Introduction
Non-small-cell lung cancer (NSCLC) is one of the
most common tumors, which presents with an increasing trend and
remains the most common cause of cancer-associated mortality
worldwide (1,2). Published literature has revealed that
tumor growth migration and invasion are the most important features
of NSCLC, which are characterized by local migration, metastasis
and reoccurrence (3). A previous
pathological study has suggested that NSCLC accounts for ~80% of
all lung cancer cases and classified adenocarcinoma, large cell
carcinoma and squamous cell carcinoma as common types of NSCLC
(4). A number of therapeutic
strategies, such as immunotherapy, gene therapy, radiotherapy and
chemotherapy, have been widely applied in clinical settings for the
treatment of NSCLC (5–7); however, poor survival rates of patients
with NSCLC have continued (8–10).
The majority of clinical patients with NSCLC are
diagnosed at an advanced stage, which is the primary reason of
rising death rate (11,12). Although previous reports have
introduced various diagnostic methods for NSCLC including
ultrasound, computerized tomography and magnetic resonance imaging,
ultrasound is the most widely used for diagnosis of NSCLC (13,14). A
previous study indicated that endobronchial ultrasound in
combination with diagnostic bronchoscopy is highly effective in the
diagnosis and staging of patients with lung cancer (15). Another previous study demonstrated
that contrast-enhanced ultrasound improved enhancement pattern and
cellular differentiation for hepatocellular carcinoma (16). In addition, contrast-enhanced
ultrasound increased the diagnostic rate via observation of
microvessel density and vascular endothelial growth factor
expression, which may be valuable in the evaluation of early-stage
breast cancer (17). In addition,
microbubble ultrasound contrast agent has been reported to enhance
the diagnostic rate for patients with suspected lung cancer in
clinical settings (18,19). These reports suggested that
microbubble ultrasound contrast not only increases reflected
signal, but also improves the diagnostic rate for patients with
suspected lung cancer in the clinic.
Reports have suggested that the expression levels of
vascular endothelial growth factor (VEGF) and VEGF receptor
(VEGFR)-3 are overexpressed in NSCLC cells and this is associated
with nodal status in operable NSCLC (20,21).
Higher expression levels of carcino-embryonic antigen (CEA) were
observed in NSCLC cells, which is regarded as a prognostic
indicator (22,23). The present study introduced a novel
targeted ultrasound contrast agent containing
chistosan/Fe3O4-parceled bispecific antibody
(TcBab) targeting of CEA and VEGFR and investigated its efficiency
for NSCLC diagnosis. The results suggested that TcBab-ultrasound
(TcBab-US) may improve accuracy and sensitivity for patients with
suspected NSCLC.
Materials and methods
Ethics statement
The present methodology was performed in strict
accordance with the recommendations of the Guide for the Care and
Use of Clinical Study of Pharmaceutical Administration Measures for
Implementation of China (24).
Employing nanoparticles loaded with superparamagnetic iron oxides
and perfluoropentane in patients has been approved by the China
Food and Drug Administration (HHRH20120514CD). The study was
approved by the Ethics Committee of Huaihe Hospital of Henan
University (Kiafeng, China). All patients were required to provide
written informed consent prior to their inclusion.
Patients
A total of 384 patients with suspected NSCLC and 20
healthy volunteers were recruited for the present study at the
Huaihe Hospital of Henan University between February 2010 and June
2016. The age of patients ranged from 32.8–67.5 years old. The
number of male (n=178) and female (n=176) patients was
approximately equal. The age of the healthy volunteers ranged from
26.9–54.1 years old (10 male and 10 female). The patients,
including those with suspected NSCLC, were diagnosed with NSCLC at
an early stage as described previously (25). Patients with lung cancer history were
excluded from this study. A 60-month follow-up was performed on all
patients. The characteristics of patients with suspected NSCLC were
summarized in Table I.
 | Table I.Characteristics of patients with
non-small cell lung cancer. |
Table I.
Characteristics of patients with
non-small cell lung cancer.
|
Characteristics | Male | Female |
|---|
| Patients, n | 178 | 176 |
| Age, years | 35.4–64.2 | 32.8–67.5 |
| Medical history of
cancer, n | 2 | 3 |
| Blood pressure,
mmHg (mean ± SD) Diagnosis (n) | 105.6±7.4 | 108.8±10.5 |
|
Ultrasound | 178 | 176 |
|
TcBab-US | 178 | 176 |
Principles and settings of
contrast-enhanced ultrasound
The ultrasound diagnosis system was used to analyze
the efficacy of a targeted ultrasound contrast agent for the
diagnosis of patients with suspected NSCLC using a preprogrammed
setting. The preprogrammed setting was optimized to obtain the
ideal image formation. The mechanical index was set at 0.2–0.4 to
avoid destruction of the fragile microbubbles containing
nano-particles. The details of principles and settings of
contrast-enhanced ultrasound were described in a previous study
(26).
RT-qPCR analysis
Total RNAs from clinical tissues and cultured cells
were extracted with TriZol® reagent (Takara Bio, Inc.,
Otsu, Japan) following the manufacturer's protocol. The RNA quality
and quantity were determined by Nanodrop® 2000 (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Reverse transcription
(RT) of first-strand cDNAs was performed using PrimeScript RT
Master mix (Takara Bio, Inc.) according to the manufacturer's
protocol. All PCR reactions were performed in an ABI PRISM 7900
Real-Time system (Thermo Fisher Scientific, Inc.) with the
SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.).
For the PCR experiments the following forward and reverse primers
were used: CEA forward, 5′-TGGCAGCAGTGACAGCAGCA-3′ and reverse,
5′-TACGGAGGTGGAGTGGGTGT-3′; VEGFR forward,
5′-AGCCGAGGAAGAACTATGAAC-3′ and reverse, 5′-ATTTGAGGGTGAGGAATGGG-3′
and GAPDH forward, 5′-CAAAGGTGGATCAGATTCAAG-3′ and reverse,
5′-GGTGAGCATTATCACCCAGAA-3′. The PCR conditions included an initial
denaturation step of 94°C for 2 min, followed by 30 cycles of 94°C
for 30 sec, 59°C for 30 sec, 72°C for 2 min and a final elongation
step at 72°C for 10 min. Taq DNA polymerase was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). GAPDH was used as
the internal control to normalize gene expression. The relative
gene expression levels were calculated using the 2-−ΔΔCq
method (27). All experiments were
repeated ≥3 times. No negative control was used.
Ultrasound nanoparticles contrast
agent
TcBab for targeting of CEA and VEGFR were provided
by the Biological Pharmaceutical Laboratory of Henan Medical
University (Kaifeng, China). The construction of
Chistosan/Fe3O4-encapsulated TcBab was
performed by using the covalent bond as described in a previous
study (28). Novel
Chistosan/Fe3O4 nanoparticles-encapsulated
TcBab was used to improve the imaging resolution ratio and
diagnostic sensitivity in early-stage NSCLC diagnosis. TcBab
contrast agent (0.2 mg/kg) was administered orally 60 min prior to
contrast-enhanced ultrasound.
ELISA
Serum was isolated from peripheral blood (10 ml)
using centrifugation at 4,000 × g for 15 min at 4°C. ELISA kits
were used to determine interleukin CEA (cat. no. DY4128) and VEGFR
(cat. no. MVR200B; both Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The procedures were performed according to the manufacturer's
protocols.
Western blotting
A549, H1650 and MRC5 cells were purchased from the
BeNa Culture Collection (Beijing Bei Na Chuanglian Biotechnology
Research Institute, Beijing, China). All cells were cultured in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) in a humidified atmosphere containing 5% CO2 at
37°C.
Total protein was harvested from A549, H1650 and
MRC5 cells cell samples (1×108) using a
radioimmunoprecipitation buffer (Sigma-Aldrich; Merck KGaA) and
protein concentrations were measured using a bicinchoninic acid
protein assay kit (Thermo Fisher Scientific, Inc.). A total of 30
µg protein was loaded per lane and separated by 10% SDS-PAGE and
transferred to a polyvinylidene difluoride membranes using a
semidry transfer system. Samples (20 µg) were blocked with 5%
bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1
h at 37°C and incubated with rabbit anti-human CEA (cat. no. 2383;
1:1,000) or VEGFR (cat. no. 2479; 1:1,000; both Cell Signaling
Technology, Inc., Danvers, MA, USA) and anti-GAPDH (1:1,000;
ab8245; Abcam, Cambridge, UK) primary antibodies for 2 h at 37°C.
The membranes were then washed with PBS three times and incubated
with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin
G secondary antibodies (1:5,000; cat. no. PV-6001; OriGene
Technologies Inc., Rockville, MD, USA) for 2 h at 37°C. Protein
expression levels were visualized using a chemiluminescence
detection system (LumiGLO; Cell Signaling Technology, Inc.).
Immunohistochemistry and histological
staining
A549 cells were cultured in six-well plates at a
density of 1×105 cells/well, in Eagle's Minimum
Essential medium (EMEM) supplemented with 10% heat-inactivated
fetal bovine serum (both Biowhittaker; Lonza Group, Ltd., Basel,
Switzerland) for 12 h at 37°C. The cells or tumor tissues were
fixed with 10% methanol for 1 h at room temperature and blocked
with phosphate buffered saline with Tween-20 containing 5% non-fat
milk for 1 h at room temperature. The cells were subsequently
incubated with rabbit anti-human CEA (1:1,000) or VEGFR (1:1,000)
primary antibodies for 2 h at 37°C. Subsequently, fluorescein
isothiocyanate-conjugated goat anti-rabbit secondary antibodies
(1:5,000; cat. no. PV-6001; OriGene Technologies Inc.) were added
for 2 h in the dark at 37°C. Cells were then counterstained with
DAPI (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 h at
37°C and imaged under fluorescence microscopy in three fields of
view (BX51; Olympus Corporation, Tokyo, Japan) at magnification,
×40. Samples without secondary antibodies were used as the negative
control for VEGFR and CEA expression. For histological staining,
the tumor tissues were fixed in 10% buffered formalin for 1 h at
37°C followed by embedding in paraffin. Sections (4-µm-thick) were
then stained with hematoxylin-eosin for 2 h at 37°C. All images
were analyzed with ImageJ 1.44p software (National Institutes of
Health, Bethesda, MD, USA). Subtypes of NSCLC were confirmed by two
clinical pathologists as described previously (29).
Treatment of NSCLC patients diagnosed
with TcBab-US
Early-stage NSCLC patients diagnosed with TcBab-US
received different treatments including radiotherapy, chemotherapy,
traditional Chinese medicine, biological therapy and comprehensive
therapy as described previously (30). The median overall survival and median
progression-free survival of NSCLC patients were analyzed in a
previous study (31).
Viability assay
NSCLC cell viability assay was assessed using a Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's protocol. NSCLC cells
(1×103) were seeded into 96-well plates and TcBab (5
mg/ml) or PBS (control) was added for 48 h at 37°C. CCK-8 reagent
was added to wells prior to the endpoint of incubation (3 h) at
37°C. NSCLC cell viability was analyzed using a microplate reader
at a wavelength of 570 nm.
Scanning electron microscopy (SEM) and
transmission electron microscopy (TEM) assays
The treated cells were fixed in 2.5% glutaraldehyde
solution for 24 h at 4°C. Following fixation, the cells were washed
in 1.2 mol/l phosphate buffer, which was changed three times within
3 h. The cells were then fixed in 1% osmic acid for 1–1.5 h and
washed in double-distilled water, which was replaced three times in
2 h. The cells were dehydrated twice at room temperature using 50,
70, 80, 90 and 100% ethanol, for 20 min at each concentration. The
ethanol solution was replaced with isoamyl acetate and the cells
were placed in a critical point drying apparatus (a high-pressure
hermetically sealed container) and liquid CO2 was added.
The cells were then dried at a critical temperature of 31.8°C and
72.8 atm, sputter-coated with platinum using an IB-5 sputter coater
with a 1.3 nm layer of platinum and observed using a Zeiss
Field-Emission-Scanning-Electron-microscope (Zeiss GmbH, Jena,
Germany) at magnification, ×400. The acceleration voltage was 1–5
kV.
The samples were fixed in 2.5% glutaric dialdehyde
for 24 h at 4°C. Following rinsing three times with
phosphate-buffered saline (PBS), the lung cancer tissue was treated
with 2% osmium tetroxide for 2 h. It was subsequently dehydrated in
a graded series of acetone following washing with PBS. Following
dehydration, the lung cancer tissue was saturated in acetone/resin
(1:1) at 37°C for 24 h, embedded in Epon, polymerized in an oven at
60°C for 24 h and cut into semi-thin sections (1-µm-thick). The TEM
assay was performed using a Varian LEO 9220 (120 kV; Carl Zeiss AG,
Oberkochen, Germany) instrument and analysis was performed using
DigitalMicrograph software version 3.7 (Gatan Inc., Pleasanton, CA,
USA) at magnification, ×200. Samples were suspended in chloroform
and sonicated at 100 kHz for 5 min at 37°C. A total of 2 µl of the
suspension was placed on a CF200-Cu-grid (Electron Microscopy
Sciences, Hatfield, PA, USA) and allowed to dry.
Pharmacodynamics of TcBab
Plasma concentration of TcBab was analyzed in
patients with suspected lung cancer after receiving TcBab contrast
agent. Blood samples were collected from 32 participators at 0, 6,
12, 18 and 24 h following administrated with TcBab contrast agent.
Plasma TcBab levels were determined via liquid
chromatography-tandem mass spectrometry as previously described
(32).
Statistical analysis
All data were presented as the mean ± standard
deviation of triplicate samples. Unpaired data was analyzed using
Student's t-test and comparisons of data between multiple groups
were analyzed using one-way analysis of variance with Tukey's post
hoc test. Kaplan-Meier was used to estimate the survival rate
during 60-months long-term observation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Affinity of TcBab for CEA and VEGFR
expression in NSCLC cells
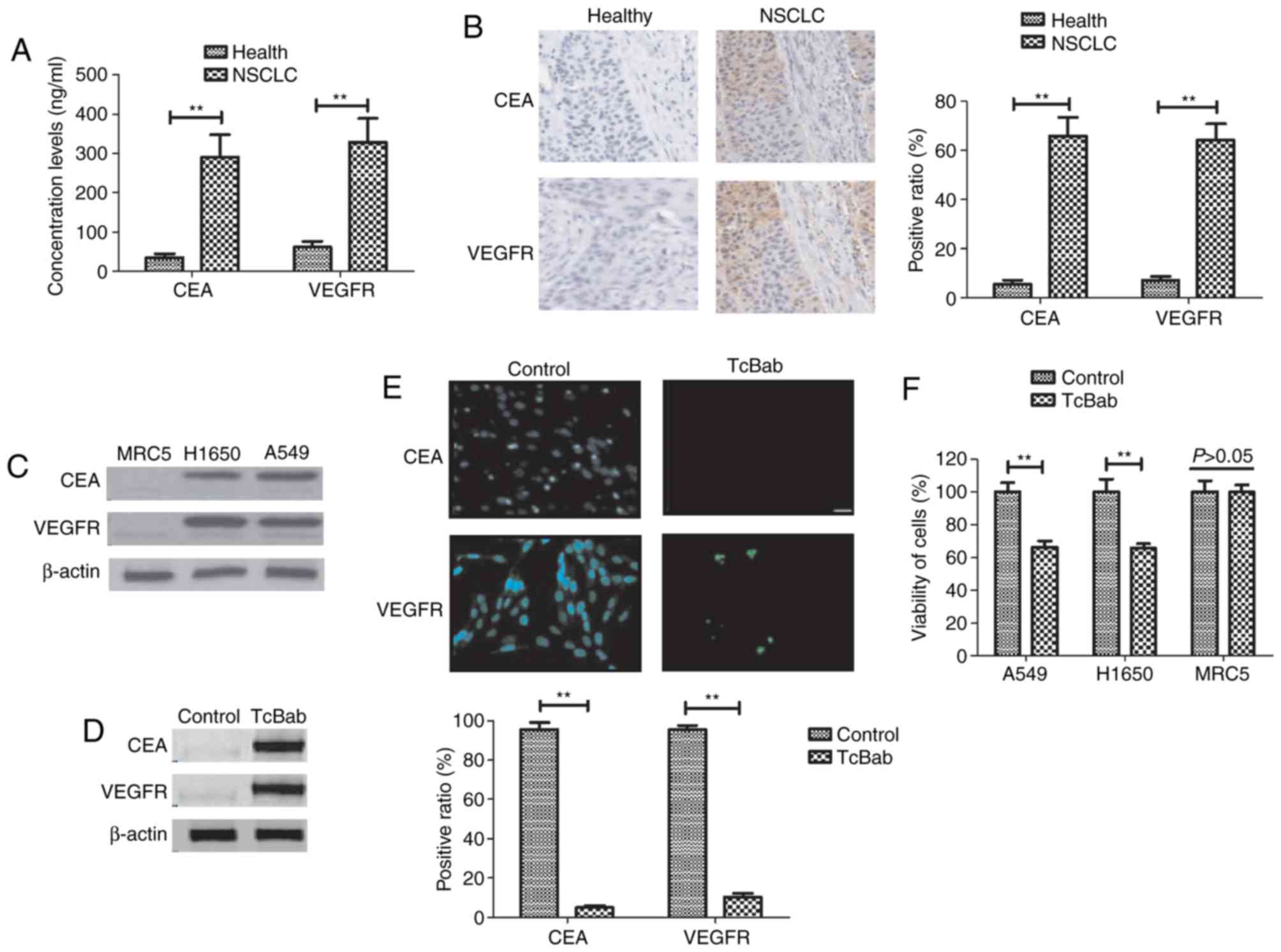
CEA and VEGFR expression levels were analyzed in
tissues isolated from patients with NSCLC and healthy controls. It
was revealed that CEA and VEGFR expression levels were
significantly upregulated in NSCLC tissues compared with healthy
tissues (Fig. 1A). It was
demonstrated that CEA and VEGFR expression levels were markedly
higher in NSCLC tumor tissue compared with normal tissue (Fig. 1B). The image gives a representation
of the VEGFR and CEA staining using one sample from each group
(Fig. 1B). Western blot analysis
demonstrated that the protein expression of CEA and VEGFR were
notably increased in A549 and H1650 cells compared to MRC5 cells
(Fig. 1C). It was demonstrated that
TcBab was able to upregulate CEA and VEGFR, as determined by
western blotting, which decreased CEA and VEGFR expression in A549
cells (Fig. 1D). Immunofluorescence
revealed that TcBab treatment decreased CEA and VEGFR expression in
A549 cells compared with the non-treated cells (Fig. 1E). TcBab treatment (5 mg/ml)
decreased the viability of NSCLC cell lines A549 and H1650, but did
not affect viability of the non-cancerous MRC5 cell line (Fig. 1F). This data suggested that CEA and
VEGFR are overexpressed in NSCLC cell lines and TcBab was able to
enhance the expression levels of both CEA and VEGFR in NSCLC
cells.
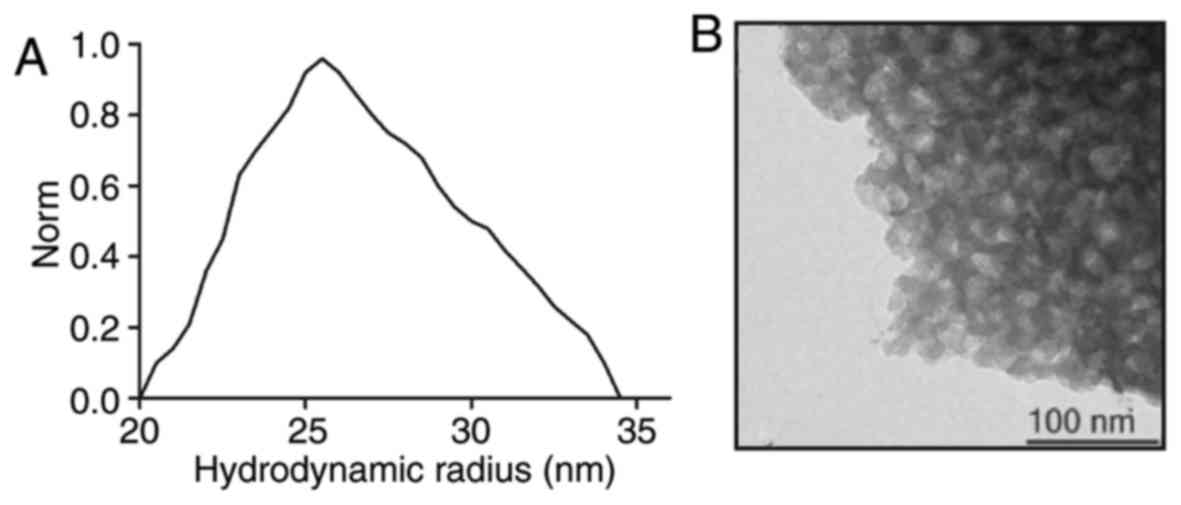
Characterization of TcBab
The hydrodynamic radius of the TcBab particles was
calculated by SEM analysis (33).
SEM revealed that a narrow particle distribution in the range from
20 to 35 nm was achieved (Fig. 2A).
TEM-images illustrated the association of pore density with TcBab
(Fig. 2B). These results indicated
that TcBab is stable.
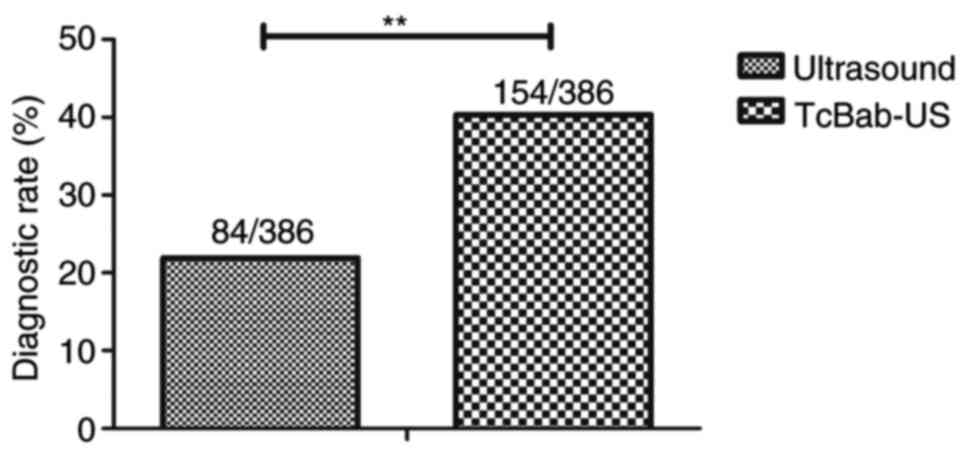
Efficacy of TcBab-US in early
diagnosis for patients with suspected NSCLC
The dose of targeting nanoparticles contrast agent
was identified as 30 mg/kg to achieve the optimum efficiency for
diagnosing patients with suspected NSCLC (Table II). Efficacy of TcBab-US for
patients with suspected NSCLC was investigated. As presented in
Fig. 3, clinical outcomes revealed
that TcBab-US diagnosed 154 suspected patients with NSCLC, whereas
ultrasound diagnosed 84 suspected patients with NSCLC out of a
total of 384 patients (P<0.01). No obvious adverse effects of
TcBab were observed in patients. These results suggested that TcBab
contributed to improved accuracy and sensitivity for diagnosing
patients with suspected NSCLC.
 | Table II.Confirmation of dosage of targeting
nanoparticles contrast agent for patients with non-small cell lung
cancer. |
Table II.
Confirmation of dosage of targeting
nanoparticles contrast agent for patients with non-small cell lung
cancer.
| Parameter | 5-15 mg/kg
(n=10) | 20-30 mg/kg
(n=14) | 35-45 mg/kg
(n=18) |
|---|
| Signal intensity
(HU) | 66.6±10.2 | 83.4±12.2 | 84.2±13.6 |
| Sensitivity
(%) | 63.5±11.8 | 84.5±10.4 | 84.6±12.1 |
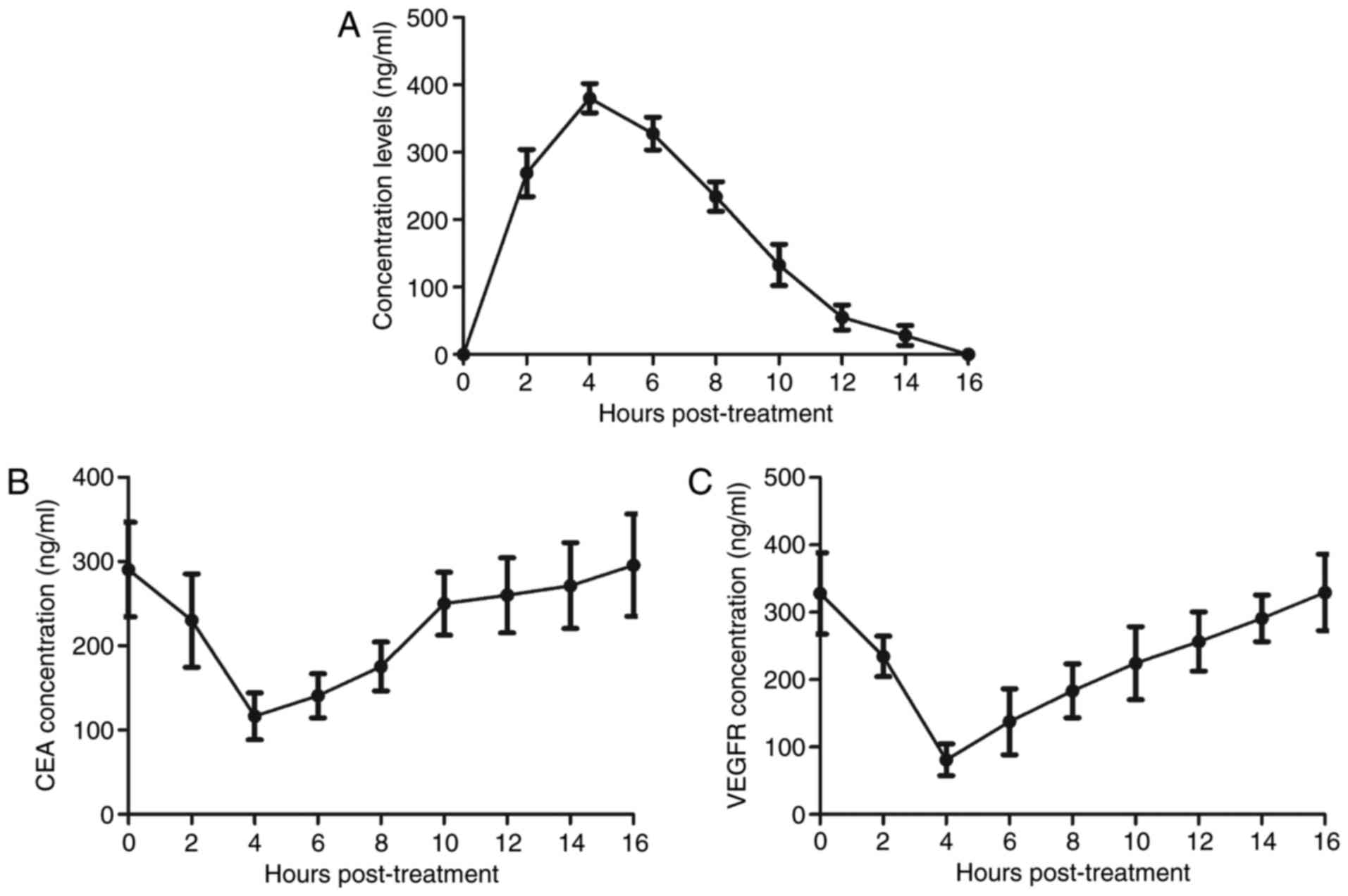
Pharmacodynamics of TcBab in plasma of
patients with suspected NSCLC
To analyze metabolism of TcBab, the pharmacodynamics
of TcBab in plasma in patients with suspected NSCLC were
investigated. As presented in Fig.
4A, plasma concentration levels of TcBab were increased within
4 h and were metabolized within 16 h. Results demonstrated that
plasma concentration levels of CEA and VEGFR were decreased within
4 h and recovered to normal levels within 16 h in patients with
suspected NSCLC (Fig. 4B and C).
This clinical data suggested that TcBab-US may be a potential
indicator for the diagnosis of early-stage patients with suspected
NSCLC.
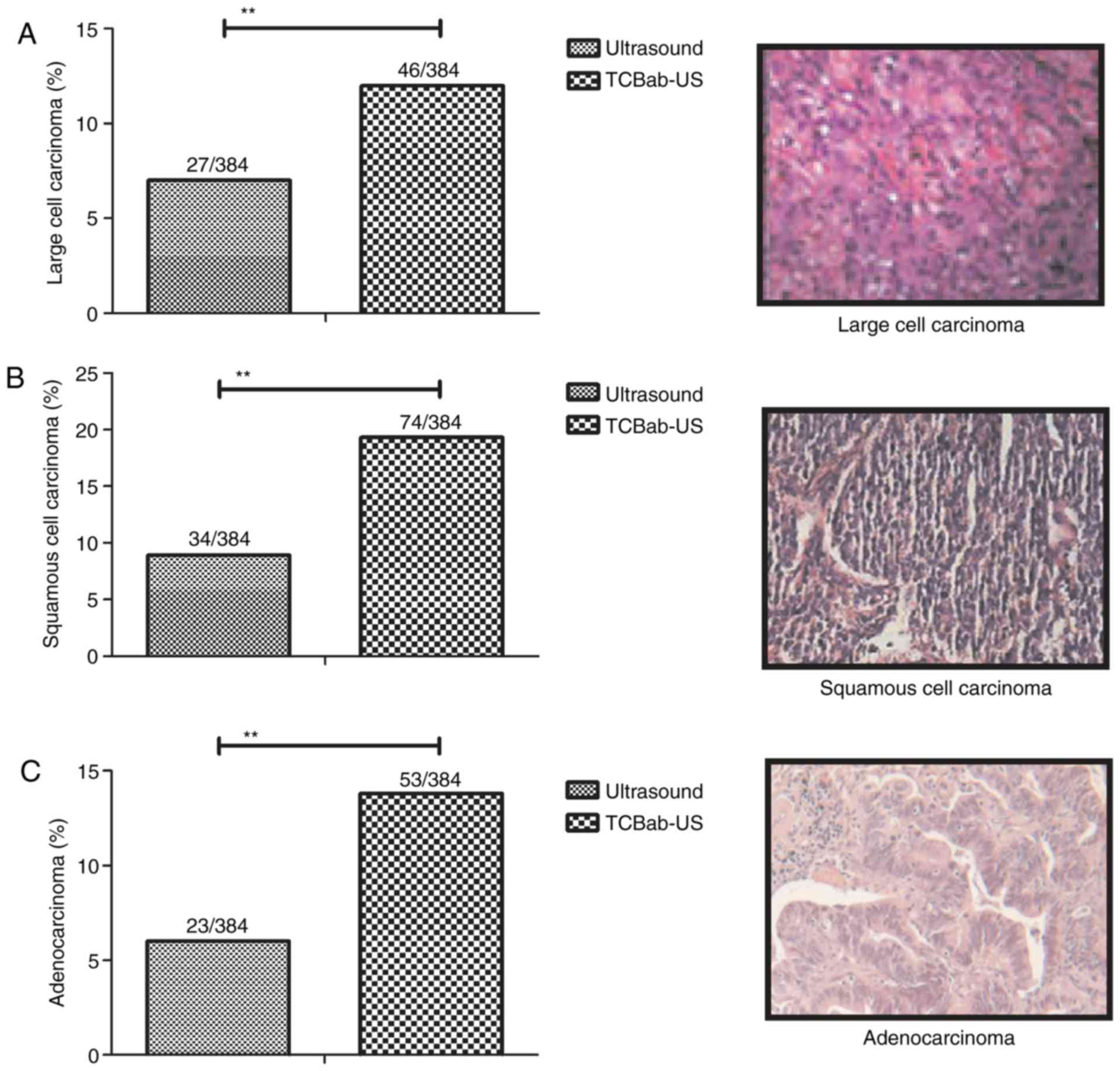
Histopathology confirms the accuracy
of TcBab-US-diagnosis in patients with NSCLC
The present study further confirmed patients with
suspected NSCLC via histopathology analysis. The representative
NSCLC tissues demonstrated that TcBab-US diagnosed 46 patients
suffering with large cell carcinoma out of a total of 384 patients,
and ultrasound diagnosed 27 patients (Fig. 5A). A total of 74 patients were
diagnosed with squamous cell carcinoma by TcBab-US, which was a
significantly greater number compared with single ultrasound
(34/384) diagnosis (Fig. 5B).
Additionally, TcBab diagnosed 53 out of 384 patients with suspected
NSCLC as having adenocarcinoma at early stage, compared with 23
patients in the ultrasound group (Fig.
5C). These data suggested that TcBab-US may be a reliable
diagnostic method in diagnosing early-stage patients with suspected
NSCLC.
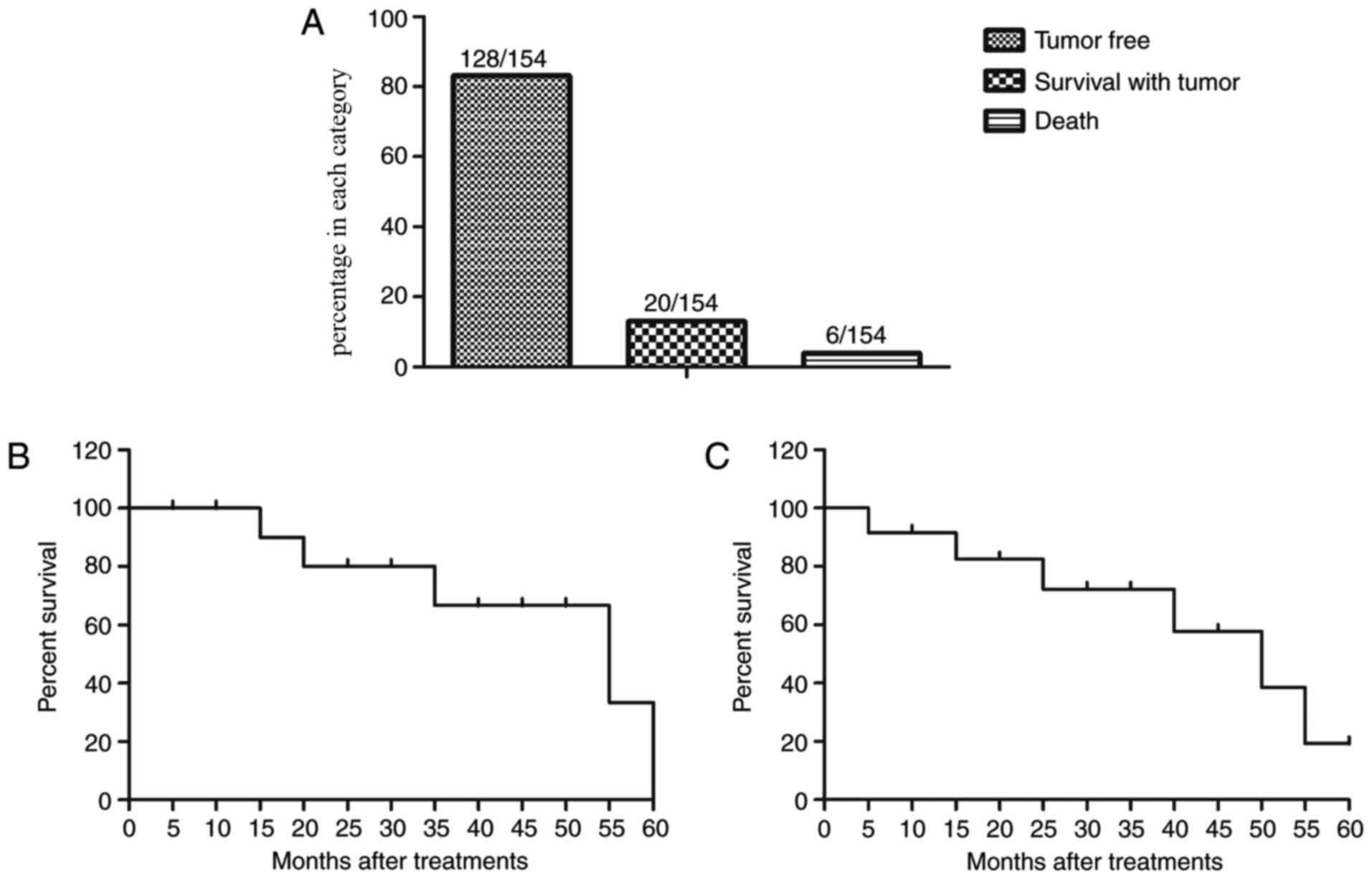
Survival rate of patients with NSCLC
diagnosed by TcBab-US
TcBab-US diagnosed early-stage NSCLC patients
received different anti-cancer treatments. A summary of the
anti-cancer treatments is presented in Table III. The results indicated that 128
patients were alive and tumor-free, 20 patients were still alive
with tumor, and 6 patients succumbed in this investigation in a
60-month follow-up (Fig. 6A). It was
also demonstrated that the median overall survival of NSCLC
patients diagnosed by TcBab-US was 54.2 months (Fig. 6B), which is significantly higher
compared with the mean survival of NSCLC patients who did not
receive TcBab as reported previously (P<0.05) (34). Notably, the median progression-free
survival was 44.8 months for patients with NSCLC (Fig. 6C). These data suggested that
early-stage diagnosis via TcBab-US prolongs median overall survival
in a 60-month follow-up for patients with NSCLC.
 | Table III.Treatment of patients with non-small
cell lung cancer diagnosed by
chistosan/Fe3O4-parceled bispecific
antibody-ultrasound. |
Table III.
Treatment of patients with non-small
cell lung cancer diagnosed by
chistosan/Fe3O4-parceled bispecific
antibody-ultrasound.
|
Characteristics | Male | Female |
|---|
| Total patients
(n) | 86 | 68 |
| Treatments (n) |
|
|
|
Radiotherapy | 10 | 10 |
|
Chemotherapy | 8 | 14 |
| Chinese
medicine | 8 | 10 |
|
Biological therapy | 12 | 8 |
|
Comprehensive therapy | 48 | 26 |
Discussion
NSCLC is one of the most prevalent malignant lung
tumors, which has become a major public health problem and the
leading cause of cancer-associated mortality worldwide (35). Data analysis has revealed that ~120
million new cases of lung cancer are diagnosed every year and that
the proportion is increasing as the tumors become more common in
younger patients (36). Notably,
>50% of patients diagnosed with NSCLC are at stage IIIB or IV
disease, which is not only difficult to treat, but also increases
pathologic material guiding systemic therapy (37). Therefore, a number of reports suggest
that early diagnosis is beneficial for the treatment of NSCLC and
may contribute to survival rate for patients in clinic (25,38).
Previous research has indicated that contrast-enhanced ultrasound
has been widely applied in NSCLC diagnosis (39). The present study investigated the
efficacy of a novel targeted ultrasound therapy that contained a
nano-scale ultrasound contrast agent for the diagnosis of
early-stage NSCLC patients. Findings demonstrated that TcBab
enhances expression of target surface antigens in NSCLC cells, and
also improves accuracy and sensitivity of ultrasound for patients
with suspected NSCLC, which prolongs median progression-free
survival and median overall survival in 60-month follow-up for
patients with NSCLC.
A previous report demonstrated that VEGFR is
overexpressed in NSCLC cell lines, which presents a potential
molecular target for the next generation of targeted therapies in
solid tumors (40). Zhang et
al (41) previously demonstrated
that a VEGFR-2 inhibitor may be regarded as an anti-cancer agent
for the treatment of thyroid cancer, glioblastoma multiforme and
NSCLC by inhibiting the activity of VEGFR. The present study
demonstrated that binding with VEGFR via nano-scale ultrasound
contrast agent TcBab enhanced ultrasonic signal feedback from lung
nodes.
CEA is one of the surface markers of NSCLC cells,
which is associated with survival in patients with stage IA-B NSCLC
(42). Cedrés et al (43) previously suggested that serum tumor
marker CEA is associated with worse prognosis in advanced NSCLC. In
addition, Fiala et al (44)
have indicated that CEA has a predictive role in patients with
advanced-stage NSCLC treated with erlotinib. Furthermore, the
diagnostic value of CEA for differentiation of early-stage NSCLC
from benign lung disease has been investigated and discussed
(45). The present study concurred
with a previous report (46) and
demonstrated that binding with CEA via nano-scale ultrasound
contrast agent TcBab enhanced ultrasonic signal feedback from lung
nidus.
At present, contrast medium has improved the
diagnostic capability of ultrasound for a large number of human
diseases (47,48). de Ziegler (49) previously demonstrated that contrast
ultrasound enhances the sensitivity and specificity assessment for
uterine pathologies in the clinic. Wang et al (50) recently indicated that microflow
imaging of contrast-enhanced ultrasound may be used to evaluate
neovascularization in peripheral lung cancer. In the p-resent
study, contrast target nano-scale ultrasound agent not only
improved the resolution of ultrasound, but also improved the
diagnostic sensitivity of ultrasound in the diagnosis of patients
with early-stage NSCLC. Notably, contrast-enhanced target contrast
agent ultrasound combined with ultrasound possesses clinical
guidance value for the assessment of patients with early-stage
NSCLC (51), which may have
important clinical implications in NSCLC diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and CX designed the study. XZ performed the
experiments and analysed the data.
Ethics approval and consent to
participate
All patients were required to provide written
informed consent prior to their inclusion. The study was approved
by the Ethics Committee of Huaihe Hospital of Henan University
(Kiafeng, China).
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ren Z, Zhou S, Liu Z and Xu S: Randomized
controlled trials of induction treatment and surgery versus
combined chemotherapy and radiotherapy in stages IIIA-N2 NSCLC: A
systematic review and meta-analysis. J Thorac Dis. 7:1414–1422.
2015.PubMed/NCBI
|
|
2
|
Tsim S, O'Dowd CA, Milroy R and Davidson
S: Staging of non-small cell lung cancer (NSCLC): A review. Respir
Med. 104:1767–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Xie Y and Xian L: Breast cancer
susceptibility gene 1 (BRCA1) predict clinical outcome in platinum-
and toxal-based chemotherapy in non-small-cell lung cancer (NSCLC)
patients: A system review and meta-analysis. J Exp Clin Cancer Res.
32:152013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Detterbeck FC, Chansky K, Groome P,
Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M
and Rami-Porta R; IASLC Staging and Prognostic Factors Committee,
Advisory Boards, and Participating Institutions: The IASLC lung
cancer staging project: Methodology and validation used in the
development of proposals for revision of the stage classification
of NSCLC in the forthcoming (Eighth) edition of the TNM
classification of lung cancer. J Thorac Oncol. 11:1433–1446. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santiago A, Barczyk S, Jelen U,
Engenhart-Cabillic R and Wittig A: Challenges in radiobiological
modeling: can we decide between LQ and LQ-L models based on
reviewed clinical NSCLC treatment outcome data? Radiat Oncol.
11:672016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gelsomino F, Ambrosini V, Melotti B,
Sperandi F and Ardizzoni A: Pitfalls in oncology: Osteoblastic
response mimicking bone progression during ceritinib treatment in
ALK-rearranged NSCLC. J Thorac Oncol. 11:e99–e101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bronte G, Passiglia F, Galvano A and Russo
A: Anti-angiogenic drugs for second-line treatment of NSCLC
patients: Just new pawns on the chessboard? Expert Opin Biol Ther.
16:1–5. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie FJ, Lu HY, Zheng QQ, Qin J, Gao Y,
Zhang YP, Hu X and Mao WM: The clinical pathological
characteristics and prognosis of FGFR1 gene amplification in
non-small-cell lung cancer: A meta-analysis. Onco Targets Ther.
9:171–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moro-Sibilot D, Smit E, de Castro Carpeño
J, Lesniewski-Kmak K, Aerts JG, Villatoro R, Kraaij K, Nacerddine
K, Dyachkova Y, Smith KT, et al: Non-small cell lung cancer
patients with brain metastases treated with first-line
platinum-doublet chemotherapy: Analysis from the European FRAME
study. Lung Cancer. 90:427–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim SH, Sun JM, Lee SH, Ahn JS, Park K and
Ahn MJ: Pembrolizumab for the treatment of non-small cell lung
cancer. Expert Opin Biol Ther. 16:397–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calabuig-Fariñas S, Lewintre EJ,
Mayo-De-Las-Casas C, Cordellat AB, Molina-Vila MA, Rosell R and
Camps C: 149P: The role of CTCs and cfDNA for diagnosis and
monitoring of EGFR mutations in advanced NSCLC patients. J Thorac
Oncol. 11(4 Suppl): S1232016. View Article : Google Scholar
|
|
12
|
Mascalchi M, Falchini M, Maddau C,
Salvianti F, Nistri M, Bertelli E, Sali L, Zuccherelli S, Vella A,
Matucci M, et al: Prevalence and number of circulating tumour cells
and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin
Oncol. 142:195–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koh MS, Tee A, Wong P, Antippa P and
Irving LB: Advances in lung cancer diagnosis and staging:
endobronchial ultrasound. Intern Med J. 38:85–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuo CH, Lin SM, Chen HC, Chou CL, Yu CT
and Kuo HP: Diagnosis of peripheral lung cancer with three echoic
features via endobronchial ultrasound. Chest. 132:922–929. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ernst A, Feller-Kopman D and Herth FJ:
Endobronchial ultrasound in the diagnosis and staging of lung
cancer and other thoracic tumors. Semin Thorac Cardiovasc Surg.
19:201–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loria F, Loria G, Basile S, Crea G,
Randazzo D and Frosina L: Contrast-enhanced ultrasound of
hepatocellular carcinoma: correlation between enhancement pattern
and cellular differentiation on histopathlogy. Updates Surg.
64:247–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Jiang Y, Dai Q, Zhu Q, Wang L and
Lu J: Peripheral enhancement of breast cancers on contrast-enhanced
ultrasound: Correlation with microvessel density and vascular
endothelial growth factor expression. Ultrasound Med Biol.
40:293–299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duguay S, Wagner JM, Zheng W, Ling J, Zhao
LC, Allen KS, North JC and Deb SJ: Ultrasound-guided needle biopsy
of neck lymph nodes in patients with suspected lung cancer: Are the
specimens sufficient for complete pathologic evaluation to guide
patient management? Ultrasound Q. 33:133–138. 2016. View Article : Google Scholar
|
|
19
|
Castro-Pocas FM, Araüjo TP, Ferreira ML
and Saraiva MM: The role of endoscopic ultrasound in a case of lung
cancer with jaundice. Endosc Ultrasound. Nov 8. 2016, (Epub ahead
of print). PubMed/NCBI
|
|
20
|
Loriot Y, Mordant P, Dorvault N, de la
motte Rouge T, Bourhis J, Soria JC and Deutsch E: BMS-690514, a
VEGFR and EGFR tyrosine kinase inhibitor, shows anti-tumoural
activity on non-small-cell lung cancer xenografts and induces
sequence-dependent synergistic effect with radiation. Br J Cancer.
103:347–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnem T, Al-Shibli K, Al-Saad S,
Delghandi MP, Busund LT and Bremnes RM: VEGF-A and VEGFR-3
correlate with nodal status in operable non-small cell lung cancer:
Inverse correlation between expression in tumor and stromal cels.
Lung Cancer. 63:277–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szturmowicz M, Rudzinski P, Kacprzak A,
Langfort R, Bestry I, Broniarek-Samson B and Orłowski T: Prognostic
value of serum C-reactive protein (CRP) and cytokeratin 19
fragments (Cyfra 21-1) but not carcinoembryonic antigen (CEA) in
surgically treated patients with non-small cell lung cancer.
Pneumonol Alergol Pol. 82:422–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai H, Liu J, Liang L, Ban C, Jiang J, Liu
Y, Ye Q and Wang C: Increased lung cancer risk in patients with
interstitial lung disease and elevated CEA and CA125 serum tumour
markers. Respirology. 19:707–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xing W, Zhigang W, Bing H, Haitao R, Pan
L, Chuanshan X, Yuanyi Z and Ao L: Targeting an ultrasound contrast
agent to folate receptors on ovarian cancer cells: Feasibility
research for ultrasonic molecular imaging of tumor cels. J
Ultrasound Med. 29:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vansteenkiste J, de Ruysscher D, Eberhardt
WE, Lim E, Senan S, Felip E and Peters S; ESMO Guidelines Working
Group: Early and locally advanced non-small-cell lung cancer
(NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 24(Suppl 6): vi89–vi98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schinkel AF, Kaspar M and Staub D:
Contrast-enhanced ultrasound: Clinical applications in patients
with atherosclerosis. Int J Cardiovasc Imaging. 32:35–48. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
29
|
Sutiman N, Tan SW, Tan EH, Lim WT,
Kanesvaran R, Ng QS, Jain A, Ang MK, Tan WL, Toh CK and Chowbay B:
EGFR mutation subtypes influence survival outcomes following
first-line gefitinib therapy in advanced Asian NSCLC patients. J
Thorac Oncol. 12:529–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou JG, Tian X, Wang X, Tian JH, Wang Y,
Wang F, Zhang Y and Ma H: Treatment on advanced NSCLC:
Platinum-based chemotherapy plus erlotinib or platinum-based
chemotherapy alone? A systematic review and meta-analysis of
randomised controlled trials. Med Oncol. 32:4712015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angelov KG, Vasileva MB, Grozdev KS,
Sokolov MB and Todorov G: Clinical and pathological
characteristics, and prognostic factors for gastric cancer survival
in 155 patients in Bulgaria. Hepatogastroenterology. 61:2421–2444.
2014.PubMed/NCBI
|
|
32
|
Granja RH, Salerno AG, de Lima AC,
Montalvo C, Reche KV, Giannotti FM and Wanschel AC: Liquid
chromatography/tandem mass spectrometry method to determine
boldenone in bovine liver tissues. J AOAC Int. 97:1476–1480.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong Y, Wang P, Liu S, Zhao G and Peng Y:
SEM analysis of the interfacial transition zone between
cement-glass powder paste and aggregate of mortar under microwave
curing. Materials (Basel). 9:pii: E7332016. View Article : Google Scholar
|
|
34
|
Gainor JF, Tan DS, de Pas T, Solomon BJ,
Ahmad A, Lazzari C, de Marinis F, Spitaleri G, Schultz K, Friboulet
L, et al: Progression-free and overall survival in ALK-Positive
NSCLC patients treated with sequential crizotinib and ceritinib.
Clin Cancer Res. 21:2745–2752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Magnuson WJ, Yeung JT, Guillod PD,
Gettinger SN, Yu JB and Chiang VL: Impact of deferring radiation
therapy in patients with epidermal growth factor receptor-mutant
non-small cell lung cancer who develop brain metastases. Int J
Radiat Oncol Biol Phys. 95:673–679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thill PG, Goswami P, Berchem G and Domon
B: Lung cancer statistics in Luxembourg from 1981 to 2008. Bull Soc
Sci Med Grand Duche Luxemb. 2:43–55. 2011.
|
|
37
|
Reck M, Popat S, Reinmuth N, de Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
25(Suppl 3): iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang S, Nan Y, Tian Y, Zhang W, Zhou B, Bu
L, Huo S, Chen G, Yu J and Zheng S: Study of distinct protein
profiles for early diagnosis of NSCLC using LCM and SELDI-TOF-MS.
Med Oncol. 25:380–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vázquez-Sequeiros E,
González-Panizo-Tamargo F, Barturen Á, Calderón Á, Esteban JM,
Fernández-Esparrach G, Gimeno-García A, Ginés A, Lariño J,
Pérez-Carreras M, et al: The role of endoscopic ultrasound guided
fine needle aspiration (EUS-FNA) in non small cell lung cancer
(NSCLC) patients: SEED-SEPD-AEG joint guideline. Rev Esp Enferm
Dig. 105:215–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pennell NA and Lynch TJ Jr: Combined
inhibition of the VEGFR and EGFR signaling pathways in the
treatment of NSCLC. Oncologist. 14:399–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Guessous F, Kofman A, Schiff D
and Abounader R: XL-184, a MET, VEGFR-2 and RET kinase inhibitor
for the treatment of thyroid cancer, glioblastoma multiforme and
NSCLC. IDrugs. 13:112–121. 2010.PubMed/NCBI
|
|
42
|
Ishikawa H, Satoh H, Yamashita YT, Ohtsuka
M and Sekizawa K: CEA and survival in patients with stage IA-B
NSCLC. Thorac Cardiovasc Surg. 50:2532002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cedrés S, Nuñez I, Longo M, Martinez P,
Checa E, Torrejón D and Felip E: Serum tumor markers CEA,
CYFRA21-1, and CA-125 are associated with worse prognosis in
advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer.
12:172–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiala O, Pesek M, Finek J, Benesova L,
Minarik M, Bortlicek Z and Topolcan O: Predictive role of CEA and
CYFRA 21-1 in patients with advanced-stage NSCLC treated with
erlotinib. Anticancer Res. 34:3205–3210. 2014.PubMed/NCBI
|
|
45
|
Chen F, Wang XY, Han XH, Wang H and Qi J:
Diagnostic value of Cyfra21-1, SCC and CEA for differentiation of
early-stage NSCLC from benign lung disease. Int J Clin Exp Med.
8:11295–11300. 2015.PubMed/NCBI
|
|
46
|
Kallmayer M, Tsantilas P, Zieger C, Ahmed
A, Söllner H, Zimmermann A and Eckstein H: Ultrasound surveillance
after CAS and CEA: What's the evidence? J Cardiovasc Surg (Torino).
55(2 Suppl 1): S33–S41. 2014.
|
|
47
|
Ignee A, Schuessler G, Cui XW and Dietrich
CF: Intracavitary contrast medium ultrasound-different
applications, a review of the literature ad future prospects.
Ultraschall Med. 34:504–525; quiz 526–528. 2013.(In German).
PubMed/NCBI
|
|
48
|
Lee SH, Kim JM, Chan V, Kim HJ and Kim HI:
Ultrasound-guided cervical periradicular steroid injection for
cervical radicular pain: Relevance of spread pattern and degree of
penetration of contrast medium. Pain Med. 14:5–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Ziegler D: Contrast ultrasound: A
simple-to-use phase-shifting medium offers saline infusion
sonography-like images. Fertil Steril. 92:369–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang S, Yang W, Fu JJ, Sun Y, Zhang H, Bai
J, Chen MH and Yan K: Microflow imaging of contrast-enhanced
ultrasound for evaluation of neovascularization in peripheral lung
cancer. Medicine (Baltimore). 95:e43612016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chopra A: 125I-Labeled
heparin-binding peptides that target heparan sulfate proteoglycans
for the in vivo imaging of peripheral amyloidosis. Molecular
Imaging and Contrast Agent Database (MICAD) [Internet]. National
Center for Biotechnology Information (US); Bethesda, MD; 2011
|