas, VA, USA) and cultured at 37°C in 5%
CO2 with Dulbecco's Modified Eagle's medium (DMEM) and
10% fetal bovine serum (FBS). Cells were plated in 24-well plates
at 3×105 cells/well 1 day prior to transfection and
cultured in 10% FBS in F12/DMEM without antibiotics. Transfection
was performed when cells reached 70% confluence. A total of 50 nM
FOX1 small interfering (si)RNA (designed by Sangon Biotech Co.,
Ltd.) was transfected into cells using Lipofectamine®
2000 Transfection Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) as previously described (20).
The sequence of FOX1 siRNA was forward,
5′-ACCCAAACCAGCUAUGAUGdTdT-3′ and reverse,
5′-CAUCAUAGCUGGUUUGGGUdTdT-3′. The sequences of the scramble siRNA
were as follows: Forward, 5′-UUCUCCGAACGUGUCACGUdTdT-3′ and
reverse, 5′-ACGUGACACGUUCGGAGAAdTdT-3′. Levels of FOXM1 mRNA and
protein were measured 48 h following transfection.
HTR8/SVneo cell proliferation
following transfection
Cells were plated in triplicate in 96-well plates at
a density of 2×103 cells/well. Subsequently, 20 µl 5 g/l
MTT solution was added at 24, 48 and 72 h. Purple crystals were
solubilized by adding 150 µl dimethyl sulfoxide and plates were
then incubated in a humidified atmosphere at 37°C for 4 h. The cell
proliferation curve was constructed by measuring the
spectrophotometric absorbance of the samples at a wavelength of 490
nm.
Flow cytometric detection of
HTR8/SVneo cell cycle and apoptosis post-transfection
At 48 h post-transfection, cells were collected.
Cells (2×105) were fixed with 75% alcohol overnight at
4°C and incubated with 1 mg/ml RNaseA for 30 min at 37°C. Cells
were stained with propidium iodide (PI) at room temperature for 15
min. Cells were also incubated with Annexin V at room temperature
for 15 min for apoptosis analysis. The cell cycle and cell
apoptosis were analyzed using a flow cytometer.
Statistical analysis
All data were analyzed using SPSS statistical
software (version 18.0; SPSS, Inc., Chicago, IL, USA) and were
tested for normal distribution. Data are expressed as mean ±
standard deviation. Multiple measurement data were tested by
one-way analysis of variance followed by the Least Significant
Difference and Student-Newman-Keuls method or Tamhane's T2, where
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
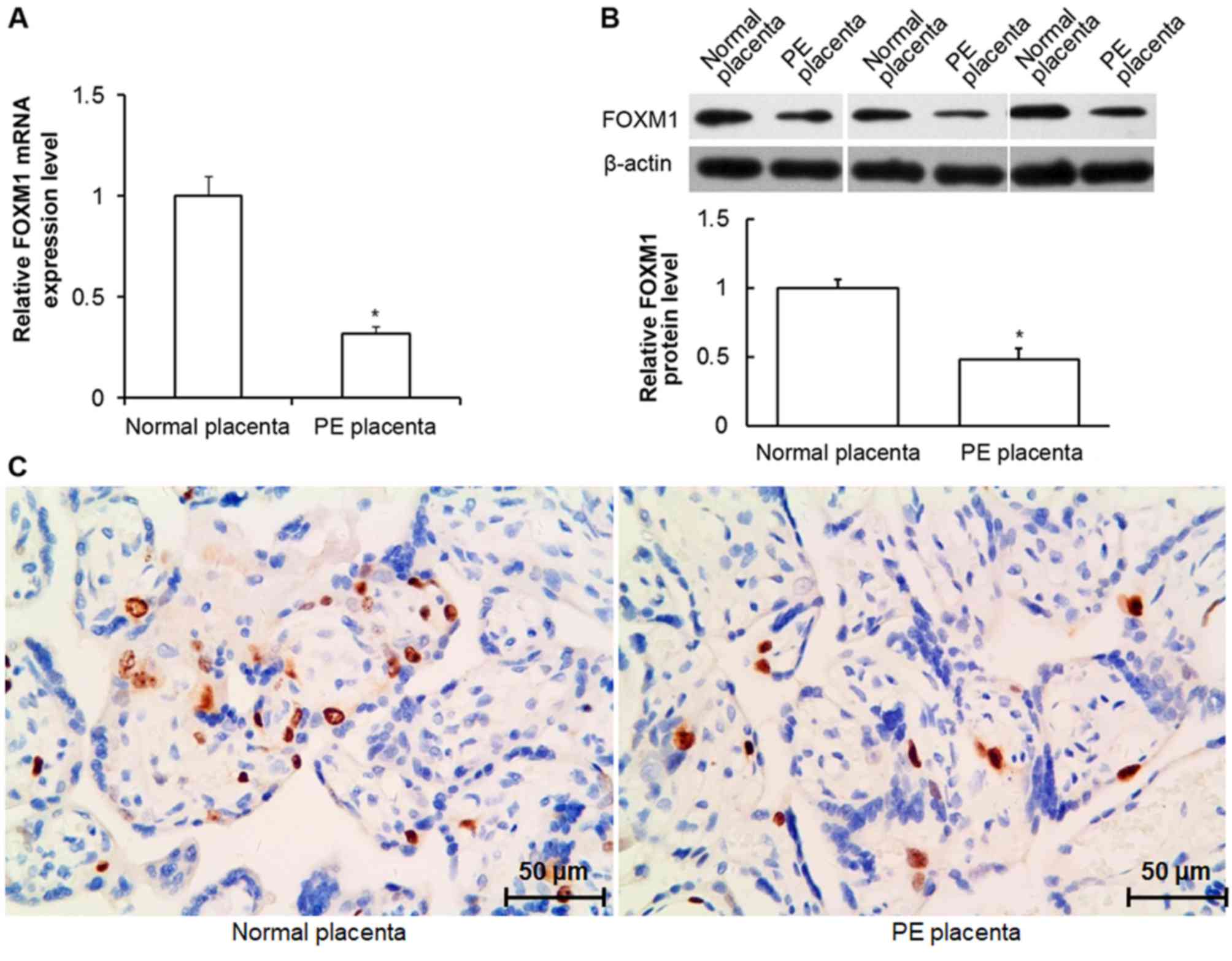
FOXM1 mRNA and protein expression is
decreased in the placentas of patients with preeclampsia
The results of RT-qPCR demonstrated that the
expression of FOXM1 mRNA in the placental tissue of patients with
preeclampsia was significantly decreased compared with the control
group (P<0.05; Fig. 1A). The
expression of FOXM1 protein in the placental tissue of patients
with preeclampsia was also significantly decreased (P<0.05;
Fig. 1B). This was supported by the
results of immunohistochemical staining, which exhibited decreased
staining for FOXM1 in the placental tissues of patients with
preeclampsia (Fig. 1C). The results
of the current study indicate that the expression of FOXM1 mRNA and
protein is downregulated in patients with preeclampsia, suggesting
that FOXM1 may serve a regulatory role in preeclampsia.
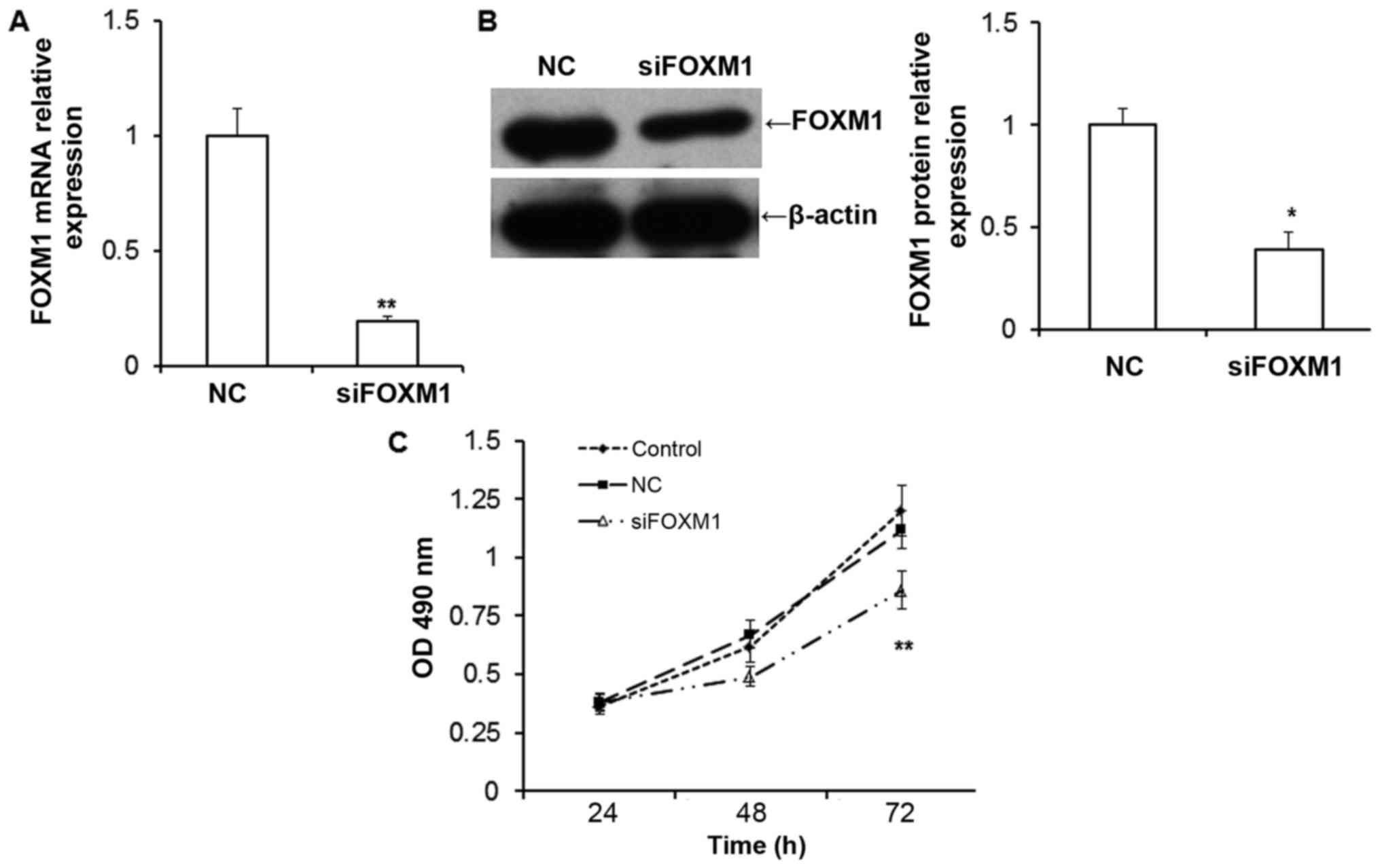
FOXM1 silencing decreases the
proliferation of HTR8/SVneo cells
To examine the function of FOXM1 in the placenta,
in vitro evaluations were conducted by silencing FOXM1
expression in HTR8/SVneo cells. The expression of FOXM1 mRNA
(P<0.01; Fig. 2A) and protein
(P<0.05; Fig. 2B) were
significantly downregulated in HTR8/SVneo cells transfected with
FOXM1 siRNA compared with negative control cells. The results of
the MTT assay demonstrated that the proliferation of HTR8/SVneo
cells was inhibited following siFOXM1 transfection compared with
cells transfected with the negative control (NC; P<0.01;
Fig. 2C). This suggests that the
downregulation of FOXM1inhibits the proliferation of HTR8/SVneo
cells.
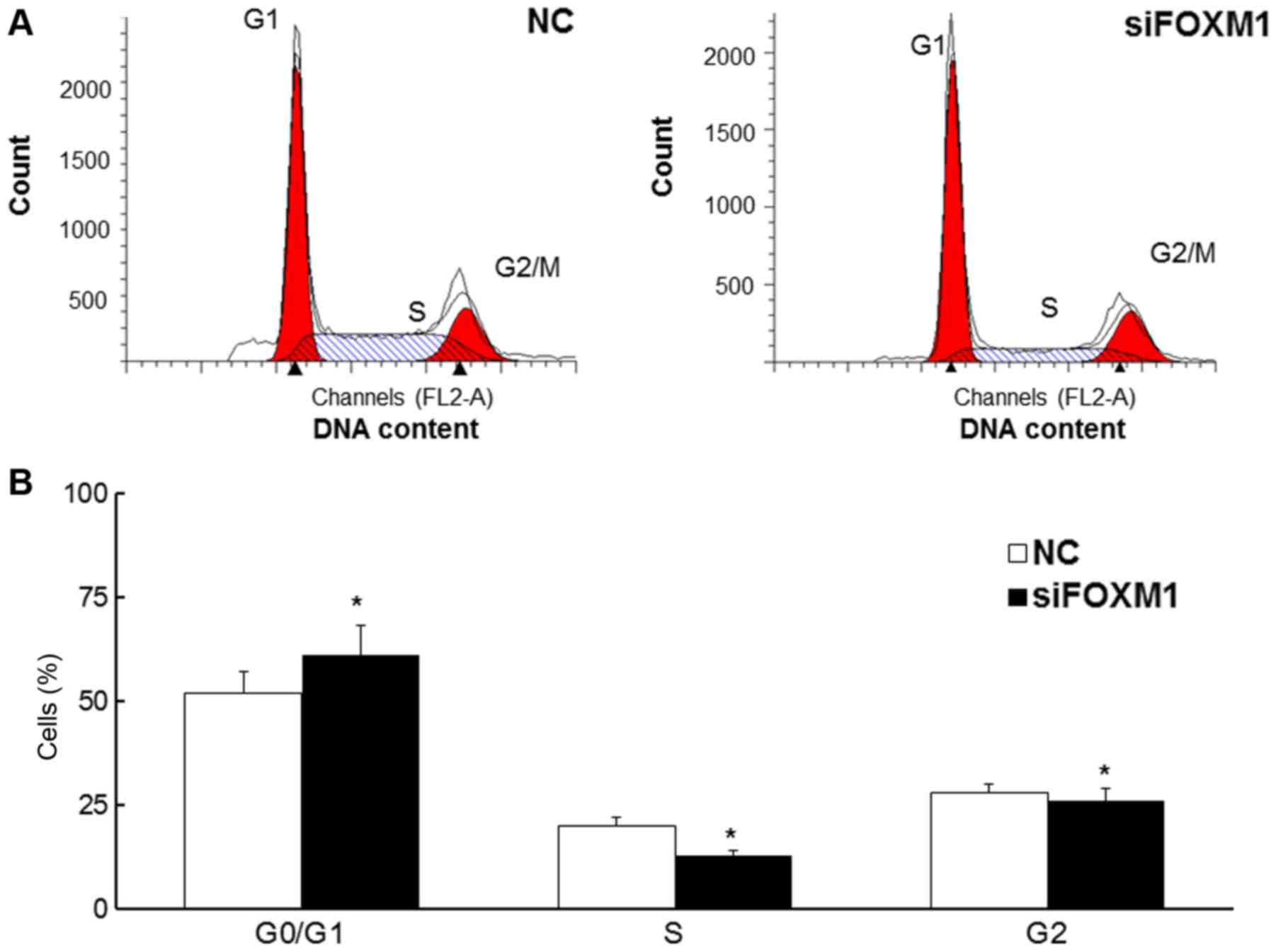
FOXM1 silencing increases the
proportion of cells in the G0/G1 phase
Flow cytometry was used to assess the proportion of
cells in each stage of the cell cycle (Fig. 3A). The results demonstrated that the
proportion of HTR8/SVneo cells in the G0/G1 phase was significantly
increased in cells transfected with FOXM1 siRNA (P<0.05),
whereas the proportion of cells in the G2 and S-phases was
decreased (all P<0.05; Fig. 3B).
These results indicate that decreased FOXM1 expression may
attenuate the cell cycle in HTR8/SVneo cells.
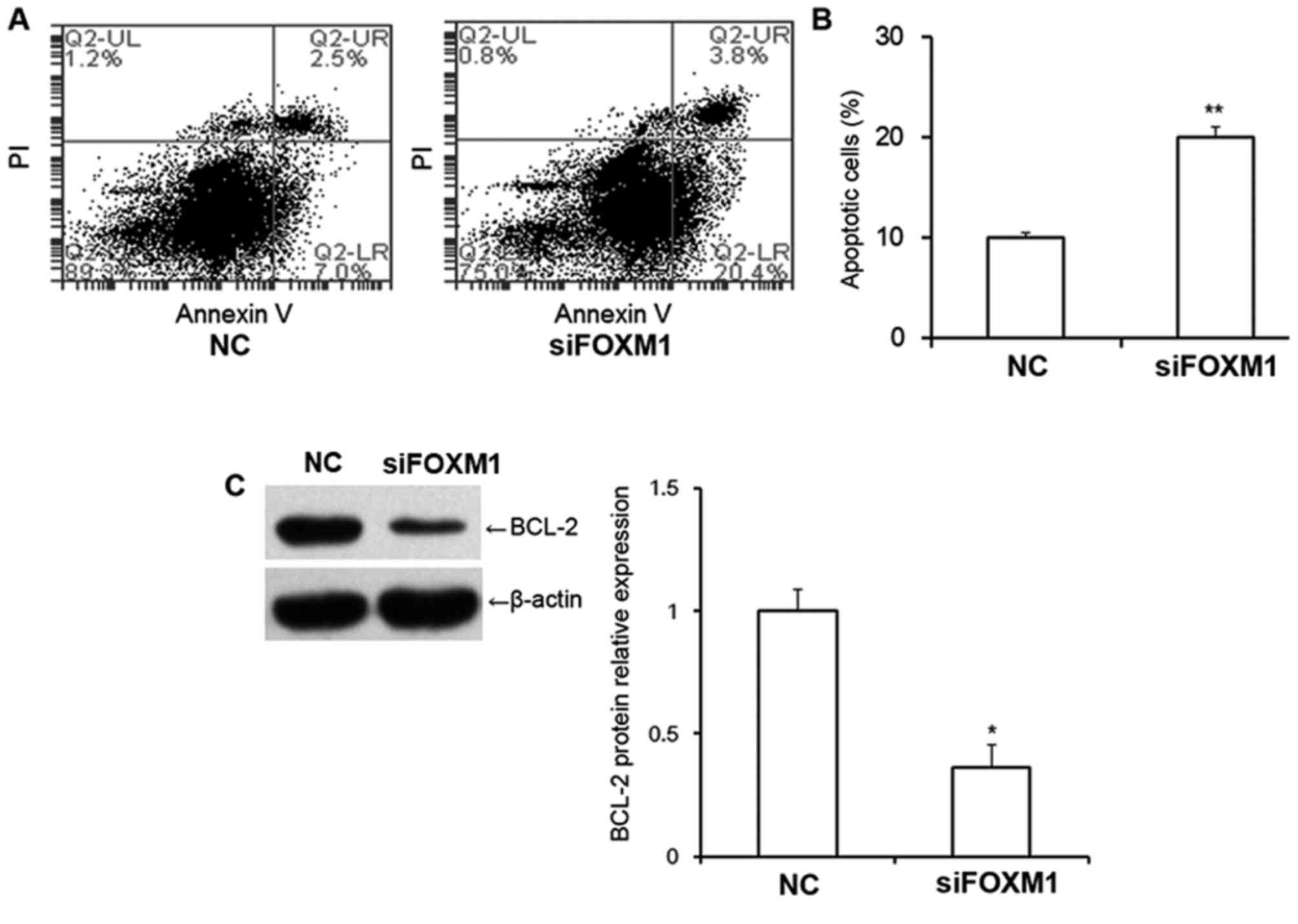
FOXM1 silencing increases the
proportion of apoptotic cells and decreases BCL-2 protein
expression
The proportion of early apoptotic cells and late
apoptotic cells in HTR8/SVneo cells was examined using flow
cytometry (Fig. 4A). The results
revealed that transfection with FOXM1 siRNA significantly increased
the proportion of apoptotic cells compared with cells transfected
with NC siRNA (P<0.01; Fig. 4B),
suggesting that decreased FOXM1 expression may increase apoptosis
in HTR8/SVneo cells. The results also revealed that BCL-2 protein
expression was significantly decreased following transfection with
FOXM1 siRNA (P<0.05; Fig. 4C),
indicating that FOXM1 is associated with BCL-2 expression.
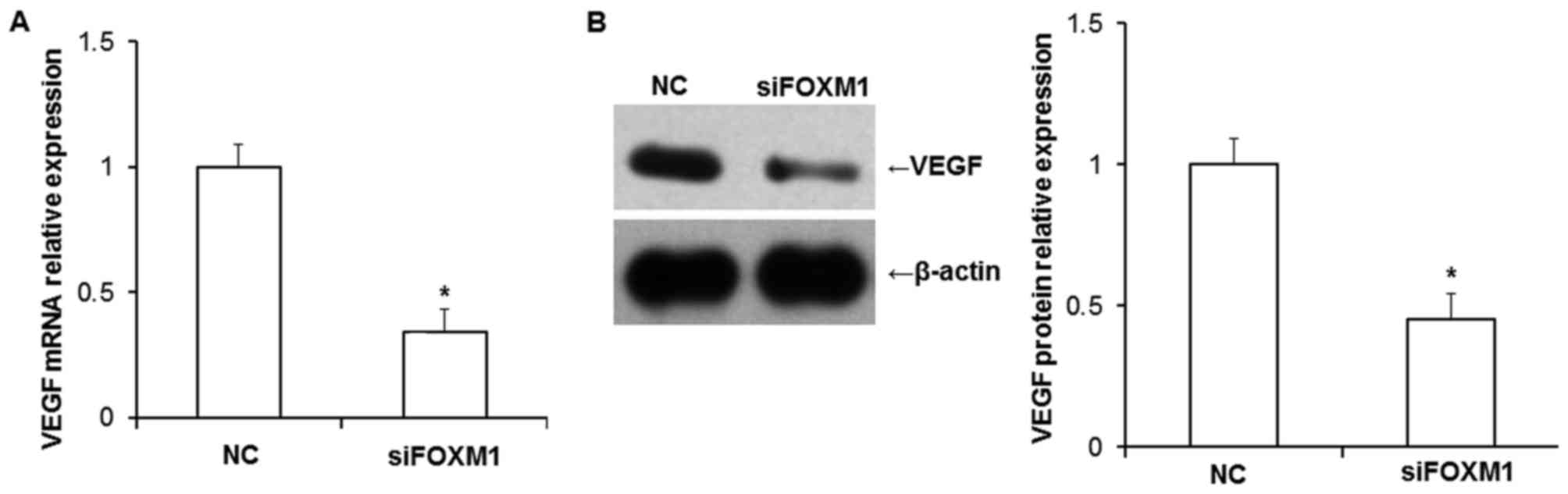
FOXM1 silencing decreases VEGF
expression
VEGF is one of the most potent angiogenic factors
and promotes angiogenesis, which, in turn increases blood supply
(21). The reduction in VEGF
expression leads to the premenstrual onset of preeclampsia,
therefore the current study examined the association between FOXM1
and VEGF. The expression of VEGF mRNA and protein in HTR8/SVneo
cells was determined by RT-qPCR and western blotting, respectively.
The results demonstrated that the decrease of FOXM1 expression
significantly downregulated the expression of VEGF mRNA and protein
(both P<0.05; Fig. 5A and B),
suggesting that FOXM1 and VEGF may function in concert and that
changes in the expression of either protein may induce vascular
dysfunction. VEGF receptor (VEGFR) expression in placental tissue
samples were also examined in a preliminary experiment by our
group, however, the results revealed no significant changes in
patients with preeclampsia compared with controls (data not
shown).
Discussion
In the present study, the role of placental FOXM1
expression in the pathogenesis of preeclampsia was investigated.
The expression of FOXM1 mRNA and protein was examined in the
placenta tissues of patients with or without preeclampsia that
underwent cesarean sections. The association between FOXM1 and VEGF
was further investigated by performing in vitro HTR8/SVneo
trophoblast cell cycle studies.
The primary manifestations of preeclampsia include
the shallow invasion of trophoblasts into the endometrium,
cessation of vascular remodeling in the uterine spiral arteries and
the decreased surface area and density of the villi. Patients with
preeclampsia often exhibit disordered vascular remodeling (22,23).
Placental hypoperfusion causes placental hypoxia, metabolism
disorders, placental sourced toxicity, vascular endothelial injury
and an imbalance of vasoactive substances, which can eventually
lead to the development of preeclampsia (24). It is widely accepted that
preeclampsia develops in two different stages (25,26).
During the first stage, vascular remodeling disorders cause a
decrease in villous trophoblast invasion and induce placental
hypoxia. In the second stage, multiple peptides produced by the
placenta are secreted into the blood circulation of the mother and
reactive oxygen species (ROS) build up in the vascular endothelium.
The imbalance between ROS production and the antioxidant defense
system exacerbates systemic small artery injury, leading to ROS
accumulation, hypertension and proteinuria (27). Previous studies have suggested that
preeclampsia may be associated with decreased placental trophoblast
invasion, ischemia and hypoxia, increased apoptosis, abnormal lipid
metabolism and other placental dysfunctions during pregnancy
(28–30). It has been reported that the
extravillous trophoblasts serve an important role in the process of
recoil in spiral arteries; the diminished invasion of extravillous
trophoblast cells leads to abnormal vascular remodeling and shallow
embryo implantation and ultimately, the development of preeclampsia
(22,31).
FOXM1 is highly expressed in a number of
malignancies and is involved in tumor cell proliferation,
apoptosis, invasion and metastasis; it is therefore a marker of
tumor development (32–35). The biological characteristics of
trophoblasts in embryos are very similar to those of cancer cells
and a number of factors involved in cancer invasion, including
human leukocyte antigen and DNA methyltransferase 3A, also serve a
role in trophoblast invasion (36,37). The
primary difference is that trophoblast invasion is a tightly
regulated process (38). FOXM1 is
expressed in the cytotrophoblast of villus and decidual tissue
during early pregnancy (11,39). FOXM1 is also expressed in the
maternal decidual cells and FOXM1 may serve as an indirect
regulator of trophoblast invasion via paracrine signaling (40). These results suggest that FOXM1 is
involved in the development and progression of preeclampsia.
In the present study, the expression of FOXM1 was
measured in normal and preeclampsia placental tissues. The
expression of FOXM1 mRNA and protein was significantly decreased in
patients with preeclampsia. This suggests that FOXM1 may indirectly
regulate trophoblast invasion through paracrine signaling, as
decreased FOXM1 expression decreases trophoblast invasion and
attenuates the invasion of trophoblast cells into the endometrium
(38,40). To the best of our knowledge, the
present study is the first to report the abnormal expression of
FOXM1 in preeclampsia placenta.
To further investigate the effect of FOXM1 on
trophoblast cells, FOXM1 siRNA was transfected into human
trophoblast cells, HTR8/Svneo. The results of the MTT assay
demonstrated that FOXM1 silencing attenuates the proliferation of
HTR8/Svneo cells. Additionally, the results of flow cytometry
demonstrated that the decreased expression of FOXM1 blocks the cell
cycle at the G0/G1 phase and increases the apoptosis of HTR8/Svneo
cells. Furthermore, the results of RT-qPCR and western blotting
revealed that decreased FOXM1 expression leads to the
downregulation of VEGF mRNA, and VEGF and BCL-2 proteins. VEGF is
one of the most effective angiogenic growth factors; it promotes
angiogenesis and increases blood supply (41). It has been demonstrated that
suppression of the VEGF signaling pathway is associated with the
development of preeclampsia (42),
which may partially explain the development of vascular remodeling
disorder in patients with preeclampsia. Silencing FOXM1 decreases
the expression of VEGF, suggesting that FOXM1 may serve its role
via the VEGF signaling pathway. VEGFR expression in tissue samples
was also examined in a preliminary experiment by our group, however
the results revealed no significant changes in patients with
preeclampsia compared with healthy patients, which may be a result
of other biological functions.
In conclusion, the results of the present study
demonstrate that the altered expression of FOXM1 may cause changes
in the expression of associated proteins, resulting in decreased
trophoblast cell proliferation, prolonged cell cycle and increased
cellular apoptosis. This demonstrates that FOXM1 serves an
important role in the development of preeclampsia by regulating the
VEGF signaling pathway.
References
|
1
|
Cetin O, Ozdemir Guzel P, Kurdoglu Z and
Sahin HG: Investigation of maternal psychopathological symptoms,
dream anxiety and insomnia in preeclampsia. J Matern Fetal Neonatal
Med. 30:2510–2515. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumwell S and Karumanchi SA:
Preeclampsia: Clinical manifestations and molecular mechanisms.
Nephron Clin Pract. 106:c72–c81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sones JL and Davisson RL: Preeclampsia, of
mice and women. Physiol Genomics. 48:565–572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brooks SA, Martin E, Smeester L, Grace MR,
Boggess K and Fry RC: miRNAs as common regulators of the
transforming growth factor (TGF)-β pathway in the preeclamptic
placenta and cadmium-treated trophoblasts: Links between the
environment, the epigenome and preeclampsia. Food Chem Toxicol.
98:50–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuckerwise L, Li J, Lu L, Men Y, Geng T,
Buhimschi CS, Buhimschi IA, Bukowski R, Guller S, Paidas M, et al:
H19 long noncoding RNA alters trophoblast cell migration and
invasion by regulating TβR3 in placentae with fetal growth
restriction. Oncotarget. 7:38398–38407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turner RJ, Bloemenkamp KW, Bruijn JA and
Baelde HJ: Loss of thrombomodulin in placental dysfunction in
preeclampsia. Arterioscler Thromb Vasc Biol. 36:728–735. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Eerden L, de Groot CJM, Zeeman GG,
Page-Christiaens GCM, Pajkrt E, Duvekot JJ, Vandenbussche FP, Oei
SG, Scheepers HCJ, van Eyck J, et al: Subsequent pregnancy outcome
after mid-trimester termination of pregnancy for preeclampsia. Aust
N Z J Obstet Gynaecol. Aug 29–2017.(Epub ahead of print).
PubMed/NCBI
|
|
8
|
Zhu H: Forkhead box transcription factors
in embryonic heart development and congenital heart disease. Life
Sci. 144:194–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Moraes Nestal G, Bella L, Zona S,
Burton MJ and Lam EW: Insights into a critical role of the
FOXO3a-FOXM1 Axis in DNA damage response and genotoxic drug
resistance. Curr Drug Targets. 17:164–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zona S, Bella L, Burton MJ, de Moraes
Nestal G and Lam EW: FOXM1: An emerging master regulator of DNA
damage response and genotoxic agent resistance. Biochim Biophys
Acta. 1839:1316–1322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao F, Bian F, Ma X, Kalinichenko VV and
Das SK: Control of regional decidualization in implantation: Role
of FoxM1 downstream of Hoxa10 and cyclin D3. Sci Rep. 5:138632015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie Y, Cui D, Sui L, Xu Y, Zhang N, Ma Y,
Li Y and Kong Y: Induction of forkhead box M1 (FoxM1) by EGF
through ERK signaling pathway promotes trophoblast cell invasion.
Cell Tissue Res. 362:421–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaswani K, Hum MW, Chan HW, Ryan J,
Wood-Bradley RJ, Nitert MD, Mitchell MD, Armitage JA and Rice GE:
The effect of gestational age on angiogenic gene expression in the
rat placenta. PLoS One. 8:e837622013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Zhu H, Klausen C, Peng B and Leung
PC: Vascular endothelial growth factor-A (VEGF-A) mediates activin
A-induced human trophoblast Endothelial-Like tube formation.
Endocrinology. 156:4257–4268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Francis VA, Abera AB, Matjila M, Millar RP
and Katz AA: Kisspeptin regulation of genes involved in cell
invasion and angiogenesis in first trimester human trophoblast
cells. PLoS One. 9:e996802014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo X, Feng L, Jia J, Chen R and Yu J:
Upregulation of VEGF by small activating RNA and its implications
in preeclampsia. Placenta. 46:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chomczynski P and Sacchi N: The
single-step method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction: Twenty-something years
on. Nat Protoc. 1:581–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan MF, Yang HL, Qian JL, Wu CS, Yuan CX,
Li XF and Zou J: Comparison of two methods for RNA extraction from
the nucleus pulposus of intervertebral discs. Genet Mol Res.
15:2016. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y, Zhang K, Li C, Yao Y, Tao D, Liu Y,
Zhang S and Ma Y: Piwil2 suppresses p53 by inducing phosphorylation
of signal transducer and activator of transcription 3 in tumor
cells. PLoS One. 7:e309992012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shibata Y, Kikuchi R, Ishii H, Suzuki S,
Harada K, Hirayama K, Suzuki A, Tatami Y, Kondo K and Murohara T:
Balance between angiogenic and anti-angiogenic isoforms of VEGF-A
is associated with the complexity and severity of coronary artery
disease. Clin Chim Acta. 478:114–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massimiani M, Salvi S, Piccirilli D,
Vecchione L, Moresi S, Ferrazzani S, Stuhlmann H and Campagnolo L:
A4. EGFL7 in placenta trophoblast and endothelial cells:
Implications in the pathogenesis of preeclampsia. J Mater Fetal
Neonatal Med. 29:42016. View Article : Google Scholar
|
|
23
|
Shah DA and Khalil RA: Bioactive factors
in uteroplacental and systemic circulation link placental ischemia
to generalized vascular dysfunction in hypertensive pregnancy and
preeclampsia. Biochem Pharmacol. 95:211–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nissaisorakarn P, Sharif S and Jim B:
Hypertension in pregnancy: Defining blood pressure goals and the
value of biomarkers for preeclampsia. Curr Cardiol Rep. 18:1312016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldman-Wohl DS and Yagel S: Examination
of distinct fetal and maternal molecular pathways suggests a
mechanism for the development of preeclampsia. J Reprod Immunol.
76:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Austgulen R: Recent knowledge on
mechanisms underlying development of preeclampsia. Tidsskr Nor
Laegeforen. 124:21–24. 2004.PubMed/NCBI
|
|
27
|
Xuan RR, Niu TT and Chen HM: Astaxanthin
blocks preeclampsia progression by suppressing oxidative stress and
inflammation. Mol Med Rep. 14:2697–2704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bokslag A, van Weissenbruch M, Mol BW and
de Groot CJ: Preeclampsia; short and long-term consequences for
mother and neonate. Early Hum Dev. 102:47–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gilani SI, Weissgerber TL, Garovic VD and
Jayachandran M: Preeclampsia and extracellular vesicles. Curr
Hypertens Rep. 18:682016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karumanchi SA and Granger JP: Preeclampsia
and pregnancy-related hypertensive disorders. Hypertension.
67:238–242. 2016.PubMed/NCBI
|
|
31
|
Liu X, Hu Y, Zheng Y, Liu X, Luo M, Liu W,
Zhao Y and Zou L: EPHB4 regulates human trophoblast cell line
HTR-8/SVneo function: Implications for the role of EPHB4 in
preeclampsia. Biol Reprod. 95:652016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Francica P, Nisa L, Aebersold DM, Langer
R, Bladt F, Blaukat A, Stroka D, Martínez MR, Zimmer Y and Medová
M: Depletion of FOXM1 via MET targeting underlies establishment of
a DNA Damage-induced senescence program in gastric cancer. Clin
Cancer Res. 22:5322–5336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu MD, Wang Y, Weng W, Wei P, Qi P, Zhang
Q, Tan C, Ni SJ, Dong L, Yang Y, et al: A positive feedback loop of
lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and
invasion. Clin Cancer Res. 23:2071–2080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sayanjali B, Christensen GJM, Al-Zeer MA,
Mollenkopf HJ, Meyer TF and Brüggemann H: Propionibacterium acnes
inhibits FOXM1 and induces cell cycle alterations in human primary
prostate cells. Int J Med Microbiol. 306:517–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maachani UB, Shankavaram U, Kramp T,
Tofilon PJ, Camphausen K and Tandle AT: FOXM1 and STAT3 interaction
confers radioresistance in glioblastoma cells. Oncotarget.
7:77365–77377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hackmon R, Pinnaduwage L, Zhang J, Lye SJ,
Geraghty DE and Dunk CE: Definitive class I human leukocyte antigen
expression in gestational placentation: HLA-F, HLA-E, HLA-C, and
HLA-G in extravillous trophoblast invasion on placentation,
pregnancy, and parturition. Am J Reprod Immunol. Feb 10–2017.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jia Y, Li T, Huang X, Xu X, Zhou X, Jia L,
Zhu J, Xie D, Wang K, Zhou Q, et al: Dysregulated DNA
Methyltransferase 3A upregulates IGFBP5 to suppress trophoblast
cell migration and invasion in preeclampsia. Hypertension.
69:356–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji L, Brkić J, Liu M, Fu G, Peng C and
Wang YL: Placental trophoblast cell differentiation: Physiological
regulation and pathological relevance to preeclampsia. Mol Aspects
Med. 34:981–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kin K, Maziarz J, Chavan AR, Kamat M,
Vasudevan S, Birt A, Emera D, Lynch VJ, Ott TL, Pavlicev M, et al:
The transcriptomic evolution of mammalian pregnancy: Gene
expression innovations in endometrial stromal fibroblasts. Genome
Biol Evol. 8:2459–2473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Knöfler M and Pollheimer J: IFPA Award in
Placentology lecture: Molecular regulation of human trophoblast
invasion. Placenta. 33 Suppl:S55–S62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roberts E, Cossigny DA and Quan GM: The
role of vascular endothelial growth factor in metastatic prostate
cancer to the skeleton. Prostate Cancer. 2013:4183402013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahmed A, Rezai H and Broadway-Stringer S:
Evidence-Based revised view of the pathophysiology of preeclampsia.
Adv Exp Med Biol. 956:355–374. 2017. View Article : Google Scholar : PubMed/NCBI
|